Abstract
We present a case of an abducens nerve palsy in a previously healthy young man in the setting of SARS-CoV-2 infection. Magnetic resonance imaging obtained 5 weeks after the onset of diplopia demonstrated an atrophic left lateral rectus muscle, which was hyperintense on T2 weighting, consistent with denervation. Although the mechanism of the nerve palsy remains unclear, it is suspected to be related to his viral illness, because the patient had no preexisting vascular risk factors or evidence of other neurologic disease on neuroimaging. Cranial nerve palsies may represent part of the neurologic spectrum of COVID-19.
Abducens nerve palsy is the most common isolated ocular motor palsy.1 In older adults, it is frequently caused by microvascular disease.2 In children, abducens nerve palsy has been associated with viral infections, including Epstein-Barr and enterovirus, as well as with vaccinations.3 Although well studied in older adults and children, abducens nerve palsy is not well studied in younger adults, in whom the incidence is low. The causes of abducens nerve palsy include vasculopathies, tumors, multiple sclerosis, and inflammatory conditions,4 with viral etiologies causing approximately 1%-10% of cases with nontraumatic etiology.2 , 5 We present the case of a young man with a unilateral abducens nerve palsy in the setting of infection with the novel coronavirus SARS-CoV-2 (COVID-19).
Case Report
A 32-year-old previously healthy man developed acute, binocular, horizontal diplopia on waking after 3 days of progressive upper respiratory illness symptoms. He denied pain with eye movements or other neurologic symptoms, such as weakness, gait abnormality, paresthesias, or anosmia. Over the following week, his respiratory symptoms worsened, and he was hospitalized for acute hypoxemic respiratory failure after testing positive for COVID-19. He underwent a 5-day course of hydroxychloroquine and supplemental oxygen, and his respiratory symptoms subsequently resolved over the next 3 weeks.
At the time of ophthalmic evaluation at the Bascom Palmer Eye Institute, 5 weeks after onset of diplopia, he reported no change in the double vision. On examination, his best-corrected visual acuity was 20/20 in each eye. Intraocular pressure was normal, and there was no afferent pupillary defect. Ocular motility examination revealed a left esotropia of 35Δ in primary gaze, 4Δ on right gaze, and 60Δ on left gaze. He had complete limitation to abduction in the left eye, consistent with a diagnosis of a complete left abducens nerve palsy. Optic nerve examination was unremarkable. There was no hypesthesia within the ophthalmic or maxillary divisions of the trigeminal nerve. Magnetic resonance imaging (MRI) was performed and revealed an atrophic left lateral rectus muscle (Figure 1 A), which was hyperintense on T2 (Figure 1B). The patient was instructed to perform monocular occlusion for alleviation of diplopia and scheduled for follow-up.
Fig 1.
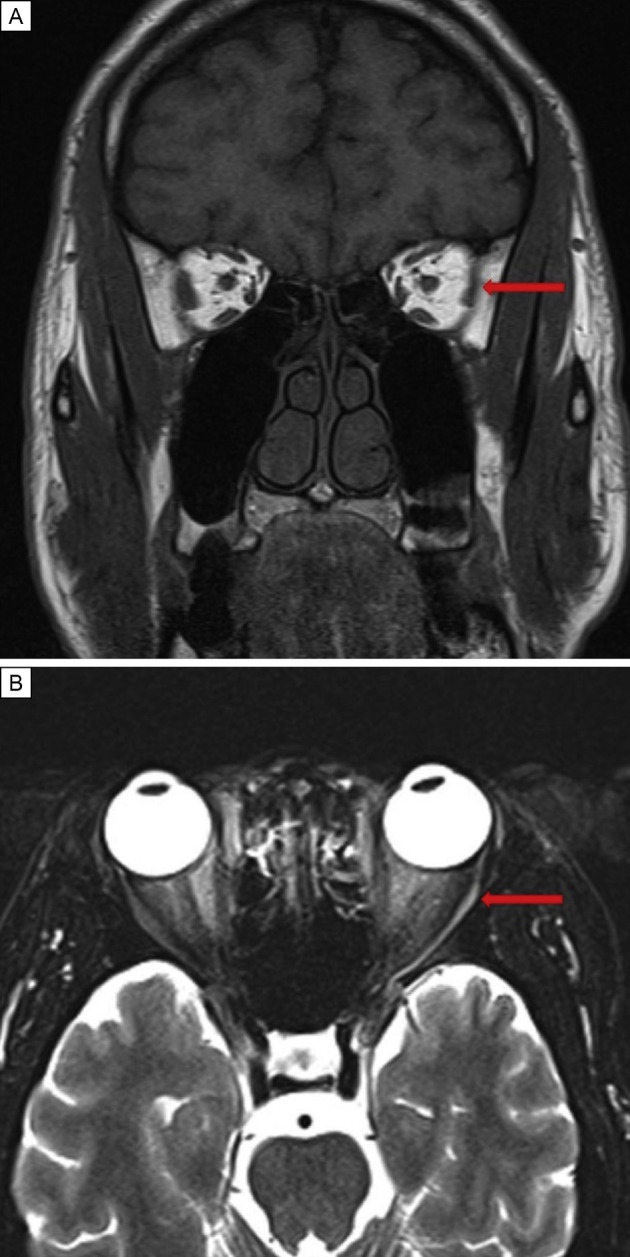
Magnetic resonance imaging of a 32-year-old man with acute-onset left abducens nerve palsy obtained 5 weeks after onset of symptoms. A, T1-weighted coronal image demonstrating volume loss of the left lateral rectus muscle. B, T2-weighted axial image showing an atrophic and hyperintense left lateral rectus muscle (red arrows).
Discussion
The clinical manifestations of COVID-19 typically include fever, cough, diarrhea, and fatigue. Neurologic manifestations of the virus, such as dizziness, headache, impaired consciousness, acute cerebrovascular disease, and impairment in smell and taste have been reported in 36.4% of hospitalized patients.4 , 6 Patients with COVID-19 infection and ophthalmoplegia in the setting of Miller Fisher syndrome have also been reported.7 , 8 In our case, the onset of diplopia 3 days following onset of COVID-19 respiratory symptoms in an otherwise healthy young patient suggests a viral etiology of the abducens nerve palsy, consistent with the report by Dinkin and colleagues.7
Prior reports of SARS-CoV infections suggest that neurologic symptoms may be due to viral involvement of the central nervous system, because post mortem analysis of infected patients with neurologic symptoms has detected viral nucleic acids in the cerebrospinal fluid and brain tissue.9 Although it has not yet been established how COVID-19 gains access to neuronal tissues, several mechanisms have been proposed. The COVID-19 virus uses a spike protein S1 to attach to host cell angiotensin-converting enzyme 2 receptors, which have been detected in various organ systems, including the respiratory, gastrointestinal, and neurologic tracts.9 , 10 Vonck and colleagues11 proposed that part of the neurologic spectrum in COVID-19 may be due to direct viral neurological injury or indirect neuroinflammatory and autoimmune mechanisms.
We hypothesize that in our patient, either a direct or indirect virally mediated insult occurred along the path of the abducens nerve. This is supported by the MRI findings, which show ipsilateral atrophy and hyperintensity of the lateral rectus muscle, consistent with denervation of the abducens nerve, a finding previously reported in patients with unilateral abducens nerve palsy.12 , 13 Additionally, muscles of patients with subacute denervation (1-12 months) have been shown to have T2 hyperintensity, consistent with the neuroimaging findings in this case.14 The normal optic nerve examination and lack of signs suggesting increased intracranial pressure or inflammation make these etiologies less likely. Furthermore, given the lack of improvement 5 weeks after the onset of symptoms in the setting of neuroimaging findings suggestive of lateral rectus denervation, we predict that the patient's abducens nerve palsy is chronic. Because the incidence of COVID-19 increases worldwide, it is important for ophthalmologists to recognize that abducens nerve palsy may represent part of the neurologic spectrum of disease.
Literature Search
PubMed was searched in May 2020 for English-language results using the following terms: coronavirus, COVID-19, cranial nerve palsy, and neurologic manifestations.
References
- 1.Elder C., Hainline C., Galetta S.L., Balcer L.J., Rucker J.C. Isolated abducens nerve palsy: update on evaluation and diagnosis. Curr Neurol Neurosci Rep [Internet] 2016;16:69. doi: 10.1007/s11910-016-0671-4. [DOI] [PubMed] [Google Scholar]
- 2.Peters G.B., 3rd, Bakri S.J., Krohel G.B. Cause and prognosis of nontraumatic sixth nerve palsies in young adults. Ophthalmology. 2002;109:1925–1928. doi: 10.1016/s0161-6420(02)01226-5. [DOI] [PubMed] [Google Scholar]
- 3.Lee M.S., Galetta S.L., Volpe N.J., Liu G.T. Sixth nerve palsies in children. Pediatr Neurol. 1999;20:49–52. doi: 10.1016/s0887-8994(98)00090-3. [DOI] [PubMed] [Google Scholar]
- 4.Moster M.L., Savino P.J., Sergott R.C., Bosley T.M., Schatz N.J. Isolated sixth-nerve palsies in younger adults. Arch Ophthalmol. 1984;102:1328–1330. doi: 10.1001/archopht.1984.01040031078029. [DOI] [PubMed] [Google Scholar]
- 5.Park K.A., Oh S.Y., Min J.H., Kim B.J., Kim Y. Cause of acquired onset of diplopia due to isolated third, fourth, and sixth cranial nerve palsies in patients aged 20 to 50 years in Korea: A high resolution magnetic resonance imaging study. J Neurol Sci. 2019;407:116546. doi: 10.1016/j.jns.2019.116546. [DOI] [PubMed] [Google Scholar]
- 6.Mao L., Jin H., Wang M. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinkin M., Gao V., Kahan J. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology. 2020 doi: 10.1212/WNL.0000000000009700. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez-Ortiz C., Méndez A., Rodrigo-Rey S. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020 doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 9.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the cns: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 10.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus: a first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vonck K., Garrez I., De Herdt V. Neurological manifestations and neuro-invasive mechanisms of the severe acute respiratory syndrome coronavirus type 2. Eur J Neurol. 2020 doi: 10.1111/ene.14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark R.A., Demer J.L. Lateral rectus superior compartment palsy. Am J Ophthalmol. 2014;157:479–487.e2. doi: 10.1016/j.ajo.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang N.Y., Demer J.L. Comparison of orbital magnetic resonance imaging in Duane syndrome and abducens palsy. Am J Ophthalmol. 2006;142:827–834. doi: 10.1016/j.ajo.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleckenstein J.L., Watumull D., Conner K.E. Denervated human skeletal muscle: MR imaging evaluation. Radiology. 1993;187:213–218. doi: 10.1148/radiology.187.1.8451416. [DOI] [PubMed] [Google Scholar]


