To the Editor,
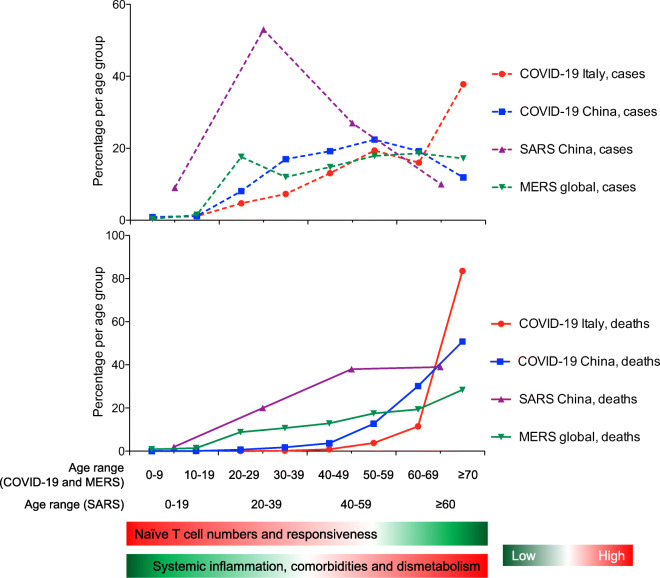
So far, little attention has been paid to the link between immunosenescence and the dramatic mortality rate of coronavirus disease 2019 (COVID-19) in older age groups. Indeed, the number of cases of COVID-19, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is very low among children and teenagers, in contrast to the increased frequency in adults and the elderly, who are also more at risk of developing very serious symptoms and death (Guan et al. 2020; Wu and McGoogan 2020). As shown in Fig. 1, a similar epidemiological profile was observed during previous coronavirus (severe acute respiratory syndrome coronavirus 1, SARS-CoV-1, and Middle east respiratory syndrome coronavirus, MERS-CoV) outbreaks (Jia et al. 2009; Salamatbakhsh et al. 2019). Notably, the same trend was also noted during West Nile virus and, with some exceptions in very young children, Ebolavirus outbreaks (Bower et al. 2016; Hayes et al. 2005). Likely this phenomenon is multifactorial. For instance, in elderly individuals with severe COVID-19, associated comorbidities are much more prevalent (Guan et al. 2020). In addition, the progressive accumulation of senescent cells during life may play a role in the vulnerability of old people to COVID-19, resulting in reduced functionality of the organs, such as the lungs, and facilitating conditions for the development of fibrosis. Moreover, senescent cells can generate a pro-inflammatory environment, referred to as SASP (for senescence-associated secretory phenotype), which includes many inflammatory cytokines (e.g., interleukin-6) and contributes to the basal hyperinflammatory status characteristic of the old person. This hyperinflammatory status might influence the expression of ACE2, CD147, cyclophilins, CD26, and other CoV-associated molecules in human tissues, thus favoring viral entry (Radzikowska et al. 2020). It likely also constitutes an already unbalanced pro-inflammatory background, on which the development of an exacerbated inflammatory response and acute respiratory distress syndrome may be facilitated upon SARS-CoV-2 infection.
Fig. 1.
Age-distribution of numbers of cases and deaths during coronavirus outbreaks and age-associated changes in immune profile. The age range for COVID-19 (Italy and China) and MERS is different from that of SARS due to a different aggregation in source datasets. Data for COVID Italy are from the COVID-19 Task Force of the Department of Infectious Diseases and the IT Service Istituto Superiore di Sanità, update of April 13, 2020; data for COVID China are from Wu et al. 2020; data for SARS China are from Jia et al. 2009; data for MERS global are from Salamatbakhsh et al. 2019
An undoubtedly common important factor for the rapid spread of these viruses is that they are emerging pathogens introduced from scratch into the human population never previously exposed to them. The induction of de novo immune responses against such viruses relies on their recognition by naïve, and not memory, T cells. Since, the pool of naïve T cells decreases with age, reaching very low numbers in the elderly (Briceno et al. 2016), we believe that this may contribute to the age-dependent development of the disease and to the greater severity of symptoms and death in the elderly, characterizing these emerging infections.
Indeed, several pieces of evidence highlight the importance of T cell responses for CoV control. Results from murine models show that virus-specific CD4+ and CD8+ T cells are essential for CoV clearance (Chen et al. 2010; Zhao et al. 2010), which is, instead, delayed in mice lacking T cells or in old animals experiencing an age-dependent decrease of virus-specific CD8+ T cells (Chen et al. 2010; Zhao et al. 2014; Zhao et al. 2011). The appearance of interferon (IFN)-γ secreting CD4+ and CD8+ T cells specific for the structural proteins of CoV has been observed in the lungs of infected mice and is associated with viral clearance (Chen et al. 2010; Zhao et al. 2009). Lung-infiltrating CoV-specific CD8+ T cells display high cytotoxic potential (Zhao et al. 2010; Zhao et al. 2009), while depletion of CD4+ T cells results in a diminished neutralizing antibody response along with higher viral titers in the lungs (Chen et al. 2010). Together, these data suggest that CD8+ T cells are important for the killing of CoV-infected cells and CD4+ T cells play a key role in the support of CoV-specific antibody responses and in the cell recruitment in the lung (Chen et al. 2010; Zhao et al. 2010; Zhao et al. 2009).
In humans, MERS-CoV, SARS-CoV-1, and SARS-CoV-2-infected patients with severe manifestations exhibit a pronounced T cell loss (Jiang et al. 2020; Li et al. 2004; Liu et al. 2020; Min et al. 2016; Qin et al. 2020; Wang et al. 2020; Wu et al. 2020) and CD4+ and CD8+ T cell numbers negatively correlate with the levels of pro-inflammatory cytokines associated with worst prognosis (Diao et al. 2020). Importantly, T cell count normalization coincides with the improvement of clinical conditions (Diao et al. 2020; Gu et al. 2005; Li et al. 2004). It has also recently been shown that activated effector CD4+ and CD8+ T cells appear in the blood of COVID-19 subjects a few days after the development of symptoms and precede their resolution, likely contributing to the infection clearance (Thevarajan et al. 2020). Moreover, the amount of virus-specific CD4+ T cells directly correlates with humoral responses (Grifoni et al. 2020; Ni et al. 2020), and a larger proportion of tissue-resident CD8+ T cells with effector potential has been reported in the bronchoalveolar lavage fluids of patients with moderate infection compared with severely ill subjects (Liao et al. 2020), whose circulating T cells are instead characterized by high expression of inhibitory checkpoints (Diao et al. 2020).
Naïve T cells represent the primary source to mount a necessary adaptive immune response against emerging viruses, such as CoV. However, the quantity of naïve T cells is progressively reduced and lymphopoiesis compromised with old age, due to thymic involution as well as defects of the lymphopoietic progenitors (Fali et al. 2018). The reduced naïve T cell pool may affect the global TCR repertoire and its diversity, and thus the capacity of an old immune system to detect neoantigens. Moreover, elderly people show a decreased number of naïve T cells, and the intrinsic properties and capacity of these cells to be activated and to differentiate are affected (Fali et al. 2019; Li et al. 2012). As a consequence, old individuals present with reduced priming capacity and impaired responses against neoantigens (Briceno et al. 2016; Nikolich-Zugich 2014). In addition, some of the comorbidities associated with aging are characterized by low naïve T cell frequencies (Rattik et al. 2019) and by metabolic alterations which may, in turn, negatively affect T cell metabolism and functionality (Nicoli et al. 2018). Overall, these observations indicate that successful generation of primary responses against novel viruses from the naïve T cell pool is compromised with aging and associated comorbidities, likely contributing to the age-related severity of emerging infections, such as that caused by SARS-CoV-2.
It is however important to highlight that the protection conferred by functional epitope-specific T cells should not be confused with the hyperactivation of the immune system that accounts for the severe tissue immune injury in COVID-19 patients with fatal prognosis. This detrimental inflammatory process, which is a further hallmark of aging, is the likely consequence of innate immune mechanisms. Although a high proportion of hyperactivated T cells have been found in COVID-19 subjects, the plasmatic cytokine profile, but not the T cell activation levels, distinguished between non-severe and severe SARS-CoV-2-infected subjects (Qin et al. 2020). Considering the relatively high median age of these patients, it is reasonable to speculate the presence of a low number of epitope-specific naïve T cells that could be primed. This suggests that most T lymphocytes infiltrating the lungs are not virus-specific but potentially harmful. If confirmed, this scenario would resemble that of other infections, such as HIV, where disease progression, although directly related with T cell hyperactivation, is inversely related with the number and functions of virus-specific T cells (Appay and Sauce 2008; Nicoli et al. 2016).
We think that the preservation of the naïve T cell pool and de novo T cell responsiveness may thus constitute a future priority to be addressed by researchers for the development of preventive strategies against emerging infections. Also, the enhancement of virus-specific T cell responses by immunotherapeutics avoiding unspecific activation and inflammation may be important in already infected patients before the onset of severe symptoms to prevent disease exacerbation and promote viral clearance. Finally, the activation of T cell responses, which have been shown to be long-lasting after SARS-CoV-1 and MERS-CoV infections, should also be considered in the development of vaccines against COVID-19. However, frail and elderly subjects have poor post-vaccination immune responses. For this reason, a great attention should be paid to mechanisms that impair SARS-CoV-2-specific immune responses, as the same mechanisms could negatively affect the effectiveness of future COVID-19 vaccines in these populations.
Code availability
Not applicable
Authors’ contributions
FN, MS, DP, PM, RG, VA, and AC: conceived the idea; FN, MS, and DP: analyzed the data; FN, VA, and AC: wrote the manuscript; FN, MS, DP, PM, RG, VA, and AC: edited and approved the manuscript.
Data availability
Not applicable
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
All authors agreed on submission of the current version for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Victor Appay and Antonella Caputo shared senior authorship.
References
- Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- Bower H, Johnson S, Bangura MS, Kamara AJ, Kamara O, Mansaray SH, Sesay D, Turay C, Checchi F, Glynn JR. Exposure-specific and age-specific attack rates for Ebola virus disease in Ebola-affected households, Sierra Leone. Emerg Infect Dis. 2016;22:1403–1411. doi: 10.3201/eid2208.160163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceno O, et al. Reduced naive CD8(+) T-cell priming efficacy in elderly adults. Aging Cell. 2016;15:14–21. doi: 10.1111/acel.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010;84:1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B, et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fali T, Fabre-Mersseman V, Yamamoto T, Bayard C, Papagno L, Fastenackels S, et al. Elderly human hematopoietic progenitor cells express cellular senescence markers and are more susceptible to pyroptosis. JCI Insight. 2018:3. 10.1172/jci.insight.95319. [DOI] [PMC free article] [PubMed]
- Fali T, Papagno L, Bayard C, Mouloud Y, Boddaert J, Sauce D, Appay V. New insights into lymphocyte differentiation and aging from telomere length and telomerase activity measurements. J Immunol. 2019;202:1962–1969. doi: 10.4049/jimmunol.1801475. [DOI] [PubMed] [Google Scholar]
- Grifoni A, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020. 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed]
- Gu J, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery SP, O’Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia N, Feng D, Fang LQ, Richardus JH, Han XN, Cao WC, de Vlas SJ. Case fatality of SARS in mainland China and associated risk factors. Trop Med Int Health. 2009;14(Suppl 1):21–27. doi: 10.1111/j.1365-3156.2008.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, et al. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J Infect Dis. 2020. 10.1093/infdis/jiaa252. [DOI] [PMC free article] [PubMed]
- Li T, Qiu Z, Zhang L, Han Y, He W, Liu Z, Ma X, Fan H, Lu W, Xie J, Wang H, Deng G, Wang A. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis. 2004;189:648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yu M, Lee WW, Tsang M, Krishnan E, Weyand CM, Goronzy JJ. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020. 10.1038/s41591-020-0901-9. [DOI] [PubMed]
- Liu Z, et al. Lymphocyte subset (CD4+, CD8+) counts reflect the severity of infection and predict the clinical outcomes in patients with COVID-19. J Infect. 2020. 10.1016/j.jinf.2020.03.054. [DOI] [PMC free article] [PubMed]
- Min CK, Cheon S, Ha NY, Sohn KM, Kim Y, Aigerim A, Shin HM, Choi JY, Inn KS, Kim JH, Moon JY, Choi MS, Cho NH, Kim YS. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020. 10.1016/j.immuni.2020.04.023. [DOI] [PMC free article] [PubMed]
- Nicoli F, Chachage M, Clowes P, Bauer A, Kowour D, Ensoli B, Cafaro A, Maboko L, Hoelscher M, Gavioli R, Saathoff E, Geldmacher C. Association between different anti-Tat antibody isotypes and HIV disease progression: data from an African cohort. Bmc Infect Dis. 2016;16:344. doi: 10.1186/s12879-016-1647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoli F, et al. Naive CD8(+) T-cells engage a versatile metabolic program upon activation in humans and differ energetically from memory CD8(+) T-cells. Front Immunol. 2018;9:2736. doi: 10.3389/fimmu.2018.02736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J. Aging of the T cell compartment in mice and humans: from no naive expectations to foggy memories. J Immunol. 2014;193:2622–2629. doi: 10.4049/jimmunol.1401174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed]
- Radzikowska U, et al. Distribution of ACE2, CD147, cyclophilins, CD26 and other SARS-CoV-2 associated molecules in human tissues and immune cells in health and disease. bioRxiv:2020.2005.2014.090332. 2020. 10.1101/2020.05.14.090332.
- Rattik S, et al. Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diab Vasc Dis Res. 2019;16:270–280. doi: 10.1177/1479164118817942. [DOI] [PubMed] [Google Scholar]
- Salamatbakhsh M, Mobaraki K, Sadeghimohammadi S, Ahmadzadeh J. The global burden of premature mortality due to the Middle East respiratory syndrome (MERS) using standard expected years of life lost, 2012 to 2019. BMC Public Health. 2019;19:1523. doi: 10.1186/s12889-019-7899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nature Medicine. 2020. 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed]
- Wang F, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020. 10.1001/jama.2020.2648. [DOI] [PubMed]
- Wu C, Chen X, Cai Y, Xia J’, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- Zhao J, Zhao J, Van Rooijen N, Perlman S. Evasion by stealth: inefficient immune activation underlies poor T cell response and severe disease in SARS-CoV-infected mice. PLoS Pathog. 2009;5:e1000636. doi: 10.1371/journal.ppat.1000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhao J, Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol. 2010;84:9318–9325. doi: 10.1128/JVI.01049-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, et al. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci U S A. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable