Abstract
Similar to other epidemics, the novel coronavirus (COVID-19) spread very fast and infected almost two hundreds countries around the globe since December 2019. The unique characteristics of the COVID-19 include its ability of faster expansion through freely existed viruses or air molecules in the atmosphere. Assuming that the spread of virus follows a random process instead of deterministic. The continuous time Markov Chain (CTMC) through stochastic model approach has been utilized for predicting the impending states with the use of random variables. The proposed study is devoted to investigate a model consist of three exclusive compartments. The first class includes white nose based transmission rate (termed as susceptible individuals), the second one pertains to the infected population having the same perturbation occurrence and the last one isolated (quarantined) individuals. We discuss the model’s extinction as well as the stationary distribution in order to derive the the sufficient criterion for the persistence and disease’ extinction. Lastly, the numerical simulation is executed for supporting the theoretical findings.
Keywords: Stochastic epidemic model, COVID-19, Threshold value, Global stability, Real data, Stationary distribution
1. Introduction
The 2019- novel coronavirus has been known to the virologist’s community as Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) [1]. The COVID-19 refers to the virus associated syndrome. SARS-CoV-2 being previously unrecognized novel-strain of the coronavirus in humans [2], [3]. Coronaviruses in general circulate among various animals with some being highly susceptible for infecting humans. Among these animals, naturally bats are thought to be proven hosts of such novel coronaviruses, nevertheless, various species of other animals are also considered an active cause for such spreads [4]. At present, the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) which is much similar to COVID-19 was spread from camels to humans, while the civet cats have been considered as source of Severe Acute Respiratory Syndrome Coronavirus-1 (SARS-CoV-1) for transmission into human. Bunch of information are presented in the ECDC factsheet on coronaviruses [4], [5].
Though the animals are understood to be a proven source, however, currently, human-to-human transmission is also considered as one of the spread source. At present, the epidemiological information are sparse for the determination of an effortless spread of this virus among the people, nonetheless, currently, on average, it is estimated that, infection in one person can cause the spread among 2-3 more people [1], [5]. The virus appears to be transferred mostly through narrow respiratory droplets by coughing, sneezing, or people’s interaction in close proximity (usually less than one meter) with each other for a certain time frame. These droplets can further be inhaled, or can stay on the surfaces being came in contact by the infected person, that can cause infection in others by touching their nose, mouth or eyes. The virus possesses ability to survive on various surfaces commencing several hours (e.g. copper, cardboard) up to a few days (e.g. plastic and stainless steel). Nonetheless, the quantity of the viable virus certainly decays over a time span and might not be present in sufficient quantity for causing the infection. It is currently estimated that the appearance of symptoms and initial infections in case of COVID-19 almost lies between 1-14 days [1], [5], [6]. Moreover till today there is no proper treatment in term of vaccine etc. However, the scientists are working faster to develop vaccine for the novel COVID-19, which will take enough time. Therefore the only way to stop the spread of this disease is to quarantine or isolate the initially infected population as showed by the Chinese Govt and also the guide line of WHO.
It could be also noted that most of the real world phenomenon are not simply deterministic, because in deterministic models, the output of the model is fully determined by the parameter values and the initial conditions. Stochastic models possess some inherent randomness. The same set of parameter values and initial conditions will lead to an ensemble of different outputs or we can say in simple world a deterministic model is one that uses numbers as inputs, and produces numbers as outputs. A stochastic model includes a random component that uses a distribution as one of the inputs, and results in a distribution for the output. These distributions may reflect the uncertainty in what the input should be (e.g. a deterministic input plus noise), or may reflect a random process (i.e. a stochastic input) [7], [8], [9].
For describing the changing behavior of several epidemic diseases in a realistic sense, the mathematical modeling is considered as an influential tool. Several epidemic models have been developed by various mathematicians and ecologists for comprehending and controlling various epidemic diseases in a region. In last twenty years, mathematical modeling is widely used for characterizing the communication of various infectious diseases (see e.g. [10], [11]. Recently various comprehensions have been made to deepen the understanding about the novel coronavirus (COVID-19) particularly grasping the valuable inferences through mathematical modeling [12], [13], [14]. The modelsdescribe the dynamics of infectious diseases, however, for modeling biological phenomenon, it is appropriate to use the stochastic differential equations due to its realistic approach. Compared to deterministic models, the stochastic models can generally result in more valuable output, by several times execution, a distribution of the expected results can be built, such as the average infections at any time t, whereas the deterministic models result in a single predicted value [15], [16], [17], [18]. Numerous approaches and methods exist for studying stochastic models (such as Binomial moment equation etc.) [19], [20].
The most basic stochastic epidemic models are those involving global transmission, meaning that infection rates depend only on the type and state of the individuals involved, and not on their location in the population. How can a model be defined explaining the sometimes-observed scenario of frequent mid-sized epidemic outbreaks? How can evolution of the infectious agent transmission rates be modeled and fitted to data in a robust way? In this paper we understand the transmission mechanism of the COVID-19 mathematically, we have formulated a model using the available literature on modeling epidemics, we propose a stochastic epidemic model for the transmission dynamics of COVID-19 virus with a varying population environment for a long-term behavior. We categorize the total population into three different classes. The first class is the susceptible individuals in which the transmission rate is distributed by white noise. The second class includes the infected individuals in which the same transmission occurs. The third class consists of the quarantine individuals with white noise.
In the recent study, we proposed a stochastic epidemic model for the transmission dynamics of the COVID-19 with a changing environment considering long term behavior. The overall population has been divided into three exclusive classes: the susceptible individuals with white noise transmission rate distribution, the infected individuals in which the same perturbation occur and quarantined individuals. Then, we will discuss the disease’ extinction and stationary distribution and develop the sufficient condition for the COVID-19. Furthermore, sample simulations are find out with the help of stochastic Runge-Kutta method for supporting the theoretical results.
2. Mathematical Model for COVID-19 model
The present section is devoted to formulation of a model based on stochastic theory for studying the transmissions dynamic of the novel virus i.e., COVID-19 pandemic. We propose a susceptible-infected-quarantined epidemic model as according to the characteristic of the disease. We also take the varying population environment to study the dynamics of the COVID-19 particularly its long-term behavior. Before to present the model, we put some assumption as given by the following assertions.
(A 1). The total population at any time t is symbolized by N(t) and it is stratified into three exclusive groups of individual: the susceptible class S(t), the COVID-19 infected people I(t) and the quarantined Q(t), i.e., which is changing with t.
(A 2). The state variables and parameters included in the model are assumed to be nonnegative.
(A 3) The initially infected individuals move to the quarantined class as performed by the Chinese in Wuhan city.
(A 4). Once the infection confirmed then the quarantined will go back to the infected compartment.
In the light of the above assumption the proposed model leads to the following stochastic epidemic problem which consist of three stochastic differential equations
| (1) |
Here in the model, Λ represents the per capita constant fecundity rate. μ 0, μ 1 and μ represents the natural mortality rate and disease-related mortality rate, respectively. γ 1 represents the constant rate at which people getting quarantined from COVID-19 infected class. B(t) is considered to be the usual Brownian motion with intensity η 1, η 2 and η 1 taken to be positive.
3. Preliminaries
Let (Ω, {Ft}t ≥ 0, P) be the complete probability space with filtration {Ft}t ≥ 0 which satisfy the normal conditions, and .
Considering a d-dimensional SDE
| (2) |
along with condition where B(t) denotes an m-dimensional usual Brownian motion. Define the operator related to (4) by
By operating on V (a function from the space ), then we have
where
By generalized It’s formula, we have
whenever .
4. The existence and uniqueness of solution to COVID-19 model
This section is about studying the existence and uniqueness of solution of the proposed stochastic COVID-19 model (1).
Theorem 1
The triplet (S(t), I(t), Q(t)) being solution of the developed stochastic COVID-19 epidemic model (1) is unique for t ≥ 0 with initial condition . Further, the solution will always remains in with unit probability, that is, almost surely (a.s).
Proof: As for initial value of the state variables the coefficients used in equations are continuous and locally lipschitz. Thus, there must exists a local unique solution (S(t), I(t), Q(t)) of the model over t ∈ [0, τe). For detail analysis of the explosion time τe one must see the references [21], [22]. To prove the global nature of the solution, we must show that a.s. Assume that we have a sufficiently large nonnegative number k 0 such that all of the initial conditions on the state lie within . Let for each positive integer k ≥ k 0, the finishing time be defined as
| (3) |
Throughout this manuscript, we must choose where ϕ stand for the null set. Definition of τk force us to say that it is increasing as k tends to ∞. Setting with τe ≥ τ ∞ a.s. Upon showing a.s., we will declare that and hence (S(t), I(t), Q(t)) will lies in a.s. ∀t ≥ 0. Thus, it is suffice to prove that a.s. In otherwise case, there must exists two positive constants ϵ from (0,1) and T such that
| (4) |
Hence there is an integer k 1 ≥ k 0, such that
Next, we will define a C 2-function in such a way that
| (5) |
It is to be noted that the H is a nonnegative function, and it can be verified from the fact that . Assume that k 0 ≤ K and 0 < T are arbitrary. Upon applying formula to Eq. (5) gives us
| (6) |
In Eq. (6), is defined by the following equation
| (7) |
Thus,
| (8) |
Setting for k ≥ k 1 and by Eq. (4), P(Ωk) ≥ ϵ. Note that for each ω from Ωk there must exist one or more than one S(τk, ω), I(τk, ω), Q(τk, ω) which equals or k. As a result H(S(τk), I(τk), Q(τk)) is no less then or . Therefore,
| (9) |
By using Eqs. (4) and (8), we can write
| (10) |
Here 1Ω(ω) represent the indicator function of Ω. Approaching k to ∞ will lead us to the contradiction showing that a.s.
Theorem 2
For any initial data (S(0), I(0), Q(0)) in the solution of the developed model (1) will remains in with unit probability, that is, for all t ≥ 0 almost surely.
Proof: Letting and assuming that a solution of the proposed stochastic COVID-19 pandemic model (1) exists in I, then for each time t ∈ I, solution of the first equation of the model (1) becomes
| (11) |
which implies that S(t) > 0. Solving the second equation of the model (1) gives us
which simply means that 0 ≤ I(t). It is handy to shown that 0 < Q(t). Hence for all t ≥ 0, which proves the conclusion.
Remark 1
Clearly, Theorems 1 and 2 guarantees that for the initial data there is a unique global solution (S(t), I(t), Q(t)) of the model (1) in almost surely. Thus
(12) By solving the differential inequality Eq. (12) yields
(13) If then a.s. Thus the desired region for the problem becomes
(14)
In upcoming study, we shall always assume that (S(0), I(0), Q(0)) ∈ Ω* unless otherwise stated.
5. The Extinction and Stationary Distribution of COVID-19 model
As for as the stochastic systems are concerned, they have no endemic equlibria. Thus, the stability analysis cannot be used as a tool for studying the disease’ persistence. As a result, one must turn his/her attention to the existence/uniqueness theory of the stationary distribution which in some sense, will work for persistence of the disease. For this purpose, we will cite a famous result from Hasminskii [23].
Let
| (15) |
Lemma 1
[16,17](Strong Law of Large Number) Letbe a continuous real valued local martingale and vanishing atthen
(16)
Lemma 2
[16,17]Assume that f ∈ C[[0, ∞) × Ω(0, ∞)] and F(t) is in C([0, ∞) × Ω, R). If there exist three positive constant λ, λ 0 and T, such that
(17)
5.1. Stationary distribution
Suppose that X(t) is a regular Markov process (time-homogeneous) in whose dynamics is given by
The diffusion matrix is of the form
Lemma 3
([16,17]).The process X(t) has the unique stationary distribution m(.) if there exist a bounded domain with regular boundary such thatclosurehaving the following properties
- 1.
In the open domain U and in its neighborhood, the least eigenvalue of A(t) is bounded away from zero.
- 2.
If x ∈ RdU, the mean time τ (at which a path starting from x reach the set U) is finite, and Sup x ∈ k Exτ < ∞ for each compact subset K ⊂ Rn. Further, if f(.) is an integrable function having measure π, then
for all x ∈ Rd.
Define a parameter
| (18) |
Theorem 3
The solution (S(t), I(t), Q(t)) of the model (1) is ergodic as well as there is a unique stationary distribution π(.) whenever .
Proof
In order to verify condition (2) of Lemma 3, we need to develop a non-negative function For this, we will first define
where c 1, c 2 are the positive constant and need to be determined later on. By using the Ito’s formula and the proposed model (1), we obtain
(19) Therefore, we have
The above implies that
Let
Namely
(20) Consequently
(21)
In addition, we can obtain
where the constant c 3 > 0 to be determined at later stages. It is handy to show that
(22) where . The next step is to prove that V 2(S, I, Q) has one and only one minimum value V 2(S 0, I 0, Q 0).
The partial derivative of V 2(S, I, Q) with respect to S, I, Q is as follow
It could be easily obtain that V 2 have unique stagnation point . Moreover, the Hesse matrix of V 2(S, I, R) at (S 0, I 0, Q 0) is
Obviously, the Hesse matrix is positive definite. Thus, V 2(S, I, Q) has a minimum value V 2(S 0, I 0, Q 0). According to Eq. (22) and from the continuity of V 2(S, I, Q), we can say that V 2(S, I, Q) has one and only one minimum value V 2(S 0, I 0, Q 0) inside .
Next, we will define a non-negative function as follows
Applying the Itô’s formula and using the proposed model, we get
(23) which leads to the following assertion
(24) where
The next step is to define the set
where δi > 0 for are infinitesimally small constants to be determined later. For the sake of simplicity, we will divide the whole into the following regions
Next, we shall prove that LV(S, I, Q) < 0 on which is the same as displaying it on the above-mentioned six regions.
Case 1. If (S, I, Q) ∈ D 1, then by Eq. (24), we get
We can choose a small constant δ 1 > 0 in such a way that so we can get for each (S, I, Q) ∈ D 1.
Case 2. If (S, I, Q) ∈ D 2, then from Eq. (24), we can obtain
Let we can select enough large c 3 > 0 and as small as possible δ 1 > 0 such that so we can get for any (S, I, Q) ∈ D 2.
Case 3. If (S, I, Q) ∈ D 3, then from Eq. (24), we can obtain
By choosing small δ 3 > 0 such that so we can get for each (S, I, Q) ∈ D 3.
Case 4. If(S, I, Q) ∈ D 4, from Eq. (24), we can obtain
We can select enough small δ 4 > 0 such that so we can get for each (S, I, Q) ∈ D 4.
Case 5. If (S, I, Q) ∈ D 5, from Eq. (24), we can obtain
We can choose sufficiently small δ 5 > 0 such that so we can get for any (S, I, Q) ∈ D 5.
Case 6. If (S, I, Q) ∈ D 6, from Eq. (24), we can obtain
We can choose sufficiently small δ 6 > 0 such that so we can get for any (S, I, Q) ∈ D 6.
Thus, we reach to the conclusion that there exist a constant W > 0 such that
Hence
(25) Assume that and τx is that time at which a path starting from x reach to the set D,
Upon integration of both sides of the inequality (25) from zero to τ (n)(t), taking expectation, and then by applying Dynkin’s formula, we obtain
Since V(x) is non-negative, therefore
Following the proof of Theorem 3 we have . Alternatively, one can say that the system (1) is regular. Thus, if we let t → ∞ and n → ∞ then we have τ(n)(t) → τxalmost surely.
Accordingly, with the help of Fatou’s lemma we get
Obviously, sup x ∈ K Eτx < ∞, where K being a compact subset of . It directly proves the condition (ii) of Lemma 3.
Moreover, the diffusion matrix for system (1) is given by
Choosing we can obtain that
It means, condition (1) of Lemma 3 also holds.
Concluding the above discussion, we can say that Lemma 3 guarantees that system (1) is ergodic as well as it has one and only one stationary distribution. Hence the proof, (Figs. 1 , 2 , 3 ). □
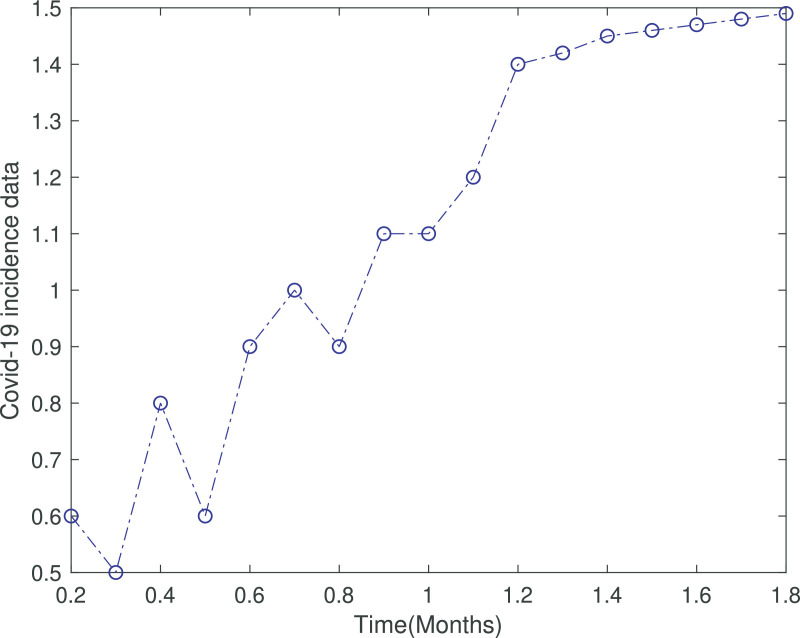
Fig. 1.
The incidence data of covid-19 from Khyber Pakhtunkhwa, Pakistan.
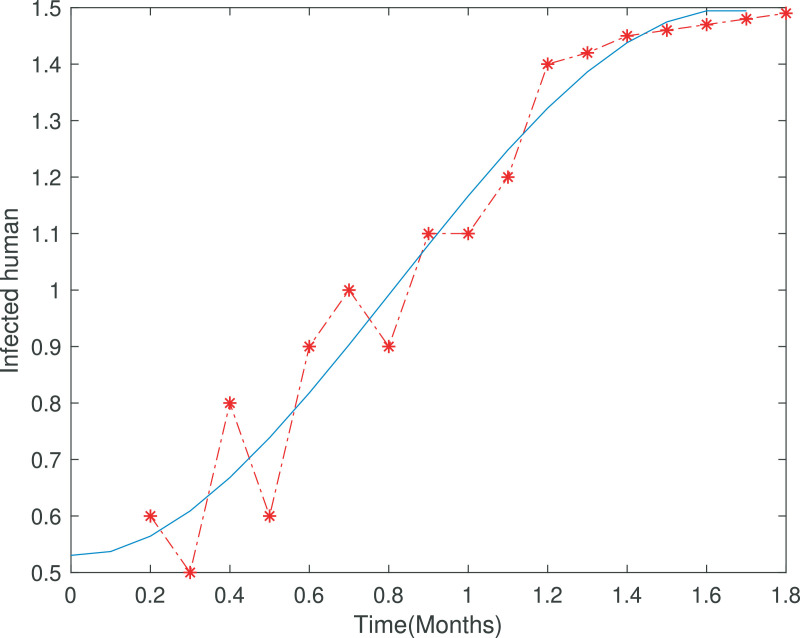
Fig. 2.
Covid-19 model comparison with real data of Khyber Pakhtunkhawa, Pakistan.
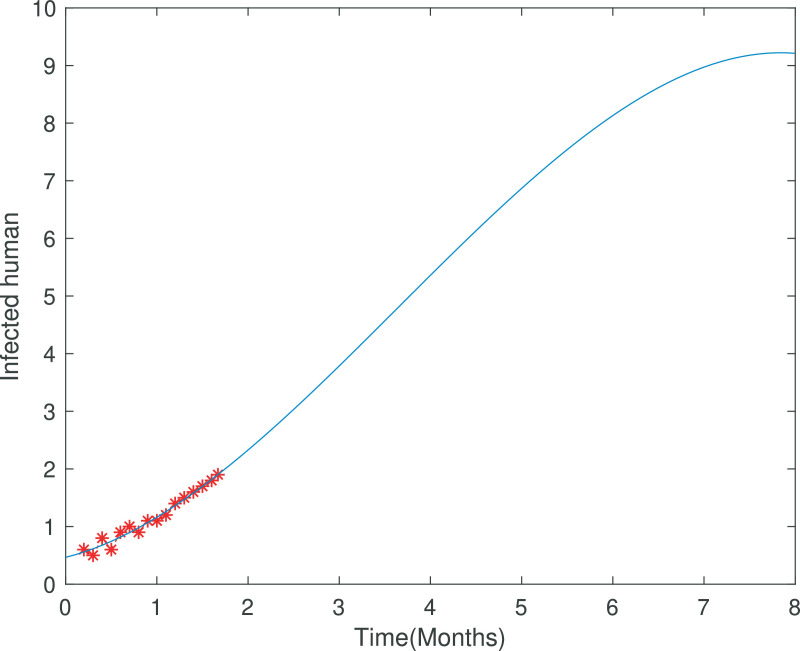
Fig. 3.
The incidence data of covid-19 from Khyber Pakhtunkhwa, Pakistan.
5.2. Extinction
Theorem 4
Assume that (S(t), I(t), Q(t)) be a solution of the developed COVID-19 model (1) along with initial data then a.s. Further
and
(26)
Proof
From the proposed model (1) we can write
(27) The integration of both sides yields
(28) We define
(29) where
(30) Clearly, M(t) is a continuous local martingale with . From relation (28), we have a.s. for all positive t. One can observe that Q(t) and A(t) are continuous adapted increasing processes on t ≥ 0 with we get limt → ∞ X(t) ≤ ∞ a.s. Thus
(31) Thus, Eq. (4) holds. Keeping in view relation (31), it is handy to show that
Setting
Because of the quadratic variation, we can write
(32) By using Lemma 1 (see for detail [16], [17], [18]) and (31), we get
Similarly, we also get
which proves Eq. (26) and hence the Lemma 4.1.
For the purpose of disease’ extinction, we have to state and prove the following theorem. □
Theorem 5
Suppose that (S(t), I(t), Q(t)) be a solution of the COVID-19 model (1) along with subsidiary conditions . If a. then
a.s., (I(t) approaches zero exponentially a.s., i.e., the COVID-19 infection will dies out from the community with unit probability). Moreover
(33)
Proof
To prove the theorem, we shall apply direct integration to the proposed stochastic COVID-19 model (1). First of all, we will apply the formula to the second equation of system (1)
(34)
(35) By integrating relation (35) from zero to t and dividing it by t leads to
(36)
(37)
(38)
(39) By using the theorem related to large number for local martingales, we obtain
By taking the limit superior of both sides
It means that whenever then
and
Now from the model (1)
(40) Adding respective sides of equations (40), we get
(41) Calculation leads to
(42) where
(43) From the last equation of system (40) we have
(44) which implies
(45) we thus obtain
consequently, (42) implies
which proves the result. □
6. Case Study and Numerical simulation
6.1. Case study of Khyber Pakhtunkhwa, Pakistan
As other provinces of Pakistan, the Khyber Pakhtunkhawa province is also effected by covid-19 virus. So we fit our model to the real data of Khyber Pakhtunkhawa (Pakistan) covid-19 cases from 9th April to 2nd June 2020. We use Matlab minimization technique and consider the following initial value in which E(0) and Q(0) are estimated while the remaining values are taken from [24] and Table 1
Table 1.
Parameters value
In Figure 1 the total cases of covid-19 has been depicted from 9th April to 2nd June 2020, which becomes one month and 24 days. In Figure 2 we fitted the real data with the infected class of our covid-19 model which clearly shows the appropriateness of behavior of the infected class. Figure 3 shows long time behavior of the covid-19 cases vs time (months). We can see that the data is accurately fit to the model curve and further, one can observe that the cases with time on long term behavior grows exponentially. This case could be alarming that the incidence may increases further in the coming months if the government not applied the proper optimal strategies.
6.2. Numerical Simulation
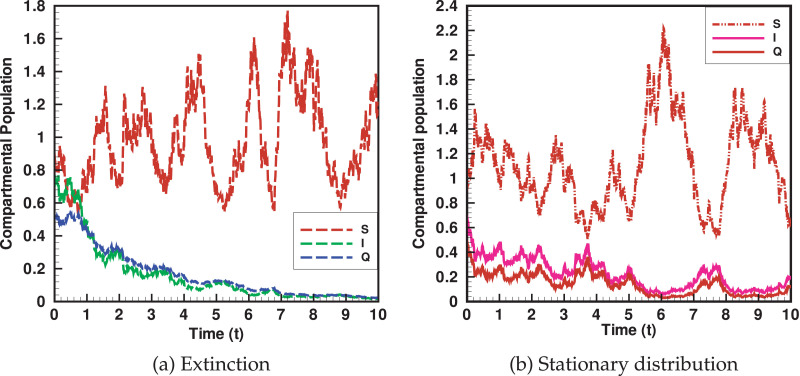
In the current section, we shall perform the numerical simulation of the developed coronavirus stochastic epidemic model. The well know stochastic Runge-Kutta (RK) method for the purposes of numerical findings will be used. This analysis will verify our derived analytical results and will show the influence and effect of noise intensity. We assume the numerical value of the parameters with biological feasibility to verify the extinction result are as: while the numerical values for the intensity of white noise are supposed to be and . Moreover, we also assume some initial sizes of populations densities i.e., and units of time 0-10. The long-term predictions and behavior of the model is presented in Fig. 4 . More, precisely Fig. 4a represent the dynamics of susceptible, infected and quarantined population. The dynamics of susceptible population is shown by red dashed line, while the infected with corona virus and quarantine are respectively represented by green dashed and blue dashed lines. Clearly we noted that the disease will extinct i.e., the infection of novel corona virus vanishes exponentially with increasing the value of white noise intensity. However there will be always susceptible population in the case of extinction. In a similar fashion, we assume the following parameter value and the strong effect of white noise to show the permanence or stationary distribution i.e., and while the initial population sizes will be taken as above. The simulation carried out for this are presented in Fig. 4b. Again the three trajectories in Fig. 4b, which represent the dynamics of susceptible (red dashed), infected (purple solid) and quarantined population (red solid), which show that the model maintain the persistence i.e., there will be always susceptible, infected and quarantine individuals. Hence it could be noted from the simulation analysis that the white noise intensity have a great influence on the dynamics of the disease: as when the value of the white noise intensity increases the infection will decreases, while on the other hand if the value of the white noise intensity decrease, the infection will increases.
Fig. 4.
The graphical results show the extinction and stationary distribution of the COVID-19 epidemic.
7. Conclusion
The novel COVID-19 is one of the severe disease in the world and till today there is no proper treatment. It could be also noted that majority of real world phenomenon are not simply deterministic, and contain randomness. With the help of stochastic theory, we developed a model for the novel COVID-19 keeping in view the characteristic of the disease to investigate the transmission dynamics with changing population environment. By adopting the idea of stochastic Lyapunov functions theory, the existence and positivity are shown. We established a suitable stochastic Lyapunov function to perform the above activity. The extinction as well as the stationary distribution have been further discussed to find the conditions that how to extinct the disease. It could be noted that the there is a great influence of noise intensity on the COVID-19 transmission. Clearly it has been observed, that the extinction of COVID-19 infected individuals increases with increasing the noise strength, while decreases disease persisting. All the above analytical findings are supported graphically with the help of numerical simulation and therefore concluded that the work reveals stochastic analysis is a better approach to study the dynamics of infectious disease particularly novel COVID-19 etc, because there are many factor which varies time to time and place to place. In future, the model can be further extended by adding an exposed class. One can also fractionalize the model by using Atangana-Baleanu, Caputo or Caputo-Fabrizo operator. Not only this can but researcher may apply optimal control technique to minimize the infected people by choosing suitable optimal control variables.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by the HEC.
References
- 1.Lai, Chih-Cheng, Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-cov-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. IntJAntimicrobAgents. 2020:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groot D., Raoul J., Baker S.C., Baric R.S., Brown C.S., Drosten C., et al. Commentary: middle east respiratory syndrome coronavirus (MERS-cov): announcement of the coronavirus study group. JVirol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group W.M.-C.R. State of knowledge and data gaps of middle east respiratory syndrome coronavirus (MERS-cov) in humans. PLoS Currents. 2013;5 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chikungunya E.C.D. C. Factsheet for health professionals available from:http://ecdc.europa.eu/en/
- 5.World Health O. Coronavirus disease 2019 (COVID-19): situation report. 2020. 67
- 6.Organization W.H. Coronavirus disease 2019 (COVID-19): situation report. 2020. 85
- 7.Perko, Lawrence . Vol. 7. Springer Science & Business Media; 2013. Differential equations and dynamical systems. [Google Scholar]
- 8.Arnold, Ludwig. Stochastic differential equations. 1974. New York.
- 9.Wu, Zunyou, McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. Jama. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.Din, Anwarud, Li Y., Liu Q. Viral dynamics and control of hepatitis b virus (HBV) using an epidemic model. Alexandria Eng J. 2020;59(2):667–679. [Google Scholar]
- 11.Khan T., Zaman G. Classification of different hepatitis b infected individuals with saturated incidence rate. Springerplus. 2016;5 doi: 10.1186/s40064-016-2706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atangana, Abdon Modelling the spread of COVID-19 with new fractal-fractional operators: can the lockdown save mankind before vaccination? Chaos Solitons Fractals. 2020;136:109860. doi: 10.1016/j.chaos.2020.109860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan, Altaf M., Abdon A. Modeling the dynamics of novel coronavirus (2019-ncov) with fractional derivative. Alexandria Eng J. 2020 [Google Scholar]
- 14.Chen, Yu, Cheng J., Jiang Y., Liu K. A time delay dynamic system with external source for the local outbreak of 2019-ncov. Appl Anal. 2020:1–12. [Google Scholar]
- 15.Gray A., Greenhalgh D., Hu L., Mao X., Pan J. A stochastic differential equation SIS epidemic model. SIAM J Appl Math. 2011;71(3):876–902. [Google Scholar]
- 16.Zhou Y., Zhang W., Yuan S. Survival and stationary distribution of a SIR epidemic model with stochastic perturbations. Appl Math Comput. 2014;244:118–131. [Google Scholar]
- 17.Lu Q. Stability of SIRS system with random perturbations. Physica A. 2009;388(18):3677–3686. [Google Scholar]
- 18.Ji C., Jiang D. Threshold behaviour of a stochastic SIR model. Appl Math Model. 2014;38(21):5067–5079. [Google Scholar]
- 19.Din, Anwarud, Liang J., Zhou T. Detecting critical transitions in the case of moderate or strong noise by binomial moments. Phys Rev E. 2018;98(1):12114. doi: 10.1103/PhysRevE.98.012114. [DOI] [PubMed] [Google Scholar]
- 20.Liang, Junhao, Din A., Zhou T. Linear approximations of global behaviors in nonlinear systems with moderate or strong noise. JChemPhys. 2018;148(10):104105. doi: 10.1063/1.5012885. [DOI] [PubMed] [Google Scholar]
- 21.Dalal N., Greenhalgh D., Mao X. A stochastic model for internal HIV dynamics. J Math Anal Appl. 2008;341(2) [Google Scholar]
- 22.Edmunds W.J., Medley G.F., Nokes D.J. The transmission dynamics and control of hepatitis b virus in the Gambia. Stat Med. 1996;15(20):2215–2233. doi: 10.1002/(SICI)1097-0258(19961030)15:20<2215::AID-SIM369>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Khasminskii R. Vol. 66. Springer Science & Business Media.; 2011. Stochastic stability of differential equations(book) [Google Scholar]
- 24.http://covid.gov.pk/.