Editor:
This report was approved by the Ethics Committee at IRCCS Ospedale San Raffaele, Milan, Italy. Among the 818 consecutive patients admitted to the emergency department between March 1 and April 30, 2020, with a positive nasopharyngeal swab test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), 16 (1.9%) experienced at least 1 severe arterial bleeding episode, defined as any imaging-proven, clinically overt sign of arterial hemorrhage coupled with a decrease in hemoglobin count ≥ 3 mg/dL and requiring intervention. A single patient (1/16; 6.2%) was excluded owing to an iatrogenic hepatic injury, bringing the incidence of spontaneous hemorrhage to 1.8% (15/818). The median time interval between onset of 2019 novel coronavirus disease (COVID-19) symptoms and occurrence of hemorrhage was 23 days (interquartile range: 19–35 d); all patients had severe, radiologically proven interstitial pneumonia and required ventilator support during their hospital stay. Thirteen of 15 patients (86.7%) were receiving prophylactic antithrombotic treatment (low-molecular-weight heparin) according to current internal guidelines; at the time of bleeding onset, no patient was above the therapeutic range of anticoagulation or had deep vein thrombosis and/or pulmonary embolism.
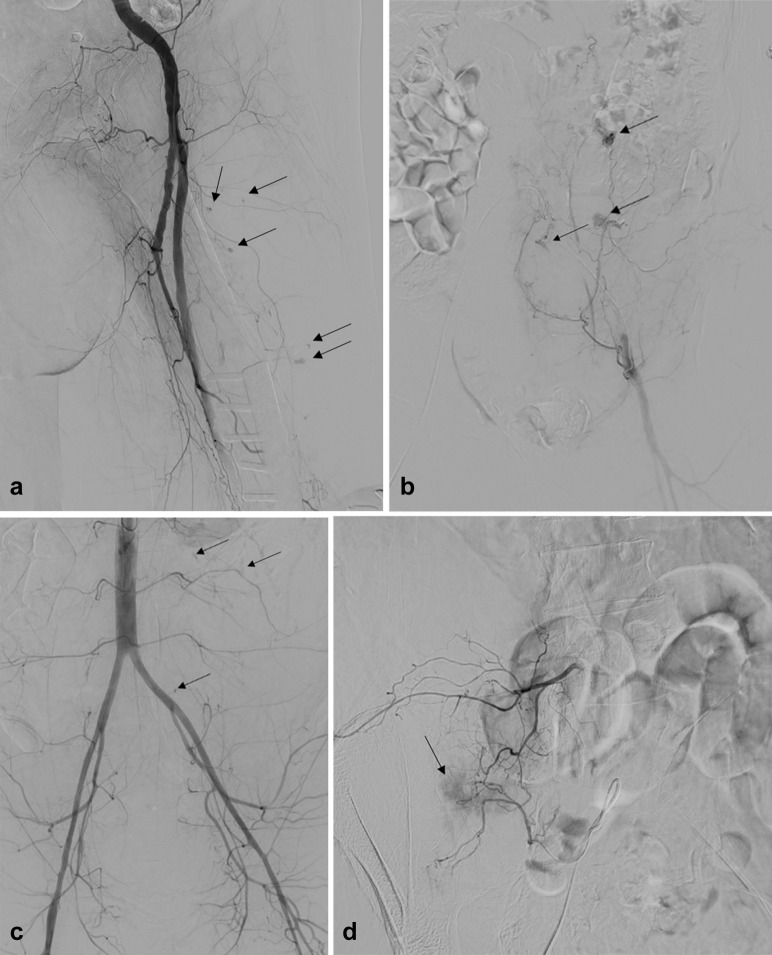
Hemorrhage was initially evaluated with contrast-enhanced, multiphase computed tomography and then treated with an interventional radiology procedure. Diagnostic angiography showed the bleeding site in all cases (lumbar/ileolumbar [8/15; 53.3%], inferior epigastric [2/15; 13.3%], inferior gluteal [1/15; 6.7%], lateral circumflex femoral [1/15; 6.7%], sigmoidal [1/15; 6.7%] right bronchial [1/15; 6.7%], and internal thoracic artery [1/15; 6.7%]). The typical angiographic pattern (found in 12 of 15 patients; 80%) consisted of multiple, tiny bleeding foci affecting distal vascular territories (Fig a–d ). Considering this peculiar bleeding pattern and the critical condition of most patients, embolization with polyvinyl alcohol particles of the entire arterial segment accounting for the hemorrhage was usually performed. Technical and complete clinical success was achieved in all patients; no procedure-related complications were recorded (1).
Figure.
Typical angiographic findings in patients with COVID-19 experiencing major spontaneous hemorrhage. Diagnostic angiography typically demonstrated multiple tiny foci of arterial bleeding (black arrows) affecting distal vascular territories of (a) left lateral circumflex femoral artery, (b) left inferior epigastric artery, (c) third and fourth left lumbar arteries, and (d) right ileolumbar arteries. In each case, successful embolization of the entire arterial segment accounting for the bleeding was achieved with polyvinyl alcohol particles of varying size: 250–355 μm in panels (a) and (b) and 355–500 μm in panels (c) and (d).
The origin of severe hemorrhage in patients with COVID-19 is unclear. Prophylactic antithrombotic treatment has been established as a well-known risk factor; however, the reported incidence of major spontaneous hemorrhage in general admission patients receiving low-molecular-weight heparin at prophylactic dosage is < 1% (2), below the disease-specific incidence observed in the present population (1.8%). A possible explanation could lie in the pathophysiology of SARS-CoV-2 infection, which is characterized by an increase of proinflammatory cytokines in serum (systemic cytokine storm), directly correlated with both disease severity and subtle coagulation disorders. Furthermore, widespread endothelial cell damage has been hypothesized to occur (3). Functional implications of this pathogenic mechanism include diffuse microvascular damage with both a substantial component of microvascular thrombosis [microCLOTS hypothesis (3)] and imbalances in platelet recruitment. The latter could then result in multiple bleeding foci typically affecting distal microcirculation, as suggested by observations in the present population and confirmed by pathologic findings (4), and occurring late in the disease course (median time to bleeding onset 23 d).
In conclusion, major spontaneous hemorrhage represents a quite uncommon, but dramatic complication of SARS-CoV-2 infection, possibly representing the other, less noted side of disease-specific coagulation disorders. Failure to acknowledge such a risk could significantly worsen the prognosis of patients with COVID-19.
Footnotes
D.P.’s E-mail: palumbo.diego@hsr.it
None of the authors have identified a conflict of interest.
References
- 1.Angle J.F., Siddiqi N.H., Wallace M.J. Quality Improvement Guidelines for Percutaneous Transcatheter Embolization: Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2010;21:1479–1486. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd N.S., Douketis J.D., Lim W., Crowther M.A. Anticoagulant prophylaxis to prevent asymptomatic deep vein thrombosis in hospitalized medical patients: a systematic review and meta-analysis. J Thromb Haemost. 2008;6:405–414. doi: 10.1111/j.1538-7836.2007.02847.x. [DOI] [PubMed] [Google Scholar]
- 3.Ciceri F., Beretta L., Scandroglio A.M. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc April. 2020;15 doi: 10.51893/2020.2.pov2. Published online. PMID: 32294809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buja L.M., Wolf D.A., Zhao B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]