Abstract
Objective
COVID-19 patients may present mild symptoms. The identification of paucisymptomatic patients is paramount in order to interrupt the transmission chain of the virus. Olfactory loss could be one of those early symptoms which might help in the diagnosis of COVID-19 patients. In this study, we aim to develop and validate a fast, inexpensive, reliable and easy-to-perform olfactory test for the screening of suspected COVID-19 patients.
Study design
Phase I was a case–control study and Phase II a transversal descriptive study.
Subjects and methods
Olfaction was assessed with the ethyl alcohol threshold test and symptoms with visual analogue scales. The study was designed in two phases: In Phase I, we compared confirmed COVID-19 patients and healthy controls. In Phase II, patients with suspected COVID-19 infection referred for testing were studied.
Results
275 participants were included in Phase I, 135 in Phase II. The ROC curve showed an AUC of 0.749 in Phase I, 0.737 in Phase II. The cutoff value which offered the highest amount of correctly classified patients was ≥ 2 (10% alcohol) for all age intervals. The odds ratio was 8.19 in Phase I, 6.56 in Phase II with a 75% sensitivity. When cases report normal sense of smell (VAS < 4), it misdiagnoses 57.89% of patients detected by the alcohol threshold test.
Conclusion
The olfactory loss assessed with the alcohol threshold test has shown high sensitivity and odds ratio in both patients with confirmed COVID-19 illness and participants with suspected SARS-CoV-2 infection.
Keywords: COVID-19, SARS-CoV-2, Olfaction, Sniff test, Olfactory impairment
Introduction
Coronaviridae can cause a wide spectrum of clinical manifestations. Previously known SARS included the Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS), but their most recent manifestation is the corona virus disease 2019 (COVID-19).
There is variation in the clinical presentation of the disease as the majority of the infected patients are asymptomatic and those who exhibit the illness may have a wide spectrum of symptoms [1]. Many patients may present mild symptoms, and up to four fifths of cases are asymptomatic [2]. Therefore, the identification of asymptomatic and paucisymptomatic patients is paramount in order to interrupt the transmission chain of the virus [3].
The availability of the SARS-CoV-2 diagnosis test is limited. Therefore, in several countries, these tests are being used only on high-risk patients, and not on possible contact or mild cases. We must rely, instead, on other screening tests in order to identify those patients who do not manifest severe symptoms but are possible carriers. Recent experience suggests that impairment of olfaction could be one of those early symptoms which might help diagnose COVID-19 patients [4].
Loss of sense of smell has been reported in the course of SARS and other coronavirus diseases [5]. In fact, the American Academy of Otolaryngology, early on in the crisis, suggested that anosmia, hyposmia and dysgeusia should be added to list of symptoms used in screening for SARS-CoV-2 infection and urged precautionary isolation for individuals with these symptoms. However, it is not clear how this should be explored [6]. Other authors, like Hopkins et al., have suggested that studies focusing on testing paucisymptomatic individuals are highly needed [7].
In the present study, we aim to develop and validate a fast, inexpensive, reliable and easy-to-perform olfactory test for the screening of suspected COVID-19 patients. Hereby we propose a modification of the alcohol sniff test developed by Davidson et al. [8].
Subjects and methods
Objectives
The study was designed in two phases: Phase I was developed in order to identify a cutoff value comparing confirmed COVID-19 patients and healthy controls. The objective of Phase II was to ascertain the validity of the test.
Sample selection
The sample was recruited from five tertiary referral hospitals in Spain (Hospital Complex of Santiago de Compostela; Hospital 12 de Octubre; Donostia University Hospital; Hospital Ramon y Cajal and Fuenlabrada Hospital).
Patients were recruited in two phases:
Phase I: Hospitalized adult patients with confirmed COVID-19 diagnosis. Controls were healthy volunteers from hospital personnel in areas not-related to COVID-19 patient caring and their healthy family members, with no symptoms of COVID-19.
Phase II: Adult patients with suspected SARS-CoV-2 infection referred to the hospital for testing. This sample comprised close contacts, patients with mild symptoms related to COVID-19 and workers in key areas (homes for the elderly, supermarkets and police force). Moderate and severe cases were not included in this phase since these cases were tested in the emergency room.
Participants were selected consecutively until the estimated goal sample size was reached. Patients not willing to participate were not included.
In both phases, participants with known olfactory impairment previous to the onset of the COVID-19 pandemic were excluded. Patients who did not exhibit sufficient cognitive skills to collaborate were also considered an exclusion criterion. Cognitive skills were assessed through the interview and the patient’s medical records. Patients with previous sinonasal inflammatory disorders, nasal surgery, traumatic brain injury or any other disease linked to olfactory dysfunction were also excluded.
Evaluation
Visual analogue scale (VAS)
The scale was read out loud for the patients by the researcher. It was used in both phases and included nasal obstruction, dysosmia (at the beginning/ currently), dysgeusia (at the beginning/ currently), rhinorrhea, nasal dryness, coughing, pharyngeal pain and headache. VAS score was rated from 0 (minimum) to 10 (maximum).
The VAS for sinonasal symptoms is currently being used in clinical practice, based on the EPOS guidelines, to classify chronic rhinosinusitis into mild (VAS 0–3), moderate (VAS > 3–7) and severe (VAS > 7–10) diseases [9]. Using this criterion, we stratified patients according to VAS smell loss: normosmic-mild olfactory loss (VAS 0–3), moderate olfactory loss (VAS 4–6) and severe olfactory loss (VAS 7–10).
Alcohol threshold test (ATT)
We propose a modification of Davidson’s alcohol sniff test [8]. In this study, Ethyl alcohol was diluted in saline solutions with five different concentrations (10%, 25%, 50%, 70% and 96%). It was prepared by the researchers using 100 ml saline bags following the dilutions included in Table 1.
Table 1.
Reference values to prepare 100 ml alcohol dilutions
| 10% | 25% | 50% | 70% | 96% | |
|---|---|---|---|---|---|
| Alcohol 70% | |||||
| Alcohol (ml) | 14.3 | 35.7 | 71.4 | 100 | Not applicable |
| Saline (ml) | 85.7 | 64.3 | 28.6 | 0 | Not applicable |
| Alcohol 96% | |||||
| Alcohol (ml) | 10.4 | 26.0 | 52.1 | 72.9 | 100 |
| Saline (ml) | 89.6 | 74.0 | 47.9 | 37.1 | 0 |
Upper table: dilutions using 70% alcohol/Lower table: dilutions using 96% alcohol
For each patient, researchers prepared five different gauzes soaked with 3–5 ml of the solution (according to the gauze size). It was presented to the patient in a solid surface. Participants were instructed to smell the gauze as many times as they needed, hold it at 3 cm of their nose and try to identify the one with the lowest concentration. This distance is estimated to be localized at the labiomental fold to avoid trigeminal irritation. Participants were instructed to smell the gauze in no particular order. The weakest concentration of alcohol a participant could detect was recorded as a threshold score (TS) of 1, 2, 3, 4 and 5 for the 10%, 25%, 50%, 70% and 96% alcohol concentrations, respectively. It was not a forced choice answer. If the participant was not able to detect 96% alcohol, a TS of 6 was recorded.
COVID-19 diagnosis
The COVID-19 patient status was diagnosed using polymerase chain reaction (PCR). It is currently considered the Gold Standard test [10].
Statistical analysis
Statistical analysis was conducted with STATA for Macintosh v. 15.1 (StataCorp ®). Normality of all the quantitative variables was assessed with Shapiro–Wilk test.
Comparability between hospitals was assessed with Anova and Kruskal–Wallis test for quantitative variables and Chi-square for qualitative variables. Data are presented as mean ± standard deviation (SD). We developed a ROC curve with subgroup analysis for age intervals for both phases. Participants were stratified according to age into three groups: 30–49, 50–69 and ≥ 70 years.
Data from VAS scores and threshold scores were compared using the Wilcoxon rank-sum test for patients/controls (Phase I) and Covid + / Covid – (Phase II). Comparison between hyposmia and dysgeusia was assessed with a Chi-square test.
Ethical considerations
The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. The study protocol was approved by the Research and Ethics Committee of the Hospital Complex of Santiago de Compostela reference 2020/189.
Results
Phase I
A total of 275 participants were included, 129 cases and 146 controls. Mean age was 55.31 ± 15.43 years (range 19–90), 47.64% male.
There were differences between hospitals regarding age (effect size 4.49; p = 0.004), but not for gender (p = 0.59) or alcohol thresholds (p = 0.07).
Under Shapiro–Wilk testing only VAS score for initial dysgeusia showed a normal distribution.
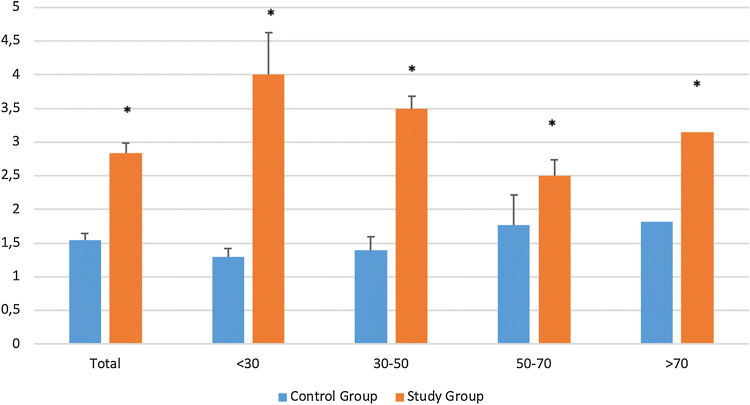
Distribution of the TS in the study group (n = 129) and control group (n = 146) is summarized in Table 2 and Fig. 1.
Table 2.
Threshold scores (TS) in the study group and control group
| TS 1 | TS 2 | TS 3 | TS 4 | TS 5 | TS 6 | |
|---|---|---|---|---|---|---|
| Study group | 27.91% | 23.26% | 15.50% | 10.85% | 14.73% | 7.75% |
| Control group | 76.03% | 9.59% | 5.48% | 3.42% | 2.74% | 2.74% |
Fig. 1.
Prevalence of threshold scores according to age. *P < 0.05 comparison between study and control groups
ROC curve – Phase I
The ROC curve analysis is shown in Fig. 2 and Table 3. The ROC curve for all included participants showed an AUC of 0.749. The cutoff value which offered the highest amount of correctly classified patients was ≥ 2 for all age intervals. The age intervals with the highest AUC was < 30 and 30–49 years. Using a TS of ≥ 2, the odds ratio was 8.19 (CI 95% 4.61–14.61).
Fig. 2.
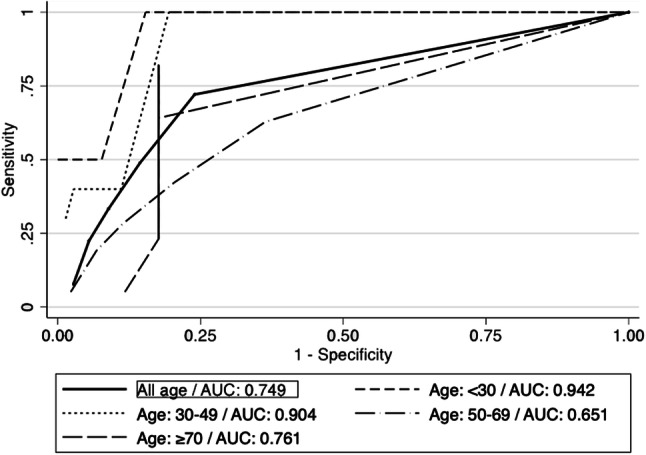
ROC curves—Phase I
Table 3.
Phase I detailed report of sensitivity, specificity, AUC stratified by cutoff value and age interval
| Thresholds | Age (years) | Area under the curve (AUC) | Sensitivity | Specificity | Correctly classified |
|---|---|---|---|---|---|
| ≥ 2 | All | 0.749 | 72.09% | 76.03% | 74.18% |
| < 30 | 0.942 | 100.00% | 84.62% | 86.67% | |
| 30–49 | 0.904 | 100.00% | 80.56% | 82.93% | |
| 50–69 | 0.651 | 62.68% | 63.64% | 63.11% | |
| ≥ 70 | 0.761 | 82.05% | 82.35% | 82.14% | |
| ≥ 3 | All | 0.749 | 48.84% | 85.62% | 68.36% |
| < 30 | 0.942 | NA | NA | NA | |
| 30–49 | 0.904 | 40.00% | 88.89% | 82.93% | |
| 50–69 | 0.651 | 42.31% | 79.55% | 55.74% | |
| ≥ 70 | 0.761 | 64.10% | 82.35% | 69.64% | |
| ≥ 4 | All | 0.749 | 33.33% | 91.10% | 64.00% |
| < 30 | 0.942 | 50.00% | 92.31% | 86.67% | |
| 30–49 | 0.904 | 40.00% | 94.44% | 87.80% | |
| 50–69 | 0.651 | 28.21% | 88.64% | 50.00% | |
| ≥ 70 | 0.853 | 41.03% | 82.35% | 53.57% |
NA not applicable
VAS for cases and controls
Means of VAS distribution for cases and controls are shown in Table 4. There were statistically significant differences for olfactory loss, dysgeusia and coughing.
Table 4.
VAS score in Phase I (means and SD)
| Olfactory loss today | Olfactory loss (initial) | Nasal obstruction | Taste loss today | Taste loss (initial) | Rhinorrhea | Nasal dryness | Cough | Pharyngeal pain | Headache | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case | 3.17 (3.69) | 4.80 (4.41) | 2.25 (3.09) | 2.46 (3.78) | 4.51 (4.32) | 1.53 (2.68) | 2.70 (3.18) | 4.79 (3.31) | 0.90 (2.02) | 1.64 (2.84) |
| Control | 0.80 (1.91) | NA | 1.69 (2.83) | 0.61 (1.52) | NA | 1.88 (2.80) | 2.49 (3.28) | 2.20 (3.10) | 1.59 (2.63) | 2.76 (3.52) |
| P value | < 0.001* | NA | 0.101 | < 0.001* | NA | 0.173 | 0.442 | < 0.001* | 0.007 | 0.008 |
NA not applicable Bold and asterisk (p < 0.05)
A Spearman correlation analysis in the study group revealed a moderate correlation between VAS for anosmia and VAS for dysgeusia (r = 0.47; p < 0001).
When cases report normal sense of smell (VAS < 4), it misdiagnoses 57.89% of patients detected by the alcohol threshold test (TS ≥ 2) (Table 5).
Table 5.
Comparison between visual analogue scale (VAS) for olfactory loss and alcohol threshold test (ATT) in cases
| ATT 1 | ATT 2 | ATT 3 | ATT 4 | ATT 5 | ATT 6 | |
|---|---|---|---|---|---|---|
| Normal–mild (VAS 0–3) (%) | 42.11 | 32.89 | 11.84 | 2.63 | 6.58 | 3.95 |
| Moderate (VAS 4–6) (%) | 16.67 | 22.22 | 5.56 | 38.89 | 11.11 | 5.56 |
| Severe (VAS 7–10) (%) | 2.86 | 2.86 | 28.57 | 14.29 | 34.29 | 34.29 |
Phase II
A total of 135 participants were included; 29 of those were tested positive for SARS-CoV-2. Mean age was 49.16 ± 16.41 years (range 19–88); 43.70% were male.
There were no differences between hospitals regarding alcohol thresholds (p = 0.42), gender (p = 0.25) or age (p = 0.10).
Under the Shapiro–Wilk test, none of the variables showed a normal distribution.
Alcohol threshold test (ATT)
The mean value of the threshold score was 3.21 ± 1.9 for SARS-CoV-2 positives and 1.8 ± 1.48 for negatives (p < 0.001).
ROC curve – Phase II
The ROC curve analysis is shown in Fig. 3 and Table 6. The ROC curve for all included participants showed an AUC of 0.737. The cutoff value which offers the highest sensitivity was ≥ 2 for all age intervals. However, contrary to Phase I, the correctly classified patients vary depending on the age intervals and thresholds used. The age interval with the highest AUC is 50–69 years.
Fig. 3.
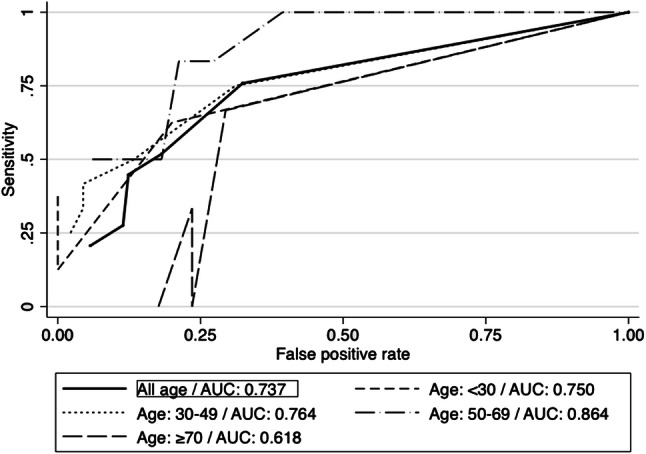
ROC curves—Phase II
Table 6.
Phase II detailed report of sensitivity, specificity, AUC stratified by cutoff value and age interval
| Thresholds | Age | Area under the curve (AUC) | Sensitivity | Specificity | Correctly classified |
|---|---|---|---|---|---|
| ≥ 2 | All | 0.737 | 75.86% | 67.62% | 69.40% |
| < 30 | 0.750 | 62.50% | 80.00% | 72.22% | |
| 30–49 | 0.764 | 75.00% | 68.89% | 70.18% | |
| 50–69 | 0.864 | 100.00% | 60.61% | 66.67% | |
| ≥ 70 | 0.618 | 66.67% | 70.59% | 70.00% | |
| ≥ 3 | All | 0.737 | 51.72% | 81.90% | 75.37% |
| < 30 | 0.750 | 37.50% | 100.00% | 72.22% | |
| 30–49 | 0.764 | 50.00% | 86.67% | 78.95% | |
| 50–69 | 0.864 | 83.33% | 72.73% | 74.36% | |
| ≥ 70 | 0.618 | NA | NA | NA | |
| ≥ 4 | All | 0.737 | 44.83% | 87.62% | 78.36% |
| < 30 | 0.750 | 25.00% | 100.00% | 66.67% | |
| 30–49 | 0.764 | 41.67% | 95.56% | 84.21% | |
| 50–69 | 0.864 | 83.33% | 78.79% | 79.49% | |
| ≥ 70 | 0.618 | 33.33% | 76.47% | 70.00% |
NA not applicable
Using the TS of ≥ 2, the odds ratio was 6.56 (CI 95% 2.38–19.75), with a sensitivity of 75% and specificity of 67.62%.
VAS score for positive and negative patients (Table 7)
Table 7.
VAS score in Phase II (means and SD): (means): NA (not applicable)
| Olfactory loss today | Olfactory loss (initial) | Nasal obstruction | Taste loss today | Taste loss (initial) | Rhinorrhea | Nasal dryness | Cough | Pharyngeal pain | Headache | |
|---|---|---|---|---|---|---|---|---|---|---|
| Positive | 4.59 (3.96) | NA | 2.38 (3.11) | 4.07 (3.98) | NA | 1.97 (2.68) | 3.17 (3.39) | 3.14 (3.08) | 1.76 (2.40) | 3.90 (3.52) |
| Negative | 1.08 (2.42) | NA | 1.69 (2.85) | 1.19 (2.35) | NA | 2.40 (3.23) | 2.81 (3.43) | 3.21 (3.42) | 2.34 (3.00) | 4.07 (3.74) |
| P value | < 0.001* | NA | 0.142 | < 0.001* | NA | 0.666 | 0.464 | 0.998 | 0.427 | 0.849 |
Bold and asterisk (statistically significant differences)
SARS-CoV-2-positive patients reported higher VAS than SARS-CoV-2-negative patients for anosmia and dysgeusia. There were no differences between positive and negative patients for the rest of VAS symptoms.
A Spearman correlation analysis between VAS for anosmia and VAS for dysgeusia in SARS-CoV2 positives revealed a 0.67 positive correlation (p < 0.001).
Discussion
In the present study, we found a strong association between hyposmia and COVID-19 infection in both confirmed and suspected COVID-19 patients. According to our results, in the current epidemiological context, patients diagnosed with hyposmia, defined as an ATT score of < 2, have a more than six times higher probability of being infected by SARS-CoV2 than healthy controls.
This test also showed a high sensitivity and specificity in Phase II (suspected patients). These data strongly point to hyposmia as a symptom of COVID-19 and suggest that testing is feasible using smell assessment methods, such as ATT.
There were no differences regarding ATT scores between hospitals neither in Phase I nor in Phase II, which can be interpreted as this test not being operator dependent. This implies that it could be implemented in any facility dealing with COVID-19 patients.
It must be noted that several objective and widely used tests are currently available [11]. In fact, the University of Pennsylvania Smell Identification Test (UPSIT) has recently been used to test COVID-19 patients and controls [12]. However, they might not be accessible in the context of a global health crisis because of delivery times, cost and the fact that they must be disposable in order to prevent contagion. Ethyl alcohol has previously been used to test the sense of smell8 and it is widely available and affordable. Furthermore, ATT is easy and fast to perform, which makes it a useful tool during this pandemic.
It should be kept in mind that ethyl alcohol can stimulate the trigeminal nerve. It is of paramount importance to perform the test with the gauze distant from the nose, to prevent excessive trigeminal stimulation. Although there are no normative data, we suggest, based on clinical practice, holding the gauze at approximately 3 cm from the nose, in the labiomental fold.
The objective behind the use of alcohol for testing in this COVID-19 health crisis is making use of its availability in any emergency room, and the ease in controlling its different concentrations.
To the best of our knowledge, this is the first study to use a smell test to explore olfaction in subjects suspected of being infected by SARS-CoV-2. Previous reports were based on anecdotal case reports or questionnaires and survey-based studies, with the exception of Moein et al. who performed the UPSIT test in a relatively small sample of 60 confirmed COVID-19 patients [12].
A Chinese cohort study reported a prevalence of anosmia of 5.1% and 5.6% of ageusia in COVID-19 patients [13, 14]. However, subsequent European series reported considerable higher values [4, 15, 16]. In particular, a large-scale survey across 12 European countries revealed a higher prevalence, reporting 85.6% and 88.0% olfactory and gustatory dysfunctions, respectively [15]. Our results are in line with those reported by Lechien et al. in their survey-based study [15]. Using the herein selected cutoff threshold value of 2, we found a 72.09% incidence of hyposmia in the Phase I study group. Interestingly, the only available American series reported similar data, with 68% self-reported olfactory loss in COVID-19 patients [17].
At the present time, there is only one published paper in which olfaction was assessed using a sniffing test [12], performed in Iran. They found that 98% of confirmed patients presented some degree of olfactory loss, compared to 18% of controls.
Differences between series may reflect a different behaviour of the SARS-CoV2 or, more probably, differences in the sampling and examination processes.
Regarding controls, according to the OLFACAT study, performed in Spain, olfactory dysfunction affects 19% of the general population, including 0.5% anosmia and 17% hyposmia [19]. This is in line with previous European published results [20] and our study (Phase I controls).
The role of questionnaires to assess olfaction
Regarding the VAS score, in Phase I there were differences between patients and controls for coughing, dysosmia and dysgeusia, but not for nose obstruction or rhinorrhea. These same results were found in Phase II with differences for dysosmia and dysgeusia but not for rhinorrhea or nasal obstruction. This fact is of paramount importance, since other sinonasal inflammatory disorders may cause olfactory loss. Nevertheless, they tend to be accompanied by rhinorrhea and nasal obstruction, which does not seem to be common in our COVID-19 sample. Similar results were found in a recent study where patients reporting sudden olfactory loss were tested for SARS-CoV-2 infection [21].
Previous survey-based studies in COVID-19 patients have not differentiated between anosmia and hyposmia [4, 22]. However, experimental infection of humans with other coronaviruses produced not only anosmia but also hyposmia [23]. Therefore, hyposmia should be specifically addressed when exploring olfaction. Previous studies have revealed that some patients may misdiagnose their ability to smell. Consequently, the authors suggest that patients should be instrumentally evaluated [24]. Moein et al. who evaluated olfaction with the UPSIT test in a case–control study, arrived at the same conclusion. They found that 29% of patients self-reported loss of smell, while it rose to 98% when assessed with the UPSIT test [12].
Our results support the examination of the smell function with psychophysical tests. In this study, we found that 57.89% of patients reporting normal sense of smell suffered olfactory loss according to a TS of less than 2, and 13.16% presented severe olfactory loss (TS of 4, 5 or 6). This is in line with previous evidence, which suggests that a significant amount of patients who suffer a loss of the sense of smell are unaware of it [25].
Gold Standard test
In this study, the reverse transcription polymerase chain reaction (RT-PCR) was assumed to be the Gold Standard test for COVID-19 diagnosis. However, early reports pose the sensitivity of this test at 72% [26]. Furthermore, it is highly dependent on the technique followed to recollect the sample, since samples with low viral dose may be classified as a false-negative result [27].
In fact, in this study, some anosmic/ hyposmic patients in Phase II, initially classified as negative, were tested positive eventually, when they developed severe symptoms and were retested.
Type of olfactory loss
Olfaction is dependent upon the first and fifth cranial nerves. While the qualitative odour sensations are conducted by the olfactory nerve, the somatosensory sensations are transmitted by the trigeminal nerve [28].
When assessing olfaction, physicians try to differentiate between both nerves. Trigeminal nerve function is explored with irritants (acetic acid, ammonia and alcohol, among others), while the olfactory nerve is explored with aromatic fragrances. The threshold needed to stimulate each nerve is different, and the nasal trigeminal system is assumed to be less sensitive than the olfactory system [29]. Apart from stimulating the trigeminal nerve, alcohol has a characteristic odour easily recognized by almost all participants. Therefore, when it is close to the nose, it stimulates the trigeminal nerve, causing a somatosensory feeling (itching) [8], and when it is presented at a prudent distance, it stimulates the olfactory nerve producing a qualitative odour sensation (alcohol odour).
Study limitations
There is a selection bias as these data do not represent the general population. This study was performed in patients with moderate severity of COVID-19 infection (Phase I), and participants with suspected COVID-19 infection (Phase II). It was not technically possible to test the general population. Therefore, these data should be interpreted with caution.
Under normal circumstances, a physical examination (anterior rhinoscopy and nasal endoscopy) would have been necessary to discard other conditions which may impair olfactory function. However, in order to ensure the safety of the examiners and given our limited resources, nasoendoscopy was not performed in this setting as there is some evidence that special protective equipment is necessary before any upper respiratory endoscopy is conducted [30].
We did not include distractors for the alcohol test. It would be advisable to include a distractor with saline to prevent false negatives.
We did not include a validated olfactory test. However, despite we have used the smell as diagnostic tool, it should be kept in mind that in this study we were validating a COVID-19 test, not an olfactory test. Therefore, we used PCR as Gold Standard test. Future well-designed studies using validated psychophysical olfactory test are needed to consider the ATT as an olfactory test. By now, it is only planned to be used as a screening test for COVID-19.
Strengths
This is the first large-scale study to use a widely available psychophysical olfactory test to assess suspected and confirmed SARS-CoV-2-infected patients. It was carefully designed in two phases to overcome the difficulties of sample selection. It is also a multicentric study encompassing five hospitals, and the results showed no differences between them, which reflects that the method is non-operator dependent. All our data are in line with previous European and North American series, both for cases and controls.
The second phase of this study was aimed at the target population, asymptomatic or paucisymptomatic patients. None of the previous studies have tested olfaction within this sample, since testing is usually limited [7].
Other authors have highlighted that survey-based studies are prone to selection bias both by age and symptoms [7]. This study overcomes this limitation.
Conclusion
Patients with SARS-CoV-2 infection have shown self-reported and instrumentally evaluated olfactory loss. The olfactory loss assessed with the alcohol threshold test showed high sensitivity and odds ratio both in patients with confirmed COVID-19 illness and participants with suspected SARS-CoV-2 infection. These data pose the alcohol threshold test as a useful tool for SARS-CoV-2 screening. It is a simple, quick and low-cost method to assess olfactory loss. It can be easily performed without any training and in almost any environment.
People with suspected SARS-CoV-2 (exposure or mild symptoms) can be easily checked with the alcohol threshold test. If the results are pathological, they should undergo further SARS-CoV-2 testing.
Funding
None.
Compliance with ethical standards
Conflict of interest
I Alobid is consultant for Roche, Menarini, GSK, MSD, Mylan and Novartis. The other authors declare no disclosure or conflict of interest. This work is part of the research completed by Byron Maldonado-Alvarado, MD, to obtain a PhD degree.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020;2(369):m1375. doi: 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- 3.Cheng MP, Papenburg J, Desjardins M, Kanjilal S, Quach C, Libman M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus-2: a narrative review. Ann Intern Med. 2020;172(11):726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaira LA, Salzano G, Deiana G, De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki M, Saito K, Min W-P, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American academy of otolaryngology head and neck surgery. Coronavirus Disease 2019: Resources [Internet]. American Academy of Otolaryngology-Head and Neck Surgery. Available from: https://www.entnet.org/content/coronavirus-disease-2019-resources
- 7.Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020;58(3):295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 8.Davidson TM, Murphy C. Rapid clinical evaluation of anosmia. The alcohol sniff test. Arch Otolaryngol Head Neck Surg. 1997;123(6):591–4. doi: 10.1001/archotol.1997.01900060033005. [DOI] [PubMed] [Google Scholar]
- 9.Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps. Rhinology. 2020;58(S29):1–464. doi: 10.4193/Rhin20.600. [DOI] [PubMed] [Google Scholar]
- 10.Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, Liu Y, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66(4):549–55. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doty RL, Smith R, McKeown DA, Raj J. Tests of human olfactory function: principal components analysis suggests that most measure a common source of variance. Percept Psychophys. 1994;56(6):701–707. doi: 10.3758/BF03208363. [DOI] [PubMed] [Google Scholar]
- 12.Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL (2020) Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. https://pubmed.ncbi.nlm.nih.gov/32301284/ [DOI] [PMC free article] [PubMed]
- 13.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q et al (2020) Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China JAMA Neurol [Internet] Available from: https://pubmed.ncbi.nlm.nih.gov/32275288/ [DOI] [PMC free article] [PubMed]
- 14.Wang Z, Yang B, Li Q, Wen L, Zhang R (2020) Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis Off Publ Infect Dis Soc Am. Available from: https://pubmed.ncbi.nlm.nih.gov/32176772/ [DOI] [PMC free article] [PubMed]
- 15.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/32253535/ [DOI] [PMC free article] [PubMed]
- 16.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L et al (2020) Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross sectional study. Clin Infect Dis Off Publ Infect Dis Soc Am [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/32215618/ [DOI] [PMC free article] [PubMed]
- 17.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS (2020) Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol [Internet]. Available from: https://pubmed.ncbi.nlm.nih.gov/32279441/ [DOI] [PMC free article] [PubMed]
- 18.Sugiura M, Aiba T, Mori J, Nakai Y. An epidemiological study of postviral olfactory disorder. Acta Oto-Laryngol Suppl. 1998;538:191–196. doi: 10.1080/00016489850182918. [DOI] [PubMed] [Google Scholar]
- 19.Mullol J, Alobid I, Mariño-Sánchez F, Quintó L, de Haro J, Bernal-Sprekelsen M et al (2012) Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open 2(6):1–13 [DOI] [PMC free article] [PubMed]
- 20.Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the skövde population-based study. Laryngoscope. 2004;114(4):733–737. doi: 10.1097/00005537-200404000-00026. [DOI] [PubMed] [Google Scholar]
- 21.Lechien J, Cabaraux P, Chiesa-Estomba C, Khalife M, Plzak J, Hans S et al (2020) Objective olfactory testing in patients presenting with sudden onset olfactory dysfunction as the first manifestation of confirmed COVID-19 infection. Available from: https://www.medrxiv.org/content/10.1101/2020.04.15.20066472v1
- 22.Gane SB, Kelly C, Hopkins C (2020) Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 58(3):299–301 [DOI] [PubMed]
- 23.Akerlund A, Bende M, Murphy C. Olfactory threshold and nasal mucosal changes in experimentally induced common cold. Acta Otolaryngol (Stockh) 1995;115(1):88–92. doi: 10.3109/00016489509133353. [DOI] [PubMed] [Google Scholar]
- 24.Adams DR, Wroblewski KE, Kern DW, Kozloski MJ, Dale W, McClintock MK, et al. Factors associated with inaccurate self-reporting of olfactory dysfunction in older US adults. Chem Senses. 2017;42(3):223–231. doi: 10.1093/chemse/bjw108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehling E, Nordin S, Espeseth T, Reinvang I, Lundervold AJ. Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2011;26(3):260–269. doi: 10.1093/arclin/acr019. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y, Long L, Zhang D, Yan T, Cui S, Yang P, et al. Potential false-negative nucleic acid testing results for Severe Acute Respiratory Syndrome Coronavirus 2 from thermal inactivation of samples with low viral loads. Clin Chem. 2020;66(6):794–801. doi: 10.1093/clinchem/hvaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cometto‐Muñiz JE, Simons C (2003) Trigeminal Chemesthesis. In: Handbook of olfaction and gustation. 2nd ed. New York: Marcel Dekker, Inc, p 1089–1112
- 29.Hummel T, Frasnelli J. The intranasal trigeminal system. Handb Clin Neurol. 2019;164:119–134. doi: 10.1016/B978-0-444-63855-7.00008-3. [DOI] [PubMed] [Google Scholar]
- 30.Van Gerven L, Hellings PW, Cox T, Fokkens W, Hopkins C, Hox V, et al. Personal protection and delivery of rhinologic and endoscopic skull base procedures during the COVID-19 outbreak. Rhinology. 2020;58(3):289–294. doi: 10.4193/Rhin20.119. [DOI] [PubMed] [Google Scholar]