Abstract
Background
Distal sensory peripheral neuropathy (DSPN) is a complication of human immunodeficiency virus (HIV). We estimate DSPN prevalence in 7 resource-limited settings (RLSs) for combination antiretroviral therapy (cART)–naive people living with HIV (PLWH) compared with matched participants not living with HIV and in PLWH virally suppressed on 1 of 3 cART regimens.
Methods
PLWH with a CD4+ count <300 cells/mm3 underwent standardized neurological examination and functional status assessments before and every 24 weeks after starting cART. Matched individuals not living with HIV underwent the same examinations once.
Associations between covariates with DSPN at entry were assessed using the χ2 test, and virally suppressed PLWH were assessed using generalized estimating equations.
Results
Before initiating cART, 21.3% of PLWH had DSPN compared with 8.5% of people not living with HIV (n = 2400; χ2(df = 1) = 96.5; P < .00001). PLWH with DSPN were more likely to report inability to work [χ2(df = 1) = 10.6; P = .001] and depression [χ2(df = 1) = 8.9; P = .003] than PLWH without DSPN. Overall prevalence of DSPN among those virally suppressed on cART decreased: 20.3%, week 48; 15.3%, week 144; and 10.3%, week 192. Incident DSPN was seen in 127 PLWH. Longitudinally, DSPN was more likely in older individuals (P < .001) and PLWH with less education (P = .03). There was no significant association between cART regimen and DSPN.
Conclusions
Although the prevalence of DSPN decreased following cART initiation in PLWH, further research could identify strategies to prevent or ameliorate residual DSPN after initiating cART in RLSs.
Keywords: HIV, peripheral neuropathy, resource-limited, antiretroviral therapy
We compared the prevalence of distal sensory peripheral neuropathy in 860 people living with human immunodeficiency virus who were antiretroviral therapy (ART) naive from multiple low-resource countries to that of 2400 HIV– matched controls. The HIV+ participants were randomized to 3 antiretroviral regimens. There was a higher prevalence of neuropathy among the HIV+ participants, which improved following the initiation of ART with subsequent viral suppression. There was no relationship with treatment arm.
Peripheral nerve disorders occur during all stages of human immunodeficiency virus (HIV) infection and are an important source of morbidity [1–3]. Distal sensory peripheral neuropathy (DSPN) is characterized pathologically by symmetrical distal-to-proximal axonal degeneration of sensory nerve fibers [4]. Worldwide DSPN prevalence in combination antiretroviral therapy (cART)–naive persons living with HIV (PLWH) varies widely, from as low as 11% to 56% [5, 6]. cART can improve CD4 count and reduce viral load, decreasing the associated risk of developing DSPN [3, 7]. However, it remains a common neurological complication of HIV [8–10].
DSPN may be asymptomatic or symptomatic [11]. It can be difficult to differentiate between HIV-associated DSPN and antiretroviral toxic neuropathy caused by dideoxynucleoside reverse transcriptase inhibitors (“d-drugs”) [5, 12, 13]. As effective and inexpensive ART, d-drugs were commonly used in resource-limited settings (RLSs) in the past. Less neurotoxic cART regimens are now more widely available. However, a study in Kenya showed a continued burden of DSPN (33.8%) among cART-experienced PLWH who were never exposed to d-drugs [14]. Exposure to protease inhibitors (amprenavir and lopinavir) has also been associated with a small risk of DSPN [15]. However, a review of PLWH initiating cART found that the risk of DSPN was only increased by protease inhibitor use if the regimen included at least 1 neurotoxic nucleoside reverse transcriptase inhibitor [9].
Additional risk factors for DSPN must be considered in the diagnosis, including older age [16], substance abuse [17], and diabetes mellitus [9]. The presence of risk factors in RLSs may differ from Western contexts due to differences in cART guidelines, nutrition, environmental exposures, use of neurotoxic medications, and endemic diseases [18]. A recent study in Zambia found higher rates of DSPN in PLWH and low dietary diversity, history of syphilis, certain drug use (ciprofloxacin and metronidazole), and history of tuberculosis (TB), while older age and lower level of education were associated with DSPN in HIV− participants [19]. TB is common among PLWH, and TB treatment can lead to neurotoxic pyridoxine deficiency [20, 21]. Micronutrient deficiencies may also be common in the context of HIV and can lead to mitochondrial toxicity, increasing the susceptibility for nerve damage among PLWH [22, 23].
As a cause of chronic neuropathic pain, DSPN contributes to unemployment, depression, and frequent medical visits [24]. A study in Rwanda found a high rate of HIV-associated DSPN (40.5%), and those with DSPN had significantly poorer scores in the physical and psychological domains of the World Health Organization Quality of Life scale [25]. Impairments in quality of life and productivity are not necessarily linearly correlated with pain and have even been shown to occur with mild neuropathic pain [3, 26]. This creates both clinical and economic incentives to improve prevention and treatment of DSPN.
The International Neurological Study (INS, AIDS Clinical Trials Group [ACTG] 5199) aimed to estimate the prevalence of DSPN before and after cART initiation. By determining the prevalence of DSPN in age-, sex-, country-, and education-matched HIV– individuals from the International Neurocognitive Normative Study (INNS, ACTG 5271) enrolled at the same sites as their HIV+ counterparts, we were able to better understand the risk of DSPN conferred by HIV itself. There were strict exclusion criteria for both cohorts that ruled out additional causes of DSPN, such as significant illnesses and substance abuse. We used standardized diagnostic measures and analytical strategies to assess the impact of DSPN and covariates.
METHODS
Study Design
HIV+ participants were enrolled in a prospective, observational study that compared the neurological and neuropsychological effects of cART in cART-naive PLWH randomly assigned to 1 of the following regimens: lamivudine/zidovudine + efavirenz (EFV; arm A), emtricitabine (FTC) + atazanavir + didanosine enteric-coated (arm B), and FTC/tenofovir + EFV (arm C). HIV– normative comparison participants were enrolled at HIV voluntary counseling and testing clinics aligned with the sites that participated in INS [27]. INS participants were assessed every 24 weeks for 192 weeks from 2006 to 2010. INNS participants were enrolled from 2011 to 2013 and underwent the same examination once at the same study sites. Baseline and longitudinal results of the neurological examination, without the comparisons between PLWH and HIV– controls and association with covariates, were previously published [28, 29].
Inclusion and Exclusion Criteria
INS participants had to be aged ≥18 years, have documented HIV-1 infection with a CD4+ count <300 cells/mm3, and have no previous use of cART. Exclusion criteria included any active severe psychiatric illness, drug abuse, serious illness within 14 days of study entry, or any other condition that would compromise the person’s ability to participate in the study. INNS participants had to be aged ≥18 years and have tested HIV– within the last 30 days of their examination in addition to the same exclusion criteria as those enrolled in INS.
Neurological Examination
INS and INNS participants underwent a standardized peripheral neuropathy assessment designed to be implemented in RLSs. Site visits were conducted to provide training and quality assurance on a regular basis by a neuropsychologist and a neurologist from the University of North Carolina. Web-based and DVD training modules were also provided, and in-person training was available to site healthcare providers who attended an annual meeting in Washington, DC. DSPN was defined by at least 1 of the following bilateral findings: less than 10 seconds of vibratory sensation using a 128-Hz tuning fork placed on the top of the distal interphalangeal joint of each great toe; failure to feel cold sensation at the base of the great toes; decreased or absent ankle stretch reflexes.
Functional status was assessed by questions on work and psychosocial factors at the beginning of the neurological examination. Functional status was interpreted from the responses to the ability to work and level of fatigue questions. Self-reported symptoms of depression and rated interest level in social activity were used to assess depression.
Statistical Analyses
All significance testing was performed at the .05 level. All reported P values are 2-sided. The associations between DSPN prevalence at entry with depression and functional status were assessed using χ2 analysis. The longitudinal analysis is restricted to PLWH who achieved virologic suppression on their first cART regimen, which allowed for drug substitution in the case of toxicity. Linear and logistic regression models using the generalized estimating equation with an autoregressive correlation structure were constructed to assess treatment effects as well as the associations of other covariates with DSPN, including education. CD4 count at entry (screening) and current CD4 count were considered as continuous variables. Pretreatment HIV-1 RNA was dichotomized as <100 000 c/mL and ≥100 000 c/mL, and as detectable and undetectable using 400 c/mL as the lower detection limit after initiating ART, as determined by the parent study, ACTG A5175. The 95% confidence intervals (CIs) around the estimated odds ratios for the covariate effect on DSPN were used.
RESULTS
Participant Demographics
A total of 860 HIV+ participants were enrolled into INS in Brazil (n = 161), India (n = 184), Malawi (n = 133), Peru (n = 62), South Africa (n = 167), Thailand (n = 73), and Zimbabwe (n = 80). Median (interquartile range) pre-cART CD4 was 173 (98, 232) cells/mm3 and median plasma log-10 HIV-1 RNA was 5.0 c/mL (4.5, 5.5). A total of 289 participants were randomized to arm A, 293 to arm B, and 278 to arm C. Median follow-up on study was 168 weeks (range: 24-192 weeks). Arm B was discontinued by the Data and Safety Monitoring Board in May 2008 because it was virologically inferior to the other regimens. Arm B participants were withdrawn from the DSPN study, which caused a significant drop-off of participants from the visit at week 72 onward.
The 2400 INNS controls were enrolled from the same sites, with a similar distribution by country. They were matched for age (median age of 34 years in INS, 35 years in INNS), sex (53% female in INS, 50% female in INNS), and education (median of 10 years). For additional information on demographics, please refer to previous publications [27–30].
Pre-cART
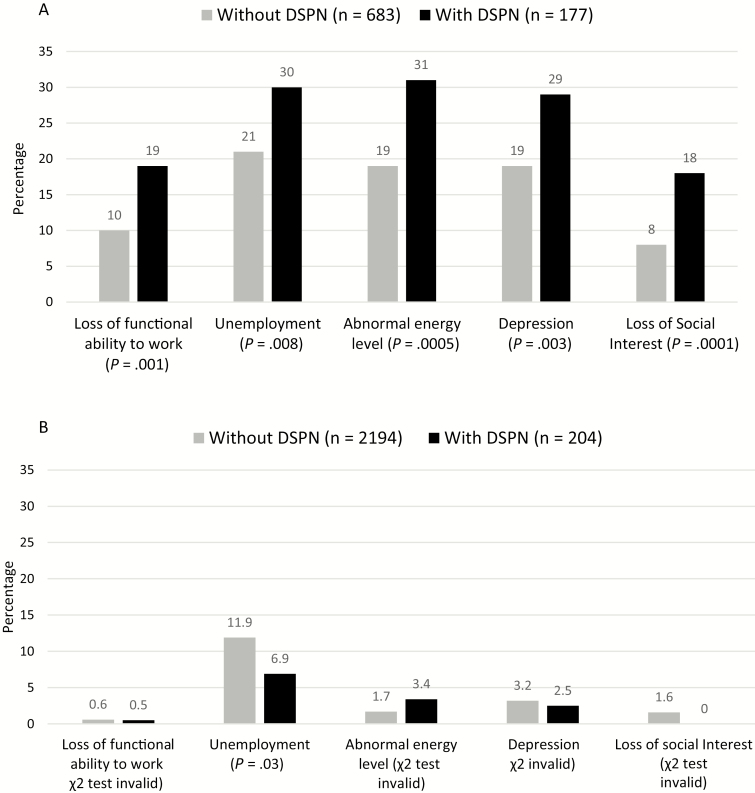
Before beginning cART, 21.3% of PLWH met examination criteria for DSPN compared with 8.5% of HIV– people [χ 2(df = 1) = 96.5; P = .008]. PLWH with prevalent DSPN were more likely to report loss of functional ability to work [χ 2(df = 1) = 10.6; P = .001], unemployment [χ 2(df = 1) = 7.0; P = .0005], and low energy levels [χ 2(df = 1) = 12.1; P = .0005] than the PLWH without DSPN before beginning cART (Figure 1A). Of those with DSPN who noted loss of energy, 38.2% described moderate to severe severity. This compared with 12.3% of PLWH without DSPN reporting moderate to severe energy loss [χ 2(df = 1) = 17.1; P = .002]. PLWH with DSPN were also more likely to report feeling depressed [χ 2(df = 1) = 8.9; P = .003] and to have a loss of social interest [χ 2(df = 1) = 14.6; P = .0001] than PLWH without DSPN.
Figure 1.
A, Proportion of people living with human immunodeficiency virus (HIV). B, people living with HIV with and without distal sensory peripheral neuropathy (DSPN) who have functional complaints and depression at study entry. Gray bars are people with no DSPN, and black bars are people with DSPN.
Figure 1B shows similar comparisons of depression and functional status symptoms between HIV– people with and without DSPN. Small numbers of individuals with DSPN precluded most statistical analysis. More individuals without DSPN were unemployed. However, the statistical significance of this association was far weaker than that seen in the PLWH group.
After cART
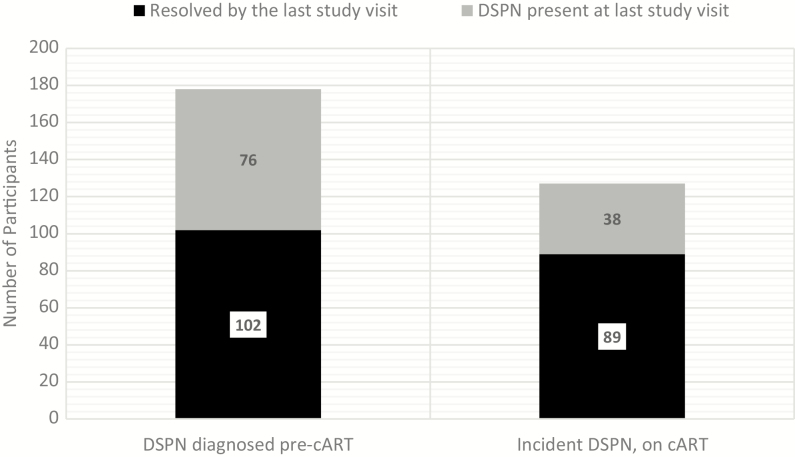
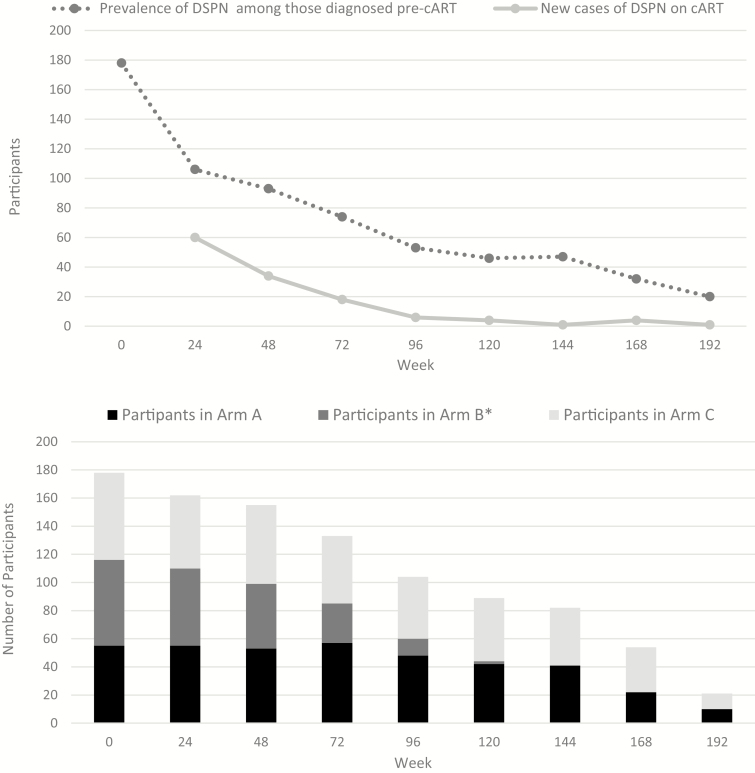
The overall prevalence of DSPN among PLWH decreased after virologic suppression on cART was achieved: 20.3% at week 48, 17.5% at week 96, 15.3% week at 144, and 10.3% at week 192 (Table 1). Of the 178 PLWH with DSPN pre-cART, 102 (57%) cases resolved by their last visit (Figure 2). Incident DSPN was seen in 127 participants during the course of the study (Figure 3A). There was no statistically significant difference between the prevalence of DSPN by arm (Figure 3B).
Table 1.
Prevalence of Distal Sensory Peripheral Neuropathy over the Course of the 192-Week Study
| Neurological dysfunction due to neuropathy over time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of participants (percentage) | |||||||||
| Week | 0 | 24 | 48 | 72 | 96 | 120 | 144 | 168 | 192 |
| No DSPN | 656 (78.7) | 648 (80.0) | 609 (79.7) | 568 (81.1) | 491 (82.5) | 449 (83.5) | 447 (84.7) | 353 (86.7) | 184 (89.8) |
| DSPN diagnosed | 178 (21.3) | 162 (20.0) | 155 (20.3) | 133 (18.9) | 104 (17.5) | 89 (16.5) | 81 (15.3) | 54 (13.3) | 21 (10.2) |
| Total no. evaluated | 834 | 810 | 764 | 701 | 595 | 538 | 528 | 407 | 205 |
| Missinga | 26 | 6 | 8 | 4 | 6 | 3 | 2 | 0 | 0 |
| Total | 860 | 816 | 772 | 706 | 601 | 541 | 530 | 407 | 205 |
Abbreviation: DSPN, distal sensory peripheral neuropathy.
aMissing = participants who did not attend the scheduled visit or did not undergo the complete clinical examination at that visit.
Figure 2.
Outcome of prevalent and incident distal sensory peripheral neuropathy (DSPN) in human immunodeficiency virus–positive participants. Black bars indicate resolution of examination evidence of DSPN, and gray bars indicate continued findings of DSPN. Abbreviations: cART, combination antiretroviral therapy.
Figure 3.
A, Number of people living with human immunodeficiency virus with distal sensory peripheral neuropathy by the initial diagnosis and (B) by treatment arm over time. Abbreviations: cART, combination antiretroviral therapy; DSPN, distal sensory peripheral neuropathy. *Arm B participants were withdrawn from the study in May 2008, coinciding with visits at week 72 and onward; see text.
Longitudinally, DSPN was more likely in older individuals and was less likely with increased time on cART and in those with a higher level of education (Table 2). Gender, cART regimen, pretreatment HIV-1 RNA, and screening CD4 or CD4 count during the course of the study did not influence the likelihood of DSPN.
Table 2.
Longitudinal Associations Between Distal Sensory Peripheral Neuropathy and Covariates
| Analysis of generalized estimating equation parameter estimates | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Estimate | Standard Error | 95% Confidence Limits | Z | Pr > |Z| | ||
| Intercept (reference group) | Comparison | 0.36 | 0.04 | 0.28 | 0.43 | 9.39 | <0.0001 |
| Countrya | Brazil | −0.29 | 0.02 | −0.33 | −0.24 | −12.44 | <0.0001 |
| Country | India | −0.37 | 0.02 | −0.41 | −0.33 | −17.92 | <0.0001 |
| Country | Malawi | −0.34 | 0.02 | −0.38 | −0.31 | −17.2 | <0.0001 |
| Country | Peru | −0.37 | 0.02 | −0.40 | −0.33 | −18.14 | <0.0001 |
| Country | South Africa | −0.33 | 0.02 | −0.38 | −0.29 | −14.57 | <0.0001 |
| Country | Zimbabwe | −0.22 | 0.03 | −0.27 | −0.17 | −8.28 | <0.0001 |
| Age | Every 10 years | 0.02 | 0.01 | 0.009 | 0.033 | 3.35 | 0.0008 |
| Sex (women = r) | Men | −0.001 | 0.01 | −0.02 | 0.02 | −0.13 | 0.89 |
| Education | Every 4 years of education | −0.01 | 0.004 | −0.02 | −0.0009 | −2.18 | 0.03 |
| Baseline DSPN | … | 0.12 | 0.06 | −0.003 | 0.23 | 1.91 | 0.06 |
| Baseline RNA | (Detected or undetected) | −0.002 | 0.005 | −0.01 | 0.007 | −0.48 | 0.63 |
| Screening CD4 count | … | −0.002 | 0.003 | −0.008 | 0.004 | −0.61 | 0.54 |
| Time on study | (Prevalence of DSPN every 24 weeks on study) | −0.007 | 0.001 | −0.009 | −0.004 | −5.28 | <0.0001 |
| Treatment (ART regimen A) | ART regimen B | 0.008 | 0.01 | −0.01 | 0.03 | 0.7 | 0.49 |
| Treatment (ART regimen A) | ART regimen C | 0.01 | 0.01 | −0.01 | 0.03 | 0.97 | 0.33 |
Negative estimates indicate that the covariate confers a decreased risk of DSPN, and positive estimates indicate an increased risk of DSPN. Statistically significant correlations are in bold and shaded.
Abbreviations: ART, antiretroviral therapy; DSPN, distal sensory peripheral neuropathy.
aParticipants from Thailand as the reference group.
DISCUSSION
This multinational study shows that there was substantial prevalence of DSPN in cART-naive PLWH in RLSs. The inclusion of age-, sex-, education-, and community-matched HIV– participants gave us the opportunity to better understand the risk of DSPN conferred by HIV itself. We found that cART-naive PLWH with DSPN were more likely to experience depression and loss of productivity than PLWH without DSPN. A key finding in our study is that the overall prevalence of DSPN among PLWH decreased substantially over the course of the study. Specifically, more than half of the PLWH diagnosed with DSPN at enrollment did not have clinical evidence of DSPN by their last visit. There were 127 participants who developed incident DSPN following cART initiation, most of whom were diagnosed at week 24 and week 48. PLWH who were older were more likely to have DSPN, and those who had a higher level of education and more time with viral suppression on cART were less likely to have DSPN. Longitudinally, DSPN was not related to cART regimen.
The relatively high prevalence of DSPN in untreated PLWH in this study is similar to what has been seen in previous studies [18, 31]. Previous work has shown that PLWH with symptomatic DSPN have increased depressive symptoms and higher self-reported lower limb dysfunction than PLWH without DSPN [32]. Our study differs from previous studies in not specifying whether DSPN was symptomatic. In contrast to the HIV+ participants, we did not find a significant association between depression and DSPN in the HIV– participants. Although this is most likely due to a low prevalence of DSPN, it may also highlight features unique to DSPN in HIV.
Previous studies have established older age as a risk factor for neuropathy [9, 16, 18]; however, the relationship with the level of education is still open to interpretation. A study in Zambia found an association between neuropathy and lower level of education in both PLWH and HIV– participants. The researchers argued that education is a marker of early childhood deprivation, which could increase the risk of adult-onset diseases and altered development [19]. Conducting this study in RLSs, we wanted to understand how disparities in wealth, education, and access to healthcare contributed to health outcomes. Education level is a useful indicator of literacy proficiency and may also influence the ability to access social resources and critical reasoning of health status. Other studies have shown that low socioeconomic status and education attainment negatively impact cART adherence and thus can lead to poor clinical outcomes [33].
Limitations of this study should be considered in interpreting our results. We used a screening examination to diagnose DSPN that has high specificity but low sensitivity [34]. As such, we may have underestimated the prevalence and incidence of DSPN in our cohort. Participants were enrolled in a clinical trial and, as such, were likely to comply with study procedures and adhere to cART over an extended period. This was confirmed with the high number of participants who maintained virologic suppression for a median over about 3 years. Furthermore, most of the sites are in urban areas and may not be representative of the regions in which many HIV+ individuals in RLSs receive their care. While we excluded PLWH and controls who had drug use and severe illnesses that might increase the likelihood of DSPN, we did not assess common non-HIV–related causes of DSPN, such as diabetes or nutritional deficiencies. Thus, we cannot comment on the role of these factors in influencing the risk of prevalent or incident DSPN in PLWH or HIV– people. However, in previous work from this study, PLWH diagnosed with incident pulmonary TB did not have an increased risk of DSPN [30]. The normative group was enrolled after the PLWH to ensure that their demographics were matched, but we do not think that this substantially influenced comparisons between the 2 groups. However, the normative group was not followed longitudinally, and we cannot comment on any longitudinal change in HIV– DSPN. We focused on virologically suppressed participants, and we cannot comment on the influence of lack of virological suppression on risks or progression of DSPN in PLWH. Individuals in arm B, which contained didanosine, were withdrawn from the study because of increased virological failure. Because we did not find a relationship between treatment regimen and prevalent or incident DSPN, we do not think that this biased our results or conclusions. The antiretroviral agents used in this study are less relevant to current regimens in both resource-rich and resource-limited settings, but we believe that our key finding that the overall prevalence of DSPN decreases in virally suppressed individuals remains important, and it is another reason to encourage cART adherence.
It is currently unknown whether early initiation of cART could prevent or alter the course of HIV-associated DSPN. DSPN has been observed in acute [35] and primary [2] HIV infection before cART initiation. Symptomatic DSPN in primary HIV infection was associated with elevated inflammatory markers compared with the asymptomatic DSPN [2]. Thus, it could be argued that although it would be difficult to prevent DSPN in acute HIV infection, early cART initiation might decrease its severity by decreasing systemic inflammation. We found that although the overall prevalence decreased following the initiation of cART, more than one-third of the virally suppressed participants still had DSPN at their last visit, suggesting that the remaining cases of neuropathy are a legacy effect of early, irreversible, HIV-induced nerve damage.
DSPN, particularly when it is asymptomatic, can be easily missed in clinical practice. Our work and the work of others has shown that it is prevalent and interferes with daily activities. Although viral suppression with cART decreases the prevalence of DSPN, the sizable number of individuals with persistent DSPN warrants further investigation and better treatment options. By creating a normative database across RLSs, we were able to uniquely estimate the burden of HIV-associated DSPN and establish covariates, some of which are socioeconomic surrogates. It is our hope that we have laid the framework to generate additional research on DSPN in RLSs and, in particular, to identify reversible risk factors. We need better strategies to prevent or to ameliorate residual DSPN in order to improve the function and daily lives of PLWH in RLSs.
Notes
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Mental Health (NIMH), the National Institutes of Health (NIH), or the institutions with which the authors are affiliated.
Financial support. The project described was supported by the NIMH and the AIDS Clinical Trials Group funded by the NIAID (award U01AI068636) and by the Statistical and Data Analysis Center (grant AI-068634). Thomas Campbell (grant support from NIAID AIDS Clinical Trail Unit [CTU], AI069450). Deise Vieira and Marcus Tulius T Silva (National Institute of Infectolgy-Oswaldo Cruz Foundation [IPEC- FIOCRUZ]; site 12101; CTU grant AI69476). Umesh Lalloo and Rosie Mngqibisa (Durban Adult HIV clinical research site [HIV CRS]; site 11201; CTU grant 5U01AI069426-03). Nagalingeshwaran Kumarasamy and Jabin Sharma (YRGCARE Medical Centre; site 11701; CTU grant AI069432). Virginia M. Kayoyo and Charity Potani (Franklin Kilembe University of North Carolina Project, Kamuzu Central Hospital, Lilongwe; site 12001; CTU grant AI069518). Mauleen Waison and Rachel Mahachi-Parirenyatwa (CRS; site 30313; CTU grant BRS-ACURE-Q-08-00173- TOOI-OOO). Cynthia Firnhaber, Sharla Faesen, and Daphne S. Radebe (Wits HIV Clinical Research Site; Helen Joseph Hospital; site 11101; CTU grant AI069463; BRS-ACURE-Q-07-00143 T006). Thira Sirisanthana and Daralak Tavornprasit (Research Institute for Health Sciences-Chiang Mai University; site 11501; CTU grant AI069399; AACTG.27.5199.06). Maria Siliprandiand Renata Londero (Hospital Nossa Senhora da Conceicao CRS; site 12201; CTU grant 5 U01 AI069401). Anjali A. Joglekar and Srikanth Prasad Tripathy (NARI Pune CRS; site 11601; CTU grant 5U01AI069417-03). Ben Kalonga and Henry Chamba (College of Medicine–Johns Hopkins Project; site 30301; CTU grant U01A1069518). Carlos Mosquera and Rosa Infante (INMENSA-Lince CRS; site 11302; CTU grant 5U01 AI069438-03; BRS- ACURE-Q-07-00141-T001-001). Jorge Sanchez and Juan Carlos Hurtado (Asociación Civil Impacta Salud y Educación; site 11301; CTU grant AI069438; BRS-ACURE-Q-08-00007-T-002). Manisha V. Ghate and Madhura Nene (NARI-NIV Clinic; site 11603; CTU grant 5U01AI069417-03). Raman Gnagakhedkar and Usha Katti (Dr Kotnis Dispensary, NARI; site 11602; CTU grant 5U01AI069417-03). H. J. was funded in part by the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group (grant 1 U01 068634). J. S. received grant support from NIAID (CTU UM1 AI06948-08). Names of AIDS Clinical Trials Group 5199 and 5271 team members who received grant funding to carry out the clinical trial are included in acknowledgement, but since they did not participate in the neuropathy analysis and manuscript writing they where not listed as name authors.
Potential conflicts of interest. R. Murphy is a consultant for Gilead. T. B. C. reports grants from Gilead and personal fees from Gilead, ViiV, and Merck. P. C. reports grants from Gilead, ViiV, and Biogen and personal fees from Gilead, Janssen Cilag, Merck, ViiV, Pfizer, and Biogen. K. R. reports nonfinancial support and personal fees from ViiV outside the submitted work. R. M. reports research grants from the Enhancing Care Foundation through NIH/NIAID. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 22nd International AIDS Conference, Amsterdam, the Netherlands, 23–27 July 2018. Abstract number: TUPEB067.
References
- 1. Centner CM, Bateman KJ, Heckmann JM. Manifestations of HIV infection in the peripheral nervous system. Lancet Neurol 2013; 12:295–309. [DOI] [PubMed] [Google Scholar]
- 2. Wang SX, Ho EL, Grill M, et al. . Peripheral neuropathy in primary HIV infection associates with systemic and central nervous system immune activation. J Acquir Immune Defic Syndr 2014; 66:303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aziz-Donnelly A, Harrison TB. Update of HIV-associated sensory neuropathies. Curr Treat Options Neurol 2017; 19:36. [DOI] [PubMed] [Google Scholar]
- 4. Simpson DM, Kitch D, Evans SR, et al. ; ACTG A5117 Study Group HIV neuropathy natural history cohort study: assessment measures and risk factors. Neurology 2006; 66:1679–87. [DOI] [PubMed] [Google Scholar]
- 5. Shurie JS, Deribew A. Assessment of the prevalence of distal symmetrical polyneuropathy and its risk factors among HAART-treated and untreated HIV infected individuals. Ethiop Med J 2010; 48:85–93. [PubMed] [Google Scholar]
- 6. Dubey TN, Raghuvanshi SS, Sharma H, Saxena R. HIV neuropathy in pre-HAART patients and it’s correlation with risk factors in Central India. Neurol India 2013; 61:478–80. [DOI] [PubMed] [Google Scholar]
- 7. Childs EA, Lyles RH, Selnes OA, et al. . Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology 1999; 52:607–13. [DOI] [PubMed] [Google Scholar]
- 8. Ellis RJ, Rosario D, Clifford DB, et al. ; CHARTER Study Group Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol 2010; 67:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Evans SR, Ellis RJ, Chen H, et al. . Peripheral neuropathy in HIV: prevalence and risk factors. AIDS 2011; 25:919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaku M, Simpson DM. HIV, antiretrovirals, and peripheral neuropathy: a moving target. Muscle Nerve 2018; 57:347–9. [DOI] [PubMed] [Google Scholar]
- 11. Saylor D. Neurologic complications of human immunodeficiency virus infection. Continuum (Minneap Minn) 2018; 24:1397–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schifitto G, McDermott MP, McArthur JC, et al. ; NEAD Consortium Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology 2005; 64:842–8. [DOI] [PubMed] [Google Scholar]
- 13. Oshinaike O, Akinbami A, Ojo O, et al. . Influence of age and neurotoxic HAART use on frequency of HIV sensory neuropathy. AIDS Res Treat 2012; 2012:961510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ndakala FN, Oyugi JO, Oluka MN, Kimani J, Jablonka A, Behrens GM. Prevalent neuropathy in a cohort of HIV-infected Kenyan sex workers using antiretroviral drugs. Pan Afr Med J 2016; 25:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis RJ, Marquie-Beck J, Delaney P, et al. ; CHARTER Group Human immunodeficiency virus protease inhibitors and risk for peripheral neuropathy. Ann Neurol 2008; 64:566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rabiu M, Ibrahim A, Yakasai AM, Hamza M, Owolabi LF. Clinical pattern of human immunodeficiency virus-associated sensory neuropathy among adults in Kano, northwestern Nigeria. N Niger J Clin Res 2018; 7:69–74. [Google Scholar]
- 17. Chai NC, McArthur JC. HIV and peripheral neuropathy. Chronic Pain HIV (eds J. S. Merlin, P. A. Selwyn, G. J. Treisman and A. G. Giovanniello) 2016:51–62. [Google Scholar]
- 18. Saylor D, Nakigozi G, Nakasujja N, et al. . Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda. Neurology 2017; 89:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kvalsund M, Chidumayo T, Hamel J, et al. . Factors associated with distal symmetric polyneuropathies in adult Zambians: a cross-sectional, observational study of the role of HIV, non-antiretroviral medication exposures, and nutrition. J Neurol Sci 2018; 388:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland: World Health Organization, 2018. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 21. Mafukidze AT, Calnan M, Furin J. Peripheral neuropathy in persons with tuberculosis. J Clin Tuberc Other Mycobact Dis 2016; 2:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Pee S, Semba RD. Role of nutrition in HIV infection: review of evidence for more effective programming in resource-limited settings. Food Nutr Bull 2010; 31:S313–44. [PubMed] [Google Scholar]
- 23. Ndakala F, Oyugi J, Oluka M. HIV-associated polyneuropathy in resource-limited settings: genetic predisposition and vitamin variations. World Journal of AIDS 2017; 7:106–21. [Google Scholar]
- 24. Lucey BP, Clifford DB, Creighton J, Edwards RR, McArthur JC, Haythornthwaite J. Relationship of depression and catastrophizing to pain, disability, and medication adherence in patients with HIV-associated sensory neuropathy. AIDS Care 2011; 23:921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Biraguma J, Rhoda A. Peripheral neuropathy and quality of life of adults living with HIV/AIDS in the Rulindo district of Rwanda. SAHARA J 2012; 9:88–94. [DOI] [PubMed] [Google Scholar]
- 26. Ellis R, Rosario D, Clifford D, et al. . Persisting high prevalence of HIV distal sensory peripheral neuropathy in the era of cART: correlates in the CHARTER study. In: CROI. Montreal, Canada. Abstract, 2009.
- 27. Robertson K, Jiang H, Evans SR, et al. ; 5271 Study Team; AIDS Clinical Trials Group International neurocognitive normative study: neurocognitive comparison data in diverse resource-limited settings: AIDS Clinical Trials Group A5271. J Neurovirol 2016; 22:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robertson K, Kumwenda J, Supparatpinyo K, et al. ; AIDS Clinical Trials Group A multinational study of neurological performance in antiretroviral therapy-naïve HIV-1-infected persons in diverse resource-constrained settings. J Neurovirol 2011; 17:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robertson K, Jiang H, Kumwenda J, et al. ; 5199 Study Team; AIDS Clinical Trials Group Improved neuropsychological and neurological functioning across three antiretroviral regimens in diverse resource-limited settings: AIDS Clinical Trials Group study a5199, the International Neurological Study. Clin Infect Dis 2012; 55:868–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson K, Oladeji B, Jiang H. HIV-1 and TB co-infection in multinational resource limited settings: increased neurological dysfunction. Clin Infect Dis 2018; 68:1739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marra CM, Boutin P, Collier AC. Screening for distal sensory peripheral neuropathy in HIV-infected persons in research and clinical settings. Neurology 1998; 51:1678–81. [DOI] [PubMed] [Google Scholar]
- 32. Galantino ML, Kietrys DM, Parrott JS, Stevens ME, Stevens AM, Condoluci DV. Quality of life and self-reported lower extremity function in adults with HIV-related distal sensory polyneuropathy. Phys Ther 2014; 94:1455–66. [DOI] [PubMed] [Google Scholar]
- 33. Birbeck GL, Kvalsund MP, Byers PA, et al. . Neuropsychiatric and socioeconomic status impact antiretroviral adherence and mortality in rural Zambia. Am J Trop Med Hyg 2011; 85:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellis RJ, Evans SR, Clifford DB, et al. ; Neurological AIDS Research Consortium; AIDS Clinical Trials Group Study Teams A5001 and A362 Clinical validation of the NeuroScreen. J Neurovirol 2005; 11:503–11. [DOI] [PubMed] [Google Scholar]
- 35. Hellmuth J, Fletcher JL, Valcour V, et al. ; SEARCH 010/RV254 Study Group Neurologic signs and symptoms frequently manifest in acute HIV infection. Neurology 2016; 87:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]