Abstract
Objective
To evaluate the predictive value of fasting blood glucose (FBG) on unfavourable outcomes and mortality in diabetes mellitus (DM) patients after acute ischaemic stroke (AIS).
Study design
A hospital-based observational cohort study was conducted. Clinical data, including sex, age, body mass index, vascular risk factors and systolic/diastolic blood pressure, were routinely collected. National Institutes of Health Stroke Scale score was used to assess stroke severity on admission. FBG was determined on the first day after fasting for at least 8 hours. The modified Rankin Scale was used to assess functional outcome at 90 days: 3–6, unfavourable outcome and 6, death.
Setting
Renmin Hospital of Wuhan University, Wuhan, China.
Participants
Patients who had AIS with DM, who were consecutively admitted within 24 hours of onset from January 2018 to June 2019.
Results
For the 568 patients, the median age was 65 years (IQR, 55–74 years). There were 377 (66.4%) men. The median FBG values were 7.37 mmol/L (IQR, 5.99–10.10 mmol/L), and the median glycated haemoglobin (HbA1c) values were 6.6 (IQR, 5.8–8.3). Multivariable logistic and Cox regression analysis of confounding factors showed that FBG at the time of admission was an independent predictor of unfavourable outcome (OR, 1.25 (1.14–1.37); p<0.0001) and mortality (HR, 1.10 (1.03–1.15); p<0.05) at 90 days after onset. Time to death was analysed by Kaplan-Meier curves based on FBG quartiles. The risk of death in the two highest quartile groups (FBG, 7.38–10.10 mmol/L; FBG, ≥10.11 mmol/L) was significantly higher than that in the two lowest quartile groups (FBG, ≤6.00 mmol/L; FBG, 6.01–7.37 mmol/L; p<0.0001).
Conclusions
Higher FBG levels are associated with unfavourable outcomes and mortality in Chinese patients who had AIS with DM. Our data contribute to the knowledge regarding the relationship between FBG and prognosis in patients with DM who had AIS.
Keywords: neurology, stroke, neuropathology, diabetic neuropathy
Strengths and limitations of this study.
This study evaluated the predictive value of fasting blood glucose at admission with regard to short-term outcome. Previous studies focusing on this evaluation are rare.
Complete follow-up was achieved in this study.
The study used routinely collected clinical data and practical statistical methods to correct the effects of confounding factors.
This study was conducted in a single centre with a limited sample size.
The data of random blood glucose as a possible meaningful predictor were not fully available in this retrospective study.
Introduction
Diabetes mellitus (DM) is recognised as an important risk factor for ischaemic stroke.1 2 Previous epidemiological investigations have confirmed that patients with DM have a high disability rate and a high risk of in-hospital death after an acute ischaemic stroke (AIS).3 4 A recent study involving a Chinese population included 10 331 patients with DM who were confirmed to have an AIS showed a high risk for in-hospital death.5 Indeed, assessment of functional outcome and mortality risk among patients who had AIS with diabetes is a common concern for both patients and clinicians.
Hyperglycaemia is common in patients who had AIS with and without DM.6 Many studies have shown that hyperglycaemia at the time of admission is associated with AIS infarct volume.7 This condition can also predict functional outcome and risk of death.8–10 However, few studies have focused on DM patients with hyperglycaemia at the time of admission. Studies have shown that high blood glucose levels in DM patients have no significant predictive value for functional outcomes and risk of death.11 Moreover, two recent studies have confirmed that high blood glucose levels are predictive of functional outcomes in diabetic and non-diabetic patients complicated by cerebral infarction.12 13 Therefore, the correlation between blood glucose levels and functional outcomes in DM patients should be defined so that clinicians can provide accurate prognosis and eventually develop glycaemic control strategies after an ischaemic stroke.
Because fasting blood glucose (FBG) can minimise the effects of diet,14 the FBG level is considered a more reliable blood glucose level detection tool than random blood glucose levels.15 Compared with random blood glucose levels, FBG level provides a stronger predictor of functional outcomes.16 17
Hence, this study aimed to investigate the predictive value of FBG on functional outcomes and mortality in Chinese patients who had AIS with DM.
Methods
Patients and study design
This retrospective observational study collected information involving patients who had AIS with DM, who were admitted to the Department of Neurology of the Renmin Hospital of Wuhan University from January 2018 to June 2019. The diagnostic criteria for acute cerebral infarction are in accordance with WHO’s standards.18 Patients with DM were defined as patients with a history of DM before admission according to their medical records or those who received drugs or insulin for hypoglycemic treatment after admission. Patients must meet the following criteria: onset was within 24 hours; age ≥18 years and, in the case of a recurrent cerebral infarction, a modified Rankin Scale (mRS) score of ≤2.19 Patients with psychoses, severe bone joint diseases and other neurological diseases that affect functional outcomes were excluded from the study.
Signed informed consent was obtained for all patients participating in the study.
Clinical variables and neuroimaging
All patients completed diagnostic testing after admission, including routine serologic testing, neuroimaging, intracranial and extravascular studies and a cardiac examination. Clinical data were routinely collected at the time of admission, included gender, age, body mass index (BMI), notation of vascular risk factors (including hypertension, DM, coronary heart disease, atrial fibrillation, hypercholesterolemia, stroke history and smoking history) and systolic/diastolic blood pressure. The severity of stroke at the time of admission was assessed using the National Institutes of Health Stroke Scale (NIHSS).20 Reperfusion therapy included intravenous thrombolysis with recombinant tissue plasminogen activator (rtPA) and endovascular therapy with intra-arterial thrombolysis or mechanical thrombectomy. The causes of stroke were grouped according to the Trial of Org 10 172 in Acute Stroke Treatment (TOAST)21 as follows: large-vessel occlusive; small-vessel occlusive; cardioembolic; and other and unknown. Patients underwent a CT scan and/or an MRI examination within 24–48 hours after admission. The diagnosis of cerebral infarction was based on these images.
End points and follow-up
The mRS score at 90 days was used as an indicator of functional outcomes as follows: ≤2, good outcome; 3–6, unfavourable outcome and 6, death. Two specially trained neurologic nurses were responsible for assessing functional outcomes of patients who had AIS by calling patients or their family members once a month.
Laboratory testing
To minimise the impact of diet on the blood glucose level, FBG levels were used as a reliable glycaemic index. Blood samples were collected at approximately 07:30 on the first day after admission after fasting for at least 8 hours. Glycated haemoglobin (HbA1c) was tested using standard test methods.
Statistical analysis
The data following normal distribution were described using the mean±SD. The data following a non-normal distribution were described using the median (quartiles). Categorical variables were described using a percentage. The Mann-Whitney U test was used to compare the non-normal distribution between two groups. The Pearson correlation coefficient and Spearman’s rank correlation coefficient were used for linear correlation analysis. The continuous variables proved to be linearly correlated with the outcomes and were brought into the regression model. We used univariable logistic regression to analyse the relationship between factors and outcomes of acute cerebral infarction at 90 days. We used univariable Cox regression to analyse the relationship between factors and mortality of acute cerebral infarction at 90 days. Factors giving a p<0.1 were re-analysed using multivariable regression analysis to determine the correlation between the FBG level and functional outcomes of cerebral infarction, as well as death. The results are expressed by ORs and 95% CIs. Moreover, we performed quartiles based on FBG levels as follows: quartile 1 (FBG, ≤6.00 mmol/L); quartile 2 (FBG, 6.01–7.37 mmol/L); quartile 3 (FBG, 7.38–10.10 mmol/L) and quartile 4 (FBG, ≥10.11 mmol/L). Kaplan-Meier survival curves were used to analyse the value of the FBG level for predicting death. SPSS V.25.0 was used for statistical analysis. A p<0.05 indicates a significant difference.
Patient and public involvement
No patients were involved with design, data provision, analysis or publication of the study.
Results
Baseline characteristics of the study cohort
A total of 568 patients who had AIS with DM, including 377 men and 191 women, were enrolled in this study and all were followed up. The median age of the patients was 65 years (IQR, 55–74 years), and the mean BMI was 24.10±2.96 kg/m2. The median NIHSS score at the time of admission was 4 (IQR, 2–10). A total of 32 of 568 patients who had AIS received reperfusion therapy, including 28 patients with intravenous rtPA thrombolysis and 7 patients with endovascular treatment. A total of 226 patients (39.8%) had unfavourable outcomes, including 58 deaths (10.2%). Of the 58 deaths in this study, 36 (62.1%) died of increased intracranial pressure; 10 (17.2%) died of cardiac diseases such as heart failure, myocardial infarction or arrhythmia; and 12 (20.7%) died of other causes such as severe pneumonia, stress ulcer bleeding or pulmonary embolism. Moreover, 14 (24.1%) had symptomatic intracerebral haemorrhages. The baseline data of all patients at the time of admission are shown in table 1.
Table 1.
Baseline characteristics of patients who had AIS
| Demographic characteristics | DM patients |
| N | 568 |
| Age (years), median (IQR) | 65 (55–74) |
| Male gender, n (%) | 377 (66.4) |
| Vascular risk factors, n (%) | |
| Hypertension | 398 (70.1) |
| Atrial fibrillation | 78 (13.7) |
| Hypercholesterolemia | 182 (32.0) |
| Coronary heart disease | 75 (13.2) |
| Previous TIA or stroke | 89 (15.7) |
| Active smoking | 205 (36.1) |
| Clinical findings | |
| BMI (kg/m2), mean±SD | 24.10±2.96 |
| Systolic blood pressure (mm Hg), median (IQR) | 148 (130–166) |
| Diastolic blood pressure (mm Hg), median (IQR) | 83 (75–93) |
| TOAST classification, n (%) | |
| Large-vessel occlusive | 177 (31.2) |
| Small-vessel occlusive | 318 (56.0) |
| Cardioembolic | 56 (9.9) |
| Other and unknown | 17 (3.0) |
| HbA1c (%), median (IQR) | 6.6 (5.8–8.3) |
| FBG (mmol/L), median (IQR) | 7.37 (5.99–10.10) |
| NIHSS score at admission, median (IQR) | 4 (2–10) |
| Reperfusion therapy, n (%) | 32 (5.6) |
| Unfavourable outcome at 3 months, n (%) | 226 (39.8) |
| Mortality at 3 months, n (%) | 58 (10.2) |
AIS, acute ischaemic stroke; BMI, body mass index; DM, diabetes mellitus; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischaemic attack; TOAST, Trial of Org 10 172 in Acute Stroke Treatment.
Main results
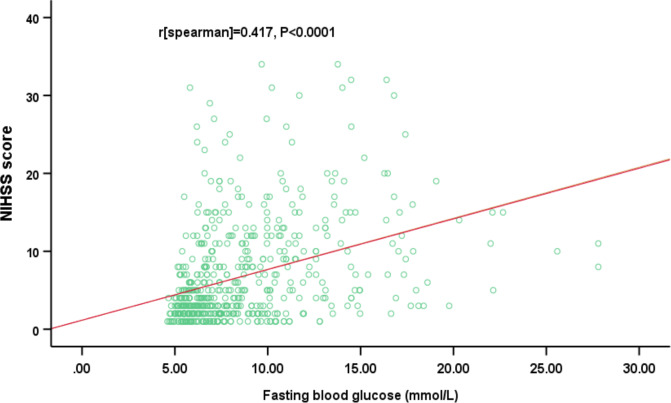
The median FBG values were 7.37 mmol/L (IQR, 5.99–10.10 mmol/L). The NIHSS scores of patients who had AIS with DM at the time of admission increased with elevation of the FBG levels. A moderately significant positive correlation was found between the NIHSS score and the FBG level (r=0.417, p<0.0001). The results are shown in figure 1. The results also showed that FBG levels had no significant correlation with other risk factors, including smoking, hypertension, coronary heart disease, hypercholesterolemia history or a history of stroke (p>0.05).
Figure 1.
The correlation between fasting blood glucose levels and the National Institutes of Health Stroke Scale (NIHSS) scores; Spearman’s analysis (r=0.417, p<0.0001).
FBG level and functional outcome at 90 days
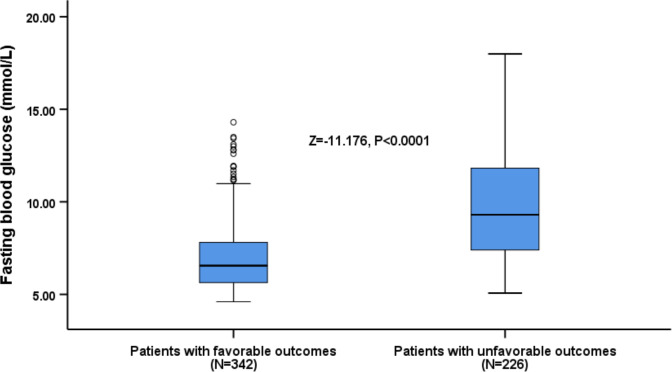
FBG levels of 226 patients with unfavourable functional outcomes at 90 days (9.64 mmol/L (IQR, 7.40–12.60 mmol/L)) were significantly higher than patients with favourable functional outcomes (6.56 mmol/L (IQR, 5.64–7.86 mmol/L); Z=−11.176; p<0.0001; figure 2). Univariable regression analysis showed that unfavourable functional outcomes were significantly correlated with age, male gender, atrial fibrillation, coronary heart disease, NIHSS score, small-vessel occlusion, HbA1c and FBG level (p<0.05). Multivariable logistic regression analysis was performed on outcome-indicating factors. The results showed that age (OR, 1.02; 95% CI 1.00 to 1.05; p=0.037), NIHSS score (OR, 1.42; 95% CI 1.31 to 1.55; p<0.0001), small-vessel occlusion (OR, 0.24; 95% CI 0.06 to 0.93; p=0.039) and FBG level (OR, 1.25; 95% CI 1.14 to 1.37; p<0.0001) were independent predictive factors of functional outcome for patients who had AIS with DM (table 2).
Figure 2.
Distribution of fasting blood glucose levels in patients with favourable and unfavourable outcomes. All data are the median and IQR. Mann-Whitney U test (Z=−11.176, p<0.0001).
Table 2.
Univariable and multivariable logistic regression analyses for unfavourable outcomes
| Parameter | Univariable analysis | Multivariable analysis | ||||
| OR | 95% CI* | P value | OR | 95% CI* | P value | |
| Age | 1.03 | 1.01 to 1.04 | <0.0001 | 1.02 | 1.00 to 1.05 | 0.037 |
| Male gender | 0.63 | 0.45 to 0.90 | 0.011 | 0.72 | 0.40 to 1.28 | 0.259 |
| Hypertension | 0.92 | 0.64 to 1.33 | 0.659 | – | ||
| Atrial fibrillation | 2.19 | 1.35 to 3.55 | 0.001 | 1.99 | 0.80 to 5.06 | 0.151 |
| Hypercholesterolemia | 0.89 | 0.62 to 1.28 | 0.530 | – | ||
| Coronary heart disease | 1.89 | 1.16 to 3.08 | 0.011 | 1.03 | 0.47 to 2.28 | 0.940 |
| Previous TIA or stroke | 1.29 | 0.82 to 2.03 | 0.280 | – | ||
| Active Smoking | 1.08 | 0.76 to 1.53 | 0.664 | – | ||
| BMI | 0.97 | 0.912 to 1.02 | 0.226 | – | ||
| Systolic blood pressure | 1.00 | 1.00 to 1.01 | 0.966 | – | ||
| Diastolic blood pressure | 1.00 | 1.00 to 1.01 | 0.875 | – | ||
| Reperfusion therapy | 0.90 | 0.43 to 1.89 | 0.785 | – | ||
| NIHSS score at admission | 1.58 | 1.46 to 1.70 | <0.0001 | 1.42 | 1.31 to 1.55 | <0.0001 |
| Large-vessel occlusive† | 1.12 | 0.38 to 3.35 | 0.839 | – | ||
| Small-vessel occlusive† | 0.07 | 0.02 to 0.19 | <0.0001 | 0.24 | 0.06 to 0.93 | 0.039 |
| Cardioembolic† | 1.25 | 0.37 to 4.72 | 0.717 | – | ||
| HbA1c (%) | 1.11 | 1.01 to 1.23 | 0.038 | 0.84 | 0.71 to 1.01 | 0.056 |
| FBG | 1.38 | 1.29 to 1.48 | <0.0001 | 1.25 | 1.14 to 1.37 | <0.0001 |
*Note that the OR corresponds to a unit increase in the explanatory variable.
†Other and unknown ischaemic stroke subtype as the reference.
BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischaemic attack.
FBG levels and mortality at 90 days
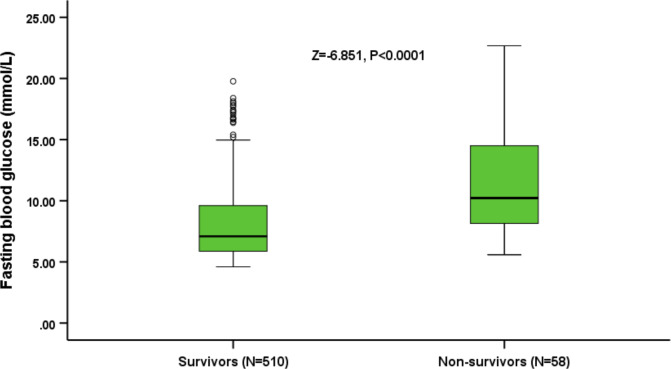
The FBG levels of 58 non-surviving patients at 90 days were significantly higher than surviving patients (10.41 mmol/L (IQR, 8.14–15.29 mmol/L vs 7.10 mmol/L (IQR, 5.88–9.65 mmol/L); Z=−6.851, p<0.0001; figure 3). Univariable Cox regression analysis of non-surviving patients showed that atrial fibrillation, coronary heart disease, NIHSS score, small-vessel occlusive disease, HbA1c and FBG level were significantly associated with death (p<0.05). Multivariable Cox regression analysis was performed on functional-outcome-indicating factors and the results showed that atrial fibrillation (HR, 2.17; 95% CI 1.20 to 3.93; p=0.011), NIHSS score (HR, 1.11; 95% CI 1.08 to 1.15; p<0.0001), small-vessel occlusion (HR, 0.07; 95% CI 0.12 to 0.38; p=0.002), HbA1c (HR, 1.32; 95% CI 1.15 to 1.51; p<0.0001) and FBG levels (HR, 1.10; 95% CI 1.03 to 1.15; p=0.004; table 3) were independent predictive factors of death for patients who had AIS with DM.
Figure 3.
Distribution of fasting blood glucose levels in survivors and non-survivors. All data are the median and IQR. Mann-Whitney U test (Z=−6.851, p<0.0001).
Table 3.
Univariable and multivariable Cox regression analyses for mortality
| Parameter | Univariable analysis | Multivariable analysis | ||||
| HR | 95% CI* | P value | HR | 95% CI* | P value | |
| Age | 1.01 | 1.00 to 1.03 | 0.252 | – | ||
| Male gender | 0.70 | 0.42 to 1.20 | 0.186 | – | ||
| Hypertension | 1.13 | 0.63 to 2.00 | 0.684 | – | ||
| Atrial fibrillation | 4.36 | 2.56 to 7.41 | <0.0001 | 2.17 | 1.20 to 3.93 | 0.011 |
| Hypercholesterolemia | 0.88 | 0.50 to 1.56 | 0.670 | – | ||
| Coronary heart disease | 1.97 | 1.06 to 3.65 | 0.031 | 1.44 | 0.76 to 2.71 | 0.262 |
| Previous TIA or stroke | 1.25 | 0.65 to 2.42 | 0.499 | – | ||
| Active smoking | 1.48 | 0.88 to 2.49 | 0.135 | – | ||
| BMI | 0.96 | 0.88 to 1.04 | 0.301 | – | ||
| Systolic blood pressure | 0.99 | 0.98 to 1.00 | 0.135 | – | ||
| Diastolic blood pressure | 1.00 | 0.98 to 1.01 | 0.620 | – | ||
| Reperfusion therapy | 1.27 | 0.46 to 3.75 | 0.659 | – | ||
| NIHSS score at admission | 1.20 | 1.14 to 1.20 | <0.0001 | 1.11 | 1.08 to 1.15 | <0.0001 |
| Large-vessel occlusive disease† | 0.88 | 0.31 to 2.46 | 0.802 | – | ||
| Small-vessel occlusive disease† | 0.02 | 0.00 to 0.13 | <0.0001 | 0.07 | 0.12 to 0.38 | 0.002 |
| Cardioembolic† | 1.05 | 0.35 to 3.19 | 0.933 | – | ||
| HbA1c (%) | 1.46 | 1.30 to 1.65 | <0.0001 | 1.32 | 1.15 to 1.51 | <0.0001 |
| FBG | 1.17 | 1.12 to 1.22 | <0.0001 | 1.10 | 1.03 to 1.15 | 0.004 |
*Note that the hazard ratio corresponds to a unit increase in the explanatory variable.
†Other and unknown ischaemic stroke subtype as the reference.
BMI, body mass index; FBG, fasting blood glucose; HbA1c, glycated haemoglobin; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischaemic attack.
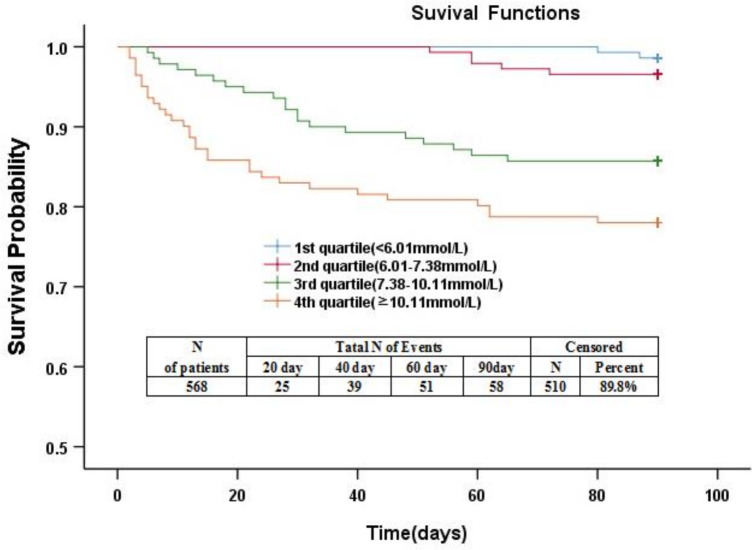
We used Kaplan-Meier curves to compare the quartiles of FBG levels and time to death after admission. The results showed that the risk of death in the two highest quartile groups (FBG, 7.38–10.10 mmol/L; FBG, ≥10.11 mmol/L) was significantly higher than the two lowest quartile groups (FBG, ≤6.00 mmol/L; FBG, 6.01–7.37 mmol/L; p<0.0001; figure 4).
Figure 4.
Kaplan-Meier survival based on fasting blood glucose (FBG) quartiles. Time to death was analysed by Kaplan-Meier curves based on FBG quartiles. Patients in the lower two quartiles (FBG, ≤6.00 mmol/L; FBG, 6.01–7.37 mmol/L) had a lower risk of mortality compared with patients with FBG levels in the higher two quartiles (FBG, >10.10 mmol/L; FBG, 7.38–10.10 mmol/L; p<0.0001).
Discussion
In this retrospective study, we found that higher FBG levels are associated with unfavourable outcomes and mortality in Chinese patients who had AIS with DM. Moreover, higher FBG was associated with higher NIHSS score on admission.
Acute stroke may be accompanied by neuroendocrine disorders and inflammation, resulting in an acute blood glucose elevation.22 Previous studies focusing on acute blood glucose elevations generally used fasting or random blood glucose levels at the time of admission as an indicator of acute blood glucose. A meta-analysis involving 32 studies showed that acute stroke patients often had high blood glucose levels, and the proportion of high blood glucose levels in acute stroke patients with and without DM reached 8%–63% and 39%–83%, respectively.6 A number of studies have shown that high blood glucose levels at the time of admission are closely related to the functional outcome of patients with AIS.9 10 23 24 Masrur et al9 studied 1408 patients who had AIS, who received intravenous thrombolysis and showed that high blood glucose levels at the time of admission increased the risk of unfavourable functional outcomes and death. Snarska et al10 and Zhao et al25 reported that a high blood glucose level in patients who had AIS at the time of admission was significantly associated with an unfavourable functional outcome and risk of in-hospital death. Moreover, several previous studies involving patients who had AIS without DM showed that high blood glucose levels were also closely related to unfavourable functional outcomes and risk of death.24 26
Because the focus of previous studies was on patients who had AIS only or patients who had AIS without DM, acute blood glucose levels at the time of admission among patients who had AIS with DM were not adequately addressed; thus, the predictive value for unfavourable functional outcomes or mortality has not been established.12 13 26–28 Yao et al24 and Hu et al28 showed that in patients who had AIS without DM, high FBG levels predicted unfavourable functional outcomes and death; however, similar levels for patients who had AIS with DM had an insignificant predictive value. Zsuga et al12 and Sung et al13 performed subgroup analysis on patients who had AIS with and without DM and showed that acute blood glucose levels in both groups had predictive power for functional outcomes. Recently, a meta-analysis incorporated 13 studies and showed no statistical difference existed in prognostic indicators between patients who had AIS with and without DM.29 In this study, we used baseline FBG levels at the time of admission as a marker for the acute blood glucose level. The results revealed additional evidence for the predictive value of high acute blood glucose levels on functional outcomes and high risk of death in patients who had AIS with DM.
The mechanism underlying the predictive value of high blood glucose levels at the time of admission on functional outcome and mortality is not fully understood; however, the correlation between a high blood glucose level after AIS and the severity of stroke and unfavourable functional outcomes may be summarised as follows. First, a high blood glucose level can affect the balance between the coagulation and fibrinolytic systems, resulting in impaired recanalisation.30 31 Second, a high blood glucose level may affect endothelium-derived nitric-oxide-mediated vasodilation, thereby reducing intracranial blood flow and reperfusion at the infarct site.32 33 In vitro studies have shown that nitric oxide synthase 3 gene expression and nitric oxide production are reduced in hyperglycaemic conditions.34 35 Clinical studies have shown that cerebral infarction tissue reperfusion is decreased and infarct volume is increased in patients with high blood glucose levels.36–38 Third, a high blood glucose level may generate oxidative stress, leading to neuroendocrine disorders and inflammatory reactions,39 40 blood–brain barrier disruption41 and eventually reperfusion injury.37 42 Two clinical studies have shown that ischaemic stroke patients with acute high blood glucose levels are at increased risk for haemorrhagic transformation,43 and cerebral haemorrhage in patients with thrombolysis leads to unfavourable functional outcomes.44 High blood glucose levels may increase the risk of vascular reperfusion injury. Fourth, patients with DM generally have insufficient insulin secretion or insulin resistance; therefore, anaerobic glycolysis may increase in patients with high blood glucose levels,45 46 resulting in brain tissue lactic acid accumulation and internal environment disorders that aggravate brain tissue damage.47 All of these pathological changes together cause severe stroke and secondary functional outcomes and death.
Strengths and limitations
This study had the following highlights. First, quite a few studies have evaluated the association between blood glucose level and outcomes of patients who had AIS without DM; however, to the best of our knowledge, no studies have evaluated these conditions in patients with DM.24 26 Furthermore, complete follow-up of all patients was achieved in this study. Third, this study used routinely collected clinical data such as gender, age, BMI, vascular risk factors, NIHSS score, systolic/diastolic blood pressure and reperfusion therapy. Additionally, practical statistical methods were used to correct the effects of confounding factors.
This study also had limitations. First, a single centre was used and sample size was limited. Second, the data of random blood glucose as a possible meaningful predictor were not fully available in this retrospective study. Therefore, we cannot investigate the predictive value of random blood glucose compared with FBG.
Conclusions
In conclusion, higher FBG levels are associated with unfavourable outcomes and mortality in Chinese patients who had AIS with DM. Our data contribute to the knowledge regarding the relationship between FBG and prognosis in patients with DM who had AIS.
Supplementary Material
Acknowledgments
We would like to acknowledge all the study participants. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Contributors: TY analysed and interpreted the results and wrote this manuscript text. TY, YZ, JS, BP, LX and QC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. TY, YZ and ZL designed the study. All authors reviewed and approved the manuscript.
Funding: This work was supported by the Guide Foundation of Wuhan University (RMYD2018M09).
Disclaimer: The funders had no role in the study design, data collection, analysis, interpretation or decision to submit the manuscript for publication.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study is approved by the Ethics Committee of Renmin Hospital of Wuhan University (No. WDRY2017-K038).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request from corresponding author.
References
- 1.Cui R, Iso H, Yamagishi K, et al. Diabetes mellitus and risk of stroke and its subtypes among Japanese: the Japan public health center study. Stroke 2011;42:2611–4. 10.1161/STROKEAHA.111.614313 [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016;388:761–75. 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 3.Andersen KK, Olsen TS. One-month to 10-year survival in the Copenhagen stroke study: interactions between stroke severity and other prognostic indicators. J Stroke Cerebrovasc Dis 2011;20:117–23. 10.1016/j.jstrokecerebrovasdis.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 4.Reeves MJ, Vaidya RS, Fonarow GC, et al. Quality of care and outcomes in patients with diabetes hospitalized with ischemic stroke: findings from get with the Guidelines-Stroke. Stroke 2010;41:e409–17. 10.1161/STROKEAHA.109.572693 [DOI] [PubMed] [Google Scholar]
- 5.Si Y, Xiao X, Xiang S, et al. Mortality-specific comorbidity among inpatients with ischemic stroke in West China. Acta Neurol Scand 2019;140:100–6. 10.1111/ane.13108 [DOI] [PubMed] [Google Scholar]
- 6.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 2001;32:2426–32. 10.1161/hs1001.096194 [DOI] [PubMed] [Google Scholar]
- 7.Baird TA, Parsons MW, Phan T, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 2003;34:2208–14. 10.1161/01.STR.0000085087.41330.FF [DOI] [PubMed] [Google Scholar]
- 8.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2018;49:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 9.Masrur S, Cox M, Bhatt DL, et al. Association of acute and chronic hyperglycemia with acute ischemic stroke outcomes post-thrombolysis: findings from get with the Guidelines-Stroke. J Am Heart Assoc 2015;4:e002193. 10.1161/JAHA.115.002193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snarska KK, Bachórzewska-Gajewska H, Kapica-Topczewska K, et al. Hyperglycemia and diabetes have different impacts on outcome of ischemic and hemorrhagic stroke. Arch Med Sci 2017;13:100–8. 10.5114/aoms.2016.61009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue W-Y, Xu Y-C, Wu Y-W, et al. Observation of elevated fasting blood glucose and functional outcome after ischemic stroke in patients with and without diabetes. Oncotarget 2017;8:67980–9. 10.18632/oncotarget.19074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zsuga J, Gesztelyi R, Kemeny-Beke A, et al. Different effect of hyperglycemia on stroke outcome in non-diabetic and diabetic patients--a cohort study. Neurol Res 2012;34:72–9. 10.1179/1743132811Y.0000000062 [DOI] [PubMed] [Google Scholar]
- 13.Sung J-Y, Chen C-I, Hsieh Y-C, et al. Comparison of admission random glucose, fasting glucose, and glycated hemoglobin in predicting the neurological outcome of acute ischemic stroke: a retrospective study. PeerJ 2017;5:e2948. 10.7717/peerj.2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moebus S, Göres L, Lösch C, et al. Impact of time since last caloric intake on blood glucose levels. Eur J Epidemiol 2011;26:719–28. 10.1007/s10654-011-9608-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of medical care in diabetes--2014. Diabetes Care 2014;37(Suppl 1):S14–80. 10.2337/dc14-S014 [DOI] [PubMed] [Google Scholar]
- 16.Osei E, Fonville S, Zandbergen AAM, et al. Impaired fasting glucose is associated with unfavorable outcome in ischemic stroke patients treated with intravenous alteplase. J Neurol 2018;265:1426–31. 10.1007/s00415-018-8866-z [DOI] [PubMed] [Google Scholar]
- 17.Cao W, Ling Y, Wu F, et al. Higher fasting glucose next day after intravenous thrombolysis is independently associated with poor outcome in acute ischemic stroke. J Stroke Cerebrovasc Dis 2015;24:100–3. 10.1016/j.jstrokecerebrovasdis.2014.07.029 [DOI] [PubMed] [Google Scholar]
- 18.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–7. 10.1161/01.STR.19.5.604 [DOI] [PubMed] [Google Scholar]
- 20.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. 10.1161/01.STR.20.7.864 [DOI] [PubMed] [Google Scholar]
- 21.Madden KP, Karanjia PN, Adams HP, et al. Accuracy of initial stroke subtype diagnosis in the TOAST study. Trial of ORG 10172 in acute stroke treatment. Neurology 1995;45:1975–9. 10.1212/wnl.45.11.1975 [DOI] [PubMed] [Google Scholar]
- 22.Dungan KM, Braithwaite SS, Preiser J-C. Stress hyperglycaemia. Lancet 2009;373:1798–807. 10.1016/S0140-6736(09)60553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang F, Jiang B, Kanesan L, et al. Higher admission fasting plasma glucose levels are associated with a poorer short-term neurologic outcome in acute ischemic stroke patients with good collateral circulation. Acta Diabetol 2018;55:703–14. 10.1007/s00592-018-1139-6 [DOI] [PubMed] [Google Scholar]
- 24.Yao M, Ni J, Zhou L, et al. Elevated fasting blood glucose is predictive of poor outcome in non-diabetic stroke patients: a Sub-Group analysis of smart. PLoS One 2016;11:e0160674. 10.1371/journal.pone.0160674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Wang L, Lu M, et al. Hyperglycemia is associated with poor in-hospital outcome in elderly patients with acute ischemic stroke. Medicine 2019;98:e16723. 10.1097/MD.0000000000016723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing L, Liu S, Tian Y, et al. C-R relationship between fasting plasma glucose and unfavorable outcomes in patients of ischemic stroke withoutDiabetes. J Stroke Cerebrovasc Dis 2019;28:1400–8. 10.1016/j.jstrokecerebrovasdis.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 27.Marulaiah SK, Reddy MP, Basavegowda M, et al. Admission hyperglycemia an independent predictor of outcome in acute ischemic stroke: a longitudinal study from a tertiary care hospital in South India. Niger J Clin Pract 2017;20:573–80. 10.4103/1119-3077.206368 [DOI] [PubMed] [Google Scholar]
- 28.Hu G-C, Hsieh S-F, Chen Y-M, et al. Relationship of initial glucose level and all-cause death in patients with ischaemic stroke: the roles of diabetes mellitus and glycated hemoglobin level. Eur J Neurol 2012;19:884–91. 10.1111/j.1468-1331.2011.03647.x [DOI] [PubMed] [Google Scholar]
- 29.Dong X-L, Guan F, Xu S-J, et al. Influence of blood glucose level on the prognosis of patients with diabetes mellitus complicated with ischemic stroke. J Res Med Sci 2018;23:10. 10.4103/1735-1995.223951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stegenga ME, van der Crabben SN, Levi M, et al. Hyperglycemia stimulates coagulation, whereas hyperinsulinemia impairs fibrinolysis in healthy humans. Diabetes 2006;55:1807–12. 10.2337/db05-1543 [DOI] [PubMed] [Google Scholar]
- 31.Gentile NT, Vaidyula VR, Kanamalla U, et al. Factor VIIa and tissue factor procoagulant activity in diabetes mellitus after acute ischemic stroke: impact of hyperglycemia. Thromb Haemost 2007;98:1007–13. 10.1160/th06-12-0719 [DOI] [PubMed] [Google Scholar]
- 32.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 2003;284:R1–12. 10.1152/ajpregu.00323.2002 [DOI] [PubMed] [Google Scholar]
- 33.Melikian N, Seddon MD, Casadei B, et al. Neuronal nitric oxide synthase and human vascular regulation. Trends Cardiovasc Med 2009;19:256–62. 10.1016/j.tcm.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Y, Vaziri ND, Coulson R, et al. Effects of simulated hyperglycemia, insulin, and glucagon on endothelial nitric oxide synthase expression. Am J Physiol Endocrinol Metab 2000;279:E11–17. 10.1152/ajpendo.2000.279.1.E11 [DOI] [PubMed] [Google Scholar]
- 35.Du XL, Edelstein D, Dimmeler S, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 2001;108:1341–8. 10.1172/JCI11235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yip PK, He YY, Hsu CY, et al. Effect of plasma glucose on infarct size in focal cerebral ischemia-reperfusion. Neurology 1991;41:899–905. 10.1212/WNL.41.6.899 [DOI] [PubMed] [Google Scholar]
- 37.Quast MJ, Wei J, Huang NC, et al. Perfusion deficit parallels exacerbation of cerebral ischemia/reperfusion injury in hyperglycemic rats. J Cereb Blood Flow Metab 1997;17:553–9. 10.1097/00004647-199705000-00009 [DOI] [PubMed] [Google Scholar]
- 38.Duckrow RB, Beard DC, Brennan RW. Regional cerebral blood flow decreases during chronic and acute hyperglycemia. Stroke 1987;18:52–8. 10.1161/01.STR.18.1.52 [DOI] [PubMed] [Google Scholar]
- 39.Bémeur C, Ste-Marie L, Montgomery J. Increased oxidative stress during hyperglycemic cerebral ischemia. Neurochem Int 2007;50:890–904. 10.1016/j.neuint.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 40.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 2002;106:2067–72. 10.1161/01.CIR.0000034509.14906.AE [DOI] [PubMed] [Google Scholar]
- 41.Kawai N, Keep RF, Betz AL, et al. Hyperglycemia induces progressive changes in the cerebral microvasculature and blood-brain barrier transport during focal cerebral ischemia. Acta Neurochir Suppl 1998;71:219–21. 10.1007/978-3-7091-6475-4_63 [DOI] [PubMed] [Google Scholar]
- 42.Kamada H, Yu F, Nito C, et al. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 2007;38:1044–9. 10.1161/01.STR.0000258041.75739.cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paciaroni M, Agnelli G, Caso V, et al. Acute hyperglycemia and early hemorrhagic transformation in ischemic stroke. Cerebrovasc Dis 2009;28:119–23. 10.1159/000223436 [DOI] [PubMed] [Google Scholar]
- 44.Lin S-F, Chao A-C, Hu H-H, et al. Hyperglycemia predicts unfavorable outcomes in acute ischemic stroke patients treated with intravenous thrombolysis among a Chinese population: a prospective cohort study. J Neurol Sci 2018;388:195–202. 10.1016/j.jns.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 45.Katsura K, Asplund B, Ekholm A, et al. Extra- and intracellular pH in the brain during ischaemia, related to tissue lactate content in normo- and hypercapnic rats. Eur J Neurosci 1992;4:166–76. 10.1111/j.1460-9568.1992.tb00863.x [DOI] [PubMed] [Google Scholar]
- 46.Schurr A. Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab 2006;26:142–52. 10.1038/sj.jcbfm.9600174 [DOI] [PubMed] [Google Scholar]
- 47.Parsons MW, Barber PA, Desmond PM, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol 2002;52:20–8. 10.1002/ana.10241 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.