Abstract
Introduction
Community-dwelling older adults living with subjective cognitive decline or mild cognitive impairment may experience decreased efficiency in their overall functional performance. This decreased cognitive efficiency may result in anxiety, low mood, perceived stress and decreased emotional well-being and quality-of-life. These psychological symptoms may further exacerbate cognitive decline.
Exploring non-pharmacological interventions such as mindfulness within primary care is vital in enabling individuals to develop strategies to manage cognitive impairment or psychological symptoms. Mindfulness-based stress reduction (MBSR) is an 8-week programme that is beneficial in alleviating psychological symptoms; however, its impact on perceived satisfaction on overall functional performance with this population has not been evaluated. The primary objective of this study is to explore the feasibility of conducting a randomised controlled trial of an occupational therapist-led MBSR programme within primary care.
Methods
Convergent mixed-methods, randomised control feasibility trial with 40 participants from an interprofessional primary care team in Toronto, Ontario. Participants are randomised into the 8-week MBSR group or wait-list control will be compared at baseline, postintervention and 4weeks follow-up. The primary aim is to determine the feasibility of the intervention with this population and setting. The secondary aim is to examine perceived satisfaction with functional performance as measured by the Canadian Occupational Performance Measure. Secondary clinical outcomes include psychological symptoms.
Analysis
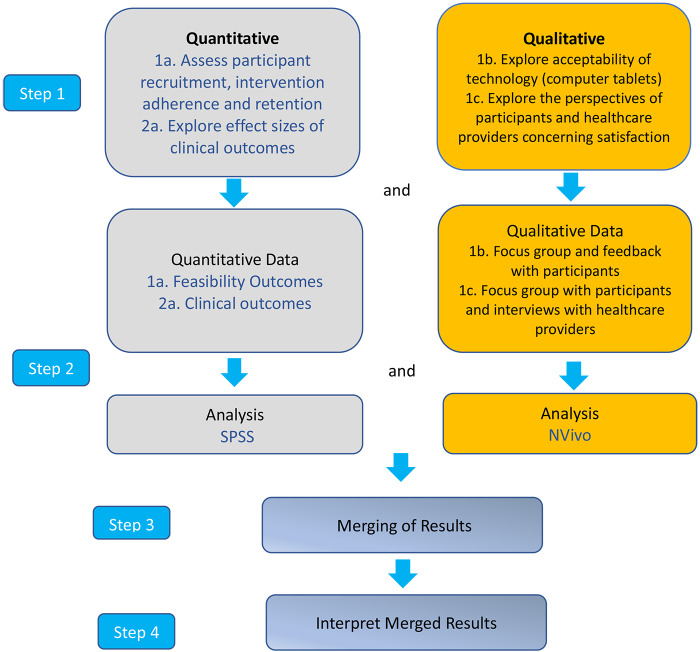
Investigators will analyse the quantitative and qualitative data strands separately. Descriptive statistics, focus group and interviews will then be merged and further analysed to best understand the feasibility and preliminary clinical outcomes from the study.
Ethics and dissemination
The study is approved by Women’s College Hospital (2017–0056-E), and Queen’s University, Kingston, Ontario (6026418). The study will follow Standard Protocol Items: Recommendations for Interventional Trials. The results will be published in peer-reviewed academic journals and disseminated to patient organisations and media.
Trial registration number
NCT03867474; Pre-results.
Keywords: primary care, mental health, anxiety disorders, delirium & cognitive disorders, depression & mood disorders, old age psychiatry
Strengths and limitations of this study.
The study will provide valuable data on feasibility and clinical outcomes to determine whether occupational therapist-led mindfulness-based stress reduction (MBSR) is appropriate for a larger clinical trial.
The first study to use the Canadian Occupational Performance Measure to evaluate perceived satisfaction on functional performance with community-dwelling older individuals living with subjective cognitive decline or mild cognitive impairment within an interprofessional primary care context.
The only study to explore the qualitative perspective of both participants and healthcare providers in terms of barriers, enablers and facilitators of implementing and delivering the MBSR programme within a primary care setting.
The study is innovative in exploring the acceptability of a tablet computer as a method of intervention delivery and data collection with this population.
The lack of an attention control comparison group and the small sample size is a study limitation.
Introduction
By 2036, approximately one-in-four Canadians will be 65 years and over,1 and an estimated one-third of community-dwelling older adults will experience memory complaints.2 The earliest sign of memory impairment is subjective cognitive decline (SCD), a self-reported decline in cognition without ‘objective evidence,’ characterised by increasing compensatory cognitive efforts and subtle cognitive decline (CD).3 If SCD is to decline further, the next stage is mild cognitive impairment (MCI), with 10%–20% of older adults developing MCI by age 65.4 MCI is clinically characterised as: (1) concern raised by the individual or an informant or clinician, (2) cognitive impairment in one or more cognitive domains relative normative data for that individual and (3) preservation of functional independence.5 6
There is a large body of evidence that demonstrates that those living with memory complaints face a decline in performance of everyday tasks, most notably in complex instrumental activities-of-daily living.7 These functional changes result in a general sense of decreased satisfaction and discontentment with their overall functional performance.8
Living with SCD or receiving a diagnosis of MCI is usually life-altering and has been found to have a negative impact on an individual’s emotional health and well-being,9 with an increased risk of depression and anxiety disorders.10 There is limited evidence that supports the use of pharmacological interventions to improve concomitant anxiety disorders11 and depression among those living with cognitive impairment.12 Medications may increase the risk of adverse side effects, especially for those with multiple comorbidities, including drug complications13 and falls.14 Exploring non-pharmacological interventions to mitigate psychosocial factors and to support functional performance is critical.10 15 Successful adaptive coping strategies to improve depression and anxiety symptoms in this population are essential to prevent and/or delay further CD.10
Evidence from the past 20 years suggests that mindfulness meditation, such as mindfulness-based stress reduction (MBSR), could benefit those living with SCD and MCI.16 17 MBSR may be neuroprotective against CD as it has been found to produce brain changes along with decreased cognitive complaints and increased memory self-efficacy.17 Furthermore, a small proof-of-concept study identified that MBSR is feasible with older adults living with MCI and that it may positively affect quality of life (QoL) and well-being.16 This study will build on these proof-of-concept and pilot studies as MBSR has demonstrated mental health benefits, including the reduction of emotional distress and worry.18 19
Other studies have demonstrated that mindfulness helps older adults with loneliness, depression, anxiety and sleep problems19–23 in general community settings and secondary care, for example, neurology clinics. However, primary care providers are often the first point of contact when older adults and their families are concerned about cognitive problems.24 There is an increasing emphasis on interprofessional primary care teams or patient medical homes to address the challenges of an ageing population. Currently, no studies to date have examined the feasibility of MBSR for those living with SCD or MCI receiving care from interprofessional primary care teams. A growing number of occupational therapists working in primary care teams are ideally positioned to support individuals with SCD and MCI through their expertise in understanding the impact of cognitive impairment on daily function. Examining effective interventions such as an occupational therapist-led, MBSR for individuals at the early stages of cognitive changes is critical to support ageing-in-place.25
The overarching purpose is to determine whether occupational therapist-led MBSR in primary care is appropriate for a larger clinical trial in the future. The study has two aims:
Primary aim
To explore the feasibility of conducting an randomised controlled trial (RCT) of an occupational therapist-led, 8-week MBSR programme in an interprofessional primary care setting. The following objectives will assess feasibility outcomes:
1(a). Assess participant recruitment, intervention adherence, and study retention (Quantitative).
1(b). Explore the acceptability of using tablet computer technology to support intervention, delivery and data collection in the MBSR programme (qualitative).
1(c). Explore the perspectives of participants and healthcare providers concerning satisfaction (eg, the intervention and its’ delivery), perceived value, and barriers and facilitators of implementation of the MBSR programme in a primary care setting (qualitative).
Secondary aim
2(a). To evaluate the effect sizes of satisfaction on functional performance as a primary clinical outcome and psychological symptoms as secondary clinical outcomes in individuals with SCD or MCI completing an 8-week MBSR programme in an interprofessional primary care setting (quantitative).
Methods
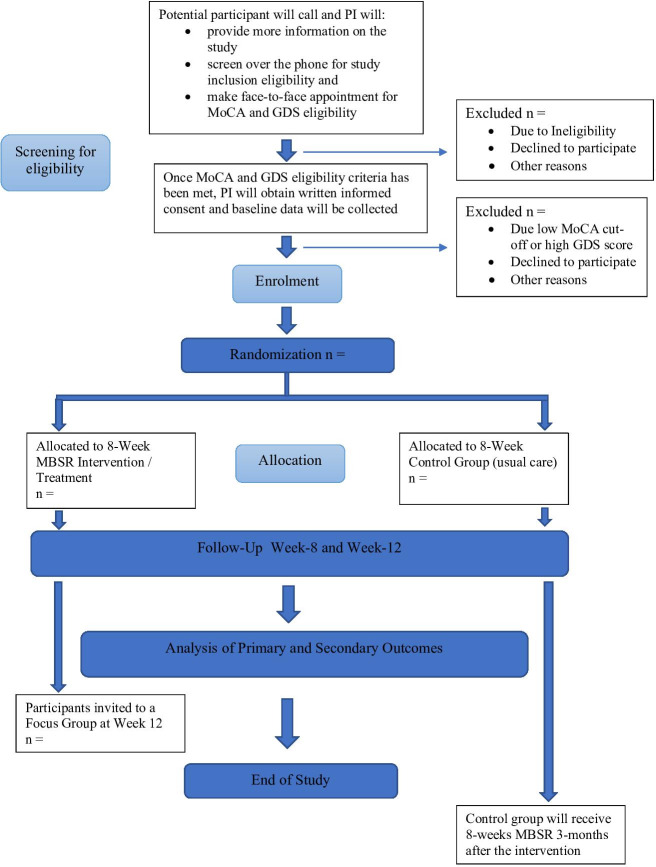
This study will use a convergent mixed-methods, single-blind RCT with two parallel groups and will follow Standard Protocol Items: Recommendations for Interventional Trials reporting26 guidelines for randomised feasibility trials (see trial design, see figures 1 and 2). There will be three assessment time points: baseline (time-1) at week 0, on completion of the intervention (time-2) at week 8 and 1-month postintervention follow-up (time-3) at week 12.
Figure 1.
SPIRIT flow diagram of participants through the study. GDS, Geriatric Depression Scale; MBSR, mindfulness-based stress reduction; MoCA, Montreal Cognitive Assessment; PI, principle investigator; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Figure 2.
Protocol flow chart.
Study setting
The study will take place at an interprofessional primary care clinic in the province of Ontario, Canada. Interprofessional team members include occupational therapy, physiotherapy, nursing, pharmacy, social work and dietetics. There are approximately 18 000 rostered patients with the clinic.
Eligibility criteria
To qualify for the study, participants will be screened using the Montreal Cognitive Assessment (MoCA), with a score of 22 or greater and a Geriatric Depression Scale (GDS) score of 6 or lower to be eligible to participate in the study. Scores of greater than 7 on the GDS and lower than 22 on the MoCA will warrant further assessment with their family physician and will be excluded from the study. The inclusion and exclusion criteria are:
Inclusion criteria
Age 60 years.
English fluency.
Living independently (non-assisted living, eg, retirement or any long-term care facility; self-report).
Have a self-reported SCD or an MCI diagnosis in their chart.
Must be a patient with the interprofessional primary care clinic.
Exclusion criteria
History of prior participation in any MBSR or other mindfulness-based interventions (MBI) in the past or having 2–3 times per week or more of either mindfulness or yoga practice.
Current history of significant medical (eg, cancer), neurological (eg, brain injury) or psychiatric condition (eg, depression with 6 or greater on the GDS), active psychosis, bereavement that significantly impacts on mood, that is, depression.
Alcoholism or other substance abuse.
Participating in other cognitive or memory training programmes in the community or is involved in another research study.
Intervention/treatment (MBSR) group
Participants randomised to the intervention arm will participate in an 8-week MBSR programme established in 1979 by Kabat-Zinn.27 Four occupational therapists, also qualified-MBSR teachers, will be involved in the delivery of the intervention group. The traditional MBSR curriculum usually has two teachers, but due to the unique population with cognitive impairment and the use of tablet computers, having two additional MBSR teachers will be beneficial to assist with any issues that may arise, including technological issues or memory challenges. The group will be 3 hours in duration (with a 15 min break) for 8 weeks, along with an orientation and one all-day retreat. Sessions will consist of: lying down (body scan), sitting (attention on the breath), and mindful movement (yoga and walking). Daily home practice will be given to be performed for 30–45 min outside of class.
We will distribute a tablet computer (mini-iPad 3 model) to each participant to access the Application (App), Insight Timer,28 for the duration of the study. Insight Timer contains guided meditation homework practices, with homework accessed by logging directly into Insight Timer. All homework data will be downloaded at the end of the 8-week programme. In addition to the App, all participants will be asked to record their home practice using pen and paper weekly logs as a backup provided by the research team. If participants have difficulty with using tablets, additional support will be provided during or after class. If any participant does not have access to Wi-Fi, we will provide them with CDs for ease of adherence for their guided homework practices, and homework will be tracked exclusively using pencil and paper sheets. Similarly, if participants have difficulty with using tablet computers, switching to CDs will be offered as an alternative low technology option.
Monitoring of adherence will include: (1) attendance records, (2) home practice logs, (3) tablet computer use (login, frequency, duration) and (4) field notes from Qualified-MBSR teachers in regard to the level of participation, engagement and group process.
Any participants who experience emotional issues (eg, increased anxiety, low mood) during the group will be referred to other healthcare professionals on the interprofessional primary care team (eg, social worker, consultant psychiatrists) for psychological support.
The control group (usual care) will be identical to the intervention group and will be offered the MBSR programme 3 months after the intervention group.
Assessment of intervention (MBSR) treatment fidelity
This study will use Gearing et al29 four major (intervention) fidelity components: Design, Training, Delivery and Receipt. The design fidelity of this feasibility RCT is to follow an existing 8-week protocol of MBSR following the authorised curriculum guide from the University of Massachusetts, Medical School, Center for Mindfulness in Medicine, Healthcare and Society. Design fidelity will be met by ensuring: a fixed number and length of sessions, following the scripted manual for the course, including external monitoring by the research team, recording any protocol deviations based on the population, monitoring of the home practice logs.
The training fidelity is significant as the teacher’s embodiment of mindfulness is central to the participant’s learning within the 8 week curriculum. To maintain training fidelity, three facilitators are qualified-MBSR teachers who have undergone training at the University of Massachusetts, Medical School; one facilitator has equivalent MBSR-qualifications from a different institution in Toronto, Canada using the same standardised MBSR treatment manuals. All qualified-MBSR Teachers have over 3 years of facilitating MBSR groups. Training fidelity will be met by: teachers meeting regularly to debrief, using the same teachers for the duration of the 8 weeks, and lastly, participant focus group inquiring about the curriculum will be used.
Delivery fidelity is the implementation of the MBSR curriculum by following both the MBSR curriculum protocol from the University of Massachusetts, Medical School and the MBI Teaching Assessment Criteria; a tool that assesses mindfulness-based teaching integrity that will be used as a guide to support the delivery of the MBSR curriculum. Delivery fidelity will also be measured by: participant focus group reflection of the teachers’ embodiment of mindfulness practice, attendance and intervention handouts provided for all participants along with tablet computers or CDs with home practice recordings.
Lastly, receipt fidelity will be achieved by attendance during the 8-week programme, in conjunction with log-ins and doing the home practices on participant’s computer tablets. Additionally, receipt fidelity will be met by: the collection of participant’s weekly handwritten home practice log sheets and inquiry discussions during the weekly sessions. This demonstrates that participants are practising the skills during the study period and are engaged and adherent to the programme. However, any missing attendance or drop-outs will be followed up with a telephone call.
Primary aim: Feasibility outcome measures
As a feasibility study, the overarching purpose is to determine whether MBSR is worthwhile for a definitive larger clinical trial for community-dwelling older adults living with SCD or MCI in an interprofessional primary care setting.
Objective 1a: Feasibility measures
Recruitment rate: will be defined as feasible for a future study if 30–40 participants are recruited within three to 4 months (May to August 2019), similar to other feasibility studies.30
Retention rate: will be deemed feasible if at least 75%–80% of participants complete six or more of the nine sessions as well as a follow-up assessment at T3 based on other feasibility studies.
Adherence rate: will be deemed to have adequate adherence for a future study if participants complete three logins per week and practice homework for at least 1.5 hours per week (duration), which would be deemed moderate adherence rate at 51–79.29 31 The treatment adherence rate is determined by the number of sessions completed in full (180 min).
Objective 1b: Acceptability of technology
Acceptability of using a tablet computer as a tool for home practice delivery will be determined through. (1) field notes by qualified-MBSR teachers documenting group participation, (2) number of participants that switch from computer tablets to low technology for the homework practices during the duration of the 8 weeks and (3) focus groups at follow-up at the end of 8 weeks (T2) examining perceived value and benefits of using technology.
Objective 1c: Satisfaction with the MBSR programme
The overall experience of the 8-week intervention will be evaluated by field notes, mid-way participants surveys, interviews with qualified-MBSR teachers (T3-week-12) and participant focus groups (T2-week-8). The dimensions of satisfaction with the programme will include length (number of weeks), difficulty (eg, pacing, workload or other challenges), and session duration (eg, too short, too long).
Secondary aim: Clinical outcome measures
Objective 2(a): Explore effect sizes of clinical outcomes
Demographic data will be collected at baseline (eg, age, education, income, physical activity, etc) along with primary and secondary clinical outcome measures.
Quantitative data
The primary clinical outcome will be the average change scores on the perceived satisfaction with functional performance as measured by the Canadian Occupational Performance Measure (COPM).32
Secondary clinical outcomes will include mood, anxiety, perceived stress, mindfulness traits, QoL and acceptance, as shown:
Patient Health Questionnaire-9 (PHQ-9).33 34
Geriatric Anxiety Inventory (GAI).35
Perceived Stress Scale (PSS).36
Cognitive and Affective Mindfulness Scale-Revised (CAMS-R).37
QoL-Alzheimer’s disease (QoL-AD).38
Acceptance and Action Questionnaire (AAQ-II).39
Time of outcome measures
Outcome measures will be assessed at baseline (time-1: week-1) on completion of the intervention at (time-2: week-8) and 1-month postintervention follow-up (time-3: week-12) (see table 1).
Table 1.
Time frame of measurement for participants in MBSR intervention
| Measures taken | (Time 1) | (Time 2) | (Time 3) | |||||||
| Item | 0 week | 1 week | 2 week | 3 week | 4 week | 5 week | 6 week | 7 week | 8 weeks (post-MBSR) | 12 weeks (follow-up) |
| Screening | ||||||||||
| (MoCA and GDS) | X | |||||||||
| Feasibility measures | X | X | X | X | X | X | X | X | X | |
| Qualitative measures | ||||||||||
| Focus group (Participants) | X | |||||||||
| Interview with MBSR teachers | X | |||||||||
| Evaluations (participants) | X | X | ||||||||
| Weekly research meeting notes | X | X | X | X | X | X | X | X | ||
| Weekly field notes | X | X | X | X | X | X | X | X | ||
| Quantitative measures | ||||||||||
| COPM (satisfaction/performance) | X | X | X | |||||||
| PHQ-9 (mood) | X | X | X | |||||||
| GAI (anxiety) | X | X | X | |||||||
| CAMS-R (mindfulness) | X | X | X | |||||||
| PSS (stress) | X | X | X | |||||||
| QoL-AD | X | X | X | |||||||
| AAQ-II (acceptance) | X | X | X | |||||||
AAQ-II, Acceptance and Action Questionnaire; CAMS-R, Cognitive and Affective Mindfulness Scale-Revised; COPM, Canadian Occupational Performance Measure; GAI, Geriatric Anxiety Inventory; GDS, Geriatric Depression Scale; MBSR, Mindfulness-Based Stress Reduction; MoCA, Montreal Cognitive Assessment; PHQ-9, Patient Health Questionnaire-9; PSS, Perceived Stress Scale; QoL-AD, Quality-of-Life in Alzheimer’s disease.
Clinical outcome measures
Primary outcome
Canadian Occupational Performance Measure
The COPM is an individualised, client-centred outcome measure. Through a semistructured interview, individuals identify areas of difficulty in the performance of everyday activities and satisfaction with their performance. Maximum of five activities can be identified, and each is rated on a 10-point scale for self-perceived performance and satisfaction for their functional performance. COPM demonstrates strong test–retest reliability for both the performance and satisfaction scores when tested a week apart40 and has demonstrated good responsiveness.41 A change of at least three points or more is recommended to distinguish between older adults who report a clinically significant change compared with those who do not.42
Secondary outcome
Patient Health Questionnaire
The PHQ-9 is a self-administered tool that scores each of the 9 Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria as ‘0’ (not at all) to ‘3’ (nearly every day), giving a total score of 27.33 PHQ-9 represents a reasonable alternative to the GDS with older adults in primary care settings.33 34 The internal reliability of the PHQ-9 is excellent, with a Cronbach’s of 0.89 in a PHQ-9 Primary Care Study, with excellent test–retest reliability. PHQ-9 has a sensitivity of 88% and a specificity of 88% for use in a population with major depression.33
Geriatric Anxiety Inventory
The GAI consists of 20 ‘agree/disagree’ items designed to assess typical common anxiety symptoms for the last week.35 GAI was developed specifically for community-dwelling older adults. The GAI has high internal consistency (α=0.76), as well as high inter-rater (r=0.89) and test–retest (r=0.86) reliability.35
Perceived Stress Scale
PSS is an assessment of the global appraisal of stress.36 The 10-item questionnaire examines stress of respondents using a 4-point scale (0-never to 4-very often). The PSS has acceptable psychometric properties, with satisfactory test–retest reliability criterion assessed at >0.70.43
The Cognitive and Affective Mindfulness Scale-Revised
CAMS-R is a brief comprehensive measure designed to capture mindfulness based on Jon Kabat-Zinn’s definition of mindfulness.37 The CAMS-R is a 10-item questionnaire with a 4-point scale (1—rarely to 4—almost always) s and has demonstrated internal consistency reliability with Cronbach’s alpha ranges from 0.61 to 0.81. The CAMS-R has also demonstrated concurrent validity with moderate to large correlation with other measures of mindfulness (r=0.5–0.67).37
Quality-of-Life in Alzheimer’s disease
The QoL-AD is a 13-item questionnaire covering multiple domains including health, mood, living situation, memory and money.44 The measure has demonstrated good test–retest reliability and strong inter-rater reliability with Cohen’s kappa values >0.70. Internal consistency is also high with a Cronbach’s alpha coefficient of 0.82.38
Acceptance and Action Questionnaire-II
The AAQ-II is a 7-item questionnaire that measures psychological flexibility-inflexibility and experiential avoidance.45 The measure has shown that psychological flexibility is a prominent factor in understanding psychological health.46 The AAQ-II has an alpha coefficient of 0.84 and demonstrates good test–retest reliability at 3 months at 0.81 and 12 months at 0.79.45 login table 1.
Sample size
The goal is to recruit approximately 40 participants (eg, 20 MBSR and 20 wait-list controls) to fit comfortably in a room. This number is feasible in the practice context and will enable examination of study objectives. To achieve this goal, 48 participants from the interprofessional primary care team will be recruited to account for an expected 20% attrition rate based on other feasibility studies.30 47
Recruitment
Participants will be recruited within the interprofessional primary care clinic. Posters will be placed in the waiting area, clinic and physician consult rooms and other interdisciplinary primary care providers may also inform potential participants about the study. Interested participants will be instructed to call the principal investigator (PI) who will explain the purpose of the research and study activities. If interested, participants will be scheduled for an intake assessment to screen for study eligibility. If eligible, the informed consent process will be reviewed with the individual, written consent obtained and then randomisation into one of the two groups will be completed.
Treatment allocation and randomisation
A block size design of four will be used to balance participants in the control or intervention groups. The block size design of four will randomly allocate two participants in the control and two in the intervention group resulting in six different possible block combinations, ideal for this feasibility study with a sample size of 40 participants. A research staff member, not involved in the trial, will design and prepare the randomisation sequence in sealed opaque envelopes to ensure allocation concealment for distribution. All research staff, including the PI, will be blinded to the randomisation list. At screening, if participants are eligible, the PI (first author) will obtain informed consent, assign participants a study number and collect baseline data. Last, a randomisation envelope with the same study number of the participant will be opened, and allocation will be to one of the two treatment groups,48 intervention (group 1) or a wait-list control (group 2). The wait-list control group will receive the MBSR intervention 3 months later when the experimental group is completed.
Blinding
The PI will assess baseline outcome measures for eligible participants at T1-week-1. A blinded independent assessor will evaluate postintervention at T2-week-8 and at T3-week-12, to minimise bias. The wait-list control (group 2) is assessed at T2-week-8 and T3-week-12, along with the intervention (group 1). To minimise unblinding, a research volunteer will provide reminder calls for the participants’ assessment date and time and will remind them not to disclose which group they are in during their assessment. Also, the independent assessor will again instruct all participants not to disclose which group they are in prior to their assessment. Due to the nature of the population with cognitive impairment, some participants may disclose their group unintentionally to the assessor. If unblinding occurs, it will be documented which participant disclosed, and it will be noted in the analysis. The qualified-MBSR teachers delivering the intervention cannot be blinded to the group allocation as they are providing the intervention being tested. Similarly, unblinding may occur if participants guess which group they are in (eg, intervention or control) however, participants are unable to confirm until after the study is completed.
Data management
The technical support department at the interprofessional primary care clinic will encrypt all computer tablets before distributing them to the intervention participants. The independent assessor will be in charge of data management including and data entry. All original hard copies of the study data, including questionnaires, teacher notes will be kept under lock and key in a secure location within the clinic. The PI will be responsible for overseeing the entire study and ensure timelines are met, data is cleaned, accurate and any missing values are identified. The committee from Queen’s University and the University of Toronto will service the role of data monitoring committee as part of PI’s Ph.D. research programme.
Qualitative data will be collected from both MBSR teachers and participants. MBSR teacher data will include weekly field notes and weekly meeting notes. A research assistant will conduct semi-structured interviews with each MBSR teacher at the completion of the intervention. Qualitative participant data will include open-ended feedback surveys at week-4 (midpoint) and week-8 (programme completion) and a focus group that will be conducted at the end of the MBSR programme. A research assistant will conduct a focus group using a guided script that will be an hour in duration. The focus group will explore satisfaction (eg, intervention and delivery), acceptability, perceived value, barriers and facilitators of the 8 week occupational therapist-led MBSR programme in primary care.
Qualitative analysis
Participant focus group and individual MBSR teacher interviews will be audio recorded and transcribed verbatim. All transcripts will be deidentified and pseudonyms will be given to each of the participants. Transcripts will be read and reread by both the PI and the research team. An inductive process of sorting, initial coding and grouping the data into broad topic-oriented categories, which is refined into fewer analytical themes, will be used.49 Critical discussion with the research staff of emerging themes will occur throughout the analysis process. The qualitative software package NVivo V.11 (QSR International) will be used to support the analysis.
To enhance trustworthiness, member checking will be used as a strategy.50 Peer debriefing, triangulation and an audit trail will be used to clarify interpretations of the data that may identify possible sources of bias. Each of these strategies will enhance trustworthiness to ensure dependability, credibility and transferability in the qualitative analysis.51
Quantitative analysis
The primary and secondary outcome measures will be analysed by the PI using IBM SPSS. A biostatistician will be consulted to provide an arms-length review of the analysis. Every attempt to minimise missing data will be implemented; however, the research team will use intention to treat (ITT), an approach that includes every participant. The ITT analysis will preserve the same sample size and reduce type I error. As a feasibility study with a small sample size, missing data is dealt with by using the last observation carried forward method, where the last available measurement for each participant at the point before withdrawal from the study, is retained and used in the analysis. In a future larger study, researchers will undertake a more sophisticated approach to allow additional factors to account for attrition.52
Baseline differences between the two groups will be tested using two-sample t-tests for normal distribution variables using the Shapiro-Wilk test and χ2 tests for categorical variables. Determining differences in clinical outcomes is not the object of this study. However, comparisons will be undertaken to investigate the estimates of the treatment effects for these potential clinical outcomes. Baseline at T1-week-1 to T2-week-8 and T1-week-1 to T3-week-12 will be analysed relative to change from baseline using one-way repeated analysis of variance for each participant and outcome measure. However, if there are any differences between the two groups, an analysis of covariance will be performed and adjustments will be made for baseline scores, as appropriate, for example, age, sex and education as possible confounders. For clinical outcome data, results will be reported as between-group mean, SD, change scores and treatment effects with a CI at 95%. Significance levels and Cohen’s d effect sizes will be reported at 95% CI.53 Similarly, feasibility and acceptability outcomes will be analysed using descriptive statistics (eg, adherence, attrition, frequency and duration logins) of intervention at baseline and the post-intervention outcome will be undertaken.
(Insight Timer - App metrics): The number of login (frequency) and length of home practice (duration) are extracted by the following: days, weeks, months and total hours overall for the duration of the MBSR programme. Descriptive statistics, including paired-sample t-tests or Wilcoxon signed-rank tests, is conducted to compare pre–post change scores on outcomes.
Benefits of participants
This protocol has been designed to explore the feasibility of conducting an RCT to determine whether an 8-week MBSR programme is feasible for a future larger clinical trial. There is growing recognition that interprofessional primary care teams are able to better support individuals with complex health conditions as compared with physician care alone. This study will be the first to explore the feasibility of an occupational therapist-led MBSR programme and provide valuable insights as to how MBSR can be best delivered with this population. In addition, this study will provide details to better implement this intervention with the use of technology, such as computer tablets to deliver the MBSR programme. Last, findings from this trial, if successful, will lay the foundation for a larger clinical trial. This study will highlight the possible benefits of MBSR and evaluation as a way to support psychological symptoms for those living with early memory issues within interprofessional primary care context.
Patient and public involvement
Patients and public members were not invited to provide feedback on the study design and the conduct of carrying out the study. The main results of the study will be disseminated to participants either through a letter or a face-to-face meeting if interested with respect to their results from baseline and end-of-study assessments.
Ethics and dissemination
Ethics permission has been granted by local and national registries. The findings of the study will be published in peer-reviewed journals and disseminated to patient organisations, national and international conferences and through social media.
Supplementary Material
Footnotes
Twitter: @todd_tran
Contributors: ToT (principal investigator, occupational therapist and Ph.D. candidate) designed this study protocol and wrote this manuscript. CD is instrumental in providing guidance on this manuscript, and without her input, this manuscript would not have been possible. EJN provided support at the inception of the design of the study and also provided helpful feedback, both verbal and written, on this manuscript. TrT provided valuable feedback around the ethics of running such a trial by providing valuable verbal and written comments at the inception and design of the current study. MF provided insightful feedback from the beginning of the design and implementation of this study protocol. MF provided a tremendous amount of guidance to allow for this study to be viable and for it to be replicable.
Funding: This study was funded by Centre for Ageing and Brain Health Innovation 3560 Bathurst Street Toronto, ON M6A 2E1 Canada And VHA Home HealthCare 30 Soudan Avenue, Suite 600 Toronto, ON M4S 1V6.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Statistics Canada Canada year book, 2011. Available: https://www150.statcan.gc.ca/n1/pub/11-402-x/2011000/chap/seniors-aines/seniors-aines-eng.htm
- 2.Zuniga KE, Mackenzie MJ, Kramer A, et al. Subjective memory impairment and well-being in community-dwelling older adults. Psychogeriatrics 2016;16:20–6. 10.1111/psyg.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–52. 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 2014;312:2551–61. 10.1001/jama.2014.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, Caracciolo B, Brayne C, et al. Mild cognitive impairment: a concept in evolution. J Intern Med 2014;275:214–28. 10.1111/joim.12190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Psychiatric Association DSMTF, American Psychiatric Association . Diagnostic and statistical manual of mental disorders: DSM-5. 5th Washington D.C: American Psychiatric Association, 2013. [Google Scholar]
- 7.Belchior P, Korner-Bitensky N, Holmes M, et al. Identification and assessment of functional performance in mild cognitive impairment: a survey of occupational therapy practices. Aust Occup Ther J 2015;62:187–96. 10.1111/1440-1630.12201 [DOI] [PubMed] [Google Scholar]
- 8.De Vriendt P, Gorus E, Cornelis E, et al. The process of decline in advanced activities of daily living: a qualitative explorative study in mild cognitive impairment. Int Psychogeriatr 2012;24:974–86. 10.1017/S1041610211002766 [DOI] [PubMed] [Google Scholar]
- 9.Joosten-Weyn Banningh L, Vernooij-Dassen M, Rikkert MO, et al. Mild cognitive impairment: coping with an uncertain label. Int J Geriatr Psychiatry 2008;23:148–54. 10.1002/gps.1855 [DOI] [PubMed] [Google Scholar]
- 10.Regan B, Varanelli L. Adjustment, depression, and anxiety in mild cognitive impairment and early dementia: a systematic review of psychological intervention studies. Int Psychogeriatr 2013;25:1963–84. 10.1017/S104161021300152X [DOI] [PubMed] [Google Scholar]
- 11.Andreescu C, Varon D. New research on anxiety disorders in the elderly and an update on evidence-based treatments. Curr Psychiatry Rep 2015;17:1–7. 10.1007/s11920-015-0595-8 [DOI] [PubMed] [Google Scholar]
- 12.Sepehry AA, Lee PE, Hsiung GYR, et al. Effect of selective serotonin reuptake inhibitors in Alzheimer's disease with comorbid depression: a meta-analysis of depression and cognitive outcomes. Drugs Aging 2012;29:793. 10.1007/s40266-012-0012-5 [DOI] [PubMed] [Google Scholar]
- 13.Kok RM, Reynolds CF. Management of depression in older adults: a review. JAMA 2017;317:2114–22. 10.1001/jama.2017.5706 [DOI] [PubMed] [Google Scholar]
- 14.Dhalwani NN, Fahami R, Sathanapally H, et al. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open 2017;7:e016358-e. 10.1136/bmjopen-2017-016358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moniz-Cook E. Psychosocial interventions through memory clinics. Nursing and Residential Care 2011;13:189–92. 10.12968/nrec.2011.13.4.189 [DOI] [Google Scholar]
- 16.Wells RE, Kerr CE, Wolkin J, et al. Meditation for adults with mild cognitive impairment: a pilot randomized trial. J Am Geriatr Soc 2013;61:642–5. 10.1111/jgs.12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smart CM, Segalowitz SJ, Mulligan BP, et al. Mindfulness training for older adults with subjective cognitive decline: results from a pilot randomized controlled trial. J Alzheimers Dis 2016;52:757–74. 10.3233/JAD-150992 [DOI] [PubMed] [Google Scholar]
- 18.Smoski MJ, McClintock A, Keeling L. Mindfulness training for emotional and cognitive health in late life. Curr Behav Neurosci Rep 2016;3:301–7. 10.1007/s40473-016-0097-y [DOI] [Google Scholar]
- 19.Geiger PJ, Boggero IA, Brake CA, et al. Mindfulness-Based interventions for older adults: a review of the effects on physical and emotional well-being. Mindfulness 2016;7:296–307. 10.1007/s12671-015-0444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creswell JD, Irwin MR, Burklund LJ, et al. Mindfulness-Based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun 2012;26:1095–101. 10.1016/j.bbi.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Splevins K, Smith A, Simpson J. Do improvements in emotional distress correlate with becoming more mindful? A study of older adults. Aging Ment Health 2009;13:328–35. 10.1080/13607860802459807 [DOI] [PubMed] [Google Scholar]
- 22.Black DS, O'Reilly GA, Olmstead R, et al. Mindfulness meditation and improvement in sleep quality and daytime impairment among older adults with sleep disturbances: a randomized clinical trial. JAMA Intern Med 2015;175:494–501. 10.1001/jamainternmed.2014.8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foulk MA, Ingersoll-Dayton B, Kavanagh J, et al. Mindfulness-based cognitive therapy with older adults: an exploratory study. J Gerontol Soc Work 2014;57:498–520. 10.1080/01634372.2013.869787 [DOI] [PubMed] [Google Scholar]
- 24.Knopman DS, Petersen RC. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc 2014;89:1452–9. 10.1016/j.mayocp.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciro CA. Maximizing ADL performance to facilitate aging in place for people with dementia. Nurs Clin North Am 2014;49:157–69. 10.1016/j.cnur.2014.02.004 [DOI] [PubMed] [Google Scholar]
- 26.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kabat-Zinn J. Stress reduction : Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. 15th anniversary ed New York, N.Y: Delta Trade Paperbacks, 2009. [Google Scholar]
- 28.Timer I Insight timer, 2019. Available: https://insighttimer.com
- 29.Gearing RE, El-Bassel N, Ghesquiere A, et al. Major ingredients of fidelity: a review and scientific guide to improving quality of intervention research implementation. Clin Psychol Rev 2011;31:79–88. 10.1016/j.cpr.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 30.Aguirre E, Stott J, Charlesworth G, et al. Mindfulness-Based cognitive therapy (MBCT) programme for depression in people with early stages of dementia: study protocol for a randomised controlled feasibility study. Pilot Feasibility Stud 2017;3 10.1186/s40814-017-0143-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perepletchikova F, Kazdin AE. Treatment integrity and therapeutic change: issues and research recommendations. Clinical Psychology: Science and Practice 2005;12:365–83. 10.1093/clipsy.bpi045 [DOI] [Google Scholar]
- 32.Law M. Canadian Association of Occupational T : In Canadian occupational performance measure. 4th Ottawa Ont: Canadian Association of Occupational Therapists, 2005. [Google Scholar]
- 33.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phelan E, Williams B, Meeker K, et al. A study of the diagnostic accuracy of the PHQ-9 in primary care elderly. BMC Fam Pract 2010;11:63. 10.1186/1471-2296-11-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pachana NA, Byrne GJ, Siddle H, et al. Development and validation of the geriatric anxiety inventory. Int Psychogeriatr 2007;19:103–14. 10.1017/S1041610206003504 [DOI] [PubMed] [Google Scholar]
- 36.Cohen S, Kamarck T. Mermelstein R. a global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 37.Feldman G, Hayes A, Kumar S, et al. Mindfulness and emotion regulation: the development and initial validation of the cognitive and affective mindfulness Scale-Revised (CAMS-R). J Psychopathol Behav Assess 2007;29:177–90. 10.1007/s10862-006-9035-8 [DOI] [Google Scholar]
- 38.Logsdon RG, Gibbons LE, Teri L, et al. Quality of life in Alzheimer's disease: longitudinal perspectives. Gerontologist 1999;39:164. [Google Scholar]
- 39.Hayes SC, Strosahl K, Wilson KG, et al. Measuring experiential avoidance: a preliminary test of a working model. Psychol Rec 2004;54:553–78. 10.1007/BF03395492 [DOI] [Google Scholar]
- 40.Carswell A, McColl MA, Baptiste S, et al. The Canadian occupational performance measure: a research and clinical literature review. Can J Occup Ther 2004;71:210–22. 10.1177/000841740407100406 [DOI] [PubMed] [Google Scholar]
- 41.Donnelly C, Carswell A. Individualized outcome measures: a review of the literature. Can J Occup Ther 2002;69:84–94. 10.1177/000841740206900204 [DOI] [PubMed] [Google Scholar]
- 42.Tuntland H, Aaslund MK, Langeland E, et al. Psychometric properties of the Canadian occupational performance measure in home-dwelling older adults. J Multidiscip Healthc 2016;9:411–23. 10.2147/JMDH.S113727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee E-H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res 2012;6:121–7. 10.1016/j.anr.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 44.Logsdon RG, Gibbons LEM, McCurry SM, et al. Quality of Life in Alzheimer’s Disease: Patient and Caregiver Reports. Aging Ment Health 1999;5:21–32. [Google Scholar]
- 45.Bond FW, Hayes SC, Baer RA, et al. Preliminary psychometric properties of the acceptance and action Questionnaire-II: a revised measure of psychological inflexibility and experiential avoidance. Behav Ther 2011;42:676–88. 10.1016/j.beth.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 46.Hayes SC, Luoma JB, Bond FW, et al. Acceptance and commitment therapy: model, processes and outcomes. Behav Res Ther 2006;44:1–25. 10.1016/j.brat.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 47.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altman DG. Avoiding bias in trials in which allocation ratio is varied. J R Soc Med 2018;111:143–4. 10.1177/0141076818764320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clarke V, Braun V. Thematic analysis. J Posit Psychol 2017;12:297–8. 10.1080/17439760.2016.1262613 [DOI] [Google Scholar]
- 50.Connelly LM. Trustworthiness in qualitative research. Medsurg Nurs 2016;25:435. [PubMed] [Google Scholar]
- 51.Hadi MA, José Closs S. Ensuring rigour and trustworthiness of qualitative research in clinical pharmacy. Int J Clin Pharm 2016;38:641–6. 10.1007/s11096-015-0237-6 [DOI] [PubMed] [Google Scholar]
- 52.Wong WK, Boscardin WJ, Postlethwaite AE, et al. Handling missing data issues in clinical trials for rheumatic diseases. Contemp Clin Trials 2011;32:1–9. 10.1016/j.cct.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 53.Evans A.Oaks T, Using basic statistics in the behavioral and social sciences. Fifth ed California: SAGE Publications Inc, 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.