Abstract
Background
Diphtheria, once a major cause of childhood morbidity and mortality, all but disappeared following introduction of diphtheria vaccine. Recent outbreaks highlight the risk diphtheria poses when civil unrest interrupts vaccination and healthcare access. Lack of interest over the last century resulted in knowledge gaps about diphtheria’s epidemiology, transmission, and control.
Methods
We conducted 9 distinct systematic reviews on PubMed and Scopus (March–May 2018). We pooled and analyzed extracted data to fill in these key knowledge gaps.
Results
We identified 6934 articles, reviewed 781 full texts, and included 266. From this, we estimate that the median incubation period is 1.4 days. On average, untreated cases are colonized for 18.5 days (95% credible interval [CrI], 17.7–19.4 days), and 95% clear Corynebacterium diphtheriae within 48 days (95% CrI, 46–51 days). Asymptomatic carriers cause 76% (95% confidence interval, 59%–87%) fewer cases over the course of infection than symptomatic cases. The basic reproductive number is 1.7–4.3. Receipt of 3 doses of diphtheria toxoid vaccine is 87% (95% CrI, 68%–97%) effective against symptomatic disease and reduces transmission by 60% (95% CrI, 51%–68%). Vaccinated individuals can become colonized and transmit; consequently, vaccination alone can only interrupt transmission in 28% of outbreak settings, making isolation and antibiotics essential. While antibiotics reduce the duration of infection, they must be paired with diphtheria antitoxin to limit morbidity.
Conclusions
Appropriate tools to confront diphtheria exist; however, accurate understanding of the unique characteristics is crucial and lifesaving treatments must be made widely available. This comprehensive update provides clinical and public health guidance for diphtheria-specific preparedness and response.
Keywords: diphtheria, outbreak, critical vaccination threshold, reproductive number, systematic review
Although largely forgotten, diphtheria remains a significant outbreak threat. Through systematic review and reanalysis, we provide a comprehensive summary of the clinical and epidemiologic aspects of diphtheria, with insights into transmission, treatment, and control.
(See the Editorial Commentary by Wiedermann on pages 98–99.)
Once a leading cause of childhood mortality, the global burden of diphtheria has fallen dramatically, from more than a million cases a year in the mid-1900s to 7097 cases reported in 2016 (Supplementary Figure 1) [1, 2]. While discovery of diphtheria antitoxin (in 1888) and penicillin (in 1928) contributed to diphtheria control, most of the reduction is attributable to diphtheria toxoid vaccine (introduced in 1923), particularly following the scale-up in 1974 of the 3-dose series of diphtheria, tetanus, and pertussis (DTP)–containing vaccines, which reached 86% global coverage in 2016 (Supplementary Figure 1, Supplementary Table 2) [3–6]. As a result of these successes, diphtheria is no longer regarded as a major public health threat and has largely been forgotten [7].
However, recent outbreaks associated with displaced populations and infrastructure failures underscore the continued threat posed by diphtheria (Supplementary Table 1). In November 2017, the largest diphtheria outbreak of this century emerged among Rohingya refugees in Kutupalong camp, Bangladesh. As of June 2019, 8640 cases and 45 deaths have been reported [8]. This is the latest in a series of large outbreaks associated with political unrest, including ongoing outbreaks in Venezuela (1904 suspected cases, 164 deaths), Yemen (1907 suspected cases, 98 deaths), and Haiti (808 probable cases, 107 deaths) [9–11]. Similar large outbreaks have exploded into multinational epidemics as recently as the 1990s, when a decade-long outbreak in eastern Europe caused 157 000 cases and 5000 deaths [12]. Further complicating the situation, global stockpiles of life-saving diphtheria antitoxin have dwindled in recent years due to expiration and discontinued production, stemming from reduced demand [13].
Recent epidemics underscore the need for renewed efforts to better understand diphtheria and enhance epidemic preparedness. Importantly, past research central to our understanding of diphtheria epidemic dynamics made inappropriate assumptions, including that vaccination confers immunity against colonization, immunity is lifelong and does not wane, and only symptomatic individuals are infectious [14–17]. This resulted in flawed estimates of key epidemiologic and disease control measures. We conducted systematic reviews of the clinical course, natural history, epidemiology, transmission dynamics, and control of diphtheria, and used extracted data to estimate key epidemiologic quantities, emend essential metrics, and explore implications for treatment and control.
METHODS
We identified key clinical and epidemiological characteristics critical to understanding and controlling diphtheria. For each, we defined a set of search terms and, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards [18], conducted 9 systematic reviews, extracted relevant data, and conducted meta-analyses of pooled data to reestimate values (see Supplementary Table 3 and the PRISMA checklist in the Supplementary Methods). Specific analyses are described below with full methods, including details of the individual meta-analyses, in the Supplementary Materials. Bayesian approaches with random effects were used for each analysis to account for heterogeneity between studies, settings, and time periods.
Literature Review
Two study members independently reviewed titles and abstracts of 6934 articles identified in PubMed and Scopus, reviewed 781 full texts, and extracted data from 266 studies (Supplementary Figure 2). We summarize the findings and use the extracted data to estimate parameters. Where data were not available, we report literature values.
Case Fatality Ratio
We estimate the case fatality ratio by age, number of vaccine doses received, antitoxin treatment status, and antitoxin delay, using mixed-effects logistic regression models, with random intercepts for studies, populations, and periods to account for heterogeneity [19] (Supplementary Materials).
Estimated Distributions From Interval-censored Data
We characterize the incubation period, serial interval, and colonization duration using distributions fit to pooled individual-level data from multiple studies using previously described methods for interval-censored data [20].
Asymptomatic Transmission
We estimate the contribution of asymptomatic infections to overall transmission using data in which contacts of both symptomatic and asymptomatic infected individuals were followed forward for 30 days.
Reproductive Number
We estimate the basic reproductive number, R0, using an adaptation of previously described methods implemented in a hierarchical Bayesian framework [21]. We estimate the effective reproductive number, R, and the serial interval individually for 23 outbreaks, then adjust for the effect of the DTP vaccine, contribution of asymptomatic individuals, waning immunity, and vaccination coverage, to estimate the range of R0 accounting for uncertainty and variability between outbreaks (see Technical Supplement, Section 3).
Diphtheria Control
We estimate the critical vaccination threshold accounting for differing transmission of asymptomatic individuals and the impact of vaccination (reduction in transmissibility and disease severity but not infection). We use estimated distributions of the effective reproductive number and the critical vaccination threshold to quantify the expected impact of antibiotics and isolation on epidemics (see Technical Supplement, Section 5).
RESULTS
Clinical Course and Natural History
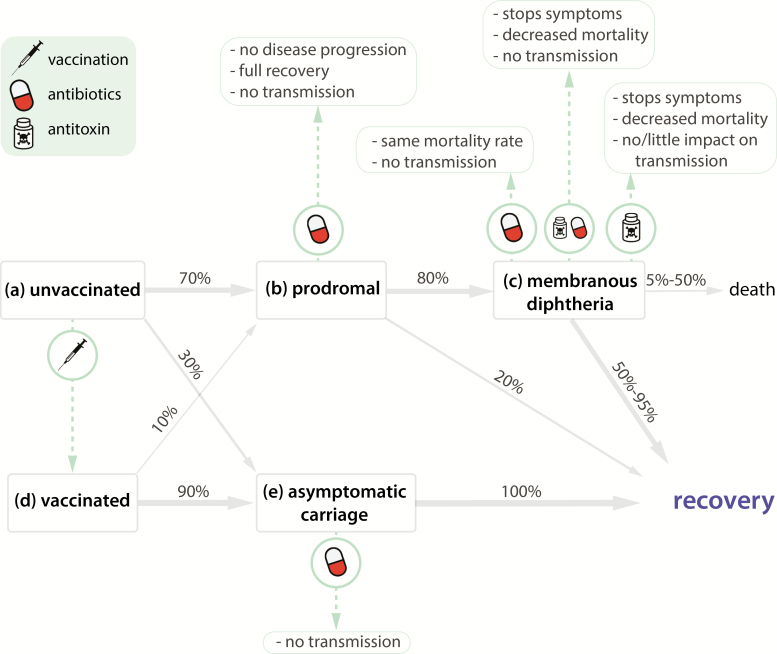
Diphtheria is caused by infection with toxigenic Corynebacterium diphtheriae or, rarely, Corynebacterium ulcerans, with disease caused by an exotoxin the bacilli produces [22]. Of the 2 common forms of diphtheria (respiratory and cutaneous), respiratory diphtheria carries a significantly higher risk of mortality and is the only form reportable to the World Health Organization (WHO). Respiratory infections typically progress from prodromal symptoms to membranous inflammation of the pharynx, tonsils, or larynx (Figure 1).
Figure 1.
Natural history of diphtheria with prevention and treatment interventions. Of the unvaccinated individuals who become infected with toxigenic Corynebacterium diphtheriae (a), 70% develop prodromal symptoms (b), whereas 30% become asymptomatic carriers (e). Eighty percent of individuals with nonspecific symptoms develop membranous diphtheria (c) whereas 20% recover. Of those with membranous diphtheria, 5%–50% of individuals die of complications, and 50%–95% recover. Vaccinated individuals can be colonized; however, the toxoid vaccine provides protection against symptoms. Thus, 90% of fully vaccinated individuals become asymptomatic carriers (e), whereas only 10% develop prodromal symptoms (b). Vaccinated individuals who develop nonspecific symptoms have lower risk of severe disease and death compared with unvaccinated individuals and are more likely to recover directly from prodromal symptoms, although they can develop severe complications (c) and die.
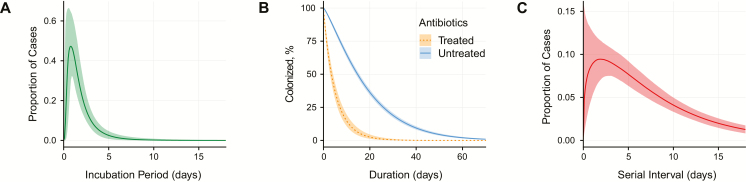
We estimate that the median time from infection to prodromal symptom onset is 1.4 days (95% credible interval [CrI], 1.0–1.9) (Supplementary Table 4, Figures 1 and 2A). An estimated 80% of untreated symptomatic cases progress to membranous diphtheria 2–3 days after symptom onset, although we could not validate these estimates using primary data [22] (Figure 1). Progression to death from asphyxia due to airway obstruction within 1–2 weeks after symptom onset is responsible for 60%–65% of deaths and is associated with laryngeal infection. Toxic cardiomyopathy occurs 7–14 days after the onset of respiratory symptoms in 10%–25% of patients and is responsible for 20%–25% of deaths [22–25]. Neurological disorders, such as hypoesthesia, polyneuropathy, and cranial neuropathies, develop weeks to months later and occur in 20%–25% of untreated cases and are responsible for up to 15% of deaths [22–25].
Figure 2.
Epidemiological characteristics of diphtheria. A, Incubation period for diphtheria (data from 4 studies). B, Time to clearance of Corynebacterium diphtheriae in cases treated with antibiotics and untreated cases. Clearance time among treated cases is from initiation of antibiotic treatment (data from 11 studies). C, Serial interval (proxy for generation time; data from 8 studies).
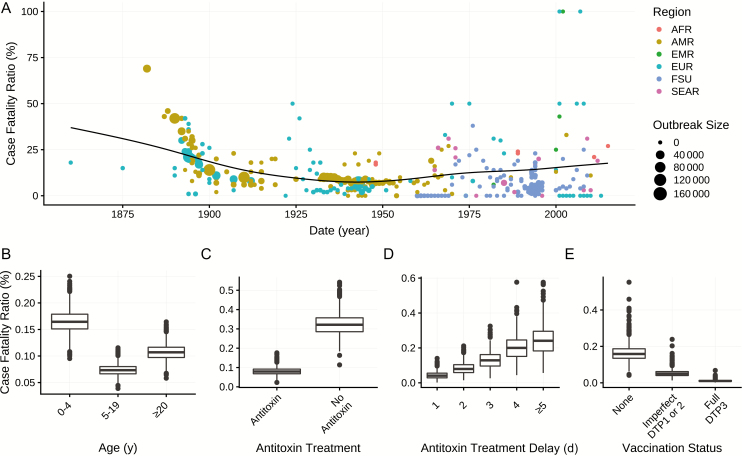
We estimate that the case fatality ratio for untreated, never-vaccinated cases is 29.0% (95% credible interval [CrI], 28.8%–29.2%). Vaccination and treatment substantially reduce the case fatality ratio (see “Treatment and Prevention” below). Children aged <5 years are more likely to die from symptomatic infection than adults >20 years of age (relative risk [RR], 1.5 [95% CrI, 1.4–1.6]), whereas children 5–19 years of age are less likely to die from infection than adults aged >20 years (RR, 0.8 [95% CrI, 0.8–0.9]) (Table 1, Figure 3B).
Table 1.
Relative Risk of Mortality Among Diphtheria Cases, by Age, Vaccination Status, and Antitoxin Receipt
| Characteristic | Relative Risk of Mortality (95% CrI) |
|---|---|
| Age, y | |
| 0–4 | 1.5 (1.4–1.6) |
| 5–19 | 0.80 (.76–.89) |
| ≥20 | Reference |
| Vaccination | |
| None | Reference |
| Partial (1–2 doses) | 0.32 (.23–.44) |
| Full (≥3 doses) | 0.07 (.04–.10) |
| Antitoxina | |
| No | Reference |
| Yes | 0.24 (.22–.28) |
Relative risks of mortality were estimated from pooled data using mixed-effects regression models that accounted for between-study variability in overall mortality rate (see Supplementary Figure 4 for data sources).
Abbreviation: CrI, credible interval.
aHospitalized cases.
Figure 3.
Case fatality ratio. A, Case fatality ratio by year, with World Health Organization (WHO) region (color), outbreak size (point size), and the weighted mean over time (black line). B, Case fatality ratio by age. C, Case fatality ratio by diphtheria antitoxin treatment. D, Case fatality ratio by diphtheria antitoxin treatment delay. E, Case fatality ratio by vaccination status. The former Soviet Union (FSU) is not a WHO region but is specific to the 1990–1998 outbreak. Abbreviations: AFR, African Region; AMR, Region of the Americas; DTP, diphtheria-tetanus-pertussis vaccine; EMR, Eastern Mediterranean Region; EUR, European Region; SEAR, South-East Asia Region.
Many colonized individuals never develop symptoms (Figure 1); we find that 31% (95% CrI, 18%–55%) of infections in unvaccinated individuals are asymptomatic. Untreated individuals remain colonized for an average of 18.5 days (95% CrI, 17.7–19.4 days), with 5% remaining colonized longer than 48 days (95% CrI, 46–51 days) (Supplementary Table 4, Figure 2B). We found no statistical evidence for different colonization times between symptomatic and asymptomatic infections, absent treatment (difference in Watanabe-Akaike information criterion, −3.4 [95% confidence interval [CI], −10.0 to 3.3]).
Epidemiology and Transmission
Since 2000, most reported outbreaks have been sporadic and small, although large outbreaks have occurred typically following reduced vaccination coverage (often associated with infrastructure failures and civil unrest) [26, 27]. Prior to 1980, adults (aged ≥20 years) comprised only 17% of cases (95% CrI, 0–81%), but subsequently 36% of reported cases (95% CrI, 0–75%) have been adults (Supplementary Figure 3).
Transmission occurs person-to-person via respiratory droplets or contact with cutaneous lesions [28]. Both symptomatic and asymptomatic individuals can transmit diphtheria, although we estimate that asymptomatic carriers cause 76% (95% CrI, 59%–87%) fewer infections than symptomatic cases over the course of carriage (Supplementary Table 4). As humans are assumed to be the sole reservoir of C. diphtheriae, cutaneous diphtheria likely plays an important role in transmission during interepidemic periods [22, 29].
The reported case fatality ratio for respiratory diphtheria has varied widely, ranging from 0 to 69% (Supplementary Table 4). The average case fatality ratio declined from 52% in the 1880s to 7% in the 1940s–1950s (Figure 3A), concurrent with the discovery and scale-up of therapies. In most recent outbreaks, mortality is concentrated among initial cases, who often receive inadequate treatment due to misdiagnosis (Supplementary Figure 4). In resource-limited settings, case fatality ratios have remained much higher (range, 3%–33%).
Across 23 outbreaks, we find that the effective reproductive number, R, during the initial growth phase of the outbreaks ranged from 1.1 to 3.2, including the Rohingya outbreak (R = 3.2 [95% CrI, 3.0–3.4]) (Supplementary Table 4). Adjusting for immunity and asymptomatic transmission, we estimate that the basic reproductive number, R0, ranged from 1.7 to 4.3 (median, 2.6) (Supplementary Table 4; lower than prior estimates of 3.5–8) [14, 15, 17, 30, 31]. We estimate that the serial interval for diphtheria (ie, the time between symptom onset in successive cases of transmission) is 7.8 days (95% CrI, 6.3–9.7 days), with 5% of intervals <0.8 days and 5% longer than 21 days (Supplementary Table 4, Figure 2C).
Treatment and Prevention
We find that postinfection administration of diphtheria antitoxin reduces mortality by 76% (RR, 0.24 [95% CrI, 0.22–0.28]) (Table 1, Figures 1C and 3C). However, because antitoxin only neutralizes circulating toxin, not intracellular toxin [23], its effectiveness depends on prompt administration relative to symptom onset: we estimate the probability of mortality increases daily, from 4.2% (95% CrI, 2.5%−7.1%) if administered within 24–48 hours, to 24% if administered on day 5 or later, approximately doubling with each day of delay (Figure 3D, Supplementary Table 4). It is not clear at what point the delay negates any benefit, although this knowledge would be valuable.
We find that patients receiving antibiotic treatment clear C. diphtheriae respiratory colonization within 5.2 days (95% CrI, 4.4–6.1 days) of initiating treatment on average, reducing the average duration of infectiousness by as much as 2 weeks (Figure 2B, Supplementary Table 4). This is contradictory to current WHO recommendations suggesting isolation for only 48 hours [32]. Longer isolation for 6 days, or until negative cultures as recommended by the Centers for Disease Control and Prevention and the American Academy of Pediatrics, may be necessary [33].
Diphtheria toxoid vaccine protects against symptomatic disease by stimulating production of antitoxin antibodies. The primary series of DTP vaccination includes doses at 6, 10, and 14 weeks, with boosters at 12–23 months, 4–7 years, 9–15 years, and every 10 years in adults [2, 27]. Through meta-analysis, we find that full vaccination (≥3 doses) with DTP vaccine is 87% (95% CrI, 68%–97%) effective against symptomatic disease, whereas incomplete vaccination (1–2 doses) is 71% (95% CrI, 17%−92%) effective (Supplementary Figure 5A, Supplementary Table 4). Previous studies found that vaccine effectiveness increases by dose, reaching 99% with 5 doses [34, 35]. Among symptomatic cases, prior history of diphtheria toxoid vaccine reduced the risk of severe disease and death: Full vaccination is 81% (95% CrI, 74%–86%) effective in preventing severe disease (defined as local and systemic symptoms plus a major complication) and 93% (95% CrI, 90%–96%) effective in preventing death; partial vaccination is 47% (95% CrI, 23%–63%) effective against severe disease and 68% (95% CrI, 56%–77%) effective against death (Table 1, Figures 1 and 3E, and Supplementary Figure 7). Diphtheria toxoid vaccine is assumed to not prevent colonization; our analysis supports this (effectiveness against colonization, −17% [95% CrI, −360% to 73%]) (Supplementary Table 4) [36].
Vaccine-derived immunity wanes over time: in populations with low uptake of booster vaccination, we find that full childhood vaccination is 96% effective against symptomatic disease among children 0–4 years old, 92% among 5- to 19-year-olds, and 63% among ≥20-year-olds (Spearman test for trend, P < .001) (Supplementary Figure 5B, Supplementary Table 4). We find similar waning immunity among individuals reporting partial vaccination (91%, 87%, and 30%; P < .001). Serological studies also demonstrate waning immunity: We find that the proportion of individuals with fully protective antibody levels (≥0.1 IU/mL) declines by 0.6% per year since vaccination (95% CI, .36%–1.52%). Similarly, from studies reporting participant age without including time since vaccination, we find that fully protective antibody levels decline by 0.8% per year of age (95% CI, .3%–1.2%), consistent with rates of decline in vaccine effectiveness (Supplementary Table 4). In contrast, prior to widespread vaccination, immunity increased with age, indicating natural infection and boosting (Supplementary Figure 6) [37, 38].
Diphtheria Control and Outbreak Response
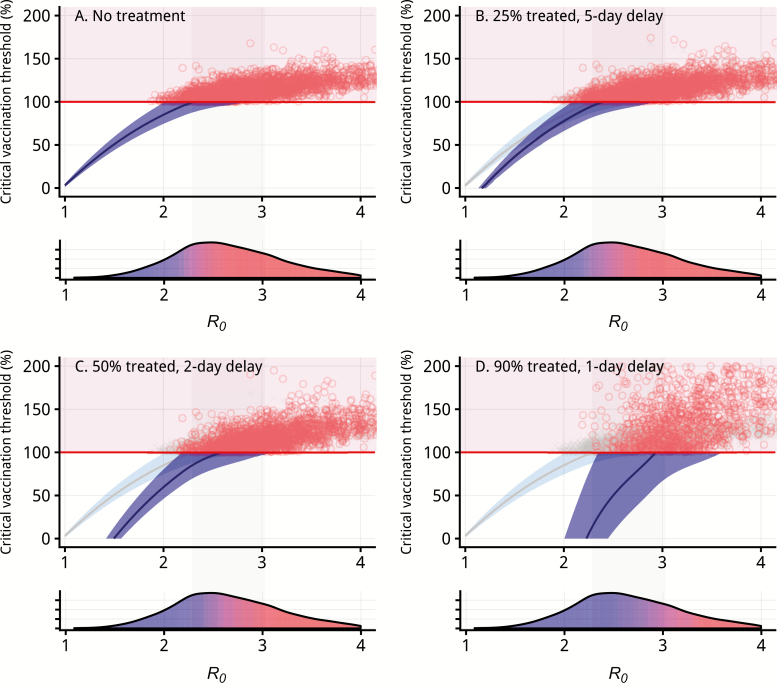
Although vaccination does not prevent colonization, we estimate it reduces transmission by 60% (95% CrI, 51%–68%), likely through reduced symptomatic shedding. Consequently, vaccination alone (100% coverage) can interrupt transmission, but only when R0 < 2.3 (95% CrI, 2.0–2.9), which we estimate to be true in 27% of outbreak settings (Figure 4A). However, through combining vaccination with antibiotics, which accelerates the clearance of colonization, consequently, we can interrupt transmission for a wider range of settings (ie, R0 ≥ 2.3). In a fully vaccinated population, antibiotic treatment of 25% of symptomatic cases an average of 5 days after fever onset can interrupt transmission when R0 < 2.4 (35% of estimated outbreak settings). Improving antibiotic treatment to 50% of cases within 2 days interrupts transmission when R0 < 2.6 (48% of settings), and with 90% treated within 1 day, transmission is interrupted when R0 < 2.9 (70% of settings) (Figure 4).
Figure 4.
Diphtheria control and outbreak response. The critical vaccination threshold, Vc, or vaccination coverage needed to achieve herd immunity, for diphtheria is dependent on the basic reproductive number, R0. Based on data from 23 outbreaks, without additional intervention or antibiotic treatment, achievable critical vaccination thresholds are only possible in 28% of simulated outbreak settings (Supplementary Table 4). The relationship between the critical vaccination threshold and basic reproductive number is shown in the top portion of each panel. The blue-shaded region indicates estimates where the critical vaccination threshold is below 100% and herd immunity is achievable through vaccination, and the red points correspond to simulated outbreaks for which herd immunity is not achievable through vaccination alone. The lower portion of each panel demonstrates the corresponding density plot of basic reproductive numbers and is shaded according to the proportion of simulated outbreaks where herd immunity is achievable (blue) compared to where herd immunity is not achievable (red). The light gray–shaded region indicates the interquartile range of our R0 estimates. A, Scenario for no treatment (replicated in B–D in light blue for reference). With each increase in the proportion of cases treated (25%, 50%, 90%), and each decrease in average delay to treatment (5-, 2-, and 1-day delays), the vaccination coverage required at each value of the basic reproductive number decreases (comparing the dark blue to the light blue wedge). The proportion of observed R0 values for which the critical vaccination threshold is achievable increases (increased blue) as treatment coverage increases and delay decreases. These scenarios demonstrate the critical importance of rapid antibiotic treatment for diphtheria outbreak prevention or response.
It follows that achieving herd immunity, whereby 100% vaccination coverage is not necessary to interrupt transmission, depends upon both R0 and antibiotic prevalence. Assuming 100% of cases are successfully treated with antibiotics eventually, an average of 7 days after fever onset, we find a critical vaccination threshold (Vc) of 91% (95% CrI, 18%–156%). This supports a hypothesis that frequent antibiotic treatment of those with prodromal symptoms likely plays an important complementary role in global control.
The effectiveness of vaccination for outbreak response is limited by the time required to deploy vaccine and develop immunity; thus, additional control measures are needed. To be most effective, we find that these measures must either interrupt transmission earlier among symptomatic individuals or also target asymptomatic individuals. In fully susceptible populations, containment requires immediate isolation of 68% (95% CrI, 50%–84%) of symptomatic cases (isolation immediately halts transmission, unlike antibiotics). For the Rohingya outbreak, with a higher R0, isolation of 78% (95% CrI, 73%–90%) of symptomatic cases is needed. Contact tracing with isolation or antibiotic prophylaxis of contacts would be similarly effective, depending on the capture rate. Alternatively, instead of a targeted approach, we find that a novel approach that includes random, mass administration of antibiotics would interrupt transmission in all but the most extreme settings, with relatively low coverage (median required coverage of 27% [95% CrI, −69% to 95%]).
DISCUSSION
Diphtheria is a reemerging infectious disease with large recent outbreaks. These outbreaks highlight the need for better understanding of the natural history, key epidemiologic parameters, the role of asymptomatic individuals in transmission, and effectiveness of control measures. Here, using historical and contemporary data, we identify knowledge gaps and misconceptions. Through novel and modern approaches, we comprehensively update clinical and epidemiological metrics to contribute to improve clinical practices and support alternative strategies for response to future diphtheria outbreaks.
Asymptomatic infection plays a critical role in both transmission and control of diphtheria, yet previous work largely ignored it [14, 15, 17, 29, 30]. We find that vaccination is highly effective at preventing symptomatic disease (>87% with 3 doses; Supplementary Table 4), yet has no effect on preventing infection, and while those with asymptomatic infections still transmit, they do so at only 24% the rate of symptomatic cases. When accounting for this, we find that diphtheria is simultaneously less transmissible than previously thought [14, 15, 17, 29, 30], but also more challenging to control through vaccination alone. Interestingly, it appears that widespread use of antibiotics likely plays a role in maintaining herd immunity through accelerated clearance of colonization, and, thus, reduced secondary cases.
As a result of asymptomatic transmission, effective outbreak response must couple vaccination with other interventions. Full vaccination coverage is only sufficient to interrupt transmission in 27% of outbreak settings. However, this improves to 70% with rapid antibiotic treatment of 90% of symptomatic cases. Efforts that target cases earlier and target asymptomatic carriers demonstrate even greater effectiveness, with lower required coverage, including contact tracing, isolation, and antibiotic prophylaxis, and should be essential tools in routine outbreaks response. However, current guidelines for isolation may be insufficient: we find that clearance after antibiotic initiation takes 5 days on average, whereas guidelines recommend isolation for only 2 days.
Our results support the idea that mass administration of antibiotics, particularly azithromycin, which reaches both symptomatic and asymptomatic individuals, could be effective for diphtheria control. This could be particularly effective if coupled with other mass healthcare activities such as vaccination. With transmission interruption achieved with only 27% population coverage, and the added benefit of substantial all-cause mortality reduction [39], this approach should be explored for rapid deployment, particularly in humanitarian crises like the Rohingya.
Prompt treatment of symptomatic cases with diphtheria antitoxin is critical to limit morbidity and mortality, especially in unvaccinated populations. Prior to antitoxin development, large outbreaks regularly produced mortality up to 69% [40], and recent outbreaks in populations lacking access have seen mortality exceeding 21% [41]. Unfortunately, complacency has led to neglect of antitoxin production; current worldwide stockpiles are only sufficient to treat 500–2500 cases, with no WHO-prequalified product on the market [42]. Given shortages, additional research is needed for improving dosage efficiency and developing diphtheria antitoxin alternatives, including human monoclonal antibodies, which have demonstrated promise and could resolve this global problem [43].
Despite extensive literature review and analysis, our findings do have limitations. Of note, our assumptions of asymptomatic transmissibility were derived from a single study performed prior to vaccine availability. We also ignore the impact of cutaneous diphtheria, which may contribute to transmission. These and other limitations discussed in the Supplementary Materials are partially the result of limited modern research on diphtheria, and renewed research interest would prove highly valuable. Despite these limitations, we were able to characterize diphtheria across 266 publications, 161 years, dozens of countries, and thousands of individuals, providing updated, complete, and generalizable estimates for numerous quantities essential for control and response.
The Rohingya diphtheria outbreak was a wake-up call for the risk diphtheria still poses. Despite early surveys finding dangerously low diphtheria vaccination coverage [44, 45], response efforts prioritized pathogens of higher perceived threat (ie, cholera, measles, polio), and few, if any, early diphtheria-specific prevention measures were taken [46]. Fortunately, when the outbreak occurred, the expeditious response undoubtedly helped slow transmission and limit mortality. The awareness of the threat diphtheria poses is improving, although substantial progress is still needed: In a 2017 survey, only 6 of 30 European Economic Area countries met the minimum criteria for diphtheria surveillance, diagnostics, and expertise [43]; other regions are likely less prepared. Updated WHO guidance on surveillance, clinical care, and outbreak response provide defined standards for filling these gaps [47], and our review complements this guidance, providing comprehensive and rigorously derived metrics.
CONCLUSIONS
The Rohingya outbreak served as a warning of the epidemic potential of diphtheria in populations with insufficient or interrupted routine immunization services. We demonstrate that although the critical vaccination threshold has been underestimated for decades, it may still be possible to achieve, with prevalent antibiotic use likely contributing to the near-elimination of diphtheria over the last century. Along with diphtheria toxoid vaccine, antibiotics and isolation are critical to interrupt transmission, and antitoxin is vital for limiting mortality, necessitating immediate action to resolve the global shortage of diphtheria antitoxin. Through comprehensive reexamination and update of clinical and epidemiological metrics, this work provides a renewed picture of diphtheria transmission and epidemiology, and a basis for future diphtheria-specific preparedness, response, and research.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Conception and design of study, and acquisition of data: S. A. T. and L. T. K. Development and/or verification of analytic methods: S. A. T., L. T. K., A. S. A., J. L. Analysis and/or interpretation of data and drafting of the manuscript: S. A. T. and L. T. K. Revision of the manuscript and approval of the final manuscript: S. A. T., L. T. K., W. J. M., L. H. C., E. M., A. S. A., J. L.
Acknowledgments. The authors thank Joshua Kaminsky for his assistance with checking their math, and all of their colleagues from the Infectious Disease Dynamics group at the Johns Hopkins Bloomberg School of Public Health for their assistance in reviewing this work and providing valuable input on its direction.
Data and code availability. All data extracted from the systematic review and relevant R, JAGS, and Stan code used for the included analyses are available at http://dx.doi.org/10.17605/OSF.IO/R2PN5.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant number R01 AI102939).
Potential conflicts of interest. The authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Diphtheria vaccine: WHO position paper. Geneva, Switzerland: World Health Organization, 2006:21–32. Available at: https://www.who.int/wer/2006/wer8103.pdf. Accessed 24 April 2019. [Google Scholar]
- 2. World Health Organization. Diphtheria Available at: http://www.who.int/immunization/monitoring_surveillance/burden/diphtheria/en/. Accessed 24 April 2019.
- 3. Grundbacher FJ. Behring’s discovery of diphtheria and tetanus antitoxins. Immunol Today 1992; 13:188–90. [DOI] [PubMed] [Google Scholar]
- 4. Fleming A. The discovery of penicillin. Br Med Bull 1944; 2:4–5. [Google Scholar]
- 5. Glenny AT, Hopkins BE. Diphtheria toxoid as an immunising agent. Br J Exp Pathol 1923; 4:283–8. [Google Scholar]
- 6. World Health Organization. Expanded Programme on Immunization Available at: https://www.who.int/immunization/programmes_systems/supply_chain/benefits_ of_immunization/en/. Accessed 26 April 2019.
- 7. Fine PEM. Herd immunity: history, theory, practice. Epidemiol Rev 1993; 15:265–302. [DOI] [PubMed] [Google Scholar]
- 8. ReliefWeb. Bi-weekly situation report #11, 9 June 2019—Bangladesh. Geneva, Switzerland: WHO, 2019. Available at: https://reliefweb.int/report/bangladesh/rohingya-refugee-crisis-who-bangladesh-bi-weekly-situation-report-11-09-june-2019. Accessed 17 June 2019. [Google Scholar]
- 9. Pan American Health Organization/World Health Organization. Diphtheria—epidemiological update. 2018 Available at: https://www.paho.org/hq/index.php?option=com_content&view=article&id=14379:24-may-2018-diphtheria-epidemiological-update&Itemid=42346&lang=en. Accessed 24 April 2019.
- 10. United Nations Children’s Fund. Yemen humanitarian situation report (June 2018) Available at: https://reliefweb.int/report/yemen/unicef-yemen-humanitarian-situation-report-june-2018-enar. Accessed 24 April 2019.
- 11. ReliefWeb. Epidemiological update: diphtheria in the Americas. Summary of the situation: 18 March 2019—Haiti Available at: https://reliefweb.int/report/haiti/epidemiological-update-diphtheria-americas-summary-situation-18-march-2019. Accessed 24 April 2019.
- 12. Dittmann S, Wharton M, Vitek C, et al. Successful control of epidemic diphtheria in the states of the former Union of Soviet Socialist Republics: lessons learned. J Infect Dis 2000; 181(Suppl 1):S10–22. [DOI] [PubMed] [Google Scholar]
- 13. Kupferschmidt K. Life-saving diphtheria drug is running out. Science 2017; 355:118–9. [DOI] [PubMed] [Google Scholar]
- 14. Trisilowati Darti I, Fitri S. On stability analysis and optimal control of an SIR epidemic model. Far East J Math Sci 2017; 102:1979–93. [Google Scholar]
- 15. Huang S-Z. A new SEIR epidemic model with applications to the theory of eradication and control of diseases, and to the calculation of R0. Math Biosci 2008; 215:84–104. [DOI] [PubMed] [Google Scholar]
- 16. Plans-Rubió P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum Vaccines Immunother 2012; 8:184–8. [DOI] [PubMed] [Google Scholar]
- 17. Anderson RM, May RM. Directly transmitted infections diseases: control by vaccination. Science 1982; 215:1053–60. [DOI] [PubMed] [Google Scholar]
- 18. Preferred Reporting Items for Systematic Reviews and Meta-Analyses. PRISMA guidelines Available at: http://www.prisma-statement.org/. Accessed 17 June 2019.
- 19. Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med 1995; 14:2685–99. [DOI] [PubMed] [Google Scholar]
- 20. Reich NG, Lessler J, Cummings DA, Brookmeyer R. Estimating incubation period distributions with coarse data. Stat Med 2009; 28:2769–84. [DOI] [PubMed] [Google Scholar]
- 21. White LF, Pagano M. A likelihood-based method for real-time estimation of the serial interval and reproductive number of an epidemic. Stat Med 2008; 27:2999–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tiwari TSP, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, eds. Plotkin’s vaccines. 7th ed. Philadelphia, PA: Elsevier, 2018:261–75.e7. [Google Scholar]
- 23. Rakhmanova AG, Lumio J, Groundstroem K, et al. Diphtheria outbreak in St. Petersburg: clinical characteristics of 1860 adult patients. Scand J Infect Dis 1996; 28:37–40. [DOI] [PubMed] [Google Scholar]
- 24. Piradov MA, Pirogov VN, Popova LM, Avdunina IA. Diphtheritic polyneuropathy: clinical analysis of severe forms. Arch Neurol 2001; 58:1438–42. [DOI] [PubMed] [Google Scholar]
- 25. Long SS, Pickering LK, Prober CG.. Principles and practice of pediatric infectious disease. Philadelphia, PA: Elsevier Health Sciences, 2012. [Google Scholar]
- 26. Quick ML, Sutter RW, Kobaidze K, et al. Risk factors for diphtheria: a prospective case-control study in the Republic of Georgia, 1995–1996. J Infect Dis 2000; 181:S121–9. [DOI] [PubMed] [Google Scholar]
- 27. Markina SS, Maksimova NM, Vitek CR, Bogatyreva EY, Monisov AA. Diphtheria in the Russian Federation in the 1990s. J Infect Dis 2000; 181(Suppl 1):S27–34. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention. Diphtheria infection 2017. Available at: https://www.cdc.gov/diphtheria/index.html. Accessed 26 April 2019.
- 29. Blattner RJ. Epidemiology of diphtheria: role of cutaneous infection. J Pediatr 1969; 74:991–3. [DOI] [PubMed] [Google Scholar]
- 30. Matsuyama R, Akhmetzhanov AR, Endo A, et al. Uncertainty and sensitivity analysis of the basic reproduction number of diphtheria: a case study of a Rohingya refugee camp in Bangladesh, November–December 2017. PeerJ 2018; 6:e4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Finger F, Funk S, White K, Siddiqui MR, Edmunds WJ, Kucharski AJ. Real-time analysis of the diphtheria outbreak in forcibly displaced Myanmar nationals in Bangladesh. BMC Med 2019; 17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. Operational protocol for clinical management of diphtheria, Bangladesh Available at: https://www.who.int/health-cluster/resources/publications/WHO-operational-protocols-diphtheria.pdf?ua=1. Accessed 20 June 2019.
- 33. American Academy of Pediatrics, Committee on Infectious Diseases; Kimberlin DW, et al. Red book: 2018–2021 report of the Committee on Infectious Diseases Available at: https://ebookcentral.proquest.com/lib/qut/detail.action? docID=5391883. Accessed 10 July 2019.
- 34. Chen RT, Hardy IRB, Rhodes PH, Tyshchenko DK, Moiseeva AV, Marievsky VF. Ukraine, 1992: First assessment of diphtheria vaccine effectiveness during the recent resurgence of diphtheria in the former Soviet Union. J Infect Dis 2000; 181:S178–83. [DOI] [PubMed] [Google Scholar]
- 35. Bisgard KM, Rhodes P, Hardy IR, et al. Diphtheria toxoid vaccine effectiveness: a case-control study in Russia. J Infect Dis 2000; 181(Suppl 1):S184–7. [DOI] [PubMed] [Google Scholar]
- 36. Doull JA, Lara H. The epidemiological importance of diphtheria carriers. Am J Epidemiol 1925; 5:508–29. [Google Scholar]
- 37. Zingher A. The Schick test performed on more than 150,000 children in public and parochial schools in New York (Manhattan and the Bronx). Am J Dis Child 1923; 25:392–405. [Google Scholar]
- 38. Eichelberger E. How long does immunity to diphtheria last? Am J Public Health Nations Health 1948; 38:1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Keenan JD, Arzika AM, Maliki R, et al. Longer-term assessment of azithromycin for reducing childhood mortality in Africa. N Engl J Med 2019; 380:2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Crum FS. A statistical study of diphtheria. Am J Public Health (N Y) 1917; 7:445–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Besa NC, Coldiron ME, Bakri A, et al. Diphtheria outbreak with high mortality in northeastern Nigeria. Epidemiol Infect 2014; 142:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. United Nations Children’s Fund. Diphtheria antitoxin: market update. Geneva, Switzerland: UNICEF, 2017. [Google Scholar]
- 43. European Centre for Disease Prevention and Control. Gap analysis on securing diphtheria diagnostic capacity in the EU/EEA. Stockholm: ECDC, 2017:33. [Google Scholar]
- 44. Médecins Sans Frontières. Health survey in Kutupalong and Balukhali refugee settlements, Cox’s Bazar, Bangladesh. Geneva, Switzerland: MSF, 2017. [Google Scholar]
- 45. Médecins Sans Frontières. Retrospective mortality, nutrition and measles vaccination coverage survey in Balukhali 2 and Tasnimarkhola camps. Geneva, Switzerland: MSF, 2017. [Google Scholar]
- 46. World Health Organization. Vaccination in humanitarian emergencies—implementation guide. 2017. Available at: https://www.who.int/immunization/documents/general/who_ivb_17.13/en/. Accessed 24 April 2019. [Google Scholar]
- 47. World Health Organization. Diphtheria: vaccine-preventable diseases, surveillance standards. Geneva, Switzerland: WHO, 2018. Available at: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_04_Diphtheria_R2.pdf?ua=1. Accessed 3 May 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.