Abstract
We assessed the association between cured tuberculosis (TB) and mortality among persons living with human immunodeficiency virus (HIV) in Latin America. We compared survival among persons with and without TB at enrollment in HIV care, starting 9 months after clinic enrollment. In multivariable analysis, TB was associated with higher long-term mortality (hazard ratio, 1.57; 95% confidence interval, 1.25–1.99).
Keywords: HIV, TB, screening, long-term mortality
Tuberculosis (TB) is the leading cause of death among persons living with human immunodeficiency virus (PLWH). Globally, 11% of PLWH with drug-sensitive TB die during treatment compared with 4% of drug-sensitive TB cases overall [1]. Data on long-term outcomes after TB cure in PLWH are limited. However, several studies in cohorts not living with HIV have found the risk of mortality is increased after TB cure [2–7]. We hypothesized that long-term mortality would also be elevated in TB survivors living with HIV. Therefore, we conducted a study to compare long-term mortality among PLWH after TB treatment completion with long-term mortality among of those without TB.
METHODS
Cohort Description
This study included data from the Caribbean, Central and South America Network for HIV Epidemiology (CCASAnet) database, which includes clinical sites in Latin America and the Caribbean and constitutes part of the International epidemiology Databases to Evaluate AIDS network. Six CCASAnet sites contributed data to this study: Instituto Nacional de Infectiologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil (FC- Brazil); Fundación Arriarán, Santiago, Chile (FA-Chile); Le Groupe Haïtien d’Etude du Sarcome de Kaposi et des Infections Opportunistes, Port-au-Prince, Haiti (GHESKIO-Haiti); Instituto Hondureño de Seguridad Social and Hospital Escuela, Tegucigalpa, Honduras (IHSS/HE-Honduras); El Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico (INCMNSZ-Mexico); and El Instituto de Medicina Tropical Alexander von Humboldt, Lima, Perú (IMTAvH-Peru). This analysis included PLWH aged ≥18 years who were antiretroviral therapy (ART)-naive at the first clinic visit between 1 January 2006 and 31 December 2015. Patients were excluded if they had initiated ART >30 days prior to enrollment or were known to have drug-resistant TB. The dataset was closed in GHESKIO-Haiti on 31 December 2015, in FC-Brazil and INCMNSZ-Mexico on 31 March 2016, in FA-Chile and IHSS/HE-Honduras on 31 May 2016, and in IMTAvH-Peru on 28 February 2017.
Statistical Analyses
Baseline demographic and clinical characteristics were compared using median (interquartile range [IQR]) or frequency (proportion), as appropriate. Data collected included TB diagnosis and treatment completion dates and TB medications. Baseline TB was defined as TB diagnosed ±30 days of clinic enrollment. Among those still alive, follow-up started 9 months after enrollment in both groups in order to exclude mortality during TB treatment. Patients were considered lost to follow-up (LTFU) if they were not known to have died and the last observed visit date was >365 days before the cohort closing date.
We compared time to death among patients with and without TB using Kaplan-Meier analysis and the log-rank test. We estimated predictors of mortality with multivariable Cox models, stratified by site and adjusting for baseline TB, sex, education, age, year of enrollment, and baseline CD4 count (cells/mm3; square root transformed). Baseline CD4 count was defined using the closest CD4 count to enrollment within a window of ±90 days. Continuous predictors were included in analyses using natural splines with 3 knots; missing data were multiply-imputed with 20 imputation replications. Analysis scripts are available at http://biostat.mc.vanderbilt.edu/ArchivedAnalyses.
RESULTS
The cohort included 19 197 PLWH; 1306 (6.8%) were diagnosed with baseline TB. Of these, 15 987 remained in care 9 months after enrollment and were included in the analysis (n = 1051 [6.6%] with baseline TB; n = 181 from FC-Brazil; n = 5 from FA-Chile; n = 630 from GHESKIO-Haiti; n = 11 from IHSS/HE-Honduras; n = 39 from INCMNSZ-Mexico; and n = 185 from IMTAvH-Peru). Among the 15 987 patients, 6635 (42%) were female, the median age was 35 years (IQR, 28–43), 2381 patients (15%) had no education or primary school only, and the median baseline CD4 count was 227 cells/mm3 (IQR, 90–386; Supplementary Table 1). Patients with TB were more likely to be male, older, less educated, with lower CD4 counts, and residing in Haiti or Peru (P < .001). The median time to completion of TB treatment was 195 days (IQR, 182–224).
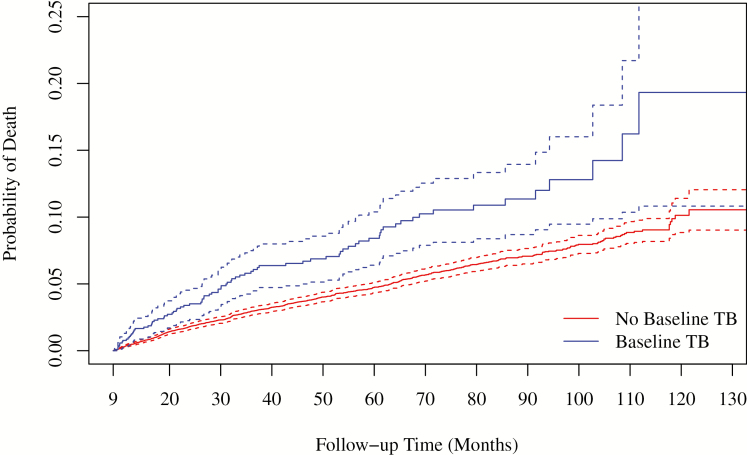
Over the study period (starting 9 months after enrollment), retention was similar among those with and without TB; 203 (19%) with baseline TB and 3075 (21%) without TB were LTFU; 83 (8%) and 704 (5%) died. Median follow-up time was 50 months (IQR, 28–79) for those with baseline TB and 54 months (IQR, 32–84) for those without TB (P < .001). Starting 9 months after enrollment (Figure 1), patients with baseline TB had higher long-term mortality compared with those without TB (P < .001). The unadjusted 5-year mortality (starting 9 months after enrollment) was 10.2% for patients with baseline TB vs 5.6% for those without TB; 10-year mortality was 19.3% vs 10.6%, respectively. In multivariable Cox models, increased mortality was associated with baseline TB (adjusted HR [aHR], 1.57; 95% confidence interval [CI], 1.25–1.99), lower CD4 count (100 vs 350 cells/mm3: aHR, 1.57; 95% CI, 1.41–1.76), older age (age 55 vs 35 years: aHR, 1.56; 95% CI, 1.33–1.84), and lower education (none vs at least secondary: aHR, 1.24; 95% CI, .92–1.67). Results were similar when limited to patients with pulmonary TB (n = 742 [71%]), mortality associated with pulmonary TB: aHR = 1.72 (95% CI, 1.31–2.26); when limited to those who initiated ART during the first 9 months (n = 857 [82%] and 10,041 [67%] with and without TB, respectively), mortality associated with TB: aHR = 1.77, 95% CI, 1.37–2.30); and with ART included as a time-varying covariate (mortality associated with TB: aHR=1.54; 95% CI, 1.22–1.94). Of those with baseline TB, 78 (7.4%) had evidence of a second episode of TB >9 months after enrollment; of those without baseline TB, 1021 (6.8%) later developed TB. Results were similar when these patients were excluded (mortality associated with baseline TB: aHR, 1.71; 95% CI, 1.33–2.18). Results were also similar when limited to those who completed TB treatment within 9 months of enrollment (n = 873 [83%]): aHR, 1.40; 95% CI, 1.07–1.83). Site-specific numbers and estimated HRs are given in Supplementary Table 2.
Figure 1.
Probability of death (95% confidence interval) after treatment completion among patients with and without baseline TB. Abbreviation: TB, tuberculosis.
DISCUSSION
We found that PLWH who present with baseline TB have an elevated risk of long-term mortality after TB treatment completion compared with people without baseline TB. The unadjusted 5- and 10-year mortality (measured from 9 months after enrollment) was nearly double among patients with baseline TB compared with those without TB, with a HR of 1.54 for baseline TB in the multivariable analysis.
There are limited data on the impact of TB on long-term mortality in PLWH, but studies in cohorts not living with HIV have also found higher long-term mortality in TB survivors. One study from the United States found that a history of treated TB is associated with a predicted average of 3.6 years of potential life loss [2]. Studies from Denmark, the United Kingdom, Israel, and the United States have reported mortality rates that are up to 7-fold higher among TB survivors compared with the general populations in the respective countries [2–6]. The causes of death reported in these studies were recurrent TB, chronic lung disease, bronchopneumonia, sepsis, cardiovascular disease, malignancy (particularly, lung cancer), and liver disease.
In a large, prospective cohort study from Vietnam, with median follow-up time of 2.9 years after TB diagnosis, the standard mortality rate was 4.0 among patients with TB compared with the control population of people living in the same households [7]. Verbal autopsies were performed for a group of randomly selected deceased patients, and the most common causes of death included recurrent TB, neoplasms, stroke, accidents, cirrhosis, pneumonia, complications of HIV, chronic obstructive pulmonary disease (COPD), and renal failure.
Behavioral factors and comorbidities that increase the risk of acquiring TB, such as smoking, alcoholism, and diabetes, likely account for at least some of the increase in long-term mortality among TB survivors. Residual lung damage may also contribute. Multiple studies in populations largely not living with HIV have documented structural damage and impaired pulmonary function after TB recovery, and a systematic review and metaanalysis found a strong association between a history of TB and COPD and bronchiectasis [8, 9]. In a study from Israel, TB survivors were more likely to die of pneumonia or sepsis, which could be related to lung impairment [3]. PLWH, already at risk of pneumococcal pneumonia, may be particularly susceptible in resource-poor settings where pneumococcal vaccination is rarely administered.
Host-level factors may also play a role. PLWH who have survived 1 episode of TB are at risk of recurrent TB. Acquisition of TB is also a marker of immune dysfunction, indicating risk of death from other causes [10]. TB may also increase HIV viral load and accelerate HIV disease progression. In addition, TB may cause persistent inflammation and immune activation, further increasing the risk of cardiovascular disease, which is already a leading cause of morbidity and mortality in PLWH [11, 12]. A large multicenter observational cohort study found that TB was associated with cardiovascular non-AIDS–associated deaths [10]. Prospective studies are needed to further assess the associations between TB, HIV, and cardiovascular disease.
Our study is limited by its retrospective design, inability to determine cause of death and TB treatment outcome, and lack of data to adjust for other clinical factors such as smoking and diabetes history. Despite these limitations, our results indicate that even successful TB outcomes may be associated with substantial long-term mortality in PLWH. These findings highlight the critical importance of TB prevention in PLWH and suggest that closer long-term follow-up may be warranted in PLWH with a history of TB. Further study is necessary to understand the long-term clinical impact of TB in PLWH.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Conference for Retroviruses and Opportunistic Infections, Seattle, WA, 6 March 2019. Abstract number 736.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID; grant R01 AI131998) and the National Institutes of Health–funded Caribbean, Central and South America Network for HIV Epidemiology, a member cohort of the International Epidemiology Databases to Evaluate AIDS (U01AI069923). This award is funded by the following institutes: the Eunice Kennedy Shriver National Institute of Child Health & Human Development, the Office of the Director, the National Institutes of Health, NIAID, the National Cancer Institute, and the National Institute of Mental Health.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Global Tuberculosis Report, 2018. World Health Organization; Available at: http://www.who.int/tb/publications/global_report/en/. Accessed 1 February 2019. [Google Scholar]
- 2. Hoger S, Lykens K, Beavers SF, Katz D, Miller TL. Longevity loss among cured tuberculosis patients and the potential value of prevention. Int J Tuberc Lung Dis 2014; 18:1347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shuldiner J, Leventhal A, Chemtob D, Mor Z. Mortality after anti-tuberculosis treatment completion: results of long-term follow-up. Int J Tuberc Lung Dis 2016; 20:43–8. [DOI] [PubMed] [Google Scholar]
- 4. Christensen AS, Roed C, Andersen PH, Andersen AB, Obel N. Long-term mortality in patients with pulmonary and extrapulmonary tuberculosis: a Danish nationwide cohort study. Clin Epidemiol 2014; 6:405–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller TL, Wilson FA, Pang JW, et al. . Mortality hazard and survival after tuberculosis treatment. Am J Public Health 2015; 105:930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tocque K, Convrey RP, Bellis MA, Beeching NJ, Davies PD. Elevated mortality following diagnosis with a treatable disease: tuberculosis. Int J Tuberc Lung Dis 2005; 9:797–802. [PubMed] [Google Scholar]
- 7. Fox GJ, Nguyen VN, Dinh NS, et al. . Post-treatment mortality among patients with tuberculosis: a prospective cohort study of 10 964 patients in Vietnam. Clin Infect Dis 2019; 68:1359–66. [DOI] [PubMed] [Google Scholar]
- 8. Pasipanodya JG, Miller TL, Vecino M, et al. . Pulmonary impairment after tuberculosis. Chest 2007; 131:1817–24. [DOI] [PubMed] [Google Scholar]
- 9. Byrne AL, Marais BJ, Mitnick CD, Lecca L, Marks GB. Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015; 32:138–46. [DOI] [PubMed] [Google Scholar]
- 10. Pettit AC, Giganti MJ, Ingle SM, et al. . Increased non-AIDS mortality among persons with AIDS-defining events after antiretroviral therapy initiation. J Int AIDS Soc 2018; 21. doi: 10.1002/jia2.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016; 30:1495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huaman MA, Kryscio RJ, Fichtenbaum CJ, et al. . Tuberculosis and risk of acute myocardial infarction: a propensity score-matched analysis. Epidemiol Infect 2017; 145:1363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.