Abstract
Background
Inflammatory mediators, including acute phase reactants and cytokines, have been reported to be associated with clinical efficacy in patients with melanoma and other cancers receiving immune checkpoint inhibitors (ICI). Analyses of patient sera from three large phase II/III randomized ICI trials, one of which included a chemotherapy arm, were performed to assess whether baseline levels of C-reactive protein (CRP), interleukin-6 (IL-6) or neutrophil/lymphocyte (N/L) ratios were prognostic or predictive.
Patients and methods
Baseline and on-treatment sera were analyzed by multiplex protein assays from immunotherapy-naïve patients with metastatic melanoma randomized 1:1 on the Checkmate-064 phase II trial of sequential administration of nivolumab followed by ipilimumab or the reverse sequence. Baseline sera, and peripheral blood mononuclear cells using automated cell counting, were analyzed from treatment-naïve patients who were BRAF wild-type and randomly allocated 1:1 to receive nivolumab or dacarbazine on the phase III Checkmate-066 trial, and from treatment-naïve patients allocated 1:1:1 to receive nivolumab, ipilimumab or both ipilimumab and nivolumab on the phase III Checkmate-067 trial.
Results
Higher baseline levels of IL-6 and the N/L ratio, and to a lesser degree, CRP were associated with shorter survival in patients receiving ICI or chemotherapy. Increased on-treatment levels of IL-6 in patients on the Checkmate-064 study were also associated with shorter survival. IL-6 levels from patients on Checkmate-064, Checkmate-066 and Checkmate-067 were highly correlated with levels of CRP and the N/L ratio.
Conclusion
IL-6, CRP and the N/L ratio are prognostic factors with higher levels associated with shorter overall survival in patients with metastatic melanoma receiving ICI or chemotherapy in large randomized trials. In a multi-variable analysis of the randomized phase III Checkmate-067 study, IL-6 was a significant prognostic factor for survival.
Keywords: cytokines; biomarkers, tumor; immunotherapy
Background
Interleukin-6 (IL-6) is a cytokine involved in immune regulation that induces acute-phase protein synthesis from the liver and plays an important role in the maintenance of hepatocytes, hematopoietic progenitor cells, elements of the skeleton, placenta, cardiovascular system and endocrine as well as nervous systems.1 IL-6 signaling requires membrane-bound glycoprotein 130 (gp130) IL-6Rß and occurs in hepatocytes, epithelial cells and leukocytes.1 Elevated levels of IL‐6 have been observed in patients with various types of cancer such as melanoma,2 ovarian,3 and colorectal cancer.4 IL‐6 signals directly to tumor cells through at least three major signaling pathways, including janus-activated kinase 1/2 (JAK1/2)/signal transactivator of transcription 3 (STAT3), rat sarcoma (RAS)/mitogen-activated protein kinase (MAPK), and phosphoinositide-3 kinase (PI3K)/AKR Thymoma (AKT), contributing to tumor promotion by expansion and survival of malignant cells, neo‐angiogenesis, and inflammation.5–11 IL‐6 can also be secreted from infiltrating myeloid cells such as tumor-associated macrophages, dendritic cells (DC) and myeloid-derived suppressor cells (MDSC),8 12 and tumor‐associated stroma such as cancer‐associated fibroblasts, endothelial, or senescent cells, contributing to a dynamic tumor-fibroblast cross-talk in the tumor microenvironment.13
High levels of IL-6 exist in many pathologic conditions including cancer and rheumatologic diseases. In a small study of patients that received ipilimumab, IL-6 and CRP were associated with poor survival. High levels of IL-6 have also been associated with toxicity from chimeric antigen receptor-T (CAR-T) cell therapy. The humanized IL-6 receptor blocking antibody tocilizumab is Food and Drug Administration (FDA)-approved for several rheumatologic conditions and has been used extensively in patients receiving CAR-T cells to treat the cytokine release syndrome.14 In one report, T-cell efficacy and function did not appear to be impaired in patients treated with tocilizumab.15 Tocilizumab has been shown to reduce the intensity and duration of immune-related colitis in patients receiving programmed death-1 (PD-1) antibodies alone or in combination with ipilimumab.16 Tocilizumab has also been used with rapid resolution of symptoms in patients treated with immune-checkpoint inhibitors (ICI) who experienced steroid-refractory immune-related adverse events.17 These clinical data suggest that IL-6 may also be associated with the etiology of immune-related side effects of immunotherapy drugs.
C-reactive protein (CRP), a prototypical acute phase reactant, is a pentameric serum protein (pentraxin) whose levels rise in inflammatory states. It is synthesized by the liver, and also to a lesser degree by smooth muscle cells, macrophages, endothelial cells, lymphocytes, and adipocytes in response to IL-6, TNF and IL-1β.18 Chronic inflammation and high CRP levels are associated with poor survival in renal cell, lung, pancreatic and breast cancers, in patients with head and neck cancer treated with radiotherapy, and the presence of CRP is associated with bony destruction in multiple myeloma.19–22 Recent studies showed a significant association of pretreatment CRP levels with progression-free and overall survival (OS) in patients with lung cancer treated with PD-1 ICI.23 24 This is consistent with published data in melanoma demonstrating that high levels of serum acute phase reactants including CRP, serum amyloid A and P, and complement components were associated with a poor clinical outcome in patients with melanoma receiving PD-1 antibodies.25
The neutrophil/lymphocyte (N/L) ratio, which may reflect systemic inflammation, has also been shown to be a significant negative prognostic factor for a variety of cancers independent of treatment.26 Its baseline value has also been shown to be associated with survival in patients treated with PD-1 blockade,27 and in patients with melanoma treated with ipilimumab28 or the combination of ipilimumab and nivolumab.29
Herein, we analyzed serum and peripheral blood mononuclear cell specimens obtained prior to therapy at baseline in patients from three randomized clinical trials of ICI in metastatic melanoma to determine whether serum IL-6, CRP and the N/L ratio were associated with OS, and to establish whether elevated levels of IL-6, CRP and/or N/L ratio were prognostic or predictive for outcome in patients receiving ICI. On-treatment levels of the two proteins were evaluated in patients receiving nivolumab or ipilimumab. We also evaluated the N/L ratio in patients on two of the randomized trials to assess its relationship to IL-6.
Methods
Patient samples
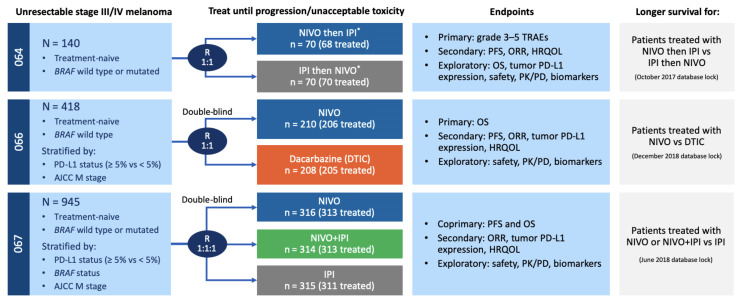
Serum samples were obtained from three independent clinical trials (Checkmate-064 (NCT01783938), Checkmate-066 (NCT01721772) and Checkmate-067 (NCT01844505)), and all protocols were approved by the Ethics Committees/Institutional Review Boards (IRBs) at the institutions where they were conducted. Serum samples from Checkmate-064 were assayed and analyzed at New York University Langone Health under an IRB-approved protocol. Samples were obtained under a Materials Transfer Agreement and coded with an anonymized five-digit number. The Checkmate-064 study was a randomized phase II study that accrued 140 patients previously untreated with immunotherapy to receive either sequential nivolumab then ipilimumab or the converse.30 The Checkmate-066 study was a randomized phase III study that accrued 418 previously untreated BRAF wild-type patients who received either nivolumab or dacarbazine.31 32 The Checkmate-067 study was a randomized phase III study that accrued 945 previously untreated patients with unresectable stage III or IV melanoma to nivolumab alone, nivolumab plus ipilimumab, or ipilimumab alone.33 34 Patient characteristics, toxicities and clinical outcomes have been described for all three studies, with long term follow-up data also published for Checkmate-066 and Checkmate-067.31 34 In all studies, responders were those with a partial or complete response defined by Response Criteria in Solid Tumors (RECIST) 1.0 at week 13 that were confirmed by subsequent imaging. The consort diagrams for all three trials have been previously published.30–32
Cytokine analyses
Serum levels of IL-6 and CRP from the Checkmate-064, Checkmate-066 and Checkmate-067 trials were assessed using Luminex multiplex assays, according to manufacturer’s instructions (R&D, Minneapolis, Minnesota, USA). For Checkmate-064, immediately prior to experiment, previously frozen samples were thawed and diluted at 1:1 and 1:100 ratios for IL-6 and CRP analysis, respectively. Experiment analyses were adjusted for dilution. For Checkmate-066 and Checkmate-067, CRP and IL-6 were assessed using customized Luminex multiplex assay panels at Myriad RBM (Austin, Texas, USA). Previously frozen samples were diluted at 1:5 and 1:5000 ratios for IL-6 and CRP analysis. Luminex 200 and MagPix instruments (Luminex Corporation, Austin, Texas, USA) were used for sample acquisition. Analyses from Checkmate-066 (77 baseline specimens) and Checkmate-067 (64 baseline specimens) were done comparing values above and below the lower limit of quantification (LLOQ). The LLOQ for Checkmate-066 was 6 pg/mL and 11 pg/mL for Checkmate-067. For Checkmate-064, analyses (109 specimens at baseline) included values above and below the median.
N/L ratio
The N/L ratio was determined by assessing absolute numbers of each cell type using an automated hematology cell analyzer at each participating institution and calculating the numeric ratio. All assays were performed within Clinical Laboratory Improvement Act-certified clinical laboratories at each institution and positive as well as negative controls were run at least daily.
Statistical analyses
Associations between baseline IL-6/CRP levels, OS and HRs were determined by Cox proportional hazards regression models. IL-6/CRP high or low groups were defined based on the median (Checkmate-064 analysis) or LLOQ value (Checkmate-066 and Checkmate-067) for the study population, depending on assay sensitivity and the number of patients with levels at LLOQ. Correlation between IL-6 and CRP levels were calculated by Spearman’s rank-order coefficient. Tests were performed in the R computing environment. Differences based on patient response were determined by Mann-Whitney test using GraphPad Prism 8 software. For all analyses, p values≤0.05 were considered statistically significant.
Results
IL-6 was associated with survival in the Checkmate-064 study
Previous work from our group has shown that baseline levels of CRP and other acute phase reactants were associated with progression-free and OS in phase II single arm studies of nivolumab and pembrolizumab.25 In order to further validate these studies and also determine if other cytokines promoting synthesis of CRP and acute phase reactants from the liver, such as IL-6, were associated with OS with ICI, we assessed cytokines and acute phase reactants in serum from patients enrolled in three randomized phase II and III studies from which survival data were available. A total of 1486 patients were treated on three randomized trials as shown in figure 1, and samples were available for analysis for 68.8% of patients in Checkmate-064, 94.2% in Checkmate-066 and 92.8% in Checkmate-067.
Figure 1.
Schemata of Checkmate-064, Checkmate-066 and Checkmate-067 clinical trials. Stage of disease, number of enrolled and treated patients, study endpoints, and survival database information are shown for the phase II randomized Checkmate-064, and phase III randomized Checkmate-066 and Checkmate-067 trials. *Switch in treatment at week 13. AJCC, American Joint Committee on Cancer; HRQOL, health-related quality of life; IPI, ipilimumab; M, metastases; NIVO, nivolumab; ORR, objective response rate; OS, overall survival; PD, pharmacodynamics; PFS, progression-free survival; PK, pharmacokinetics; R, randomized; TRAE, treatment-related adverse event.
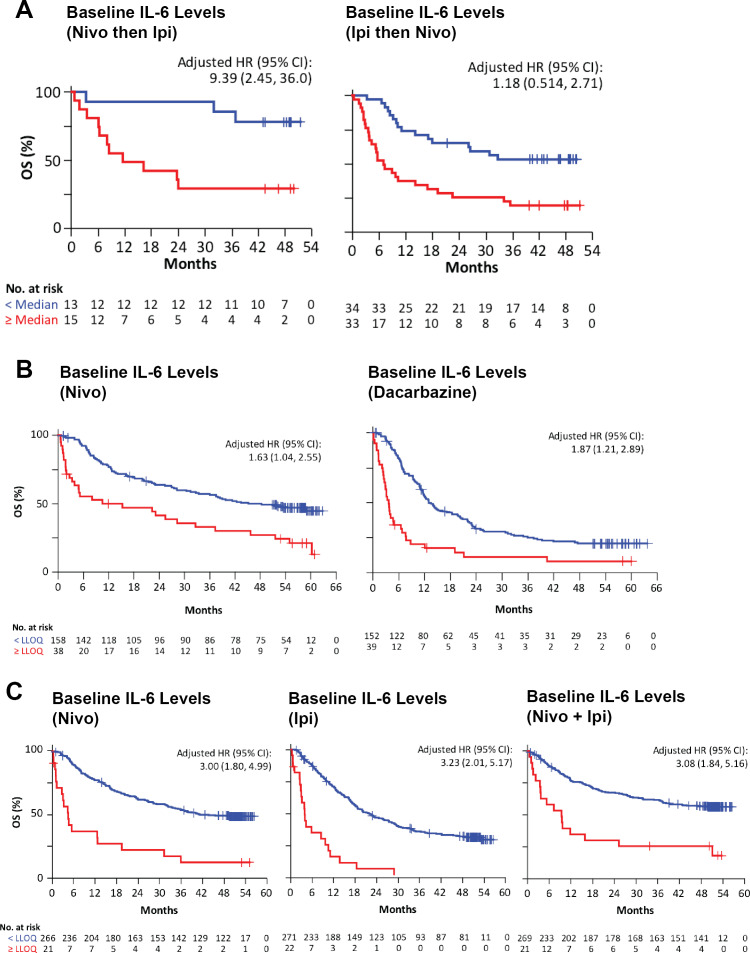
Baseline serum samples from the randomized phase II Checkmate-064 study were analyzed and an assessment of association with response and OS was performed. Low levels of IL-6 at baseline for cohort A, and at week 13 for both cohorts were associated with complete and partial response (online supplementary figure 1). IL-6 below the median value (15.1 pg/mL) was associated with better OS in Checkmate-064 for patients who received sequential nivolumab for 12 weeks then ipilimumab for 12 weeks (cohort A) with an adjusted HR for IL-6 of 5.46 (95% CI 1.52 to 19.7, figure 2A). IL-6 below the median value (12.7 pg/mL) was also associated with better OS for patients treated with ipilimumab then nivolumab (cohort B), with an adjusted HR for IL-6 of 3.07 (95% CI 1.65 to 5.72, figure 2A). We also analyzed both cohorts combined, for which IL-6 below the median value (13.3 pg/mL) was associated with better OS compared with above the median, with a HR of 3.029 (95% CI 1.765 to 5.200) (online supplementary figure 1C).
Figure 2.
Baseline serum IL-6 levels are associated with overall survival (OS). Kaplan-Meier survival curves were determined for three independent clinical trials for patients stratified according to the median baseline serum levels or the lower limit of quantification (LLOQ). Patients below the median or LLOQ are represented by blue lines and those above by red lines. (A) Patients in Checkmate-064 received sequential nivolumab then ipilimumab (cohort A) or sequential ipilimumab then nivolumab (cohort B); median IL-6, 13.3 pg/mL. (B) Patients in Checkmate-066 were treated with nivolumab or dacarbazine; LLOQ IL-6, 6 pg/mL. (C) Patients in Checkmate-067 received nivolumab or ipilimumab alone, or concurrent nivolumab and ipilimumab; LLOQ IL-6, 11 pg/mL. HR adjusted for ECOG, BRAF, M stage, baseline LDH, and melanoma subtype. IL, interleukin; M, metastases; ECOG, Eastern Cooperative Oncology Group; LDH, Lactate Dehydrogenase.
jitc-2020-000842supp001.pdf (384.4KB, pdf)
IL-6 was associated with survival in the Checkmate-066 and Checkmate-067 studies
Baseline serum samples were obtained from 196 patients who received nivolumab and 191 who received dacarbazine in Checkmate-066. Sera at baseline were obtained from 287 patients who received nivolumab, 293 patients who received ipilimumab and 290 patients who received ipilimumab combined with nivolumab in Checkmate-067. Analysis of baseline Checkmate-066 specimens showed that for nivolumab-treated patients, relatively high IL-6 was associated with poor OS with an adjusted HR of 1.63 (95% CI 1.04 to 2.55) (univariate HR of 2.43, 95% CI 1.60 to 3.70) and for dacarbazine-treated patients, IL-6 was associated with poor OS with an adjusted HR of 1.87 (95% CI 1.21 to 2.89) (univariate HR of 2.91, 95% CI 1.99 to 4.27) (figure 2B).
Analysis of the baseline Checkmate-067 specimens demonstrated that for nivolumab-treated patients, IL-6 was associated with poor OS with an adjusted HR of 3.00 (95% CI 1.80 to 4.99) (univariate HR of 4.34, 95% CI 2.65 to 7.10). For ipilimumab-treated patients, IL-6 was associated with poor OS with an adjusted HR of 3.23 (95% CI 2.01 to 5.17) (univariate HR of 4.93, 95% CI 3.12 to 7.79). For patients treated with ipilimumab combined with nivolumab, IL-6 was associated with poor OS with an adjusted HR of 3.08 (95% CI 1.84 to 5.16) (univariate HR of 3.68, 95% CI 2.21 to 6.12) (figure 2C).
IL-6 was a prognostic factor in multivariable analyses for the Checkmate-066 and Checkmate-067 studies
A multivariable analysis was performed for known prognostic factors for metastatic melanoma, including substage M1a versus M1b versus M1c, performance status 0 versus 1, N/L ratio or LDH above or below the upper limit of institutional normal (table 1). In all three arms of the Checkmate-067 study, IL-6 was a significant independent variate for OS, with HR 2.54 and p=0.0058 for nivolumab, HR=3.9 and p=7.71E−08 for ipilimumab, and HR=2.07 and p=0.015 for the combination-treated arm. In the Checkmate-066 study, IL-6 did not reach significance as an independent variate for OS (p=0.061), while LDH and N/L ratio were significant in the dacarbazine and nivolumab arms. CRP was not included in this analysis as it is highly correlated with IL-6 and resulted in multicollinearity.
Table 1.
IL-6 multivariate analysis of Checkmate-066 and -067
| CheckMate -066a | CheckMate -067b | |||||||||
| NIVO | DTIC | NIVO | NIVO+IPI | IPI | ||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| IL-6, LLOQ ≥vs < | 1.58 (0.977 to 2.578) | 0.061 | 1.39 (0.864 to 2.257) | 0.17 | 2.54 (1.31 to 4.92) | 0.0058 | 2.07 (1.15 to 3.71) | 0.015 | 3.9 (2.38 to 6.41) | 7.71E−08 |
| LDH, ULN>vs ≤ | 1.87 (1.22 to 2.87) | 0.004 | 1.92 (1.30 to 2.83) | 9.58E−04 | 1.52 (1.05 to 2.20) | 0.026 | 1.66 (1.15 to 2.38) | 0.0067 | 1.63 (1.21 to 2.20) | 0.0011 |
| ECOG, 1 vs 0 | 1.4 (0.895 to 2.190) | 0.14 | 1.23 (0.86 to 1.77) | 0.25 | 1.4 (0.971 to 2.042) | 0.071 | 1.82 (1.26 to 2.63) | 0.0013 | 1.48 (1.09 to 2.02) | 0.012 |
| M stage, M1c vs M0/M1a/M1b | 0.96 (0.630 to 1.487) | 0.88 | 1.4 (0.972 to 2.017) | 0.071 | 1.7 (1.18 to 2.44) | 0.0039 | 1.49 (1.02 to 2.18) | 0.041 | 1.57 (1.16 to 2.11) | 0.0033 |
| BRAF status, mutated vs wildtype | – | – | – | – | 0.76 (0.525 to 1.104) | 0.15 | 0.73 (0.499 to 1.093) | 0.13 | 0.8 (0.594 to 1.104) | 0.18 |
| N/L ratio | 1.11 (1.05 to 1.16) | 1.27E−04 | 1.13 (1.08 to 1.19) | 1.49E−06 | 1.02 (0.987 to 1.056) | 0.23 | 1.06 (1.01 to 1.11) | 0.017 | 1.01 (0.998 to 1.035) | 0.076 |
DTIC, dacarbazine; IL, interleukin; IPI, ipilimumab; LLOQ, lower limit of quantification; M, metastases; NIVO, nivolumab; N/L, neutrophil/lymphocyte; ULN, Upper limit of normal.
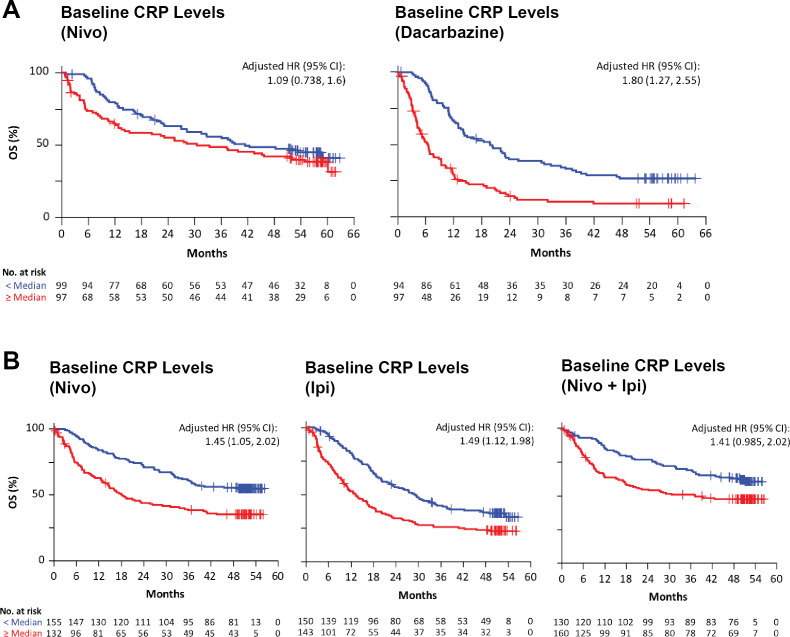
CRP was associated with survival in the Checkmate-066 and Checkmate-067 studies
For patients treated with nivolumab in Checkmate-066, CRP was not significantly associated with OS with an adjusted HR of 1.09 (95% CI 0.738 to 1.6) (univariate HR of 1.34, 95% CI 0.927 to 1.95) as shown in figure 3A. However, for patients treated with dacarbazine chemotherapy, high CRP was associated with poor OS with an adjusted HR of 1.80 (95% CI 1.27 to 2.55) (univariate HR of 2.46, 95% CI 1.78 to 3.40) also shown in figure 3A. Analysis of baseline Checkmate-067 specimens showed that CRP was also associated with poor OS for nivolumab, with an adjusted HR of 1.45 (95% CI 1.05 to 2.02) (univariate HR of 1.98, 95% CI 1.44 to 2.72), for ipilimumab with an adjusted HR of 1.49 (95% CI 1.12 to 1.98) (univariate HR of 1.72, 95% CI 1.30 to 2.26), and for ipilimumab combined with nivolumab, with an adjusted HR of 1.41 (95% CI 0.985 to 2.02) (univariate HR of 1.71, 95% CI 1.21 to 2.44) as shown in figure 3B. Baseline CRP was also associated with poor OS in both cohorts of Checkmate-064.35
Figure 3.
Baseline serum CRP levels are associated with overall survival (OS). Kaplan-Meier survival curves were determined in two independent clinical trials for patients stratified according to the median baseline serum levels. Patients below the median or LLOQ are represented by blue lines and those above by red lines. (A) Patients in Checkmate-066 were treated with nivolumab or dacarbazine; median CRP, 5.3 ug/mL. (B) Patients in Checkmate-067 received nivolumab or ipilimumab alone, or concurrent nivolumab and ipilimumab; median CRP, 5.75 ug/mL. HR adjusted for ECOG, BRAF, M stage, baseline LDH, and melanoma subtype. CRP, C-reactive protein; LLOQ, lower limit of quantification; M, metastases.
Baseline N/L ratio was associated with survival in the Checkmate-066 and Checkmate-067 studies in a multivariable analysis and was highly correlated with IL-6 and CRP.
Analysis of Checkmate-066 specimens showed that the N/L ratio was significantly but modestly associated with OS for nivolumab (HR=1.11, 95% CI 1.05 to 1.16) with p=1.27E−4 and for dacarbazine (HR=1.13 (95% CI 1.08 to 1.19) with p=149E−6 (table 1). For patients treated in Checkmate-067, the N/L ratio was only significantly associated with OS for the NIVO+IPI combination arm (HR=1.06, 95% CI 1.01 to 1.11) with p=0.017 (table 1). The N/L ratio was significantly associated with IL-6 for patients treated in Checkmate-066 and Checkmate-067 with p values of 1.5e−10 and 4.19e−18 respectively (online supplementary figure 2A). N/L ratio was also correlated with CRP for patients treated in Checkmate-066 and Checkmate-067 with p values of 1.6e−10 and 1.95e−23 respectively (online supplementary figure 2B).
On-treatment levels of IL-6 and CRP were associated with survival in the checkmate-064 study
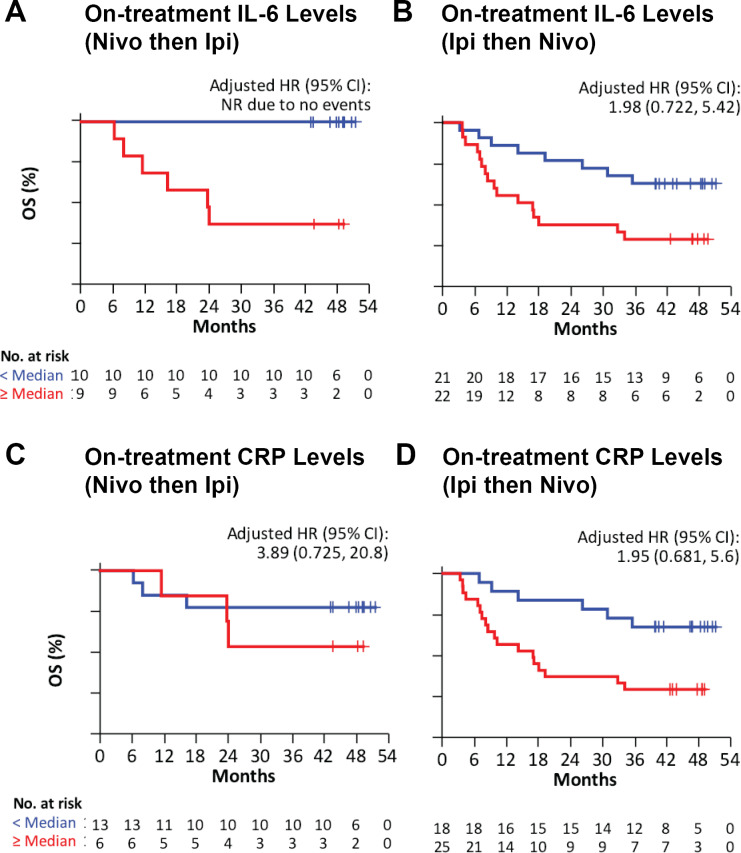
Checkmate-064 serum samples from patients receiving treatment with sequential nivolumab then ipilimumab (cohort A) or ipilimumab then nivolumab (cohort B) were analyzed for IL-6 and CRP at week 13, after the last dose of the first agent and prior to the second cycle of treatment. For IL-6 below the median (11.4 pg/mL) in cohort A, no patients had died, and therefore a HR was not calculated (figure 4A). In cohort B, for IL-6 below the median (13.9 pg/mL) there was an adjusted HR of 1.98 (95% CI 0.722 to 5.42) for OS (figure 4B). The combined cohorts of Checkmate-064 were also assessed, for which IL-6 below the median value (13.6 pg/mL) was associated with better OS, with a HR of 4.097 (95% CI 1.974 to 8.500) (online supplementary figure 1D).
Figure 4.
Increased serum IL-6 and CRP levels are associated with reduced survival after nivolumab or ipilimumab treatment in Checkmate-064. Kaplan-Meier survival curves were calculated for patients stratified according to the median serum levels at week 13 (after first treatment) of (A) IL-6 in patients receiving sequential nivolumab then ipilimumab (cohort A); (B) IL-6 in patients receiving sequential ipilimumab then nivolumab (cohort B); (C) CRP in cohort A; and (D) CRP in cohort B. Median IL-6, 13.6 pg/mL. Median CRP, 15.8 ug/mL. HR adjusted for ECOG, BRAF, M stage, baseline LDH, and melanoma subtype. Patients below the median or LLOQ are represented by blue lines and those above by red lines. CRP, C-reactive protein; IL, interleukin; LLOQ, lower limit of quantification; M, metastases; OS, overall survival.
For CRP below the median, there was an HR of 3.89 (95% CI 0.725 to 20.8) for cohort A, and an HR of 1.95 (95% CI 0.681 to 5.6) for OS in cohort B (figure 4C, D).
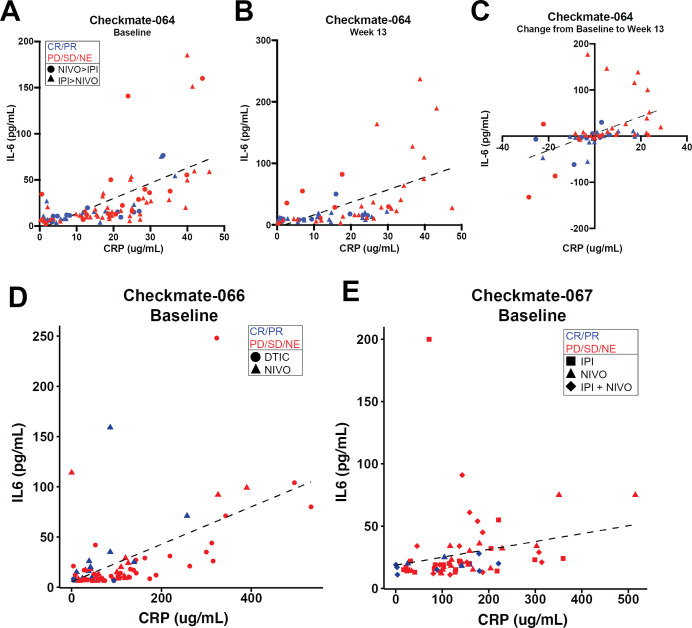
IL-6 and CRP serum levels were correlated
Serum IL-6 and CRP levels were highly correlated with one another in Checkmate-064 at baseline (figure 5A, p=2.152E−13, r2=0.529) and after nivolumab or ipilimumab treatment (figure 5B, p=1.487E−08, r2=0.47). Changes in IL-6 and CRP from baseline to week 13 (after nivolumab or ipilimumab) were also correlated (figure 5C, p=2.325E−06, r2=0.381). For evaluable patients in Checkmate-066 with IL-6 levels>LLOQ, baseline IL-6 and CRP were highly correlated (figure 5D, p<0.001, r2=0.54), and for Checkmate-067, baseline IL-6 and CRP were also highly correlated (figure 5E, p<0.001, r2=0.47). For patients from both Checkmate-066 and Checkmate-067 with IL-6 levels<LLOQ, CRP levels were significantly lower compared with patients with IL-6 levels>LLOQ (online supplementary figure 2C).
Figure 5.
IL-6 and CRP serum levels are correlated at baseline and week 13 in Checkmate-064 and at baseline in Checkmate-066 and Checkmate-067 patients. Correlation of serum IL-6 and CRP levels collected from all evaluable patient samples. (A) Checkmate-064 at baseline (p<0.001; r2=0.529) or (B) after nivolumab or ipilimumab treatment (week 13) (p<0.001; r2=0.47). (C) Correlation of changes in IL-6 and CRP levels from baseline to week 13 in Checkmate-064 were graphed (p=2.325E−06; r2=0.381). (D) Checkmate-066 at baseline (p<0.001; r2=0.54; n=77). (E) Checkmate-067 at baseline (p<0.001; r2=0.47; n=64). Patients with partial response (PR) or complete response (CR) are represented in blue and those with progressive disease (PD), stable disease (SD) or not evaluable (NE) are shown in red. CRP, C-reactive protein; DTIC, dacarbazine; IL, interleukin.
On-treatment decreases in serum IL-6 were associated with better OS in the Checkmate-064 study
Changes in serum IL-6 and CRP levels from baseline to post-nivolumab or ipilimumab were assessed in Checkmate-064. Decreases in IL-6 were associated with longer OS compared with those with increasing levels, with a HR of 2.531 (95% CI 1.165 to 5.500) (online supplementary figure 1E). No significant difference was found in OS or changes in CRP levels (HR 1.341, 95% CI 0.6204 to 2.897) (online supplementary figure 1F).
Discussion
In this study, we demonstrated that baseline serum cytokine IL-6 and acute phase reactant CRP as well as the peripheral blood N/L ratio were associated with OS in three randomized trials. All three markers were prognostic factors for outcome since an association with OS was observed for IL-6, CRP and N/L ratio in patients treated with ICI as well as the chemotherapy agent dacarbazine. In a multivariable analysis of the Checkmate-067 study, IL-6 was an independent prognostic factor, along with substage, LDH and performance status. On-treatment levels of IL-6 and CRP at week 13 were also associated with OS with sequential nivolumab or ipilimumab in one randomized phase II study (Checkmate-064).
There is a significant body of evidence from small phase II trials suggesting that high levels of both IL-6 and CRP are associated with a poor outcome with ICI, particularly ipilimumab,36–38 but also nivolumab.24 25 These data support our more extensive experience in the current work derived from three randomized trials in patients with immunotherapy-naïve metastatic melanoma. There are also indirect data suggesting that IL-6, and by extension CRP, are associated with the immunotherapy-related cytokine release syndrome that can occur with ICI, and similar conclusions can be inferred from increasing evidence on the use of the IL-6 receptor blocking antibody tocilizumab to reverse steroid-refractory immune-related adverse events in ICI-treated patients.16 17 39 40 A high N/L ratio has also been shown in small numbers of selected patients treated with ipilimumab and PD-1 blocking antibodies to be associated with poor survival.41 42
Blockade of IL-6 has been associated with control of immune-related adverse events with ipilimumab, nivolumab or the combination of both agents,16 17 39 40 strongly suggesting an immune role for IL-6, especially in patients receiving ICI. Further substantiation for the immune role for IL-6 is shown by its impact on CD4+T cells, promoting expression of the T helper (Th)2 associated cytokine IL-443 while suppressing IFNγ expression.44 IL-6, in the presence of transforming growth factor beta (TGFβ), is also critical for the polarization of Th17 T-cells.45 In myeloid cells, IL-6 signaling promotes the expression of vascular endothelial growth factor (VEGF) and arginase, while down regulating expression of CD80, CD86 and IL-12.46 IL-6 also promotes the polarization of M2 macrophages, an anti-inflammatory subset of macrophages associated with poor survival in a variety of cancers.47 48
While the results of this study demonstrate that serum levels of IL-6 are associated with poor patient outcomes, the cellular source(s) contributing to this remain to be elucidated. IL-6 is known to be produced by a wide variety of cells including T-cells, neutrophils, various other immune cells, fibroblasts, and tumor cells.49 While beyond the scope of the present study, preliminary data from our lab have suggested a contribution of T-cells in the differential levels of IL-6 between responding and non-responding patients (data not shown).
A therapeutically important immune modulatory role for IL-6 is supported by data showing that IL-6 blockade, in combination with suppression of CD40 signaling, TGFβ blockade or PD-1 signaling, has been associated with increased antitumor activity in mouse models of cancer.42 50–52 Combined blockade of IL-6 and PD-L1 induced tumor regression in mice bearing subcutaneous pancreatic tumors that was associated with increased intratumoral T-cells. CD8-depleting but not CD4-depleting antibodies eliminated the antitumor activity of combined IL-6 and PD-L1 blockade in mice. This combination also promoted significant antitumor activity in mice bearing spontaneously arising pancreatic tumors, prolonging survival associated with increased T-cell infiltration.42 These data support the immune suppressive function of IL-6 and its downstream mediators such as CRP and serum amyloid.53 54 IL-6 has also been suggested as the cause of an elevated N/L ratio.55
The mechanism of action by which elevated IL-6, which promotes synthesis of acute phase reactants by the liver, is associated with worse survival in melanoma and other cancers, and possibly associated with toxicity in patients receiving ICI is unclear. IL-6 and CRP levels are highly associated in a variety of studies,41 were also highly correlated in the Checkmate-064 samples, and may play an indirect and a direct immune suppressive role, respectively. In addition to a potential immune suppressive function, IL-6 has pleiotropic activities that are tumor promoting.6–12 High levels of IL-6 are also associated with the formation of desmoplastic tumor stroma and can also stimulate the generation of MDSC cells in coordination with TGF-β. IL-6 induces tumor cell expression of STAT3 and its downstream target genes, which encode proteins that can drive tumor proliferation like cyclin D1 and/or survival like the BCL2-like protein 1 (BCL-xL). STAT3 promotes IL6 gene expression which can then result in a positive feedback autocrine loop.6 IL-6 clearly has important immune regulatory activities through induction of STAT3, which also induces expression of angiogenic molecules, including VEGF, factors that control invasiveness and/or metastasis such as matrix metalloproteinases, and immune suppressive cytokines such as TGF-β.56 Induction of STAT3 by high levels of IL-6 can drive immune suppression via negative regulation of neutrophil and NK cell function, induction of PD-1 expression on T-cells, inhibition of effector T-cell function, inhibition of DC maturation and function, and expansion of regulatory T-cells and MDSC in the tumor microenvironment.57–60 IL-6 may also suppress ketogenesis through its transcriptional master regulator, peroxisome proliferator-activated receptor alpha (PPAR-alpha). This tumor-associated alteration in hepatic metabolism magnifies the host stress response which leads to glucocorticoid levels that suppress tumor immunity.61
CRP was also associated with a poor outcome in patients treated with ICI and chemotherapy in this study. Its role in adaptive immunity has not been well documented, but it has been shown to have an immunosuppressive role in experimental murine encephalomyelitis62 and can diminish antigen presentation in vitro.63 Recent data have also shown that CRP binds to T-cells of patients with melanoma and suppresses their function in a dose dependent manner at the earliest stages of T-cell activation.64 Therefore, both IL-6 and its downstream molecule CRP may have direct immune suppressive roles accounting for the poor outcome in patients that have high levels of both molecules.
Based on the work described herein, and the published body of work described above, blockade of IL-6 and CRP synthesis and/or activity in combination with immune checkpoint therapies may enhance response and survival rates in patients with cancers, including melanoma, and may be associated with a lower rate of immune-related adverse events. This rationale will be tested in an ongoing phase II trial of ICI in which the IL-6 receptor blocking antibody tocilizumab will be added to the combination of nivolumab and ipilimumab (NCT03999749). Endpoints of that trial are immune-related toxicity as well as response rate and progression-free survival.
Conclusions
In patients with metastatic melanoma receiving ICI or chemotherapy in large randomized trials, IL-6, CRP and the N/R ratio are interdependent prognostic factors with higher levels associated with shorter OS. In a multi-variable analysis of the randomized phase III Checkmate-067 study, IL-6 remained a significant independent variate for survival. These data suggest that IL-6 may be an immune target in patients with melanoma receiving checkpoint inhibition.
Acknowledgments
We wish to thank the patients and families who made these studies possible; the clinical investigators, sites, and study teams who participated in the CheckMate-064, CheckMate-066, and CheckMate-067 studies; colleagues from StemScientific who provided assistance with preparation of figures, and colleagues at Bristol-Myers Squibb (Princeton, New Jersey) and ONO Pharmaceutical Company Ltd. (Osaka, Japan).
Footnotes
ASL and DW contributed equally.
Contributors: ASL: designed, conducted, analyzed and interpreted data, prepared manuscript. DW: conducted, analyzed and interpreted data, edited manuscript. MV: conducted experiments. XQ: conducted, analyzed and interpreted data, edited manuscript. HT: conducted, analyzed and interpreted data, edited manuscript. MW-R: conducted, analyzed and interpreted data, edited manuscript. JW: interpreted data, conceived and supervised project, prepared and edited manuscript.
Funding: This study was funded by R01 CA175732-01, K99/R00 CA230201-01, Bristol-Meyers Squibb.
Competing interests: ASL: received grant funding from NextCure. Employee and stock ownership in Moderna Therapeutics. DW: has stock in Bristol-Myers Squibb, Merck, GlaxoSmithKline, Seattle Genetics, Moderna Therapeutics, Iovance Biotherapeutics, Cue Biopharma, Fate Therapeutics, Atra Biotherapeutics, and Fortress Biotech. XQ: receives salary from BMS. HT: receives salary from BMS. MW-R: receives salary from BMS and has stock in BMS. JW: has stock or other ownership in Altor BioScience, Celldex, Biond and CytomX Therapeutics. Has honoraria in Bristol-Myers Squibb, Merck, Genentech, AbbVie, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Eisai, Altor BioScience, Amgen, Roche, Ichor Medical Systems, Celldex, CytomX Therapeutics, Nektar, Novartis, Array, WindMIL, Takeda and Sellas. Has consulting or advisory role in Celldex, Ichor Medical Systems, Pieris Pharmaceuticals, Altor BioScience, Bristol-Myers Squibb, Merck, Genentech, Roche, Amgen, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, AbbVie, Eisai, CytomX Therapeutics, Nektar, Novartis, Array, WindMIL, Takeda and Sellas. Received research funding (to the Institution) from Bristol-Myers Squibb, Merck, GlaxoSmithKline, Genentech, Astellas Pharma, Incyte, Roche and Novartis. Received 1 year of grant funding from Acetylon Pharmaceuticals. Received travel, accommodations, expenses from Bristol-Myers Squibb, GlaxoSmithKline, Daiichi Sankyo, Roche, Celldex, Amgen, Merck, AstraZeneca, Genentech, Novartis, Incyte, WindMIL and Takeda.
Patient consent for publication: Not required.
Ethics approval: All patients signed informed consent approved by the NYU Institutional Review Board for the use of the serum and peripheral blood samples from the three trials described in this work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol 1997;15:797–819. 10.1146/annurev.immunol.15.1.797 [DOI] [PubMed] [Google Scholar]
- 2. Hoejberg L, Bastholt L, Johansen JS, et al. Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Res 2012;22:287–93. 10.1097/CMR.0b013e3283550aa5 [DOI] [PubMed] [Google Scholar]
- 3. Lutgendorf SK, Weinrib AZ, Penedo F, et al. Interleukin-6, cortisol, and depressive symptoms in ovarian cancer patients. J Clin Oncol 2008;26:4820–7. 10.1200/JCO.2007.14.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. Int J Colorectal Dis 2010;25:135–40. 10.1007/s00384-009-0818-8 [DOI] [PubMed] [Google Scholar]
- 5. Mohapatra P, Prasad CP, Andersson T. Combination therapy targeting the elevated interleukin-6 level reduces invasive migration of BRAF inhibitor-resistant melanoma cells. Mol Oncol 2019;13:480–94. 10.1002/1878-0261.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang C, Xin H, Zhang W, et al. CD5 binds to interleukin-6 and induces a feed-forward loop with the transcription factor STAT3 in B cells to promote cancer. Immunity 2016;44:913–23. 10.1016/j.immuni.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linnskog R, Jönsson G, Axelsson L, et al. Interleukin-6 drives melanoma cell motility through p38α-MAPK-dependent up-regulation of WNt5a expression. Mol Oncol 2014;8:1365–78. 10.1016/j.molonc.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsukamoto H, Nishikata R, Senju S, et al. Myeloid-derived suppressor cells attenuate TH1 development through IL-6 production to promote tumor progression. Cancer Immunol Res 2013;1:64–76. 10.1158/2326-6066.CIR-13-0030 [DOI] [PubMed] [Google Scholar]
- 9. Rice SJ, Liu X, Zhang J, et al. Advanced NSCLC patients with high IL-6 levels have altered peripheral T cell population and signaling. Lung Cancer 2019;131:58–61. 10.1016/j.lungcan.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 10. Tsukamoto H, Fujieda K, Hirayama M, et al. Soluble IL6R expressed by myeloid cells reduces tumor-specific Th1 differentiation and drives tumor progression. Cancer Res 2017;77:2279–91. 10.1158/0008-5472.CAN-16-2446 [DOI] [PubMed] [Google Scholar]
- 11. Ara T, Nakata R, Sheard MA, et al. Critical role of STAT3 in IL-6-mediated drug resistance in human neuroblastoma. Cancer Res 2013;73:3852–64. 10.1158/0008-5472.CAN-12-2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 2011;19:456–69. 10.1016/j.ccr.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 13. Karakasheva TA, Lin EW, Tang Q, et al. IL-6 mediates cross-talk between tumor cells and activated fibroblasts in the tumor microenvironment. Cancer Res 2018;78:4957–70. 10.1158/0008-5472.CAN-17-2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Betts BC, St Angelo ET, Kennedy M, et al. Anti-IL6-receptor-alpha (tocilizumab) does not inhibit human monocyte-derived dendritic cell maturation or alloreactive T-cell responses. Blood 2011;118:5340–3. 10.1182/blood-2011-06-363390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uemura M, Trinh VA, Haymaker C, et al. Selective inhibition of autoimmune exacerbation while preserving the anti-tumor clinical benefit using IL-6 blockade in a patient with advanced melanoma and Crohn's disease: a case report. J Hematol Oncol 2016;9:81. 10.1186/s13045-016-0309-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019;25:551–7. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 18. Du Clos TW. Function of C-reactive protein. Ann Med 2000;32:274–8. 10.3109/07853890009011772 [DOI] [PubMed] [Google Scholar]
- 19. Katano A, Takahashi W, Yamashita H, et al. The impact of elevated C-reactive protein level on the prognosis for oro-hypopharynx cancer patients treated with radiotherapy. Sci Rep 2017;7:17805. 10.1038/s41598-017-18233-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agnoli C, Grioni S, Pala V, et al. Biomarkers of inflammation and breast cancer risk: a case-control study nested in the EPIC-Varese cohort. Sci Rep 2017;7:12708. 10.1038/s41598-017-12703-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yasuda Y, Saito K, Yuasa T, et al. Early response of C-reactive protein as a predictor of survival in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Int J Clin Oncol 2017;22:1081–6. 10.1007/s10147-017-1166-2 [DOI] [PubMed] [Google Scholar]
- 22. Hang J, Xue P, Yang H, et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci Rep 2017;7:2993. 10.1038/s41598-017-03153-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pastorino U, Morelli D, Leuzzi G, et al. Baseline and postoperative C-reactive protein levels predict mortality in operable lung cancer. Eur J Cancer 2017;79:90–7. 10.1016/j.ejca.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 24. Akamine T, Takada K, Toyokawa G, et al. Association of preoperative serum CRP with PD-L1 expression in 508 patients with non-small cell lung cancer: a comprehensive analysis of systemic inflammatory markers. Surg Oncol 2018;27:88–94. 10.1016/j.suronc.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 25. Weber JS, Sznol M, Sullivan RJ, et al. A serum protein signature associated with outcome after anti-PD-1 therapy in metastatic melanoma. Cancer Immunol Res 2018;6:79–86. 10.1158/2326-6066.CIR-17-0412 [DOI] [PubMed] [Google Scholar]
- 26. Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 27. Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer 2018;6:74. 10.1186/s40425-018-0383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer 2015;112:1904–10. 10.1038/bjc.2015.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rosner S, Kwong E, Shoushtari AN, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med 2018;7:690–7. 10.1002/cam4.1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weber JS, Gibney G, Sullivan RJ, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–55. 10.1016/S1470-2045(16)30126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ascierto PA, Long GV, Robert C, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol 2019;5:187–94. 10.1001/jamaoncol.2018.4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 33. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hodi FS, Chiarion-Sileni V, Gonzalez R, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:1480–92. 10.1016/S1470-2045(18)30700-9 [DOI] [PubMed] [Google Scholar]
- 35. Yoshida T, Ichikawa J, Giuroiu I, et al. C reactive protein impairs adaptive immunity in immune cells of patients with melanoma. J Immunother Cancer 2020;8:e000234. 10.1136/jitc-2019-000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tjin EPM, Krebbers G, Meijlink KJ, et al. Immune-escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol Res 2014;2:538–46. 10.1158/2326-6066.CIR-13-0097 [DOI] [PubMed] [Google Scholar]
- 37. Hardy-Werbin M, Rocha P, Arpi O, et al. Serum cytokine levels as predictive biomarkers of benefit from ipilimumab in small cell lung cancer. Oncoimmunology 2019;8:e1593810. 10.1080/2162402X.2019.1593810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bjoern J, Juul Nitschke N, Zeeberg Iversen T, et al. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with ipilimumab. Oncoimmunology 2016;5:e1100788. 10.1080/2162402X.2015.1100788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horisberger A, La Rosa S, Zurcher J-P, et al. A severe case of refractory esophageal stenosis induced by nivolumab and responding to tocilizumab therapy. J Immunother Cancer 2018;6:156. 10.1186/s40425-018-0481-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rotz SJ, Leino D, Szabo S, et al. Severe cytokine release syndrome in a patient receiving PD-1-directed therapy. Pediatr Blood Cancer 2017;64:e26642 10.1002/pbc.26642 [DOI] [PubMed] [Google Scholar]
- 41. Szalai AJ, van Ginkel FW, Dalrymple SA, et al. Testosterone and IL-6 requirements for human C-reactive protein gene expression in transgenic mice. J Immunol 1998;160:5294–9. [PubMed] [Google Scholar]
- 42. Eriksson E, Milenova I, Wenthe J, et al. IL-6 signaling blockade during CD40-mediated immune activation favors antitumor factors by reducing TGF-β, collagen type I, and PD-L1/PD-1. J Immunol 2019;202:787–98. 10.4049/jimmunol.1800717 [DOI] [PubMed] [Google Scholar]
- 43. Rincón M, Anguita J, Nakamura T, et al. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med 1997;185:461–70. 10.1084/jem.185.3.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diehl S, Anguita J, Hoffmeyer A, et al. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity 2000;13:805–15. 10.1016/S1074-7613(00)00078-9 [DOI] [PubMed] [Google Scholar]
- 45. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008;9:641–9. 10.1038/ni.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tsukamoto H, Fujieda K, Senju S, et al. Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity. Cancer Sci 2018;109:523–30. 10.1111/cas.13433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jackute J, Zemaitis M, Pranys D, et al. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol 2018;19:3. 10.1186/s12865-018-0241-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xue Y, Tong L, LiuAnwei Liu F, et al. Tumorinfiltrating M2 macrophages driven by specific genomic alterations are associated with prognosis in bladder cancer. Oncol Rep 2019;42:581–94. 10.3892/or.2019.7196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014;6:a016295. 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mace TA, Shakya R, Pitarresi JR, et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018;67:320–32. 10.1136/gutjnl-2016-311585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bialkowski L, Van der Jeught K, Bevers S, et al. Immune checkpoint blockade combined with IL-6 and TGF-β inhibition improves the therapeutic outcome of mRNA-based immunotherapy. Int J Cancer 2018;143:686–98. 10.1002/ijc.31331 [DOI] [PubMed] [Google Scholar]
- 52. Liu H, Shen J, Lu K. IL-6 and PD-L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun 2017;486:239–44. 10.1016/j.bbrc.2017.02.128 [DOI] [PubMed] [Google Scholar]
- 53. Shiels MS, Katki HA, Hildesheim A, et al. Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J Natl Cancer Inst 2015;107. 10.1093/jnci/djv199. [Epub ahead of print: 28 Jul 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Findeisen P, Zapatka M, Peccerella T, et al. Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol 2009;27:2199–208. 10.1200/JCO.2008.18.0554 [DOI] [PubMed] [Google Scholar]
- 55. Erreni M, Mantovani A, Allavena P, et al. Tumor-associated macrophages (TAM) and inflammation in colorectal cancer. Cancer Microenviron 2011;4:141–54. 10.1007/s12307-010-0052-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798–809. 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gotthardt D, Putz EM, Straka E, et al. Loss of STAT3 in murine NK cells enhances NK cell-dependent tumor surveillance. Blood 2014;124:2370–9. 10.1182/blood-2014-03-564450 [DOI] [PubMed] [Google Scholar]
- 58. Herrmann A, Kortylewski M, Kujawski M, et al. Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res 2010;70:7455–64. 10.1158/0008-5472.CAN-10-0736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Iwata-Kajihara T, Sumimoto H, Kawamura N, et al. Enhanced cancer immunotherapy using STAT3-depleted dendritic cells with high Th1-inducing ability and resistance to cancer cell-derived inhibitory factors. J Immunol 2011;187:27–36. 10.4049/jimmunol.1002067 [DOI] [PubMed] [Google Scholar]
- 60. Kujawski M, Zhang C, Herrmann A, et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res 2010;70:9599–610. 10.1158/0008-5472.CAN-10-1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Flint TR, Janowitz T, Connell CM, et al. Tumor-Induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab 2016;24:672–84. 10.1016/j.cmet.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang L, Liu S-H, Wright TT, et al. C-reactive protein directly suppresses Th1 cell differentiation and alleviates experimental autoimmune encephalomyelitis. J Immunol 2015;194:5243–52. 10.4049/jimmunol.1402909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jimenez RV, Wright TT, Jones NR, et al. C-Reactive protein impairs dendritic cell development, maturation, and function: implications for peripheral tolerance. Front Immunol 2018;9:372. 10.3389/fimmu.2018.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weber JS, Tang H, Hippeli L, et al. Serum IL-6 and CRP as prognostic factors in melanoma patients receiving single agent and combination checkpoint inhibition. J Clin Oncol 2019;37:100 10.1200/JCO.2019.37.15_suppl.100 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000842supp001.pdf (384.4KB, pdf)