Abstract
Background
The natural history of anti-interferon-γ (IFN-γ) autoantibody-associated immunodeficiency syndrome is not well understood.
Methods
Data of 74 patients with anti-IFN-γ autoantibodies at Srinagarind Hospital, Thailand, were collected annually (median follow-up duration, 7.5 years). Annual data for 19 patients and initial data for 4 patients with anti-IFN-γ autoantibodies at the US National Institutes of Health were collected (median follow-up duration, 4.5 years). Anti-IFN-γ autoantibody levels were measured in plasma samples.
Results
Ninety-one percent of US patients were of Southeast Asian descent; there was a stronger female predominance (91%) in US than Thai (64%) patients. Mycobacterium abscessus (34%) and Mycobacterium avium complex (83%) were the most common nontuberculous mycobacteria in Thailand and the United States, respectively. Skin infections were more common in Thailand (P = .001), whereas bone (P < .0001), lung (P = .002), and central nervous system (P = .03) infections were more common in the United States. Twenty-four percent of Thai patients died, most from infections. None of the 19 US patients with follow-up data died. Anti-IFN-γ autoantibody levels decreased over time in Thailand (P < .001) and the United States (P = .017), with either cyclophosphamide (P = .01) or rituximab therapy (P = .001).
Conclusions
Patients with anti-IFN-γ autoantibodies in Thailand and the United States had distinct demographic and clinical features. While titers generally decreased with time, anti-IFN-γ autoantibody disease had a chronic clinical course with persistent infections and death. Close long-term surveillance for new infections is recommended.
Keywords: anti-interferon-γ autoantibodies, adult-onset immunodeficiency, anticytokine autoantibodies, disseminated nontuberculous mycobacterial infection, opportunistic infection
Patients with anti-interferon-γ autoantibodies in Thailand and the United States had distinct clinical features. While titers decreased with time, 24% of Thai patients died during 7.5 years of follow-up. None of the US patients died during a median follow-up period of 4.5 years.
Nontuberculous mycobacteria (NTM) are ubiquitous, weakly virulent environmental bacteria. Disseminated NTM infections mostly occur in the immunocompromised, such as patients living with advanced human immunodeficiency virus (HIV) and those with Mendelian defects in the interleukin-12/interferon-γ (IFN-γ) pathway [1, 2]. In the past decade and a half, anti-IFN-γ autoantibodies have been strongly associated with severe disseminated NTM infections and other disseminated opportunistic infections (Salmonella, Histoplasma, and Cryptococcus) in previously healthy adults, predominantly in or from Southeast and East Asia [3–21]. While numerous reports have been published since 2004, longitudinal follow-up data are scarce, leaving the natural history of this acquired immunodeficiency syndrome poorly understood. Therefore, we returned to the largest described prospective cohort of patients in Thailand [16] and a different retrospective cohort of patients in the United States to describe clinical manifestations and long-term clinical and laboratory outcomes in patients with anti-IFN-γ autoantibodies.
METHODS
Thai Cohort
Seventy-four patients not living with HIV with anti-IFN-γ autoantibodies at Srinagarind Hospital in northeastern Thailand were enrolled in an institutional review board (IRB)-approved protocol beginning in 2010 and followed annually until December 2018. The participants included 56 patients previously reported [16] and 18 new patients. Patients were both diagnosed from incident cases at Srinagarind Hospital and referred from across Thailand with suspected diagnoses. Demographic and clinical data were recorded on standardized forms, and plasma samples were obtained annually where possible. All participants provided written informed consent.
US Cohort
Twenty-three patients not living with HIV with anti-IFN-γ autoantibodies seen at the National Institutes of Health (NIH) were enrolled in either of 2 IRB-approved protocols. The first patient was accrued in 1998 and accrual remains open. Patients were referred from across the United States with suspected diagnoses of anti-IFN-γ autoantibodies. Nineteen patients had annual follow-up visits through March 2019. Demographic and clinical data were collected by reviewing electronic chart records. Plasma samples were obtained from a repository. All participants provided written informed consent.
Measurement of Anti-IFN-γ Autoantibody Levels
Anti-IFN-γ autoantibody levels in plasma samples were measured for 67 Thai patients and 23 US patients using a particle-based assay as previously described [22] (details are described in the Supplementary Materials). Plasma samples were unavailable for 7 Thai patients. Only 1 sample collected at the initial presentation was available for 5 US patients. Based on the availability of the samples, up to 4 time points including the initial and the last visit were chosen for analysis. All samples were tested at first thaw. Each sample was analyzed at 7 dilutions (1:100, 1:1000, 1:4000, 1:16 000, 1:64 000, 1:256 000, 1:1 024 000). A standard curve using a commercial anti-human IFN-γ antibody (R&D Systems) was run on each plate. From the compiled standard curve data, the dynamic (linear) range of the assay was determined (fluorescence intensity [FI] values between 870 and 22 100 arbitrary units). FI values within the dynamic range were multiplied by the corresponding dilution factor to yield neat FI (NFI). The average NFI for each sample was used for statistical analysis.
Statistical Analyses
To determine the prevalence of infections during follow-up, we calculated the percentage of follow-up visits with infection for each patient and averaged these values for the Thai and US cohorts. For clinical data, group differences were analyzed using the Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. Statistical tests were performed at the 0.05 level. Analyses were performed using Prism, version 8.0 (Graphpad Software, Inc).
To determine the time to infection clearance, the first visit at which there were no signs of infection was defined as the clearance time point. We excluded 18 patients who had resolved infections prior to study enrollment. For patients who received cyclophosphamide or rituximab in addition to antibiotics, the time to infection clearance was calculated from the visit at which they first received cyclophosphamide or rituximab.
For the analysis of anti-IFN-γ autoantibody levels, longitudinal trajectories of NFI (in log-10 scale) were assessed by fitting a linear mixed model adjusting for age, sex, the presence of active infection (model 1), antibiotic use alone (model 2), and cyclophosphamide or rituximab use within the past year (model 3). We included terms for fixed and random effects in order to obtain estimates of the overall mean and individual trajectories, respectively, across time. We assessed whether autoantibody levels at a previous visit and current autoantibody levels informed the presence of infection at the incident visit (model 4). We also determined whether antibiotics (model 5) or cyclophosphamide or rituximab use (model 6) predicted infection at future visits. For these analyses, we implemented a model that included a term for lagged NFI or treatment (ie, NFI or treatment at a previous visit). Given that we had multiple measurements for each patient, we used generalized estimating equations [23], adjusting for a small sample [24]. All analyses were performed using R version 3.4.4. Specific functions are lme(package nlme [25]) and geese(package saws [24]).
RESULTS
Demographic Features of the Thai Cohort
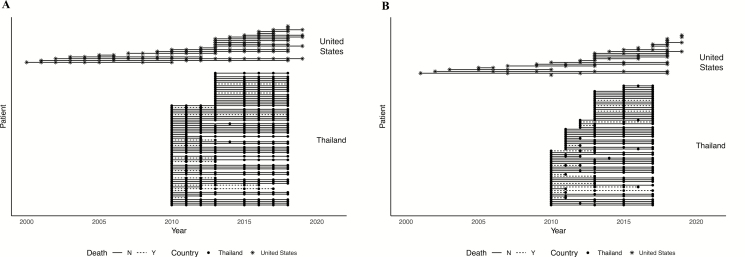
In Thailand, 37 patients (50%) were followed for 8 years and 16 patients (22%) were followed for 6 years. Figure 1A and 1B show the follow-up collection time points for clinical data analysis and titer analysis, respectively. The median (interquartile range [IQR]) age at presentation of the 74 Thai patients was 50 (46, 56) years; 47 (64%) were female (Table 1).
Figure 1.
Data collection time points. Clinical data collection timepoints (A) and anti-IFN-γ autoantibody level analysis time points (B) in the Thai and US cohorts. Abbreviation: IFN, interferon.
Table 1.
Demographic Features of Patients in Thailand and the United States
| Demographic | Thailand (n = 74) | United States (n = 23) | P Valuea |
|---|---|---|---|
| Age (years) | |||
| Median (interquartile range) | 50 (46, 56) | 45 (35, 59) | ns |
| Mean (standard deviation) | 50.4 (11.0) | 46.2 (15.7) | … |
| Range | 18–83 | 12–74 | … |
| Sex | .01 | ||
| Male | 27 (36%) | 2 (9%) | |
| Female | 47 (64%) | 21 (91%) | |
| Race | ns | ||
| Asian | 74 (100%) | 21 (91 %) | |
| Thai | 74 (100%) | 3 (14%) | |
| Filipino | 7 (33%) | ||
| Vietnamese | 3 (14%) | ||
| Laotian | 3 (14%) | ||
| Taiwanese | 3 (14%) | ||
| Cambodian | 1 (5%) | ||
| Singaporean | 1 (5%) | ||
| White | 2 (9%) |
Abbreviation: ns, not significant.
aThe Fisher exact test was used to determine the P values.
Demographic Features of the US Cohort
The US participants were accrued over a longer period, with 5 patients (21%) having data collected for more than 8 years (Figure 1). The median (IQR) age at presentation of the 23 US patients was 45 (35, 59) years. Twenty-one (91%) were female, 21 (91%) were of Southeast Asian descent, and 2 (9%) were US-born white (Table 1).
Clinical Features in Thailand
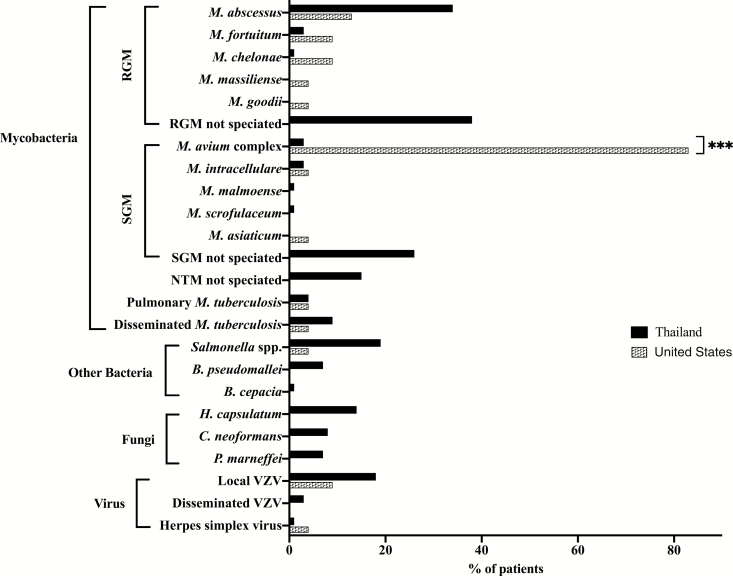
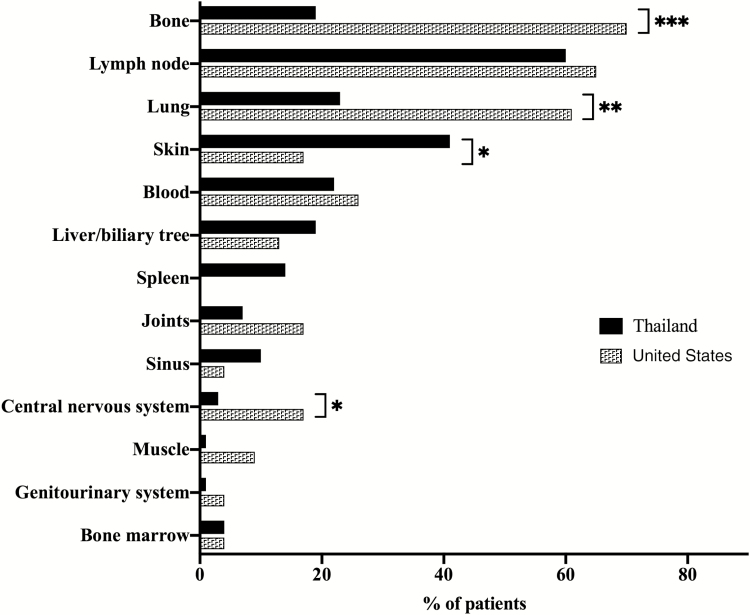
Table 2 shows the types of infections and disease-associated conditions at presentation. In Thailand, 47 patients (64%) presented with disseminated NTM infection, 17 (23%) presented with disseminated NTM and another opportunistic infection (disseminated Salmonella, Histoplasma, Cryptococcus), and 10 (14%) presented with another opportunistic infection alone. Mycobacterium abscessus was the most commonly isolated NTM species (34%), but definitive speciation was unavailable for a significant number of rapid-growing mycobacteria. Among other opportunistic infections, herpes zoster (21%) and salmonellosis (19%) were most common (Figure 2, Supplementary Table 1). In Thailand, lymph nodes were the most commonly involved site (60%), followed by skin (41%; Figure 3, Supplementary Table 2). Forty-eight patients (65%) had disease-associated conditions at presentation, the most common of which was Sweet syndrome, seen in 28 patients (38%; Table 2).
Table 2.
Clinical Features of Patients in Thailand and the United States
| Clinical Feature | Thailand (n = 74) | United States (n = 23) | P Valuea |
|---|---|---|---|
| Initial clinical presentation | |||
| Disseminated NTM infection | 47 (64%) | 20 (87%) | ns |
| Other opportunistic infectionb | 27 (36%) | 3 (13%) | .04 |
| Disseminated NTM and another opportunistic infection | 17 (23%) | 2 (9%) | ns |
| Other opportunistic infection alone | 10 (14%) | 1 (4%) | ns |
| Associated conditions at presentation | |||
| Total number (%) of patients | 48 (65%) | 9 (39%) | ns |
| Sweet syndrome | 28 (38%) | 2 (9%) | .009 |
| Lymphatic obstruction | 11 (15%) | 1 (4%) | ns |
| Erythema pustulosis | 12 (16%) | ns | |
| Chronic pain | 6 (8%) | 4 (17%) | ns |
| Hypercalcemia | 6 (8%) | ns | |
| Erythema nodosum | 4 (5%) | 1 (4%) | ns |
| Pustular psoriasis | 2 (3%) | ns | |
| Neuropathy | 2 (3%) | 2 (9%) | ns |
| Infections during follow-up | |||
| Mean % of follow-up visits with active infection | 29 | 14 | .02 |
Abbreviations: ns, not significant; NTM, nontuberculous mycobacteria.
aFisher exact test was used to determine the P values.
bWith or without disseminated NTM infections.
Figure 2.
Isolated organisms at presentation in Thailand and the United States. Abbreviations: B, Burkholderia; C, Cryptococcus; H, Histoplasma; M, mycobacterium; NTM, nontuberculous mycobacteria; P, penicillium; RGM, rapid-growing mycobacteria; SGM, slow-growing mycobacteria; VZV, varicella-zoster virus.
Figure 3.
Sites of infections at presentation in Thailand and the United States. * P < .05, ** P < .01, *** P < .001.
Clinical Features in the United States
In the United States, 20 patients (87%) presented with disseminated NTM infection, 2 (9%) presented with disseminated NTM and another opportunistic infection, and 1 (4%) presented with disseminated M. tuberculosis (Table 2). Other presenting opportunistic infections were significantly less common in the United States than in Thailand (P = .04). Mycobacterium avium complex (MAC) was the most commonly isolated NTM species (83%) and was significantly more common in the United States than in Thailand (P < .0001). Only 1 US patient (4%) had salmonellosis, and none of the patients had infections with Burkholderia, Histoplasma, Cryptococcus, or Talaromyces (Penicillium) marneffei (Figure 2). Bone was the most common site of infection (70%), followed by lymph nodes (65%; Figure 3, Supplementary Table 2). Infections involving the bone (P < .0001), lung (P = .002), and central nervous system (P = .03) were significantly more common in the United States than in Thailand, while skin infections were significantly less common in the United States (P = .048). Two US patients (9%) had Sweet syndrome, which was significantly less common in the United States than in Thailand (P = .009; Table 2).
Clinical Outcomes in Thailand
In Thailand, 66 patients (89%) were treated with antibiotics alone. Eight patients (11%) received cyclophosphamide with antibiotics due to progressive disease despite more than 3 courses of parenteral antibiotics within 12 months. Upon clinical improvement, patients in Thailand discontinued therapy without secondary antibiotic prophylaxis. The mean percentage of follow-up visits with infection was 29% (Table 2).
Of the 37 Thai patients who were followed for 8 years, 29 (78%) were infection-free and 7 (19%) were still being treated for infection after 8 years. Of the 16 patients followed for 6 years, 13 (81%) were infection-free and 2 (13%) were being treated for infection. Eighteen patients (24%) died during follow-up, with 9 of those deaths due to infection, sepsis, or disease progression (Supplementary Table 3). Of those 18 who died, 14 (78%) had had NTM or other opportunistic infections at their last follow-up visit. One patient died after cyclophosphamide therapy. Seventeen of the 18 patients who died (94%) had been treated with antibiotic therapy alone.
Clinical Outcomes in the United States
In the United States, 13 patients (57%) were treated with antibiotics alone, 9 patients (39%) received rituximab at the physician’s discretion due to progressive infections despite long-term antibiotic therapy, and 1 patient received rituximab for systemic lupus erythematosus. Upon clinical improvement, patients were maintained on indefinite secondary antibiotic prophylaxis with daily azithromycin. Nineteen US patients were followed for a median (IQR) duration of 54 (13, 109) months (range, 4–214 months); 4 were diagnosed within the last year of the study and were without follow-up visits. The mean percentage of follow-up visits with infection in the US cohort was 14%, which was lower than that in Thailand (P = .02; Table 2). None of the 19 US patients with follow-up died.
Infection Clearance
The median time to infection clearance was 3 years (95% confidence interval [CI], 2, 4), 4 years (95% CI, 2, not achieved), and 5 years (95% CI, 2, 4) in patients who received antibiotics only, rituximab and antibiotics, and cyclophosphamide and antibiotics, respectively. The upper limit of 95% CI for the rituximab group could not be calculated due to limited data.
Anti-IFN-γ Autoantibody Levels in Thailand
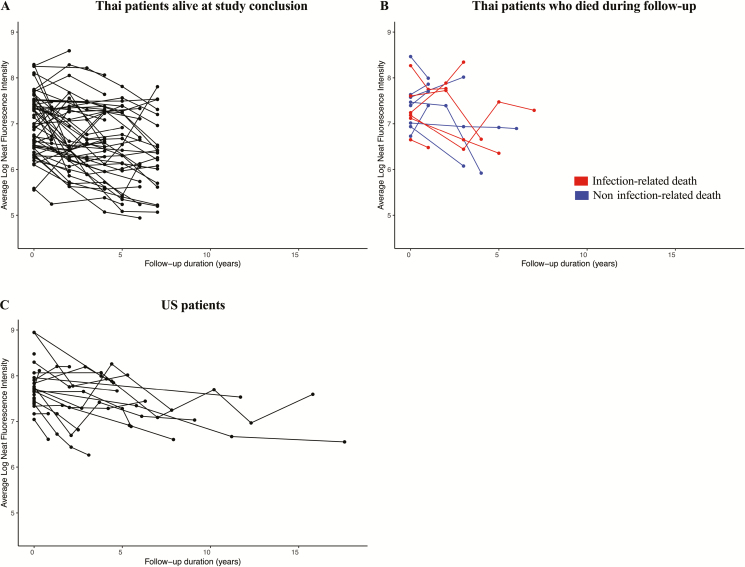
Figure 4A and 4B shows anti-IFN-γ autoantibody levels over time in Thailand. Overall, there was a significant decrease in autoantibody levels over time (P < .001; Table 3A). Figure 5A shows the yearly change in autoantibody levels in individual patients. There were no differences in initial autoantibody levels or trends in autoantibody levels over time based on sex or age at enrollment (Table 3A). Anti-IFN-γ autoantibody levels were significantly higher in those with active infections than in those with resolved infections (P < .001). The mean autoantibody levels at each time point by infection status are shown in Supplementary Figure 1. There were no differences in autoantibody levels with antibiotic use (Table 3A). Autoantibody levels significantly decreased with cyclophosphamide therapy (P = .01; Table 3A).
Figure 4.
Anti-interferon (IFN)-γ autoantibody levels in Thailand and the United States. Anti-IFN-γ autoantibody levels at up to 4 time points were measured using a particle-based assay [22] for 67 patients in Thailand (A, B) and for 23 patients in the United States (C). A, Thai patients who were alive at the end of the follow-up period. B, Thai patients who died during the study. Each patient is represented by a separate line on the graph.
Table 3.
Analysis of Anti-Interferon-γ Autoantibody Levels in the Thai and US Cohorts
| A. Model Results for the Thai Cohort | B. Model Results for the US Cohort | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 | Value | SE | P Value | Model 1 | Value | SE | P Value |
| Intercept | 7.29 | 0.35 | <.001 | Intercept | 7.26 | 0.29 | <.001 |
| Annual change | −0.07 | 0.02 | <.001 | Annual change | −0.04 | 0.02 | .02 |
| Infection | 0.26 | 0.08 | <.001 | Infection | 0.29 | 0.12 | .02 |
| Age at enrollment | −0.01 | 0.01 | .19 | Age at enrollment | 0.01 | 0.01 | .31 |
| Male | 0.21 | 0.14 | .15 | Male | −0.49 | 0.31 | .13 |
| Model 2 | Value | SE | P Value | Model 2 | Value | SE | P Value |
| Intercept | 7.26 | 0.35 | <.001 | Intercept | 7.31 | 0.32 | <.001 |
| Annual change | −0.06 | 0.02 | <.001 | Annual change | −0.04 | 0.02 | .02 |
| Infection | 0.25 | 0.09 | <.001 | Infection | 0.30 | 0.12 | .02 |
| Age at enrollment | −0.01 | 0.01 | .19 | Age at enrollment | 0.01 | 0.01 | .31 |
| Male | 0.21 | 0.14 | .15 | Male | −0.48 | 0.32 | .15 |
| Antibiotics | 0.03 | 0.10 | .79 | Antibiotics | −0.06 | 0.15 | .68 |
| Model 3 | Value | SE | P Value | Model 3 | Value | SE | P Value |
| Intercept | 7.45 | 0.36 | <.001 | Intercept | 7.39 | 0.30 | <.001 |
| Annual change | −0.07 | 0.02 | <.001 | Annual change | −0.04 | 0.01 | <.001 |
| Infection | 0.23 | 0.08 | <.001 | Infection | 0.18 | 0.11 | .10 |
| Age at enrollment | −0.01 | 0.01 | .10 | Age at enrollment | 0.01 | 0.01 | .31 |
| Male | 0.23 | 0.15 | .13 | Male | −0.46 | 0.32 | .17 |
| Cyclophosphamide | −0.43 | 0.17 | .01 | Rituximab | −0.37 | 0.11 | .001 |
Longitudinal trajectories of anti-interferon-γ autoantibody levels were assessed by fitting a linear mixed model adjusting for age, sex, and the presence of infection (model 1), antibiotic use alone (model 2), and cyclophosphamide or rituximab use (model 3).
Abbreviation: SE, standard error.
Figure 5.
Individual trends in anti-interferon (IFN)-γ autoantibody levels over time. A linear mixed model adjusting for sex, age at presentation, and presence of infection was used to determine the individual trends in autoantibody levels over time. The y-axis shows the annual change in anti-IFN-γ autoantibody levels for each patient in Thailand (A) and the United States (B). Dotted lines represent the overall group trend for the Thai and US cohorts.
Anti-IFN-γ Autoantibody Levels in the United States
Figure 4C shows autoantibody levels over time in the United States, and Figure 5B shows the yearly change in autoantibody levels in individual patients. Overall, there was a significant decrease in autoantibody levels over time (P = .017; Table 3B). There were no differences in initial autoantibody levels or trends in autoantibody levels over time based on sex or age at enrollment. Anti-IFN-γ autoantibody levels were higher in those with active infections (P = .017). There were no differences in autoantibody levels with antibiotic use (Table 3B). Autoantibody levels significantly decreased with rituximab therapy (P = .001; Table 3B).
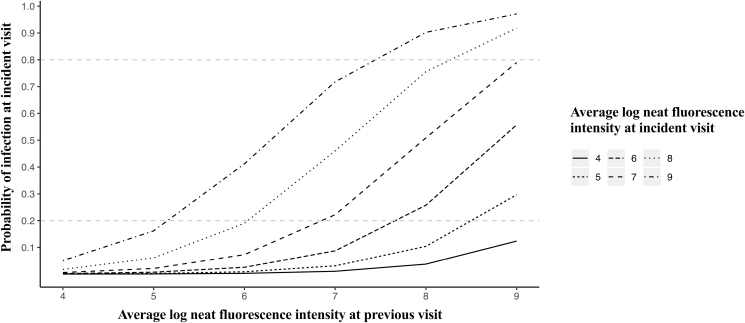
Prediction of Infection in Thailand
Figure 6 represents the probability of infection based on anti-IFN-γ autoantibody levels at 2 consecutive visits in the Thai cohort. A 1-log increase in autoantibody levels at the previous visit increased the odds of having an infection at the next visit 3.63 times (P = .01; Table 4A). The odds of having an infection at a given visit increased 2.98 times with a 1-log increase in autoantibody levels (P = .01). Continued antibiotic use at a previous visit increased the odds of persistent infection at the next visit (P < .001). Cyclophosphamide use at a previous visit had no effect on the presence of infection at the subsequent visit (Table 4A).
Figure 6.
Probability of infection based on anti-interferon-γ autoantibody levels at 2 consecutive visits in the Thai cohort. A model including a term for lagged neat fluorescence intensity was used to assess whether autoantibody levels at 2 consecutive visits inform the presence of infection at the latter visit in the Thai cohort. The y-axis shows the probability of infection at the incident visit based on the autoantibody levels at a previous visit (x-axis) and the incident visit (lines on the graph).
Table 4.
Prediction of Infection in the Thai and US Cohorts
| A. Model Results for the Thai Cohort | B. Model Results for the US Cohort | ||||||
|---|---|---|---|---|---|---|---|
| Model 4 | Estimate | Odds | P Value | Model 4 | Estimate | Odds | P Value |
| Intercept | −17.93 | 0.00 | .00 | Intercept | −25.11 | 0.00 | .00 |
| 1-log increase in NFI at previous visit | 1.29 | 3.63 | .01 | 1-log increase in NFI at previous visit | 0.76 | 2.14 | .32 |
| 1-log increase in NFI at current visit | 1.09 | 2.98 | .01 | 1-log increase in NFI at current visit | 2.38 | 10.85 | .01 |
| Model 5 | Estimate | Odds | P Value | Model 5 | Estimate | Odds | P Value |
| Intercept | −2.85 | 0.06 | .00 | Intercept | −38.33 | 0 | .00 |
| Antibiotics at previous visit | 2.14 | 8.54 | .00 | Antibiotics at previous visit | −8.88 | 0.0001 | .00 |
| Model 6 | Estimate | Odds | P Value | Model 6 | Estimate | Odds | P Value |
| Intercept | −1.27 | 0.28 | .00 | Intercept | −1.77 | 0.17 | .00 |
| Cyclophosphamide at previous visit | −0.34 | 0.71 | .79 | Rituximab at previous visit | 1.35 | 3.84 | .04 |
| Cyclophosphamide at current visit | 1.27 | 3.57 | .17 | Rituximab at current visit | 0.60 | 1.83 | .48 |
The odds of infection based on anti-interferon-γ autoantibody levels (model 1), antibiotic use alone (model 2), and cyclophosphamide or rituximab use (model 3) was assessed using a model that included a term for lagged NFI or treatment (NFI or treatment at a previous visit).
Abbreviation: NFI, neat fluorescence intensity.
Prediction of Infection in the United States
In US patients, the odds of having an infection increased 10.85 times with a 1-log increase in autoantibody levels at a given visit (P = .01). Antibiotic therapy at a previous visit decreased the odds of an infection at the next visit (P < .001). Rituximab therapy at the previous visit increased the odds of an infection at the next visit (P = .04; Table 4B).
DISCUSSION
The natural history of anti-IFN-γ autoantibody-associated immunodeficiency syndrome is poorly understood. Here, we report on a prospective longitudinal study in Thailand and a retrospective cohort of US patients, recognizing that data collection time points and follow-up durations were different. At both sites, cases were referred for suspected anti-IFN-γ autoantibodies based on infection history and clinical presentation.
The majority of US patients (91%) were of Southeast Asian descent, most of whom were born in Asia. While the median ages at presentation were similar, there was a markedly stronger female predominance (91%) in the United States compared to Thailand (64%). This is consistent with the published literature, which reflects males and females being similarly affected in Southeast Asia [5, 10, 26], while the majority of cases outside of Asia [9, 27–29] are women born in Southeast Asia.
Patients in Thailand and the United States had somewhat different clinical manifestations. While M. abscessus was the most common NTM in Thailand, MAC was the predominant NTM in the United States. At presentation, other opportunistic infections (salmonellosis, histoplasmosis, cryptococcosis) were more common in Thailand than in the United States (P = .04), which is likely due to a higher chance of exposure to these pathogens in Thailand. Kham-Ngam and colleagues [30] reported that the most common NTM species causing extrapulmonary infections in northeast Thailand was M. abscessus (25%) followed by MAC (15%). Interestingly, they also report that in patients living with HIV in northeast Thailand, MAC infection was more common than M. abscessus infection [30]. These data suggest that mycobacterial infections associated with anti-IFN-γ autoantibodies in Thailand may be distinct from those encountered in advanced HIV. Infections involving the bone (P < .0001), lung (P = .002), and central nervous system (P = .03) were more common in the United States, while infections of the skin were more common in Thailand (P = .048). These differences in infection sites could be due to the different optimal temperatures for growth of MAC and M. abscessus, the predominant NTM species in the United States and Thailand, respectively [26, 31]. The fact that more than 90% of US patients were of Southeast Asian descent and potentially similar to those affected in Thailand suggests that infections with different NTM species at different sites are dependent on geographic location and environmental exposure.
Twenty-four percent of Thai patients died during follow-up, while none of the US patients did, although follow-up durations differed between and even within the cohorts. Thai patients who died from carbapenem-resistant Klebsiella pneumonia infection, phaeohyphomycosis, and Acinetobacter baumannii pneumonia had been treated with antibiotics alone without cyclophosphamide. Their long-term antibiotic therapy in addition to their underlying immunodeficiency syndrome could have predisposed them to developing severe, drug-resistant infections. One potential contributor to the significant mortality rate in Thailand may be the high rate of infection with M. abscessus, a resistant and pathogenic organism [32], as well as the high rate of other severe opportunistic infections. Interestingly, the mean percentage of follow-up visits with infection in the Thai cohort (29%) was higher than in the US cohort (14%; P = .02), although whether this was related to the use of secondary antibiotic prophylaxis in the United States is unknown.
Initial anti-IFN-γ autoantibody levels were similar and titers significantly decreased over time in both Thailand and the United States, even in the absence of specific immunomodulatory therapy. Given the very different demographics of anti-IFN-γ autoantibody disease in the United States and Europe (overwhelmingly female from Southeast Asia), it was interesting that we found neither age nor sex to be discriminating factors in autoantibody levels at presentation or over time.
The exact pathogenesis of anti-IFN-γ autoantibody-associated immunodeficiency syndrome remains elusive. Human leukocyte antigen–associated molecular mimicry triggered by the noc2 gene of Aspergillus has been proposed and is well demonstrated in the mouse [33]. However, in the human context, the driving force behind titers is unclear. Do higher levels of anti-IFN-γ autoantibodies cause infections or do autoantibody levels increase as a result of infections? Our data show that anti-IFN-γ autoantibody levels are strongly associated with disease activity and likely correlate with the biological activity of the anti-IFN-γ autoantibodies. While intrapatient autoantibody titers helped predict disease activity and clinical improvement, we were unable to identify absolute values that predicted specific outcomes.
Rituximab and cyclophosphamide significantly lowered autoantibody titers. The antibody-directed therapy used in Thailand (cyclophosphamide) [34] was used in a small number of participants (11%) and was not predictive of future infection status. In US patients, there was a high rate of use of rituximab (43%), which was associated with an increased probability of persistent infection at the next visit. This could reflect higher antecedent disease severity in patients who received rituximab. Rituximab therapy has been reported to effectively reduce autoantibody titers and improve IFN-γ signaling [35–38]. In our cohort, we did not observe any rituximab toxicities beyond infusion reactions, but further research is needed to determine whether there are potential harmful effects of rituximab therapy in patients with anti-IFN-γ autoantibodies. The median time to infection clearance was 3, 4, and 5 years in patients who received antibiotics alone, rituximab and antibiotics, and cyclophosphamide and antibiotics, respectively. These results show that patients have chronic, persistent infections, suggesting the importance of long-term therapy.
There are important limitations to our study. First, the number of participants was small, reflecting the fact that anti-IFN-γ autoantibody disease is still relatively rarely recognized. Second, all of the Thai participants were from a single tertiary university hospital in northeastern Thailand and all of the US participants were referred to the NIH, which are referral patterns that may contain selection biases that limit generalizability. Third, we still do not know what the time course of autoantibody positivity is before infection presentation, nor is it clear whether there is a specific threshold below which infection does not occur. Despite these limitations, this first description of the natural history and evolution of anti-IFN-γ autoantibody-associated immunodeficiency syndrome suggests areas for future research.
Patients with anti-IFN-γ autoantibodies in Thailand and United States had distinct demographic and clinical features. While titers generally decreased with time, anti-IFN-γ autoantibody-associated immunodeficiency syndrome has a chronic clinical course with persistent infections and death. Close long-term follow-up and surveillance for recurrent or new infections seems prudent. A prospective randomized trial would clarify the role of cyclophosphamide or rituximab in the treatment of this syndrome.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This research was made possible through the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID) and the National Institutes of Health (NIH) Medical Research Scholars Program, a public–private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation (grant 2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, Elsevier, and other private donors.
Potential conflicts of interest. P. C. reports grants from the NIH, Gilead, MSD, GSK, the Kirby Institute, and Emory University. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Al-Muhsen S, Casanova JL. The genetic heterogeneity of Mendelian susceptibility to mycobacterial diseases. J Allergy Clin Immunol 2008; 122:1043–51; quiz 1052–3. [DOI] [PubMed] [Google Scholar]
- 2. Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis 2015; 15:968–80. [DOI] [PubMed] [Google Scholar]
- 3. Browne SK, Holland SM. Anticytokine autoantibodies in infectious diseases: pathogenesis and mechanisms. Lancet Infect Dis 2010; 10:875–85. [DOI] [PubMed] [Google Scholar]
- 4. Browne SK, Holland SM. Immunodeficiency secondary to anticytokine autoantibodies. Curr Opin Allergy Clin Immunol 2010; 10:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chetchotisakd P, Mootsikapun P, Anunnatsiri S, et al. Disseminated infection due to rapidly growing mycobacteria in immunocompetent hosts presenting with chronic lymphadenopathy: a previously unrecognized clinical entity. Clin Infect Dis 2000; 30:29–34. [DOI] [PubMed] [Google Scholar]
- 6. Döffinger R, Helbert MR, Barcenas-Morales G, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis 2004; 38:e10–4. [DOI] [PubMed] [Google Scholar]
- 7. Höflich C, Sabat R, Rosseau S, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 2004; 103:673–5. [DOI] [PubMed] [Google Scholar]
- 8. Patel SY, Ding L, Brown MR, et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol 2005; 175:4769–76. [DOI] [PubMed] [Google Scholar]
- 9. Kampmann B, Hemingway C, Stephens A, et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest 2005; 115:2480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chetchotisakd P, Kiertiburanakul S, Mootsikapun P, Assanasen S, Chaiwarith R, Anunnatsiri S. Disseminated nontuberculous mycobacterial infection in patients who are not infected with HIV in Thailand. Clin Infect Dis 2007; 45:421–7. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka Y, Hori T, Ito K, Fujita T, Ishikawa T, Uchiyama T. Disseminated Mycobacterium avium complex infection in a patient with autoantibody to interferon-gamma. Intern Med 2007; 46:1005–9. [DOI] [PubMed] [Google Scholar]
- 12. Baerlecken N, Jacobs R, Stoll M, Schmidt RE, Witte T. Recurrent, multifocal Mycobacterium avium-intercellulare infection in a patient with interferon-gamma autoantibody. Clin Infect Dis 2009; 49:e76–8. [DOI] [PubMed] [Google Scholar]
- 13. Koya T, Tsubata C, Kagamu H, et al. Anti-interferon-gamma autoantibody in a patient with disseminated Mycobacterium avium complex. J Infect Chemother 2009; 15:118–22. [DOI] [PubMed] [Google Scholar]
- 14. Tang BS, Chan JF, Chen M, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol 2010; 17:1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kampitak T, Suwanpimolkul G, Browne S, Suankratay C. Anti-interferon-γ autoantibody and opportunistic infections: case series and review of the literature. Infection 2011; 39:65–71. [DOI] [PubMed] [Google Scholar]
- 16. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012; 367:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hase I, Morimoto K, Sakagami T, Kazumi Y, Ishii Y, van Ingen J. Disseminated Mycobacterium gordonae and Mycobacterium mantenii infection with elevated anti-IFN-γ neutralizing autoantibodies. J Infect Chemother 2015; 21:468–72. [DOI] [PubMed] [Google Scholar]
- 18. Nishimura T, Fujita-Suzuki Y, Yonemaru M, et al. Recurrence of disseminated Mycobacterium avium complex disease in a patient with anti-gamma interferon autoantibodies by reinfection. J Clin Microbiol 2015; 53:1436–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asakura T, Namkoong H, Sakagami T, Hasegawa N, Ohkusu K, Nakamura A. Disseminated Mycobacterium genavense infection in patient with adult-onset immunodeficiency. Emerg Infect Dis 2017; 23:1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aoki A, Sakagami T, Yoshizawa K, et al. Clinical significance of interferon-γ neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis 2018; 66:1239–45. [DOI] [PubMed] [Google Scholar]
- 21. Chi CY, Chu CC, Liu JP, et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood 2013; 121:1357–66. [DOI] [PubMed] [Google Scholar]
- 22. Ding L, Mo A, Jutivorakool K, Pancholi M, Holland SM, Browne SK. Determination of human anticytokine autoantibody profiles using a particle-based approach. J Clin Immunol 2012; 32:238–45. [DOI] [PubMed] [Google Scholar]
- 23. Bible J, Albert PS, Simons-Morton BG, Liu D. Practical issues in using generalized estimating equations for inference on transitions in longitudinal data: what is being estimated? Stat Med 2019; 38:903–16. [DOI] [PubMed] [Google Scholar]
- 24. Fay MP, Graubard BI. Small-sample adjustments for Wald-type tests using sandwich estimators. Biometrics 2001; 57:1198–206. [DOI] [PubMed] [Google Scholar]
- 25. Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1–137. 2018. Available at: https://CRAN.R-project.org/package=nlme. Accessed May 2019. [Google Scholar]
- 26. Hase I, Morimoto K, Sakagami T, Ishii Y, van Ingen J. Patient ethnicity and causative species determine the manifestations of anti-interferon-gamma autoantibody-associated nontuberculous mycobacterial disease: a review. Diagn Microbiol Infect Dis 2017; 88:308–15. [DOI] [PubMed] [Google Scholar]
- 27. O’Connell E, Rosen LB, LaRue RW, et al. The first US domestic report of disseminated Mycobacterium avium complex and anti-interferon-γ autoantibodies. J Clin Immunol 2014; 34:928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanitsh LG, Löbel M, Müller-Redetzky H, et al. Late-onset disseminated Mycobacterium avium intracellulare complex infection (MAC), cerebral toxoplasmosis and salmonella sepsis in a German caucasian patient with unusual anti-interferon-gamma IgG1 autoantibodies. J Clin Immunol 2015; 35:361–5. [DOI] [PubMed] [Google Scholar]
- 29. van de Vosse E, van Wengen A, van der Meide WF, Visser LG, van Dissel JT. A 38-year-old woman with necrotising cervical lymphadenitis due to Histoplasma capsulatum. Infection 2017; 45:917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kham-Ngam I, Chetchotisakd P, Ananta P, et al. Epidemiology of and risk factors for extrapulmonary nontuberculous mycobacterial infections in Northeast Thailand. PeerJ 2018; 6:e5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Griffith DE, Aksamit T, Brown-Elliott BA, et al. ; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 32. Tung YJ, Bittaye SO, Tsai JR, et al. Risk factors for microbiologic failure among Taiwanese adults with Mycobacterium abscessus complex pulmonary disease. J Microbiol Immunol Infect 2015; 48:437–45. [DOI] [PubMed] [Google Scholar]
- 33. Lin CH, Chi CY, Shih HP, et al. Identification of a major epitope by anti-interferon-γ autoantibodies in patients with mycobacterial disease. Nat Med 2016; 22:994–1001. [DOI] [PubMed] [Google Scholar]
- 34. Chetchotisakd P, Anunnatsiri S, Nanagara R, Nithichanon A, Lertmemongkolchai G. Intravenous cyclophosphamide therapy for anti-IFN-gamma autoantibody-associated mycobacterium abscessus infection. J Immunol Res 2018; 2018:6473629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012; 119:3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Czaja CA, Merkel PA, Chan ED, et al. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-gamma autoantibody. Clin Infect Dis 2014; 58:e115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naik R, Cortes JA. Persistent Mycobacterium abscessus infection secondary to interferon-γ autoantibodies. Ann Allergy Asthma Immunol 2016; 116:461–2. [DOI] [PubMed] [Google Scholar]
- 38. Pruetpongpun N, Khawcharoenporn T, Damronglerd P, et al. Disseminated Talaromyces marneffei and Mycobacterium abscessus in a patient with anti-interferon-gamma autoantibodies. Open Forum Infect Dis 2016; 3:ofw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.