Abstract
Before we have reported lamprey PHB2 could enhance the cellular oxidative-stressed tolerance, here the aim was to explore its mechanisms. We used flow cytometry analysis to identify a Lampetra morii homologue of PHB2 (Lm-PHB2) that could significantly decrease the levels of ROS generation in HEK293T cells. According to confocal microscopy observations, Lm-PHB2 contributed to maintain the mitochondrial morphology of HEK293T cells, and then both cellular nuclear location and translocation from the nucleus to mitochondria of Lm-PHB2 were also examined in HEK293T cells under oxidative stress. We also examined the expressions and locations of various Lm-PHB2 deletion mutants and the amino acid mutant by confocal microscopy and the results showed that the translocation of Lm-PHB2 into mitochondria was dependent on the Lm-PHB21-50aa region and the 17th, 48th and 57th three arginines (R) of N-terminal were very critical. In addition, the analyses of QRT-PCR and Western blot demonstrated that Lm-PHB2 increased the expression levels of OPA1 and HAX1 in HEK293T cells treated with H2O2. The analyses of immunofluorescence and immunoprecipitation showed that Lm-PHB2 could interact with OPA1 and HAX1, respectively. The above mentioned results indicate that Lm-PHB2 could assist OPA1 and HAX1 to maintain mitochondrial morphology and decrease ROS levels by the translocation from the nucleus to mitochondria under oxidative stress.
Keywords: Lamprey, PHB2, Translocation, Mitochondria, Oxidative stress
Abbreviations: PHB2, prohibitin 2; ROS, reactive oxygen species; HEK293T, Human embryonic kidney cells 293T; QRT-PCR, quantitative real-time polymerase chain reaction; OPA1, optic atrophy associated protein 1; HAX1, HCLS1-associated protein X-1; HCLS1, hematopoietic lineage cell-specific protein; IF, immunofluorescence; IP, immunoprecipitation
Highlights
-
•
Lamprey, as one of the most primitive jawless vertebrate, the PHB2 protein of lamprey can translocalize from the nucleus to mitochondria in HEK293T cell lines under oxidative stress.
-
•
Under oxidative stress lamprey PHB2 protein maintains mitochondrial stability by interacting with OPA1 and HAX1 in mitochondria.
1. Introduction
In humans, oxidative stress plays an important role in many diseases, including cardiovascular diseases, neurodegenerative diseases (such as Alzheimer's and Parkinson's [1]), autoimmune diseases (such as systemic lupus erythematosus and cancer [2,3]) and probably novel coronavirus-infected pneumonia (COVID-19) that an ongoing new challenge we are facing. Furthermore, numerous studies have demonstrated the extensive involvement of oxidative stress in ageing itself [4]. Oxidative stress represents an imbalance between toxic reactive oxygen species (ROS) and antioxidant systems, and high levels of ROS induce inflammation and cytotoxicity, which damages proteins, lipids, and DNA and subsequently disrupts mitochondrial structure and functions, ultimately resulting in cell death [[5], [6], [7]]. At present, most experts think it is related to the sudden onset of systemic inflammatory response syndrome in COVID-19 severe patients [8].
Prohibitins (PHBs) assemble into large ring complexes in the mitochondrial inner membrane to maintain mitochondrial dynamics and morphogenesis, associated with multiple neurodegenerative disease [9,10], autoimmune diseases [11], cancer [12,13] and aging [10,14,15]. Prohibitin 2 (PHB2), one of two homologous prohibitin proteins, comprises a conserved and ubiquitously expressed protein family with prohibitin 1 (PHB1) [16]. Recently, PHB2, a pleiotropic protein reportedly essential for nucleus-mitochondria communications, cell proliferation and development in eukaryotes [17,18], was reported to play important roles in enhancing oxidative stress tolerance in mammalian cells [19,20]. Some reports also suggested other functions of PHB2, such as transcriptional regulation in the nucleus and cell signalling in the plasma membrane [21,22]. The C-terminal domain is sufficient for the ERα-dependent nuclear translocation of human PHB2 in yeast and C. elegans, and PHB proteins have been reported to possess a non-cleavable leader peptide at their N termini [16,21]. In contrast to the extensive studies on mammalian PHB2, few studies on the PHB2 from lamprey as one of original jawless vertebrata have been performed. While we first demonstrated that the PHB2 homologue from the Chinese northeast lamprey (Lampetra morii, Lm-PHB2) enhances the oxidative stress tolerance of Chang (CHL) cells in 2015 [20], the detailed mechanisms have not been discussed in any previous studies. The present study aimed at analyzing the translocation of Lm-PHB2 and its effect on mitochondrial morphology and cellular ROS levels under oxidative stress. This was conducted as part of the effort to provide a new clue for treating diseases induced by oxidative stress.
2. Materials and methods
2.1. Plasmid construction and transfection
According to a previous study reported by Li et al. the Lm-PHB2 coding region, flanked by the EcoRI and BamHI restriction sites, was amplified and then subcloned into the eukaryotic expression vector pEGFP-N1. The recombinant plasmid was extracted using the TaKaRa MiniBEST Plasmid Purification Kit Ver. 4.0 (TaKaRa, China), and endotoxins were removed using Endotoxin-Be-Gone (Sangon Biotech, China). Transfection was performed on cells that were at least 70% confluent using the Translipid Transfection Reagent (TransGen Biotech, China) according to the manufacturer's instructions. After 24 h, the cells were washed with PBS, and the medium was replaced with fresh, normal growth medium with or without 0.5 mmol/L (mM) H2O2 for 3 h for subsequent quantitative real-time PCR (QRT-PCR) and Western blot assays.
2.2. Cloning, expression and purification of the lamprey PHB2 protein
According to a previous study reported by Li et al. the Lm-PHB2 coding sequence, flanked by the EcoRI and HindIII restriction sites, was amplified and subcloned into pET32a to produce a His-tagged fusion protein. Recombinant Lm-PHB2 (rLm-PHB2) was expressed in E. coli. Rosetta blue cells and induced with 1 mM IPTG at 30 °C for 5 h. The soluble supernatant was collected and applied to a Ni-NTA His-Bind resin column (Sangon Biotech, China) equilibrated with binding buffer (20 mM Tris-HCl (pH 8.0), 500 mM NaCl, 20 mM imidazole). After washing the column with wash buffer (20 mM Tris-HCl (pH 8.0), 500 mM NaCl, 30 mM imidazole), the recombinant protein rLm-PHB2 was collected in elution buffer comprising 20 mM Tris-HCl (pH 8.0), 500 mM NaCl, and 80 mM imidazole. The concentration of rLm-PHB2 was measured using a bicinchoninic acid (BCA) protein assay kit (Beyotime, China). The purified rLm-PHB2 protein was analysed by 12% SDS-PAGE and stored at −80 °C.
2.3. Cell culture
HEK293T cells line was purchased from ATCC. The HEK293T cells were cultured in dulbecco's modified eagle's medium (DMEM) (GIBCO, USA) supplemented with 10% (vol/vol) fetal bovine serum (GIBCO, USA) in a humidified incubator with 5% CO2 at 37 °C.
2.4. QRT-PCR
HEK293T cells were transfected with the pEGFP-N1 or pEGFP-N1-Lm-PHB2 plasmids for 24 h and total RNA was then isolated from the cells using RNAiso Plus (Takara, China). The RNA was subjected to reverse transcription using the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, China). QRT-PCR was performed with the SYBR Premix ExTaq™ II Kit (Takara, China) according to the manufacturer's protocol using GAPDH as an internal control. Primers used for QRT-PCR are listed in Table 1 .
Table 1.
Primers used for QRT-PCR.
| Name | Sequence |
|---|---|
| GAPDH (upstream) | 5′-CAGGAGGCATTGCTGATGAT-3′ |
| GAPDH (downstream) | 5′-GAAGGCTGGGGCTCATTT-3′ |
| OPA1(upstream) | 5′-TGGGTCCGATTCTTCCAGTA-3′ |
| OPA1(downstream) | 5′-TGAGGGTTATTCAACACAATGC-3′ |
| HAX1 (upstream) | 5′-GAACCAACGTCCCAGGAATA-3′ |
| HAX1 (downstream) | 5′-ACAGTAACCCGACACGAAGC-3′ |
2.5. Western blot
HEK293T cells were seeded into 6-well plates and incubated at 37 °C for 24 h and subsequently transfected with the pEGFP-N1 or pEGFP-N1-Lm-PHB2 plasmids at 37 °C for an additional 24 h. After centrifugation, the cell pellets were collected and lysed with cell lysis buffer (Beyotime, China) containing phenylmethanesulfonyl fluoride (PMSF). The concentrations of the total proteins were also measured by the BCA kit (Beyotime, China) with bovine serum albumin (BSA) as the standard. The protein samples were electrophoresed by 12% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Germany). After blocking with 5% non-fat dairy milk (Yili, China) in Tris-buffered saline tween (TBST) buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.05% Tween-20) for 2 h, the membranes were incubated with the following primary antibodies at room temperature for 5 h: anti-OPA1 (optic atrophy associated protein 1) (Proteintech, China; 1:1000), anti-HAX1(HCLS1-associated protein X-1) (Proteintech, China; 1:1000) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Bioworld, China; 1:2000). The membranes were washed with TBST buffer five times to remove non-specifically bound proteins and then incubated with the appropriate peroxidase-conjugated goat anti-rabbit secondary antibody (Bioworld, China) at a ratio of 1:5000 at room temperature for 1 h. After washing with TBST buffer five times, the membranes were visualized with the BeyoECL Plus Detection Kit (Beyotime, China).
2.6. ROS measurement
2′7′-dichlorofluorescein diacetate (DCF-DA) was used to measure the ROS levels. HEK293T cells (1 × 105 cells/well in 6-well plates) were treated with 10 and 100 μg/mL of extract, 1 and 10 μg/mL of cyanidin-3-O-galactoside, trolox (positive control, 50 μmol/L (μM)) and 2 mM glutamate for 8 h. Then, the cells were washed with phosphate buffered solution (PBS) and incubated in 10 μM DCF-DA in DMEM without phenol red for 30 min. Cells were washed twice with PBS. Fluorescence was measured at an excitation wavelength of 490 nm and an emission wavelength of 525 nm.
2.7. Confocal microscopy
The eukaryotic expression vector pEGFP-N1 expresses green fluorescent protein (GFP). First, we transfected the recombinant pEGFP-N1-Lm-PHB2 plasmid into HEK293T cells and examined the cellular localization of Lm-PHB2 using confocal microscopy (Zeiss LSM800, Germany); cells transfected with the empty vector were used as a control. Subcellular organelles and Lm-PHB2 with pEGFP were visualized by Hoechst 33258 (Beyotime, China; nuclear DNA, blue, 369 nm and 460 nm for absorbance and emission, respectively), MitoTracker Red (Invitrogen, USA; mitochondria, red, 578 and 599 nm for absorbance and emission, respectively), and GFP (Lm-PHB2, green, 495 nm and 519 nm for absorbance and emission, respectively). The cells were washed twice with PBS and then re-examined in response to oxidative stress (0.5 mM H2O2 treatment for 1 h); unstimulated cells transfected with the recombinant plasmid were used as a control.
2.8. Co-immunoprecipitation
The pEGFP-N1 and pEGFP-N1-Lm-PHB2 plasmids were transiently transfected into HEK293T cells and incubated at 37 °C for 24 h. After centrifugation, the cell pellets were collected and lysed with cell lysis buffer (Beyotime, China) containing PMSF. Protein G agarose beads (18 μL per reaction) were then added, and 700 μL of PBS was used to wash the agarose. The samples were centrifuged at low speed and washed four times. Next, 0.7 mL of the cell lysates and 1 μg of the purified antibody were added to new microcentrifuges, and the samples were incubated for 1 h on a flat platform. After incubation, the cell lysates were transferred to the washed Protein G beads in the spin columns and incubated overnight at 4 °C on a flat platform. Each column was inserted into a supplied 2-mL microcentrifuge tube and spun at 12,000 g for 30 s at 4 °C. The beads in the spin column were washed with 700 μL of 1 × IP buffer six times and then with 0.1 × IP buffer. The spin columns were centrifuged at 12,000 g for 30 s. Next, 40–50 μL of 1 × loading buffer was added to the beads and mixed gently, and the samples were then heated to 95 °C for 5 min. The columns were inserted into new microcentrifuge tubes and centrifuged at 12,000 g for 30 s. The eluted immunoprecipitants were then subjected to SDS-PAGE.
2.9. Statistical analysis
The data shown represent the mean ± SD of at least three independent experiments, performed in triplicate. Student's t-test was used to analyze the differences between PBS and rLm-PHB2 treated groups. A statistical significance was shown as follows: *P < 0.05, **P < 0.01 and ***P < 0.001.
3. Results
3.1. Recombinant Lm-PHB2 protein decreased ROS levels in HEK293T cells
To explore the roles of rLm-PHB2 expressed in prokaryotes in regulating cellular ROS levels, we measured intracellular ROS levels in HEK293T cells treated with rLm-PHB2 of different concentrations using flow cytometry. As shown in Fig. 1 (A and B), the levels of ROS in HEK293T cells were significantly decreased when treated with 6 μM rLm-PHB2 under H2O2 stimulation, indicating that rLm-PHB2 could inhibit ROS production in H2O2-stimulated HEK293T cells. In addition, confocal microscopic images (Fig. 1C) showed the rLm-PHB2 could enter HEK293T cells and localize to the cytoplasm. Which suggested that Lm-PHB2 might play roles of decreasing ROS levels in HEK293T cells by the mitochondria in the cytoplasm, because cellular H2O2 stimulation is known to induce mass ROS generation during mitochondrial-dependent apoptotic processes [23].
Fig. 1.
rLm-PHB2 decreased the levels of ROS in HEK293T cells. (A) After digestion, HEK293T cells treated with PBS or rLm-PHB2 were stained with DCFH-DA and analysed by flow cytometry. The mean fluorescence intensity of HEK293T cells in the P2 region is shown. (B) Histogram indicating the decreased ROS levels in HEK293T cells treated with rLm-PHB2. Each treatment was repeated three times. Significant differences between the PBS- and rLm-PHB2-treated groups are indicated with asterisks *P < 0.05, respectively. (C) Confocal microscopic images of the rLm-PHB2 protein entering HEK293T cells and localizing to the cytoplasm. After 24 h, rLm-PHB2 cultures were immunostained with a fluorescent antibody (red), mitochondria were stained with MitoTracker (green), and nuclei were stained with Hoechst 33258 (blue). The scale bar represents 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Lm-PHB2 is required for maintaining mitochondrial morphology in HEK293T cells
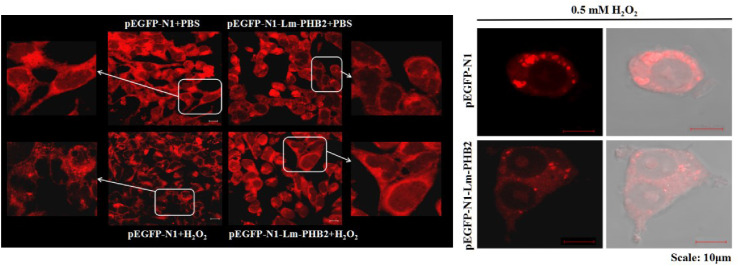
To explore the effect of Lm-PHB2 on mitochondrial morphology, we conducted pEGFP-N1-Lm-PHB2 plasmids and transfected HEK293T cells. The mitochondrial morphology of HEK293T cells treated with PBS and 0.5 mM H2O2 with the pEGFP-N1-Lm-PHB2 or pEGFP-N1 plasmids was examined using confocal microscopy. The results showed that the mitochondria in HEK293T cells transfected with pEGFP-N1-Lm-PHB2 and stimulated with H2O2 did not appear fragmentation, a large fraction of mitochondria in the cells that were transfected with pEGFP-N1 and treated with H2O2 were fragmented (Fig. 2 ). Therefore, Lm-PHB2 is required for maintaining mitochondrial morphology in HEK293T cells.
Fig. 2.
Lm-PHB2 is required for maintaining mitochondrial morphology in HEK293T cells. HEK293T cells treated with PBS and 0.5 mM H2O2 were transfected with the pEGFP-N1-Lm-PHB2 or pEGFP-N1 plasmids, after transfecting the mitochondria were stained with MitoTracker Red (red) and their mitochondrial morphologies were examined by confocal microscopy. The scale bar represents 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Lm-PHB2 protein was translocated from the nucleus to mitochondria under oxidative stress
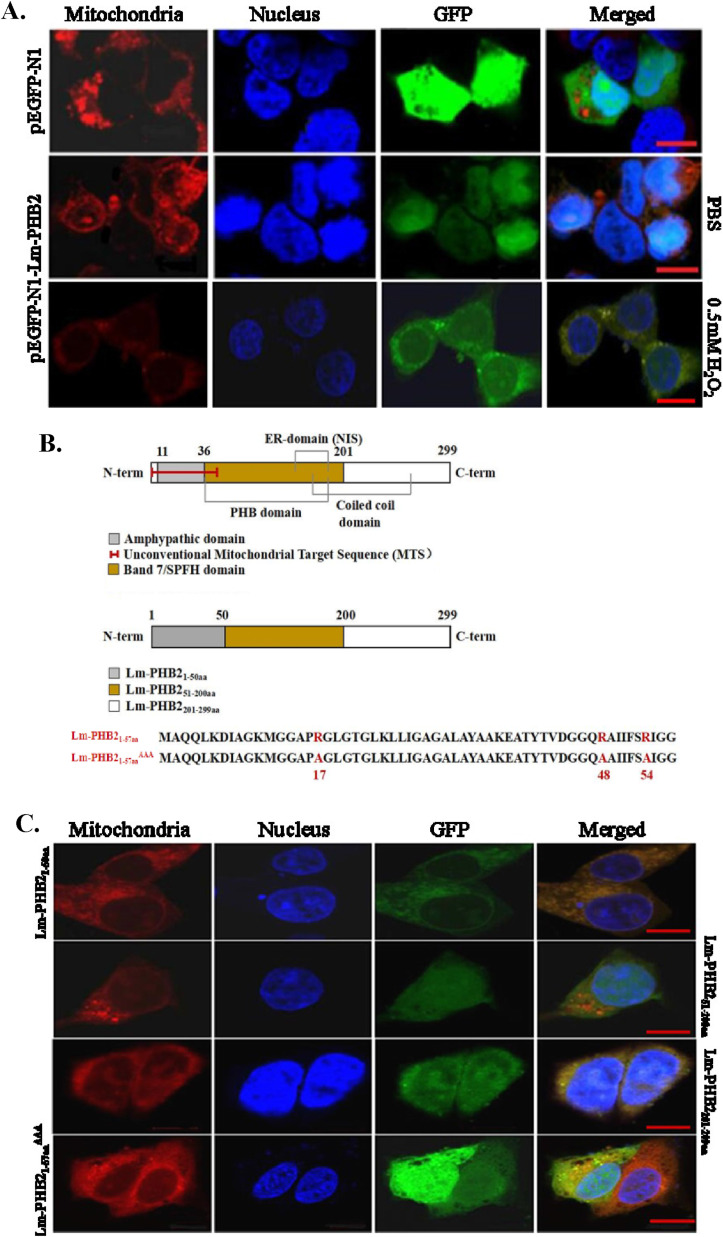
Before we found that the cellular oxidative stress tolerance enhanced by Lm-PHB2 was closely associated with the translocation of Lm-PHB2 from the nucleus to the mitochondria in CHL cells during oxidative stress. To further establish Lm-PHB2 could protect the mitochondria by the translocation of Lm-PHB2 from the nucleus to the mitochondria during oxidative stress, we constructed the pEGFP-N1-Lm-PHB2 recombinant plasmids in a previous study. Subsequently, the pEGFP-N1-Lm-PHB2 recombinant plasmid and pEGFP-N1 plasmid were transfected into HEK293T cells, respectively, and the subcellular localization and translocation of Lm-PHB2 protein under oxidative stress were examined by confocal microscopy. Interestingly, exogenous Lm-PHB2 also specifically localized to the nucleus and was translocated almost from the nucleus to the mitochondria in HEK293T cells after stimulation with 0.5 mM H2O2 for 1 h (Fig. 3 A).
Fig. 3.
Translocation of Lm-PHB2 to mitochondria in HEK293T cells in response to oxidative stress. (A)The Lm-PHB2 protein was translocated from the nucleus to the mitochondria in HEK293T cells under oxidative stress. Subcellular organelles and the Lm-PHB2 protein were visualized by Hoechst 33258 (blue, nucleus), GFP (green, Lm-PHB2), and MitoTracker Red (red, mitochondria). The scale bar represents 10 μm. (B)Lm-PHB2 deletion mutants and a mitochondria-targeted signal-mutated version of Lm-PHB2 proteins were respectively constructed in this study. (C)The translocation of Lm-PHB2 into mitochondria might be dependent on the Lm-PHB21-50aa region and the 17th, 48th and 57th three arginines(R) were very critical. Subcellular organelles and the protein mutants of Lm-PHB21-50aa, Lm-PHB251-200aa, Lm-PHB2201-299aa and Lm-PHB21-57aaAAA were visualized by Hoechst 33258 (blue, nucleus), GFP (green, Lm-PHB2 mutants), and MitoTracker Red (red, mitochondria). The scale bar represents 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To investigate the mechanism of Lm-PHB2 translocation, we examined the expression of various Lm-PHB2 deletion mutants (Lm-PHB21-50aa, Lm-PHB251-200aa and Lm-PHB2201-299aa) and a mitochondria-targeted signal-mutated version (Lm-PHB21-57aa AAA) (Fig. 3B). Confocal microscopy was used to show that Lm-PHB21-50aa localized to the mitochondria and cytoplasm, while Lm-PHB21-57aa AAA localized similarly to the Lm-PHB251-200aa and Lm-PHB2201-299aa and failed to localize to the mitochondria (Fig. 3C). The results indicated that the translocation of Lm-PHB2 into mitochondria might be dependent on the Lm-PHB21-50aa region and the 17th, 48th and 57th three arginines(R) were very critical.
3.4. Lm-PHB2 protects mitochondria via interactions with OPA1 and HAX1
To explore the role of Lm-PHB2 after translocation to mitochondria, the expression levels of OPA1 and HAX1 was examined by QRT-PCR and Western blot. Lm-PHB2 increased the expression levels of OPA1 and HAX1 in HEK293T cells treated with 0.5 mM H2O2 (Fig. 4 A–C). We also examined the interactions of Lm-PHB2 with OPA1 and HAX1 using co-immunoprecipitation (co-IP) and immunofluorescence (IF) assays, respectively. Lm-PHB2 was mainly localized in the nucleus in the PBS treatment group, as determined by IF, however, under H2O2 treatment, Lm-PHB2 was translocated to mitochondria that co-localized with OPA1 or HAX1 (Fig. 4D and E). The co-IP results showed that Lm-PHB2 interacted with OPA1 and HAX1 to a greater extent in H2O2-stimulated (0.5 mM) HEK293T cells transfected with pEGFP-N1-Lm-PHB2 compared with that in cells transfected with pEGFP-N1 (Fig. 4F). The above results indicated that Lm-PHB2 might maintain mitochondrial morphology via interacting with OPA1 or HAX1.
Fig. 4.
Lm-PHB2 protects mitochondria by interacting with OPA1 and HAX1. (A,B,C) The relative expression levels that OPA1 or HAX1 compared to the internal control GAPDH in HEK293T cells transfected with the pEGFP-N1-Lm-PHB2 plasmids were up-regulated after treatment with 0.5 mM H2O2 for 2 h by QRT-PCR (A) and Western blot (B) analysis. K:pEGFP-N1 group, Q:pEGFP-N1-Lm-PHB2 group, K + H:pEGFP-N1+H2O2 group, Q + H:pEGFP-N1-Lm-PHB2+H2O2 group. The histogram (C) compares the intensities of the various bands shown in the blot. Control:PBS-treated group, H2O2:H2O2-treated group. Asterisks indicate significant differences from PBS-treated cells at the *P < 0.05 or **P < 0.01 level, respectively. (D,E) Lm-PHB2 co-localization with OPA1 (D) or HAX1 (E) in HEK293T cells transfected with the pEGFP-N1-Lm-PHB2 plasmids when cultivated in the presence of 0.5 mM H2O2 for 2 h, as analysed by IF observations. Nuclear was stained with Hoechst 33258. GFP antibody was used to capture the Lm-PHB2 protein. The scale bar represents 10 μm. (F,G) Co-IP analysis of Lm-PHB2 interacting with OPA1 (F) and HAX1 (G) in HEK293T cells transfected with the pEGFP-N1-Lm-PHB2 plasmids after treatment with 0.5 mM H2O2 for 2 h. K:pEGFP-N1 group, Q:pEGFP-N1-Lm-PHB2 group.
4. Discussion
Many diseases are associated with increased levels of ROS, such as cardiovascular, Parkinson's and Alzheimer's diseases [24]. However, clinical trials exploring the efficacies of antioxidant therapies for disease treatments have failed to show any benefits [25,26]. ROS arise from diverse intracellular and extracellular sources in the vascular wall, and there appears to be a fine balance between the physiological roles of the stable ROS hydrogen peroxide, observed at low H2O2 concentrations, and the pathological effects of higher ROS concentrations [27,28]. In 2015, Li et al. first demonstrated that lamprey PHB2 enhances the oxidative stress tolerance of CHL cells, most likely due to the presence of the protein in the mitochondria after its translocation from the nucleus during oxidative stress. Based on our data, lamprey PHB2 shares more than 75% sequence homology with human PHB2, and their sequences are mainly divergent in the N and C termini [20]. The C-terminal domain is sufficient for the endoplasmic reticulum (ER) α-dependent nuclear translocation of human PHB2. Thus, human PHB2 localizes to the mitochondria via the mitochondrial transmembrane sequence (MTS) at its N terminus and can localize to the nucleus via its C-terminal domain, suggesting that PHB2 is shuttled between the two organelles [16,17]. Human PHB2 is mainly involved in the functionality of the mitochondrial inner membrane as a protein-lipid scaffold [29]. Regrettably, the mechanisms underlying the enhanced oxidative stress tolerance effects of Lm-PHB2 were not discussed in detail.
4.1. ROS generation is inhibited by Lm-PHB2 in HEK293T cells
Some reports have also suggested other functions of PHB2, such as transcriptional regulation in the nucleus and cell signalling in the plasma membrane [30]. In contrast to the extensive studies on PHB2 in jawed vertebrates, little is known about PHB2 and its biological activities and physiological roles in jawless lampreys. Molecular cloning and characterisation of a PHB2 homologue from Chinese northeast lamprey (Lampetra morii) was molecularly cloned and characterized in a past study. Recently, we used a flow cytometry assay to demonstrate that Lm-PHB2 could inhibit ROS generation induced by H2O2 in HEK293T cells.
4.2. Lm-PHB2 is required for maintaining mitochondrial morphology induced by OPA1
In most eukaryotic cells, mitochondria are responsible for providing energy by generating ATP via oxidative phosphorylation and controlling the levels of oxidative stress [31]. The dynamin-like GTPase OPA1 is required for both the maintenance of normal cristae in the inner membrane and cristae remodelling during mitochondria-mediated apoptosis [32,33]. Therefore, PHB proteins have been suggested to participate in the regulation of mitochondrial morphology because they genetically interact with Mdm12p, a protein required for normal mitochondrial morphology and function [34]. According to our confocal microscopy assay, mitochondrial fragmentation was decreased in HEK293T cells transfected with pEGFP-N1-Lm-PHB2 plasmid after treatment with H2O2 compared with that in cells transfected with pEGFP-N1 plasmid, indicating that Lm-PHB2 is required for maintaining mitochondrial morphology.
Mitochondrial fragmentation is associated with deficient mitochondrial fusion activity [28], and we identified the molecular mechanism by which Lm-PHB2 maintains mitochondrial morphology. According to QRT-PCR and Western blot analyses, Lm-PHB2 increased the expression level of OPA1 in HEK293T cells treated with H2O2, and our IF and Co-IP results showed that Lm-PHB2 was capable of interacting with OPA1. This indicates that the increased OPA1 expression in HEK293T cells might also help maintain mitochondrial morphology via the interaction with Lm-PHB2.
4.3. Lm-PHB2 interacts with the HAX1 protein in mitochondria
An imbalance between excessive ROS production and impaired protective mechanisms acting to eliminate ROS can cause tissue damage and cell apoptosis, which is considered oxidative stress [6,35]. HAX1 represents a novel protein that regulates apoptosis and promotes cell survival due to its homology and perceived structural similarities to the anti-apoptotic protein Bcl-2 [36]. HAX1, which was initially identified as an HS1-binding protein and as an inhibitor of apoptosis, was found to directly associate with PHB2 in mitochondria [37,38]. According to QRT-PCR and Western blot analyses, Lm-PHB2 increased the expression level of HAX1 in HEK293T cells treated with H2O2, and our IF and Co-IP results showed that Lm-PHB2 was capable of interacting with HAX1. This result indicates that the increased HAX1 expression in HEK293T cells might also help protect the mitochondria via the interaction with Lm-PHB2. Treatment with H2O2 induces significant cell death in a dose- and time-dependent manner [39], and Hax-1 reportedly interacts with an increasingly diverse array of proteins, indicating that it might exert its anti-apoptotic activities via different pathways. Interestingly, HAX1 expression was not significantly increased in H2O2-treated HEK293T cells transfected with pEGFP-N1 plasmid [39], however, the reasons underlying this phenomenon require further investigation.
The above mentioned results indicate that Lm-PHB2 significantly inhibits ROS generation by translocation to the mitochondria and protecting the mitochondria induced by OPA1 and HAX1 (Fig. 5 ). These provide a new perspective for the treatment of diseases caused by oxidative stress. These results also provide a new direction for the prevention and treatment of the immune dysfunction caused by oxidative stress.
Fig. 5.
Summary of the translocalization and mitochondrial protection of Lm-PHB2. Lm-PHB2 localizes in the mitochondria and nucleus. In mitochondria, Lm-PHB2 decreased the levels of ROS via forming a complex with HAX1 or OPA1. In the presence of ERα and E2, mitochondrial PHB2 translocates into the nucleus, wherein it represses ERα-dependent transcription. Lm-PHB2 also possesses a non-cleavable N-terminal MTS, indicating possible shuttling between the two organelles.
4.4. PHB2 was a vital shuttling protein between the nucleus and mitochondria
PHB2 represents a novel class of protein that shuttles between the nucleus and mitochondria as a communicator and coordinator where it assumes completely unrelated functions to coordinate a single response, making it viable targets for the treatment of diverse pathologies [40]. The over-expression of a PHB2 (S91A) mutant was highly indicative of mitochondrial dysfunction and very similar to findings reported following the expression of PHB mutants lacking or containing alterations in the MTS domain, critical for mitochondrial localization [32,41,42]. Lm-PHB2 could trans-localizate from the nucleus to mitochondria under oxidative stress. The phosphorylation of nuclear PHB2 on S91 might promote nuclear export of Lm-PHB2 and mitochondrial localization. However, this still needs further evidence.
5. Conclusion
Lm-PHB2 could significantly decrease the levels of ROS generation in HEK293T cells. Both cellular nuclear location and translocation from the nucleus to mitochondria of Lm-PHB2 were also examined in HEK293T cells under oxidative stress. The translocation of Lm-PHB2 into mitochondria was dependent on the Lm-PHB21-50aa region and the 17th, 48th and 57th three arginines (R) of N-terminal were very critical. In addition, Lm-PHB2 increased the expression levels of OPA1 and HAX1 in HEK293T cells under oxidative stress and then Lm-PHB2 could interact with OPA1 and HAX1, respectively. Lm-PHB2 could assist OPA1 and HAX1 to maintain mitochondrial morphology and decrease ROS levels by the translocation from the nucleus to mitochondria under oxidative stress. Lm-PHB2 may represent a breakthrough in the understanding of the anti-oxidative stress mechanism of lower vertebrates, which is simpler and more effective than that in mammals. These results are also helpful to understand PHB2 represents a novel class of protein that shuttles between the nucleus and mitochondria as a communicator and coordinator, and also provide a new direction for the prevention and treatment of the immune dysfunction caused by oxidative stress.
Author contributions
TS L conceived and designed all the experiments and analysed all the data. Y S carried out the cell culture work, Western blot, QRT-PCR and statistical analyses as well as writing the manuscript. Q L also wrote the manuscript. F S carried out all the confocal microscopy analyses. CY Zh, SN M and D Q were involved in the preparation of the all experimental materials. QW L oversaw the preparation of the final version of the manuscript.
Funding sources
This work was supported by grants from National Natural Science Foundation of China (No. 31501907), Scientific and Technological Research Projects of Liaoning Provincial Department of Education (No. L2015287). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CRediT authorship contribution statement
Ying Shi: Writing - original draft, Formal analysis. Qing Li: Writing - original draft, Writing - review & editing. Feng Sun: Formal analysis. Tiesong Li: Conceptualization, Formal analysis.
Declaration of competing interest
The authors declare that they have no competing interests.
Contributor Information
Qingwei Li, Email: liqw@263.net.
Tiesong Li, Email: sally_ts_li@163.com.
References
- 1.Rogers N.M., Ghimire K., Calzada M.J., Isenberg J.S. Matricellular protein thrombospondin-1 in pulmonary hypertension: multiple pathways to disease. Cardiovasc. Res. 2017;113:858–868. doi: 10.1093/cvr/cvx094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawmiller D., Li S., Mori T., Habib A., Rongo D., Delic V., Bradshaw P.C., Shytle R.D., Sanberg C., Bickford P., Tan J. Beneficial effects of a pyrroloquinolinequinone-containing dietary formulation on motor deficiency, cognitive decline and mitochondrial dysfunction in a mouse model of Alzheimer's disease. Heliyon. 2017;3 doi: 10.1016/j.heliyon.2017.e00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Dalmazi G., Hirshberg J., Lyle D., Freij J.B., Caturegli P. Reactive oxygen species in organ-specific autoimmunity. Auto Immun Highlights. 2016;7:11. doi: 10.1007/s13317-016-0083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Z., Song J., Tian R., Yang Z., Yu G., Lin L., Zhang G., Fan W., Zhang F., Niu G., Nie L., Chen X. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew Chem. Int. Ed. Engl. 2017;56:6492–6496. doi: 10.1002/anie.201701181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moritani C., Kawakami K., Fujita A., Kawakami K., Hatanaka T., Tsuboi S. Anti-oxidative activity of hydrolysate from rice bran protein in HepG2 cells. Biol. Pharm. Bull. 2017;40:984–991. doi: 10.1248/bpb.b16-00971. [DOI] [PubMed] [Google Scholar]
- 6.Birben E., Sahiner U.M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal A., Aponte-Mellado A., Premkumar B.J., Shaman A., Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol. 2012;10:1477–7827. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu L.L., Wang W.J., Zhu Q.J., Yang L. Novel coronavirus pneumonia related liver injury: etiological analysis and treatment strategy. Chin. J. Hepatol. 2020;28:E001. doi: 10.3760/cma.j.issn.1007-3418.2020.02.001. 0. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 9.Merkwirth C., Martinelli P., Korwitz A., Morbin M., Brönneke H.S., Jordan S.D., Rugarli E.I., Langer T. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet. 2012;8(11) doi: 10.1371/journal.pgen.1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijtmans L.G., Artal S.M., Grivell L.A., Coates P.J. The mitochondrial PHB complex: roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cell. Mol. Life Sci. 2002;59:143–155. doi: 10.1007/s00018-002-8411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xun Yiping, Chen Peng, Yan Hai, Weikang, Shi Lili, Chen Guangyu, Du Hongwu. Identification of prohibitin as an antigen in Behcet's disease. Biochem. Biophys. Res. Commun. 2014;451:389–393. doi: 10.1016/j.bbrc.2014.07.126. [DOI] [PubMed] [Google Scholar]
- 12.Mishra S., Nyomba B.G. Prohibitin- at the crossroads of obesity-linked diabetes and cancer. Exp. Biol. Med. 2017;242(11):1170–1177. doi: 10.1177/1535370217703976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., Faller D.V. Roles of prohibitin in growth control and tumor suppression in human cancers. Transl. Oncogenomics. 2008;3:23–37. [PMC free article] [PubMed] [Google Scholar]
- 14.Artal-Sanz M., Tavernarakis N. Opposing function of mitochondrial prohibitin in aging. Aging. 2010;2:1004–1011. doi: 10.18632/aging.100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H., Arnouk H., Sripathi S., Chen P., Zhang R., Bartoli M., Hunt R.C., Hrushesky W.J., Chung H., Lee S.H., Jahng W.J. Prohibitin as an oxidative stress biomarker in the eye. Int. J. Biol. Macromol. 2010;47(5):685–690. doi: 10.1016/j.ijbiomac.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasashima K., Ohta E., Kagawa Y., Endo H. Mitochondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropic human prohibitin 2. J. Biol. Chem. 2006;281:36401–36410. doi: 10.1074/jbc.M605260200. [DOI] [PubMed] [Google Scholar]
- 17.Bavelloni A., Piazzi M., Raffini M., Faenza I., Blalock W.L. Prohibitin 2: at a communications crossroads. Int Union of Bioch and Mol Bio. 2015;67(4):239–254. doi: 10.1002/iub.1366. [DOI] [PubMed] [Google Scholar]
- 18.Kowno M., Watanabe-Susaki K., Ishimine H., Komazaki S., Enomoto K., Seki Y. Prohibitin 2 regulates the proliferation and lineage-specific differentiation of mouse embryonic stem cells in mitochondria. PloS One. 2014;9 doi: 10.1371/journal.pone.0081552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chowdhury D., Kumar D., Bhadra U., Devi T.A., Bhadra M.P. Prohibitin confers cytoprotection against ISO-induced hypertrophy in H9c2 cells via attenuation of oxidative stress and modulation of Akt/Gsk-3βsignaling. Mol. Cell. Biochem. 2017;425:155–168. doi: 10.1007/s11010-016-2870-3. [DOI] [PubMed] [Google Scholar]
- 20.Li T., Wang Y., Gao Y., Li Q. Identification and characterisation of the anti-oxidative stress properties of the lamprey prohibitin 2 gene. Fish Shellfish Immunol. 2015;42:447–456. doi: 10.1016/j.fsi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Mishra S., Murphy L.C., Murphy L.J. The Prohibitins: emerging roles in diverse functions. J. Cell Mol. Med. 2006;10:353–363. doi: 10.1111/j.1582-4934.2006.tb00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thuaud F., Ribeiro N., Nebigil C.G., Desaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chem. Biol. 2013;20:316–331. doi: 10.1016/j.chembiol.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon S.J., Lee J.H., Moon K.D., Jeong I.Y., Ahn D.U., Lee M.K. Induction of apoptosis by isoegomaketone from Perilla frutescens L. in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent, -independent pathway. Food Chem. Toxicol. 2014;65:97–104. doi: 10.1016/j.fct.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Lachén-Montes M., González-Morales A., Zelaya M.V., Pérez-Valderrama E., Ausín K., Ferrer I. Olfactory bulb neuroproteomics reveals a chronological perturbation of survival routes and a disruption of prohibitin complex during Alzheimer's disease progression. Sci. Rep. 2017;7:9115. doi: 10.1038/s41598-017-09481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ising C., Koehler S., Brähler S., Merkwirth C., Höhne M., Baris O.R. Inhibition of insulin/IGF-1 receptor signaling protects from mitochondria-mediated kidney failure. EMBO Mol. Med. 2015;7:275–287. doi: 10.15252/emmm.201404916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshimaru T., Komatsu M., Tashiro E., Imoto M., Osada H., Miyoshi Y. Xanthohumol suppresses oestrogen-signalling in breast cancer through the inhibition of BIG3-PHB2 interactions. Sci. Rep. 2014;4:7355. doi: 10.1038/srep07355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osman C., Merkwirth C., Langer T. Prohibitins and the functional compartmentalization of mitochondrial membranes. J. Cell Sci. 2009;122:3823–3830. doi: 10.1242/jcs.037655. [DOI] [PubMed] [Google Scholar]
- 30.Pabona J.M., Velarde M.C., Zeng Z., Simmen F.A., Simmen R.C. Nuclear receptor co-regulator Krüppel-like factor 9 and prohibitin 2 expression in estrogen-induced epithelial cell proliferation in the mouse uterus. J. Endocrinol. 2009;200:63–73. doi: 10.1677/JOE-08-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theiss A.L., Sitaraman S.V. The role and therapeutic potential of prohibitin in disease. Biochim. Biophys. Acta. 2011;1813:1137e43. doi: 10.1016/j.bbamcr.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merkwirth C., Dargazanli S., Tatsuta T., Geimer S., Löwer B., Wunderlich F.T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22:476–488. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olichon A., Baricault L., Gas N., Guillou E., Valette A., Belenguer P. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J. Biol. Chem. 2003;278:7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 34.Berger K.H., Yaffe M.P. Prohibitin family members interact genetically with mitochondrial inheritance components in saccharomyces cerevisiae. Mol. Cell Biol. 1998;18:4043–4052. doi: 10.1128/mcb.18.7.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Theiss A.L., Idell R.D., Srinivasan S., Klapproth J.M., Jones D.P., Merlin D. Prohibitin protects against oxidative stress in intestinal epithelial cells. Faseb. J. 2007;21:197e206. doi: 10.1096/fj.06-6801com. [DOI] [PubMed] [Google Scholar]
- 36.Yap S.V., Koontz J.M., Kontrogianni-Konstantopoulos A. HAX-1: a family of apoptotic regulators in health and disease. J. Cell. Physiol. 2011;226:2752–2761. doi: 10.1002/jcp.22638. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y., Demoliere C., Kitamura D., Takeshita H., Deuschle U., Watanabe T. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J. Immunol. 1997;158:2736–2744. [PubMed] [Google Scholar]
- 38.Cilenti L., Soundarapandian M.M., Kyriazis G.A., Stratico V., Singh S., Gupta S. Regulation of HAX-1 anti-apoptotic protein by Omi/HtrA2 protease during cell death. J. Biol. Chem. 2004;279:50295–50301. doi: 10.1074/jbc.M406006200. [DOI] [PubMed] [Google Scholar]
- 39.Koontz J., Kontrogianni-Konstantopoulos A. Competition through dimerization between antiapoptotic and proapoptotic HS-1-associated protein X-1 (Hax-1) J. Biol. Chem. 2014;289:3468–3477. doi: 10.1074/jbc.M113.536151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bavelloni A., Piazzi M., Raffini M., Faenza I., Blalock W.L. Prohibitin 2: at a communications crossroads. IUBMB Life. 2015;67(4):239–254. doi: 10.1002/iub.1366. [DOI] [PubMed] [Google Scholar]
- 41.Kasashima K., Ohta E., Kagawa Y., Endo H. Mitochondrial functions and estrogen receptor-dependent nuclear translocation of pleiotropichuman prohibitin 2. J. Biol. Chem. 2006;281:36401–36410. doi: 10.1074/jbc.M605260200. [DOI] [PubMed] [Google Scholar]
- 42.Bavelloni A., Piazzi M., Faenza I., Raffini M., D'Angelo A. Prohibitin 2 represents a novel nuclear AKT substrate during all-trans retinoic acid-induced differentiation of acute promyelocytic leukemia cells. Faseb. J. 2014;28:2009–2019. doi: 10.1096/fj.13-244368. [DOI] [PubMed] [Google Scholar]