Abstract
Agricultural workers who have concurrent exposure to pesticides and noise are at increased risk of hearing loss. We recruited 163 Thai conventional and 172 organic farmers to answer our questionnaires about personal demographics, agricultural activities, and pesticide and agricultural machinery use. This information was used to calculate the years of conventional (pesticide use) farming and the years of agricultural noise exposure, and to estimate semiquantitative metrics for pesticide exposure (cumulative intensity score-years) and cumulative noise exposure (dB(A)-years) for each conventional farmer. All participants underwent pure tone audiometric testing. The mean hearing threshold in the low-frequency band (0.5–2 kHz) and high-frequency band (3–6 kHz) were used for analysis. Years involved in conventional farming and years using agricultural machinery were associated with an increase in the average hearing threshold for the high-frequency band after controlling for age, ever exposed to industrial noise and cigarette smoking. The highest category of cumulative insecticide exposure (score-years), cumulative organophosphates exposure (score-years) and cumulative noise exposure (dB(A)-years) were also associated with an increased high-frequency band hearing threshold among conventional farmers. Results from the full cohort and the subcohort of conventional farmers support each other and the hypothesis that pesticide and noise have an additive effect on hearing, since no model interactions were significant.
Keywords: agriculture, hearing loss, noise, pesticide
Introduction
Agricultural workers are more likely to have hearing loss than other occupations. A study conducted in the USA found a higher prevalence of hearing loss in farmers than construction laborers (farmers 17 to 79%, laborers 11 to 64% depending on the frequency tested) (Kerr et al., 2003). Another study among adult farmers in Brazil, also found an association between agricultural work and hearing loss in both the low and high frequencies (Kós et al., 2014). Audiometric testing of 376 US farm workers and farm residents found the prevalence of hearing loss was 9 to 47% depending on the frequency tested, the highest prevalence (47%) was found for the average threshold at 3–8 kHz frequency (Gomez et al., 2001). Meanwhile in Thailand, the Ministry of Public Health reported the highest incidence of work-related noise-induced hearing loss was among agricultural workers. This incidence has been shown to be almost double that of construction laborers and has rapidly increased over time since 2013 (Rattanarak et al., 2018). A hearing assessment of 253 farmers in Thailand found a prevalence of 88% for hearing abnormalities in any tested frequency (Suwanno et al., 2008), which was much higher than a study conducted among US farmers (Humann, 2011). Since the hearing loss in farmers was most commonly observed in the high frequencies, exposure to noise from agricultural practices was proposed as the potential cause of the hearing loss (Plakke and Dare, 1992; Gomez et al., 2001; Harris, 2005; Humann et al., 2012). It should be noted that not all the studies reported earlier define hearing loss in the same way. Nevertheless these reports are consistent in identifying agricultural work as a risk factor for hearing loss.
Various types of machinery are commonly used in Thai agriculture, including hand and riding tractors, mowers, millers, and motorized backpack sprayers. Studies have shown that the amount of time driving field tractors and working around grain dryers was significantly associated with hearing loss (Beckett et al., 2000; McBride et al., 2003). Meanwhile, some rural- or farm-related activities, such as years of hunting or target shooting and years living on a farm have also been associated with hearing loss, especially in the high frequencies (Humann et al., 2012).
Some studies have also suggested an association between hearing loss and pesticide exposures. The US Agricultural Health Study reported hearing loss was associated with their exposure score for insecticides, and specifically with the highest category of exposure to organophosphates among licensed pesticide applicators (Crawford et al., 2008). In other studies, workers exposed to organophosphates showed significant worsening of their hearing thresholds in the higher frequencies (Guida et al., 2010; Delecrode et al., 2012). In Suphan Buri, Thailand, an association was found between organophosphate use and hearing loss. Participants who reported current use of organophosphates had a significantly elevated odds ratio (OR 6.8, 95% CI 2.3 to 20.3) of having high-frequency hearing loss, compared to those who had never used organophosphates, after controlling for age, smoking and alcohol drinking status (Choochouy et al., 2016). The European Agency for Safety and Health at Work and the Nordic Expert Group conducted ototoxicity reviews of several pesticides. Although they described poor/questionable human data, they reported that there were some animal data showing auditory effects at levels above current occupational exposure limits, for organophosphates alone or together with pyrethroids, and suggested that these pesticides could be ototoxic to the human hearing system (Campo et al., 2009; Johnson and Morata, 2010). The possible underlying mechanism of pesticide-induced hearing loss may be partially be explained from the results of animal studies. The injury of stereocilia in the organ of Corti was observed under a scanning electron microscope after organophosphates were administered intraperitoneally to guinea pigs (Körbes et al., 2010; Finkler et al., 2012). A dose–response relationship was also established. However, no cellular mechanism was suggested. No studies have suggested a mechanism by which co-exposure to pesticides and noise could impact hearing loss. Although some studies have shown an association between pesticide exposure and hearing loss, and some studies have shown an association between agricultural occupations or use of agricultural machinery and hearing loss, none have looked at concurrent exposures to both. Therefore, the goal of this study was to determine the association between both pesticide and noise exposures and hearing thresholds among Thai agricultural workers.
Materials and methods
Participants and data collection
This cross-sectional study was conducted in three agricultural areas in Thailand; Phitsanulok in the lower-north, Nakhon Sawan in the central and Yasothon in the northeast region. All participants were age 18–60 years at the time of recruitment and working on a conventional (pesticide using) or organic farm. If they were a conventional farmer they had to spray pesticides or be hired to spray pesticides for other farmers during the year before data collection began. Organic farmers had to be certified as organic and could not have used pesticides in the past year. Recruitment started in 2016 and 175 conventional farmers and 176 organic farmers agreed to participate.
Questionnaires were administered to participants by a trained interviewer and covered general demographic characteristics, agricultural activities and noise-related activities. Conventional farmers were then asked to record their pesticide use via diary each day for 12 months. The field staff checked with the farmer and collected the diaries once a month. This study protocol was approved by the Ethical Review Committee for Human Research, Faculty of Public Health, Mahidol University, Institutional Review Board. All study participants were informed about the research procedures and signed written informed consent.
Before the hearing examination began the participants answered an additional screening questionnaire that asked about occupational history, medical history, symptoms related to hearing health, and military service history. Among the recruited participants, several were excluded including 10 (2.9%) who reported ear pain on the day of examination, 6 (1.7%) who had a diagnosed ear disease such as chronic otitis media or used a hearing aid. With these exclusions, there were 335 participants available for analysis, 163 conventional farmers and 172 organic farmers.
Audiometric data
Prior to the exam, participants were asked to avoid exposure to routine working noise or recreational noise for the 12 h preceding the exam. At the time of health examination, standard pure tone audiometry (PTA) was conducted following the modified Hughson–Westlake procedure with ascending–descending approach (Carhart and Jerger, 1959), recommended by American National Standards Institute guidelines ANSI S3.21–1978 (ANSI, 1997). The calibrated audiometers (MADSEN Micromate 304; GN Otometrics, Denmark and Model AC50 C; Sibelmed, Spain) were used in a soundproof room that met the US Occupational Safety and Health Administration limits for background noise (Occupational Safety and Health Administration, 1970). Testing was conducted at 0.5, 1, 2, 3, 4, and 6 kHz, with a retest at 1 kHz to check the repeatability of the participants’ response. Subjects underwent otoscopic examination by well-trained research staff to evaluate impacted earwax. Tests at each frequency were characterized separately for the right and left ears.
For purpose of data analysis, pure tone thresholds were combined into two continuous variables (in units of dB HL) representing the average hearing threshold of the low and high-frequency bands. These measurements were used as the outcome in the data analysis.
The low-frequency band was calculated as the arithmetic mean of the hearing threshold at 0.5, 1, and 2 kHz and the high-frequency band was calculated as the arithmetic mean of the hearing threshold at 3, 4 and 6 kHz (Humann et al., 2012). These frequency combinations were recommended by either the International Organization for Standardization (ISO, 1990) or the American National Standards Institute (ANSI, 1996). A hearing threshold of >25 dB has been commonly used to indicate clinical hearing loss (World Health Organization, 2018). In our dichotomous statistical analyses this cutoff (25 dB HL) was used to define hearing loss in either the low- or the high-frequency bands.
Estimating cumulative noise exposure
An interview about agricultural machinery use was conducted for each subject and the data collected about the type of agricultural machinery used and the amount of time used (years) and frequency of use (days/crop cycle × number of crops per year) for each machine type. Visits to the farms of each participant were made and short-term noise measurements were collected in the hearing zone of the participants during use of equipment they commonly used. The noise level was measured by type 2 sound level meter (Model LxT1; Larson Davis, PCB Piezotronics, USA) with A weighting, slow response mode setting. The most common machinery used by all participants were small/large hand and riding tractors and hand grass mowers, whereas the most common machinery used by conventional farmers were backpack sprayers and rice blowers. The common agricultural machinery noise levels are presented in Table 1.
Table 1.
Agricultural machinery Leq noise level
| Agricultural machinery noise level, dB(A) | Motorized backpack sprayer (n = 147) | Battery backpack sprayer (n = 44) | Rice blower (n = 35) | Hand tractor (n = 144) | Riding tractor (n = 58) | Hand grass mower (n = 121) |
|---|---|---|---|---|---|---|
| Median | 90.8 | 64.8 | 90.2 | 82.1 | 83.3 | 90.7 |
| 25th Percentile | 82.5 | 61.8 | 89.1 | 79.9 | 81.6 | 88.2 |
| 75th Percentile | 91.2 | 68.3 | 92.6 | 85.3 | 86.6 | 92.7 |
By combining the report of years of equipment use and the measurement data the cumulative exposure to noise was calculated for each participant. The equation was based on that used by Davies et al. (2009):
where j is an agricultural machinery type used by the farmer, n is number of agricultural machinery types used by the farmer, Tj is lifetime days of use the machine type j defined as the frequency of use (days/year assuming a full day of use times the years of use), and Leqj is the noise exposure measured for machine j. The calculated noise exposure was then categorized into tertiles.
Estimating cumulative pesticide exposure for conventional farmers
Pesticide use practices were obtained from the diary collected from the conventional farming participants. Pesticide use was classified separately for insecticides, herbicides, and fungicides. Insecticides were further categorized as organophosphates, carbamates or cypermethrins, whereas herbicides and fungicides were not further categorized. The intensity level was calculated as described by Dosemeci et al. (2002) using their general algorithm which included pesticide mixing practices, types of spraying equipment used and personal protective equipment used. This was combined with the duration (years of use) and frequency of use (days/year) collected from the diaries to estimate a cumulative pesticide exposure score:
at least 40% of the conventional farmers were exposed to insecticides and/or herbicides and for these pesticides; the cumulative exposure scores were categorized into tertiles plus those having a zero cumulative exposure serving as the reference category, resulting in four exposure categories. When ˂40% of the conventional farmers were exposed (fungicides, organophosphates and pyrethroids), these cumulative exposure scores were dichotomized by using the median. So, including those with zero cumulative exposure score there were three categories of exposure. For carbamates there were ˂10% of conventional farmers exposed, so any exposure was then compared to with those zero exposure (two categories).
Data analysis
Seven potential confounding variables i.e., age, cigarette smoking, alcohol consumption, ever exposed to industrial noise (y/n), ever exposed to industrial solvents (y/n), ever served in the military (y/n) and currently having a second job (y/n) were identified. Age was selected because of the known association with hearing loss (Howarth and Shone, 2006; Kaewboonchoo et al., 2007). Cigarette smoking (Nakanishi et al., 2000), alcohol consumption (Curhan et al., 2011; Curhan et al., 2015), military service (Wells et al., 2015) and industrial noise or solvent exposure (Vyskocil et al., 2012; Prakairungthong and Kerdmuang, 2017) were included because previous studies have shown an association with hearing loss. Meanwhile, second job was selected as an indicator of possibly having a noisy job, other than farming. However, gender was not considered as a potential confounder in this study because it was considered a surrogate for other confounders, especially smoking, since in this cohort all current smokers or ex-smokers were male and no females reported smoking currently or in the past.
Owing to the high proportion of subjects with hearing loss defined as an average hearing threshold of >25 dB HL in one of the frequency bands, Poisson models were used to investigate predictive factors associated with the dichotomous risk of having hearing loss. A number of authors have recommended using Poisson models to estimate the relative risk (RR) because of the increasing differential between the OR and RR when the outcome incidence exceeds 10% (McNutt et al., 2003; Greenland, 2004; Zou, 2004).
To determine the association between pesticide and noise variables and the average hearing threshold in each frequency band, linear regression models were developed for each ear independently (see Supplementary material, available at Annals of Work Exposures and Health online). In addition, to examine the importance of ‘ear’ as a predictor the right and left ear data were combined and a Generalized Estimating Equation (GEE) model with a robust covariance estimator was used to adjust for the within subject correlations. The years involved in conventional farming (use of pesticides) and the years of agricultural machinery use were selected as pesticide–noise indicators for all participants. Meanwhile, the cumulative pesticide (score-years) and cumulative noise exposure (dB(A)-years) were used as a semiquantitative exposure matrix among the subset of conventional farmers. Thus, there were three final models. The first GEE model included data from all the participants (years of pesticide use and years of agricultural machinery use), whereas the second and third GEE models included data only from conventional farmers (cumulative pesticide exposure score-years and cumulative noise exposure dB(A)-years).
The seven potential confounders previously mentioned were first examined in univariate models of hearing threshold (dB HL) for the low and high-frequency bands. Then including all statistically significant covariates, a backward elimination method was used to develop the final models, which also included the exposure variables (either years of pesticide use and agricultural machinery use or cumulative exposure to noise and pesticides).
Independent t-tests or chi-square tests were also used to determine the difference of means or proportions between farmer groups. All analyses were performed using IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA).
Results
General characteristics
The general characteristics of the participants are presented in Table 2. There were slightly more male than female participants (57% versus 43%). Most of the participants never smoked, but over half are current alcohol drinkers. About a quarter have worked in industrial jobs with noise exposure, but few have served in the military. Many (42%) also have second jobs, in addition to their agricultural work.
Table 2.
General characteristic of study participants
| Characteristics n (%) or mean (SD) | Total (n = 335) | Conventional farmers (n = 163) | Organic farmers (n = 172) | P-value |
|---|---|---|---|---|
| Gender, n (%) | <0.001* | |||
| Male | 190 (56.7) | 118 (72.4) | 72 (41.9) | |
| Female | 145 (43.3) | 45 (27.6) | 100 (58.1) | |
| Age (years), mean (SD) | 47.9 (8.6) | 47.3 (9.7) | 48.4 (7.4) | 0.218 |
| Cigarette smoking, n (%) | 0.035* | |||
| Never | 249 (74.3) | 116 (71.2) | 133 (77.3) | |
| Former | 21 (6.3) | 7 (4.3) | 14 (8.1) | |
| Current | 65 (19.4) | 40 (24.5) | 25 (14.5) | |
| Alcohol consumption, n (%) | <0.001* | |||
| Never | 127 (37.9) | 52 (31.9) | 75 (43.6) | |
| Former | 26 (7.8) | 1 (0.6) | 25 (14.5) | |
| Current | 182 (54.3) | 110 (67.5) | 72 (41.9) | |
| Industrial noise ever, n (%) | 0.009* | |||
| No | 250 (74.6) | 132 (81.0) | 118 (68.6) | |
| Yes | 85 (25.4) | 31 (19.0) | 54 (31.4) | |
| Industrial solvent ever, n (%) | 0.558 | |||
| No | 315 (94.0) | 152 (93.3) | 163 (94.8) | |
| Yes | 20 (6.0) | 11 (6.7) | 9 (5.2) | |
| Military services ever, n (%) | 0.325 | |||
| No | 311 (92.8) | 149 (91.4) | 162 (94.2) | |
| Yes | 24 (7.2) | 14 (8.6) | 10 (5.8) | |
| Second job, n (%) | <0.001* | |||
| No | 195 (58.2) | 123 (75.5) | 72 (41.9) | |
| Yes | 140 (41.8) | 40 (24.5) | 100 (58.1) | |
| Years of agricultural machinery use, mean (SD) | 8.6 (8.4) | 11.9 (7.7) | 5.5 (7.9) | <0.001* |
| Years involved in conventional farming, mean (SD) | 19.0 (10.9) | 21.9 (10.2) | 16.3 (11.0) | <0.001* |
| Cumulative agricultural machinery noise exposure (dB(A)-years), mean (SD)a | 62.7 (39.5) | 86.2 (10.9) | 40.4 (43.7) | <0.001* |
| Cumulative herbicides exposure (score-years), mean (SD)a | — | 3.89 (5.54) | — | |
| Cumulative fungicides exposure (score-years), mean (SD)a | — | 0.91 (2.76) | — | |
| Cumulative insecticides exposure (score-years), mean (SD)a | — | 1.80 (3.31) | — | |
| Cumulative organophosphates exposure (score-years), mean (SD)a | — | 0.58 (1.62) | — | |
| Cumulative carbamates exposure (score-years), mean (SD)a | — | 0.25 (1.18) | — | |
| Cumulative pyrethroids exposure (score-years), mean (SD)a | — | 0.36 (0.94) | — |
aData only calculated from conventional farmers.
*Significant at P-value 0.05.
Conventional farmers have, on average, worked longer in agriculture than organic farmers, but their age was not significantly different. This in part explains why conventional farmers, on average, reported more years using agricultural machinery, and also had larger cumulative exposures to agricultural noise than organic farmers. However, organic farmers were more likely to have reported having industrial noise exposure and to having second jobs in addition to farming. Most (89.5%) of the organic farmers reported using pesticides in the past, however the total years of use was greater for conventional farmers. For classes of insecticides, the highest exposure in cumulative exposure score-years was observed for the organophosphates.
Audiometry results
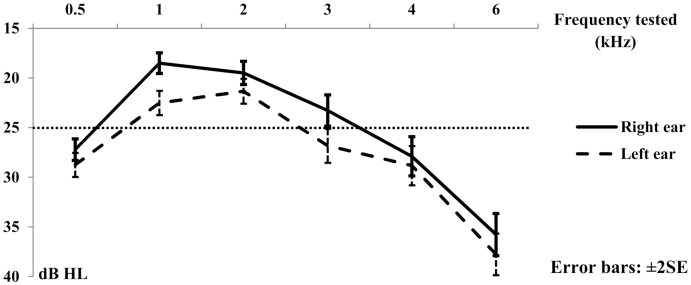
The average hearing threshold for each frequency in each ear is shown in Fig. 1. The average hearing threshold of the left ear was higher than the right ear in every frequency tested. The pattern of the average hearing thresholds was fairly similar in both ears. When summarized by low (0.5–2 kHz) and high (3–6 kHz) frequency bands, the prevalence of abnormal hearing (>25 dB HL average loss) was higher in the high-frequency band than the low-frequency band (Table 3). However, 93 and 78% of those with high frequency loss also had low frequency loss in right and left ear, respectively.
Figure 1.
Average hearing threshold (dB HL) of each frequency in both ears for all study participants.
Table 3.
Prevalence of abnormal hearing (>25 dB HL) as the average loss in the low and high-frequency bands in the right and left ear (n = 335)
| Abnormal hearing | Low frequency (0.5–2 KHz), n (%) | High frequency (3–6 kHz), n (%) | P-value |
|---|---|---|---|
| Right ear | 70 (20.9) | 169 (50.4) | <0.001* |
| Left ear | 122 (36.4) | 184 (54.9) | <0.001* |
*Significant at P-value 0.05.
Risk factors for hearing loss
Poisson models for the risk of having hearing loss or not, based on the >25 dB average loss in the high-frequency band, found that years involved in conventional farming and years using agricultural machinery were both significant predictive factors. In univariate models, there was an increasing risk of having hearing loss in the high-frequency band of both ears for each year of work in conventional farming (RR of 1.03 95% CI 1.02 to 1.05 for both ears). There was also an increasing risk of having hearing loss in the high-frequency band for each year using agricultural machinery (RR of 1.02 95% CI 1.01 to 1.04 for right ear RR of 1.02 95% CI 1.004 to 1.04 for left ear, respectively). No significant relationship was found between years in conventional agriculture or years of machinery use and hearing loss in the low-frequency band for either ear. No other covariates or potential confounders were significant predictors of hearing loss in any of these models.
When models were developed to predict the average hearing threshold (in units of dB HL) for each frequency band, a number of factors were significant predictors of the threshold level in univariate models. In anticipation issues of collinearity we examined the correlation of years using pesticides, years using agricultural machinery, age in years and found that age and years in conventional farming were moderately correlated (r = 0.4). All other comparisons were much less correlated. Since age is an important predictor of hearing loss regardless of occupational exposures, we included this in the models.
In the full models for the low-frequency band, hearing threshold was not significantly associated with either years of pesticide use or years of agricultural machinery use. However, age and the variable for ‘ear’ remained significant. The full models for the high-frequency band showed that the years involved in conventional (pesticide using) agriculture and the years using agricultural machinery were significantly associated with a higher hearing threshold in the high-frequency band, even after accounting for age, ever being exposed to industrial noise and smoking status (Table 4). In this model age, ‘ear’ and current or former cigarette smoking were also significant predictors of hearing loss.
Table 4.
Final GEE model of the average hearing threshold (in units of dB HL) among full cohort of all farmers for the low and high-frequency bands (n = 670 ears)
| Variables | Low frequency (0.5–2 kHz) | High frequency (3–6 kHz) | ||||
|---|---|---|---|---|---|---|
| β | SE | P-value | β | SE | P-value | |
| Model 1: years of exposure | ||||||
| Intercept | 11.310 | 2.082 | <0.001* | 1.075 | 3.502 | 0.759 |
| Years using pesticides | 0.047 | 0.050 | 0.355 | 0.223 | 0.078 | 0.004* |
| Years using agricultural machinery | −0.115 | 0.046 | 0.062 | 0.231 | 0.089 | 0.009* |
| Ear | ||||||
| Right | 0.00 | — | — | 0.00 | — | — |
| Left | 2.488 | 0.443 | <0.001* | 1.876 | 0.570 | 0.001* |
| Age (years) | 0.259 | 0.049 | <0.001* | 0.444 | 0.084 | <0.001* |
| Industrial noise ever | ||||||
| Never | 0.00 | — | — | 0.00 | — | — |
| Ever | 0.311 | 1.032 | 0.763 | 3.535 | 1.904 | 0.063 |
| Cigarette smoking | ||||||
| Never | 0.00 | — | — | 0.00 | — | — |
| Former | −0.396 | 1.031 | 0.701 | 4.419 | 1.960 | 0.024* |
| Current | 3.535 | 2.854 | 0.216 | 9.244 | 3.994 | 0.021* |
*Significant at P-value 0.05.
Although we examined an interaction term for years involved in pesticide use agriculture and years using agricultural machinery, it was not statistically significant in any model. In addition, no interaction was observed between those pesticide and noise use variables and any of the other covariates, including the ‘ear’ variable.
Hearing loss predictors for conventional (pesticide using farmers)
On the basis of more detailed historical pesticide use and agricultural machinery use questionnaires among the subset of conventional farmers, we were able to investigate the impact of a semiquantitative estimate of cumulative pesticide exposure (score-years) and cumulative noise exposure (dB(A)-years) on changes in average hearing threshold. Cumulative herbicides and fungicide metrics (score-years) were not significantly associated with hearing threshold in either the high or low-frequency bands (data not shown). Unlike the low-frequency band, the highest exposure categories for cumulative insecticide exposures (score-years) and cumulative noise exposures (dB(A)-years) were significantly associated with an increase in the hearing threshold in the high-frequency band, after accounting for the covariates of age, industrial noise exposure and smoking (Table 5).
Table 5.
Final GEE model of the average hearing threshold (in units of dB HL) among subcohort of conventional farmers for the low and high-frequency bands (n = 326 ears)
| Variables | Low frequency (0.5–2 KHz) | High frequency (3–6 kHz) | ||||
|---|---|---|---|---|---|---|
| β | SE | P-value | β | SE | P-value | |
| Model 2: type of pesticides exposure | ||||||
| Cumulative insecticide exposure (score-years) | ||||||
| None | 0.00 | — | — | 0.00 | — | — |
| 0.07–0.945 | 2.485 | 1.226 | 0.059 | 2.884 | 2.595 | 0.266 |
| 0.946–2.407 | 0.108 | 1.253 | 0.931 | 2.970 | 2.290 | 0.195 |
| >2.407 | 0.846 | 1.811 | 0.640 | 5.280 | 2.795 | 0.046* |
| Cumulative noise exposure (dB(A)-years) | ||||||
| 0–72.4 | 0.00 | — | — | 0.00 | — | — |
| 72.4–88.9 | 2.312 | 1.921 | 0.229 | 5.856 | 3.378 | 0.083 |
| >88.9 | −0.608 | 1.903 | 0.749 | 9.199 | 3.475 | 0.008* |
| Ear | ||||||
| Right | 0.00 | — | — | 0.00 | — | — |
| Left | 1.222 | 0.689 | 0.076 | 0.358 | 0.856 | 0.676 |
| Model 3: type of insecticides exposure | ||||||
| Cumulative organophosphate exposure (score-years) | ||||||
| None | 0.00 | — | — | 0.00 | — | — |
| 0.09–1.118 | 2.171 | 1.291 | 0.093 | 3.513 | 2.409 | 0.145 |
| >1.118 | 0.951 | 2.022 | 0.638 | 10.459 | 3.133 | 0.001* |
| Cumulative noise exposure (dB(A)-years) | ||||||
| 0–72.4 | 0.00 | — | — | 0.00 | — | — |
| 72.4–88.9 | 2.415 | 1.955 | 0.217 | 5.670 | 3.282 | 0.084 |
| >88.9 | −0.398 | 1.891 | 0.833 | 8.011 | 3.428 | 0.019* |
| Ear | ||||||
| Right | 0.00 | — | — | 0.00 | — | — |
| Left | 1.222 | 0.689 | 0.076 | 0.358 | 0.856 | 0.676 |
All models controlling for age, ever being exposed to industrial noise and smoking status.
*Significant at P-value 0.05.
Looking in more detail at the type of insecticides that might be associated with this significant relationship between pesticide use and hearing threshold, we found that the cumulative carbamate or pyrethroid exposure (score-years) was not significantly associated with the hearing threshold (data not shown). Only the cumulative organophosphate exposure for the highest exposure category of score-years was significantly associated with hearing loss in the high-frequency band, but not in the low-frequency band. In addition, cumulative noise exposure in highest category was significantly associated with a higher hearing threshold in the high-frequency band, but not for the low-frequency band (Table 5). Although age and current smoking were significant predictors of hearing loss in the high frequency, the ‘ear’ variable was not significant.
The interaction terms between cumulative insecticide or organophosphate exposure and cumulative noise exposure were not statistically significance for either hearing frequency band. In all models age remained a significant predictor of hearing threshold, with increasing age increasing the risk of abnormal hearing. In these subcohort analyses current smoking was a significant predictor of hearing loss in the high-frequency band. Both ever exposed to industrial noise and the ear variable were nonsignificant predictors for all subcohort models.
Discussion
A very high prevalence of clinical (> 25 dB HL threshold) hearing loss was observed in this study (50–55%) in the high-frequency band (3–6 kHz). Other studies and reports have also found that agricultural workers are at risk of hearing loss (Gomez et al., 2001; Kerr et al., 2003; Suwanno et al., 2008). Thus, it is necessary to understand what work-related factors are contributing to this high prevalence of hearing loss in Thai agricultural workers in order to develop effective interventions and policies.
As expected, we found that aging seems to be the most important predictor in all of our models. With no other factors in the model agricultural workers would reach the clinical hearing threshold of 25 dB HL in the high-frequency band of the right ear at age 62 years. This is not surprising since age-related hearing loss is one of the most typical conditions among older adults (National Institute on Deafness and Other Communication Disorders, 2016).
We also found a significant association between the hearing threshold and smoking, mostly in high-frequency band. Others have also reported an impact of smoking on hearing (Chang et al., 2016; Pezzoli et al., 2017) and especially in workers who are exposed to noise (Sung et al., 2013; Wang et al., 2017). We also found that previous exposure to industrial noise was of borderline significance in some models.
We found a consistency of results in the models that included the full cohort of conventional farmers and organic farmers (most of whom had previously used pesticides) and the models that used only the subcohort of conventional farmers. All models suggested that increased use of pesticides (years or cumulative exposure score-years) is a risk factor for hearing loss. More specifically, the cumulative exposure score-years of insecticide use or organophosphate use increased the risk of higher hearing thresholds. Likewise, all models suggested that increased use (years or cumulative noise exposure in dB(A)-years) of noisy agricultural machinery increased the risk of higher hearing thresholds. Humann et al. (2012) observed an association between high-frequency hearing thresholds and years living on a farm, as well as years spent on various agricultural activities. Crawford et al. (2008) reported an association between hearing loss and noise exposures and pesticide exposure scores to insecticides, which was similar to the findings in our study. In contrast, a study conducted by Kós et al. (2014) found an association for both low and high-frequency band hearing loss and agricultural work. However, our study only found an association in the high-frequency band. This could be because of differences in the hearing threshold calculation method. In summary though, the results of this and other studies support the finding of an increase in the hearing threshold of the high-frequency band in both ears due to increasing years or cumulative exposure to pesticides and noise. One possible hypothesis proposed for organophosphate toxicity to cochlea is the generation of reactive oxygen species that induce cellular damage (Clerici et al., 1995, 1996).
The significance of the ‘ear’ variable in the full cohort models of years of exposure suggests a greater impact on the left ear than the right ear in both low and high-frequency band models. We suspect that this asymmetric effect may be caused by years of noise exposure rather than years of pesticide exposure, although there was no significant interaction between the noise and ‘ear’ variables in the model. The participants’ handedness might make them more susceptible to noise exposures in the left ear (Le et al., 2017). For instance, if noise source is closer to one side than the other it might play a role in this effect. The head shadow effect was suggested as differentially shielding the right ear from noise at the other side. But a study by Berg et al. (2014) examining agricultural machinery noise exposures was not consistent with our findings since they only observed the asymmetric pattern in men. Information about participants’ handedness or the side of machinery used was not available in this study. Alternatively, the left ear might more inherently susceptible to noise exposure compared with right ear (Nageris et al., 2007; Sturman et al., 2018). This might be because of individual differences in ear anatomy and physiology or differences in recovery after exposure to noise (Le et al., 2017).
However, the ‘ear’ variable in the subcohort model of conventional farmers was not significant which suggests that there was a bilateral effect of cumulative exposure to pesticides and noise in two ears. Morata and Lemasters (1995) suggested that the characteristics of a chemically induced hearing loss include bilateral loss, irreversible loss and a high frequency onset. One explanation for a bilateral impact is that chemicals are absorbed and distributed through the blood circulation throughout the body and can reach the cochlea of both ears. Since there have been no previous studies that looked at concurrent exposures to both pesticide and noise and we found inconsistent results in our models, we cannot clearly conclude whether exposure to pesticides and noise effect hearing in a bilateral or asymmetric manner.
Potential synergism between noise and pesticide exposures has been suggested in the literature (Guida et al., 2010; Delecrode et al., 2012). Since the interaction term was not significant of any of the models this implies that the impact of pesticide and noise exposures on the hearing threshold among agricultural workers is not a synergistic/multiplicative effect but rather an additive one. This suggests that either insecticides, especially organophosphates, or noise exposures could independently cause changes in hearing threshold of the high-frequency band or could work together in concert to increase the risk of hearing loss.
Strengths and limitations
This study recruited both conventional and organic agricultural workers, enabling us to investigate the impact on hearing of agricultural noise which is experienced by both groups and pesticides which are only currently used by the conventional farmers. Previous studies recruited workers who currently use pesticides and compared them with those who did not (Crawford et al., 2008; Kós et al., 2014). These studies used a simple yes/no categorization for noise exposure and included it as a covariate in their models, but did not report the results. A strength of our study was the estimation of the cumulative noise and pesticide exposure for the subcohort of conventional farmers. This enabled a more detailed examination of the impact of these semiquantitative measures of noise and pesticide exposures, but resulted in a lower sample size, which reduced our statistical power.
The pesticide diary used for collecting the data about the pesticide use and for calculating the intensity score and frequency of use in a year is believed to have less recall bias than retrospective questionnaires that have been previously used (Crawford et al., 2008). However, the diaries could have been incomplete or inaccurate in the amount of pesticide applied. We did not conduct a validity study to verify the accuracy of the diaries.
In comparison to self-reported hearing loss, this study used audiometric examination of hearing loss to reduce misclassification (Gomez et al., 2001). Measurement error was controlled by using standard procedures, calibrated equipment, and trained personnel. However, any audiometric error is expected to be non-differential and to affect to all participants equally so it would not introduce misclassification. Although this study used PTA, which is the gold standard method used to investigate the hearing threshold among participants, some authors have recommended complimentary audiologic tests such as high-frequency audiometry, otoacoustic emission or central auditory processing tests to describe the pathologies of the hearing system in more detail (Morata and Little, 2002; Teixeira et al., 2002; Nandamudi et al., 2012; Alcarás et al., 2013; Ashok Murthy and Visweswara Reddy, 2014).
Other limitations were the lack of detailed historical data on the use of hearing protection and the subject’s history of hunting or shooting which might have modified the effect of noise exposure. However, to our knowledge the use of hearing protection while operating agricultural machinery and hunting or shooting has never been common in Thai agricultural workers.
Conclusions
This study found an association in high-frequency band between both pesticide exposure (years of use and exposure score-years of pesticide or organophosphate use) and noise exposure (years of use or dB(A)-years) and an increase in the hearing threshold among Thai agricultural workers, while accounting for age, industrial noise ever and smoking status. These findings support previous studies examining hearing loss from pesticide exposure or noise exposures alone. This study did not find an interaction between the effect of noise exposure and pesticide exposure on hearing but instead found that these are additive effects. In addition, we did identify current smoking as one of factor that strongly contributes to hearing loss. A public health education program on hearing protection is needed among Thai agricultural workers who experience a very high prevalence of clinical hearing loss.
Funding
This study was supported in part by the grant from Thammasat University, Thailand. The research instrument was supported by the Office of Disease Prevention and Control 4 Ratchaburi, Royal Thai Ministry of Public Health. In addition, partial funding for this project was provided by the Fogarty International Center and the National Institute of Environmental Health Sciences of the National Institutes of Health, and the National Institute for Occupational Safety and Health of the US Centers for Disease Control and Prevention; under Grant the Global Environmental and Occupational Health Program Awards (U01TW010091 and U2RTW010088).
Disclaimer
The authors declare no conflict of interest relating to the material presented in this article. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors.
Supplementary Material
References
- Alcarás PA, Larcerda AB, Marques JM (2013) Study of evoked otoacoustic emissions and suppression effect on workers exposed to pesticides and noise. Codas; 25: 527–33. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute (ANSI) (1996) Determination of occupational noise exposure and estimation of noise-induced hearing impairment. New York: American National Standards Institute. [Google Scholar]
- American National Standards Institute (ANSI) (1997) Methods for manual pure-tone threshold audiometry. New York: American National Standards Institute. [Google Scholar]
- Ashok Murthy V, Visweswara Reddy YJ (2014) Audiological assessment in organophosphorus compound poisoning. Indian J Otolaryngol Head Neck Surg; 66: 22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett WS, Chamberlain D, Hallman E et al. (2000) Hearing conservation for farmers: source apportionment of occupational and environmental factors contributing to hearing loss. J Occup Environ Med; 42: 806–13. [DOI] [PubMed] [Google Scholar]
- Berg RL, Pickett W, Linneman JG et al. (2014) Asymmetry in noise-induced hearing loss: evaluation of two competing theories. Noise Health; 16: 102–7. [DOI] [PubMed] [Google Scholar]
- Campo P, Maguin K, Gabriel S et al. (2009) Combined exposure to noise and ototoxic substances. Luxembourg: European Agency for Safety and Health at Work. [Google Scholar]
- Carhart R, Jerger JF (1959) Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Disord; 24: 330–345. [Google Scholar]
- Chang J, Ryou N, Jun HJ et al. (2016) Effect of Cigarette smoking and passive smoking on hearing impairment: data from a population-based study. PLoS One; 11: e0146608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choochouy N, Kongtip K, Nankongnab N et al. (2016) Effect of occupational exposure to organophosphate pesticide on chemical-induced hearing loss. Asia Journal of Public Health; 7: 34–47. [Google Scholar]
- Clerici WJ, DiMartino DL, Prasad MR (1995) Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear Res; 84: 30–40. [DOI] [PubMed] [Google Scholar]
- Clerici WJ, Hensley K, DiMartino DL et al. (1996) Direct detection of ototoxicant-induced reactive oxygen species generation in cochlear explants. Hear Res; 98: 116–24. [DOI] [PubMed] [Google Scholar]
- Crawford JM, Hoppin JA, Alavanja MC et al. (2008) Hearing loss among licensed pesticide applicators in the agricultural health study. J Occup Environ Med; 50: 817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan SG, Eavey R, Shargorodsky J et al. (2011) Prospective study of alcohol use and hearing loss in men. Ear Hear; 32: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curhan SG, Eavey R, Wang M et al. (2015) Prospective study of alcohol consumption and self-reported hearing loss in women. Alcohol; 49: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies HW, Teschke K, Kennedy SM et al. (2009) A retrospective assessment of occupational noise exposures for a longitudinal epidemiological study. Occup Environ Med; 66: 388–94. [DOI] [PubMed] [Google Scholar]
- Delecrode CR, de Freitas TD, Frizzo AC et al. (2012) Prevalence of tinnitus in workers exposed to noise and organophosphates. Int Arch Otorhinolaryngol; 16: 328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemeci M, Alavanja MC, Rowland AS et al. (2002) A quantitative approach for estimating exposure to pesticides in the Agricultural Health Study. Ann Occup Hyg; 46: 245–60. [DOI] [PubMed] [Google Scholar]
- Finkler AD, Silveira AF, Munaro G et al. (2012) Otoprotection in guinea pigs exposed to pesticides and ginkgo biloba. Braz J Otorhinolaryngol; 78: 122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MI, Hwang SA, Sobotova L et al. (2001) A comparison of self-reported hearing loss and audiometry in a cohort of New York farmers. J Speech Lang Hear Res; 44: 1201–8. [DOI] [PubMed] [Google Scholar]
- Greenland S. (2004) Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol; 160: 301–5. [DOI] [PubMed] [Google Scholar]
- Guida HL, Morini RG, Cardoso AC (2010) Audiological evaluation in workers exposed to noise and pesticide. Braz J Otorhinolaryngol; 76: 423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DA. (2005) An Epidemiological assessment of Ohio farmers’hearing sensitivity. Ohio: University of Cincinnati. [Google Scholar]
- Howarth A, Shone GR (2006) Ageing and the auditory system. Postgrad Med J; 82: 166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humann MJ. (2011) Hearing loss and task-based noise exposures among agricultural populations. Iowa: The University of Iowa. [Google Scholar]
- Humann MJ, Sanderson WT, Gerr F et al. (2012) Effects of common agricultural tasks on measures of hearing loss. Am J Ind Med; 55: 904–16. [DOI] [PubMed] [Google Scholar]
- International Organization for Standardization (ISO) (1990) Determination of Occupational Noise Exposure and Estimation of Noise-Induced Hearing Impairment. Geneva, Switzerland: International Organization for Standardization. [Google Scholar]
- Johnson AC, Morata TC (2010) 142. Occupational exposure to chemicals and hearing impairment. In Torén K, editor The Nordic expert group for criteria documentation of health risks from chemicals. Sweden: Occupational and Environmental Medicine at Sahlgrenska Academy, University of Gothenburg. [Google Scholar]
- Kaewboonchoo O, Saleekul S, Jaipukdee S (2007) Age related changes in hearing level among Thai people. J Med Assoc Thai; 90: 798–804. [PubMed] [Google Scholar]
- Kerr MJ, McCullagh M, Savik K et al. (2003) Perceived and measured hearing ability in construction laborers and farmers. Am J Ind Med; 44: 431–7. [DOI] [PubMed] [Google Scholar]
- Körbes D, Silveira AF, Hyppolito MA et al. (2010) Organophosphate-related ototoxicity: description of the vestibulocochlear system ultrastructural aspects of guinea pigs. Braz J Otorhinolaryngol; 76: 238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kós MI, Miranda MF, Guimarães RM et al. (2014) Evaluation of the auditory system of farm workers exposed to pesticides. Revista CEFAC; 16: 941–948. [Google Scholar]
- Le TN, Straatman LV, Lea J et al. (2017) Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg; 46: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DI, Firth HM, Herbison GP (2003) Noise exposure and hearing loss in agriculture: a survey of farmers and farm workers in the Southland region of New Zealand. J Occup Environ Med; 45: 1281–8. [DOI] [PubMed] [Google Scholar]
- McNutt LA, Wu C, Xue X et al. (2003) Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol; 157: 940–3. [DOI] [PubMed] [Google Scholar]
- Morata TC, Lemasters GK (1995) Epidemiologic considerations in the evaluation of occupational hearing loss. Occup Med; 10: 641–56. [PubMed] [Google Scholar]
- Morata TC, Little MB (2002) Suggested guidelines for studying the combined effects of occupational exposure to noise and chemicals on hearing. Noise Health; 4: 73–87. [PubMed] [Google Scholar]
- Nageris BI, Raveh E, Zilberberg M et al. (2007) Asymmetry in noise-induced hearing loss: relevance of acoustic reflex and left or right handedness. Otol Neurotol; 28: 434–7. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Okamoto M, Nakamura K et al. (2000) Cigarette smoking and risk for hearing impairment: a longitudinal study in Japanese male office workers. J Occup Environ Med; 42: 1045–9. [DOI] [PubMed] [Google Scholar]
- Nandamudi S, Murthy VA, Ramakrishna Y et al. (2012) Effect of deliberate ingestion of organophosphate pesticide on distortion product oto-acoustic emissions (DPOAE). International Journal of Clinical Medicine. 3: 5. [Google Scholar]
- National Institute on Deafness and Other Communication Disorders (2016) Age-Related Hearing Loss Available at https://www.nidcd.nih.gov/health/age-related-hearing-loss Accessed 11 December 2018.
- Occupational Safety and Health Administration (1970). Occupational safety and health standards: Occupational health and environmental control—Audiometric test rooms (Standard No. 1910.95 App D) Available at https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.95AppD Accessed 15 November 2018.
- Pezzoli M, Lofaro D, Oliva A et al. (2017) Effects of smoking on eustachian tube and hearing. Int Tinnitus J; 21: 98–103. [DOI] [PubMed] [Google Scholar]
- Plakke BL, Dare E (1992) Occupational hearing loss in farmers. Public Health Rep; 107: 188–92. [PMC free article] [PubMed] [Google Scholar]
- Prakairungthong J, Kerdmuang S (2017) Factors associated with hearing loss among workers in auto part manufacturing industry in Suphanburi province. Journal of Nursing and Health Care; 35: 98–108. [Google Scholar]
- Rattanarak A, Intarapintuwat M, Wangsan K et al. (2018) State of occupational noise induce hearing loss situation in Thailand and other countries. KKU Journal for Public Health Research; 10: 1–10. [Google Scholar]
- Sturman CJ, Frampton CM, Ten Cate WJF (2018) Hearing loss asymmetry due to chronic occupational noise exposure. Otol Neurotol; 39: e627–34. [DOI] [PubMed] [Google Scholar]
- Sung JH, Sim CS, Lee CR et al. (2013) Relationship of cigarette smoking and hearing loss in workers exposed to occupational noise. Ann Occup Environ Med; 25: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanno S, Pongmuttasaya P, Jankhonkaen S et al. (2008) Prevalence of accident and hearing loss of machine handling by agricultural workers in responsible area of The Office of Disease Prevention and Control 7th Ubon Ratchatani Province. Thailand: The Office of Disease Prevention and Control 7th Ubon Ratchatani Province. [Google Scholar]
- Teixeira CF, Giraldo Da Silva Augusto L, Morata TC (2002) Occupational exposure to insecticides and their effects on the auditory system. Noise Health; 4: 31–9. [PubMed] [Google Scholar]
- Vyskocil A, Truchon G, Leroux T et al. (2012) A weight of evidence approach for the assessment of the ototoxic potential of industrial chemicals. Toxicol Ind Health; 28: 796–819. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang Z, Zhou M et al. (2017) The combined effect of cigarette smoking and occupational noise exposure on hearing loss: evidence from the Dongfeng-Tongji Cohort Study. Sci Rep; 7: 11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells TS, Seelig AD, Ryan MA et al. (2015) Hearing loss associated with US military combat deployment. Noise Health; 17: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018) Deafness and hearing loss Available at http://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss Accessed 15 November 2018.
- Zou G. (2004) A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol; 159: 702–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.