This secondary analysis of cross-sectional studies assesses the relative mortality trends associated with mammographic screening and postsurgical adjuvant endocrine therapy and chemotherapy among women with early breast cancer in Victoria, Australia.
Key Points
Question
Is population-based mammographic screening or endocrine therapy and chemotherapy (adjuvant therapy) after curative surgery for early or operable breast cancer associated with the decline in breast cancer mortality in Victoria, Australia?
Findings
In this secondary analysis of cross-sectional studies of breast cancer mortality, stages at diagnosis and adjuvant therapy uptake were compared in Victoria. Advanced breast cancer incidence doubled from 1986 to 2013, and crude breast cancer mortality declined by 30% after 1994; by 1999, most women were receiving adjuvant therapy, which may be associated with this decline.
Meaning
These findings suggest that adjuvant therapy, rather than mammographic screening, is associated with the decline in breast cancer mortality in Victoria, Australia.
Abstract
Importance
Diagnosis of early breast cancer (EBC) in women by mammographic screening and postsurgical adjuvant endocrine therapy and chemotherapy (termed adjuvant therapy) began simultaneously in many countries in the 1990s. Subsequent breast cancer mortality declines were variously attributed to mammographic screening and/or adjuvant therapy.
Objective
To determine the relative mortality reductions associated with these 2 interventions in women with EBC who had been exposed to both.
Design, Setting, and Participants
This secondary analysis of cross-sectional studies assessed groups of women with invasive breast cancer in the State of Victoria, Australia, from January 1, 1982, to December 31, 2013, who were included in the Victorian Cancer Registry (VCR). The population consisted of participants in population-based studies of female breast cancer from 1986 to 2013 using data from 4 VCR population-based surveys of breast cancer treatment from 1986 to 1999; VCR data on breast cancer incidence, mortality, and TNM stage at diagnosis from 1986 to 2013; and Victorian mammographic screening program (BreastScreen Victoria) data from 1992 to 2007. Breast cancer incidence and mortality data were analyzed for all 76 630 women registered with invasive breast cancer with the VCR from January 1, 1982, to December 31, 2013, and breast cancer treatment and screening data were analyzed additionally for the groups of surveyed women as described above.
Exposures
Participation in BreastScreen Victoria and receipt of adjuvant therapy after surgery for EBC.
Main Outcomes and Measures
Data were analyzed for associations between crude breast cancer mortality trends and uptake of adjuvant therapy and downstaging by mammographic screening.
Results
Of all 76 630 women registered with breast cancer with the VCR from January 1, 1982, to December 31, 2013. Joinpoint analyses of the time trend in crude mortality showed an increase from 31.6 per 100 000 women in 1982 to 34.3 per 100 000 women in 1994, with a single joinpoint at 1994, followed by a significant declining trend to 23.9 per 100 000 women in 2013 (annual percentage change, −1.3%; 95% CI, −1.6% to −0.9%). By 1999, 74% of all Victorian women with EBC (737 of 1001) had commenced adjuvant endocrine therapy, and 72% (187 of 260) of premenopausal and 29% (215 of 741) of postmenopausal women with EBC had commenced adjuvant chemotherapy. Crude incidence of advanced-stage breast cancer almost doubled from 12.2 per 100 000 women in 1986 to 23.9 per 100 000 women in 2013.
Conclusions and Relevance
This study found that mammographic screening did not downstage breast cancer in Victoria from advanced to early, so population mortality benefit is lacking. Adjuvant therapy uptake was associated with all of the decline in Victorian breast cancer mortality since 1994. Given these findings, monitoring the relative contributions of mammographic screening and adjuvant therapy for EBC to breast cancer mortality reductions in populations of women exposed to both should be mandatory.
Introduction
Since 1988, the Early Breast Cancer Trialist Collaborative Group (EBCTCG) has been conducting systematic reviews of the effects of endocrine therapy and cytotoxic chemotherapy (adjuvant therapy), administered after surgical removal, on mortality due to breast cancer confined to the breast and adjacent axilla (early breast cancer [EBC]).1,2 The fifth EBCTCG review in 20052 reported that, throughout the next 15 years, breast cancer–specific mortality in women with EBC would be approximately halved by 6 months of anthracycline-based chemotherapy followed by 5 years of adjuvant tamoxifen therapy and, for middle-aged women with estrogen (ER)-positive cancer, administering this adjuvant chemotherapy to premenopausal women for more than 1 year and adjuvant tamoxifen to all women for more than 2 years would significantly reduce their cumulative mortality due to breast cancer.2
To reduce breast cancer mortality by screening, cancer must be detected at an early stage when cure by treatment is possible.3 Therefore, monitoring mammographic screening in populations should include measurement of the stages at diagnosis to determine whether screening is reducing the incidence of advanced breast cancer, defined by the American Joint Cancer Commission (AJCC) as stage III breast cancer locally invasive beyond the breast and/or with metastatic breast cancer in fixed axillary lymph nodes and any lymph node metastases in regional nonaxillary lymph nodes or as stage IV breast cancer with hematogenous metastases to organs and tissues distant to the breast,4 compared with EBCTCG EBC,2 which is AJCC stages I and II (downstaging).3 Unfortunately, analyses of long-term trends of advanced breast cancer incidence for decades are only available today for populations screened by mammography in New South Wales (NSW), Australia,5 the United States,3 Norway,6 and the Netherlands.7 All 4 countries reported that advanced breast cancer incidence either remained stable3,6,7 or increased5 after mammographic screening began, so no downstaging to EBC was detected.
A 2012 analysis of age-specific breast cancer incidence and mortality trends from 1986 to 2007 in Australia concluded that most of the 28% decline in age-specific breast cancer mortality since 1994 could be attributed to adjuvant therapy uptake by women with EBC, not to the Australian mammographic screening program (BreastScreen).8 Stage-specific crude incidences of female breast cancer in the State of Victoria are now available from 1986 to 2013, so the objective of this study was to compare their trends with trends in adjuvant therapy uptake in the female population from 1986 to 1999 and trends in breast cancer mortality from January 1, 1982, to December 31, 2013.
Methods
This secondary analysis of cross-sectional studies compares trends in breast cancer mortality among Victorian women, stage at diagnosis, and uptake of adjuvant therapy by women with EBC. Each individual study met the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline when published.9 This report follows the STROBE guidelines for discussion, including presentation of key results, study limitations, interpretation, and generalizability. All of the epidemiological data in this report are in the public domain and have been published and/or can be obtained by request to cancer registries or calculated from publications10,11,12,13,14,15 (eMethods 1 in the Supplement) using Australian Bureau of Statistics data.16 Ethics permission was sought and obtained as necessary by the original studies.4,10,11,12,13,14,15 The present study was exempted from review post hoc by the Deakin University Human Research Ethics Committee for the use of deidentified data and meeting the definition of negligible-risk research. This study followed the ethical principles of the Declaration of Helsinki.17
In Australia since 1982, all new cases of cancer (invasive malignant neoplasia), except basal and squamous cell skin cancers, have been registered by law with the 8 state and territory cancer registries.8 By 2000, 10 population-based management studies (treatment surveys) of female breast cancer were undertaken in Australia, including 9 state-based and 1 national survey in 1995,4,10,11,12 most of which focused on operable breast cancer, which is AJCC stages I and II EBC.4 The national survey also reported the survey methods in detail and a summary of 7 of the state surveys.4
For this study based in Victoria, relevant data were obtained directly and/or by calculation from all 3 state surveys and the Victorian component of the national surveys on adjuvant therapy commencement (uptake) for all premenopausal (or younger than 50 years) and all postmenopausal (or 50 years or older) women with EBC and the TNM stage at diagnosis for all women diagnosed with breast cancer in 1986, 1990, 1995, and 1999.10,11,12 Crude Victorian data on breast cancer stages at diagnosis were published for 2006 and 200712 and subsequently for 2006 to 2013 in the Victorian Cancer Registry (VCR) 2014 annual Cancer in Victoria report.13 This 2014 report stated that the proportions of breast cancer stages were stable from 2006 to 2013. However, crude breast cancer incidence rose progressively during that period13 (Figure 1), so the years 2006, 2007, 2010, and 2013 within that period were also used for staging. Of note, Victorian age-standardized staging data have never been published.13
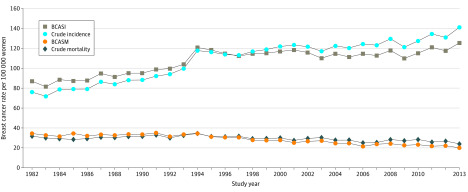
Figure 1. Incidence of Breast Cancer and Mortality Trends in Victoria, Australia: 1982-2013.
BCASI indicates breast cancer age-standardized incidence; BCASM, breast cancer age-standardized mortality.
We used the above staging data to calculate trends of crude incidence of Victorian stages I and II EBC and advanced stages III and IV (III/IV) breast cancer from 1986 to 2013 (Table 1).16 These crude incidences were all corrected for missing data to 16% of women in 1986 (eMethods 1 in the Supplement), 11% in 1990, 4% in 1995, 9% in 1999, 4% in 2006 to 2007, and 6% in 2006 to 201310,11,12,13,14,15 by assuming that the proportions of Victorian women with various stages of breast cancer in the missing data were the same as those for whom data were available. Of note, missing data were only 4% in 1995 and 6% in 2006 to 2013, respectively,12,14 so sensitivity analyses to test missing data assumptions were not indicated.
Table 1. Crude Incidence Rates for Breast Cancer Stratified by Early Cancer and Stage.
| Survey | Total No. of cases | Early breast cancer, No. (%) | Stage I | Stage II | Stages III/IV | |||
|---|---|---|---|---|---|---|---|---|
| No. (%) | Crude incidence rate | No. (%) | Crude incidence rate | No. (%) | Crude incidence rate | |||
| Victoria 1986a | 799 | 671 (84) | 436 (55) | 41.5 | 235 (29) | 22.4 | 128 (16) | 12.2 |
| Victoria 1990a | NA | 856 (NA) | 471 (55)b | 42.8 | 265 (31)b | 24.1 | NA | NA |
| Victoria 1995a | 1316 | 1158 (88) | 776 (59) | 67.5 | 382 (29) | 33.2 | 158 (12) | 13.7 |
| Victoria 1999a | NA | 1181 (100) | 744 (63) | 62.0 | 437 (37) | 36.4 | NA | NA |
| Staging project 2006c | 2974 | 2500 (84) | 1407 (47) | 54.2 | 1093 (37) | 42.2 | 474 (16) | 18.3 |
| Staging project 2007c | 2990 | 2466 (82) | 1343 (45) | 50.7 | 1123 (38) | 42.8 | 524 (18) | 19.2 |
| Victoria 2010c | 3499 | 2904 (83) | 1645 (47) | 58.7 | 1260 (36) | 45.0 | 595 (17) | 21.3 |
| Victoria 2013c | 4075 | 3382 (83) | 1915 (47) | 66.0 | 1467 (36) | 50.6 | 693 (17) | 23.9 |
Abbreviation: NA, not available.
The 1990 proportions are those available for early breast cancer and are not corrected for missing data because the total registered was not reported.
Breast cancer mortality rates are derived from data collected from death certificates and validated by VCR staff.10,14 The crude incidence and mortality rates of Victorian female breast cancer from 1982 to 2013 were provided by the VCR (eTable in the Supplement), and published age standardized to year 2001 breast cancer incidence (BCASI) and mortality (BCASM) rates from the VCR were also used14 (eTable in the Supplement). The relative reduction in breast cancer mortality between any 2 periods was calculated as the difference in the mortality rate between the 2 periods divided by the mortality rate for the first period.8
The 1995 national survey reported that 25% of Australian women 20 years or older resided in Victoria in 1995 and that more than 90% of breast cancer in 1995 was diagnosed in women 40 years or older.4 In 2011, 25% of Australia’s 11.2 million women (2.8 million) resided in Victoria.16
The Australian government offers 2 yearly free population-based mammographic screenings for women 40 years or older, with women aged 50 to 69 years invited biennially (BreastScreen).18 The BreastScreen program, funded in 1991, is implemented by the 6 state and 2 territory governments. The Victorian component, BreastScreen Victoria, was developed from 1 regional service with 14 screening sites in 1991 to 8 regional services with 59 screening sites in 1995 and reported on its first decade of screening (1992-2002) in 2003.18 Participation in BreastScreen Victoria by women aged 40 to 79 years increased from 4.8% in 1992 to 1993 to a plateau of approximately 37% from 1998, which was also the national participation rate; that rate continued to 2010.18,19 For the women aged 50 to 69 years who were invited, participation increased from 48.3% in 1994 to 1995 to 57.5% in 1996 to 199718 and then plateaued at the national level of 53% to 57%.18,19 From 5% to 10% of Victorian women were undergoing mammographic screening outside BreastScreen before 1992 and after.8,18 Rescreening responses to the biennial invitation are a BreastScreen quality indicator; the 2005-2006 BreastScreen Australia report shows that approximately 60% of Victorian women who attend a first screening attend a second screening, and 80% of those women attend third and subsequent mammographic screenings.20
Statistical Analysis
Data were analyzed from January 1, 1982, to December 31, 2013. Joinpoint analysis of the crude breast cancer mortality trend was undertaken using version 4.8.0.1 of the software developed by the US National Cancer Institute.21 This analysis objectively identifies (by permutation analysis) joinpoints as those points where a significant change in the linear slope of the mortality trend (log scale) was detected during the study period22 and presents the segments of the mortality trend on each side of a joinpoint as annual percentage changes (APCs) with 95% CI.21 This presentation allows identification of statistically significant changes in the mortality trend and, by identifying the year of the change, associates these changes with interventions, such as the introduction of screening or adjuvant therapy. The joinpoint analyses used 2-sided hypothesis tests with P = .05 as identifying statistical significance.21,22
Results
These analyses include breast cancer data collected by law in the VCR for all 76 630 women registered with invasive breast cancer in Victoria from 1982 to 2013; treatment and stage at diagnosis from 4 published VCR-based surveys of 6 months each in 1986, 1990, 1995, and 1999; and stage at diagnosis from all women registered with the VCR from 2006 to 2013. Breast cancer crude incidence and BCASI rates increased from 1982 to 2013, and the corresponding crude mortality and BCASM rates decreased (Figure 1). The crude incidence rate increased by 100 per 100 000 population from 1986 and 1992 and then in 1994 peaked at 120 per 100 000 above the 1982-1992 trend, declining to a fluctuating increasing trend without a subsequent compensatory drop below the 1986-1992 trend (Figure 1), as shown by fitting a linear trend to the data for 1982 to 1991 by joinpoint and using it to extrapolate to 2013, giving predicted values of 73.85 in 1982 and 127.97 in 2013,21 evidence of overdiagnosis of EBC based on mammographic screening.3
Joinpoint analyses of the time trend in crude mortality showed that annual crude mortality increased from 1982 to 1994, but this increase was not statistically significant (APC, 0.7%; 95% CI, −0.1% to 1.5%). A single joinpoint occurred at 1994 (best-estimate 95% CI, 1990-1997), followed by a significant declining trend to 2013 (Figure 2) (APC, −1.3%; 95% CI, −1.6% to −0.9%), a relative reduction in breast cancer mortality of 30% since 1994 (Figure 1). Importantly, there is no second joinpoint in this crude mortality trend, so no evidence of a statistically significant association of mammographic screening with the Victorian breast cancer mortality decline after 1994 was found.
Figure 2. Crude Mortality Due to Breast Cancer and Stage Incidence Trends in Victoria, Australia: 1982-2013.
The crude incidence of stage I breast cancer in Victoria increased by 3% from 1986 to 1990 (Table 1 and Figure 2). This initial increase was followed by a large increase of 62% from 1990 to 1995, when 30% or more of Victorian women 40 years or older were screened.18 Victorian stage II crude incidence more than doubled after rising above the 1986-1990 trend. Crude incidence of advanced stages III/IV breast cancer increased by 96% from 12.2 to 23.9 per 100 000 women from 1986 to 2013, ruling out a direct association of mammographic screening with breast cancer mortality (Table 1 and Figure 2).
In 1986 in Victoria, only 127 of 635 women (20%) with EBC began endocrine therapy, which consisted of tamoxifen in 94% to 100% of women. However, this proportion had almost doubled by 1990 (298 of 764 [39%]) and more than tripled by 1999 (737 of 1001 [74%]) (Table 2). Adjuvant chemotherapy uptake for premenopausal women increased from 40 of 190 (21%) in 1986 to 187 of 260 (72%) in 1999 and for postmenopausal women, from 20 of 445 (4%) in 1986 to 215 of 741 (29%) in 1999 (Table 2). By 1995, the standard combination adjuvant therapy of combined cyclophosphamide, methotrexate sodium, and fluorouracil had changed to a combination of cyclophosphamide and the anthracycline adriamycin.12 The crude breast cancer mortality peaked from 31.6 per 100 000 women in 1982 to 34.3 per 100 000 women in 1994 (Figure 2) and then fell continuously to 23.9 per 100 000 women in 2013. In 1995, 57% of premenopausal women began adjuvant chemotherapy and 62% of all women with EBC began tamoxifen, and in 1999, adjuvant premenopausal chemotherapy uptake had increased to 72% and adjuvant tamoxifen uptake had increased to 74% (Table 2), consistent with the EBCTCG findings that adjuvant therapy uptake could decrease breast cancer mortality after 1 year.2
Table 2. Victorian Population Surveys of Adjuvant Therapy Uptake by Women With Early Breast Cancer10,11,12.
| Study year | Adjuvant therapy uptake, No./total No. (%) | All women, tamoxifen uptake, No./total No. (%) | |||
|---|---|---|---|---|---|
| Premenopausal women or younger than 50 y | Postmenopausal women or 50 y or older | ||||
| Tamoxifen | Chemotherapy | Tamoxifen | Chemotherapy | ||
| 1986 | 11/190 (6) | 40/190 (21) | 116/445 (26) | 20/445 (4) | 127/635 (20) |
| 1990 | NA | NA | NA | NA | 298/764 (39) |
| 1995 | 58/235 (25) | 134/235 (57) | 599/832 (72) | 141/832 (17) | 657/1067 (62) |
| 1999 | 159/260 (61) | 187/260 (72) | 578/741 (78) | 215/741 (29) | 737/1001 (74) |
Abbreviation: NA, not available.
Adjuvant therapy uptake for EBC in Victoria increased more than 3-fold from 1986 to 1999 (Table 2). When analyzed using EBCTG 2005 data for adjuvant tamoxifen therapy2 and EBCTCG 2012 data for adjuvant anthracycline and cyclophosphamide therapy23 as described previously7 (eMethods 2 in the Supplement), the 1999 adjuvant therapy uptake (Table 2) can account for the entire 30% decline in Victorian crude breast cancer mortality after 1994. By comparing the crude mortality trend from 1984 to 1994 extrapolated by the 95% CI bound of the APC of 1.5% (above) with the observed trend, we calculated that as many as 3688 Victorian women could have had their deaths due to breast cancer prevented by this adjuvant therapy from 1994 to 2013 (eTable in the Supplement).
Discussion
A 2013 Victorian VCR–based cohort study indicated that most women who begin oral adjuvant endocrine therapy persist with it.24 Using the same calculation strategy (eMethods 2 in the Supplement) and the national survey data,4 the adjuvant therapy that Australian women with EBC commenced in 1995 may account for 23% of the observed 28% mortality decline in breast cancer nationally and for all of the decline if the 1999 Victorian adjuvant therapy data are used (Table 2).12 In Western Australia, crude breast cancer mortality fell by 24% from 1999 to 2014.25 Using this same calculation strategy (eMethods 2 in the Supplement), the adjuvant therapy that Western Australian women with EBC commenced in 199926 could account for all of that mortality decline. Furthermore, axillary lymph node metastases in women with EBC in Western Australia increased by more than 70% in postmenopausal women with ER-positive EBC from 1990 to 1999,26 indicating that mammographic screening was not associated with reduced stages II and III breast cancer incidence in that decade.
In the 1999 Victorian management survey, with approximately one-third of women aged 50 to 69 years diagnosed via BreastScreen Victoria,12 the proportions of women with ER-positive breast cancer commencing adjuvant tamoxifen therapy were almost the same for women diagnosed clinically as via BreastScreen (95% vs 92%). For adjuvant chemotherapy uptake, the proportions were similar for women with ER-negative and ER-positive cancer (71% vs 64%) (Vicki White, PhD; unpublished data from the 1999 survey; received by email November 1, 2019). However, more women with ER-positive cancer diagnosed clinically commenced adjuvant chemotherapy than women diagnosed via BreastScreen (39% vs 22%). Therefore, no evidence suggests that diagnosis via BreastScreen was associated with greater access to adjuvant therapy and subsequently reduced breast cancer mortality. Unfortunately, often overlooked in evaluating mammographic screening is the extent to which improvements in adjuvant therapy given after surgery for EBC have reduced the potential of screening mammography to have a direct effect on breast cancer mortality.27,28
A 2016 International Agency for Research on Cancer systematic review of breast cancer screening for 72 countries,29 where more than half of all of the world’s women live, reported that screening mammography was available to some or all populations of women. However, no trend data for advanced-stage breast cancer were reported for any country,29 so the effectiveness of that screening cannot be evaluated from that report. A 2018 systematic review of the effect of mammographic screening on advanced breast cancer incidence in Europe30 could not reach a conclusion because, together with other problems, staging did not have a consistent definition and was not ascertained consistently. A systematic review of 8 of the original randomized clinical trials of population mammographic screening vs controls for whom staging data were available31 found that the effect of early detection on reducing the incidence of advanced-stage breast cancer accounted for most of its effect on breast cancer mortality and that monitoring the incidence of advanced-stage breast cancer as an early indicator of the effect of service screening was indicated.
The age-standardized incidence of advanced metastatic breast cancer (Surveillance, Epidemiology, and End Results distant disease3) for women in NSW, Australia’s most populous state (3.6 million women in 2011), increased by 67% from 4.3 to 7.2 per 100 000 population from the 1988-1995 to the 2006-2012 periods,5 suggesting that mammographic screening was not downstaging breast cancer in that state. Crude Australian data for advanced breast cancer (AJCC stages III/IV) are available for 1995 (1210 of 10 081 women [12%])4 and 2011 (2403 of 14 569 women [17%]),32 resulting in calculated annual crude national incidence rates of stages III/IV breast cancer of 13.3 per 100 000 women in 1995 and 21.7 per 100 000 women in 2011, a 63% increase. These NSW and Australian increases in advanced-stage cancer are consistent with the 74% Victorian increase in incidence of advanced stages III/IV crude breast cancer from 1995 to 2013 (Table 1 and Figure 2). For metastatic stage IV breast cancer in Australia (1995: 403 of 10 081 women [4%]4; 2011: 583 of 14 591 women [4%]32), there was a 45% increase per 100 000 population. Therefore, mammographic screening also appears to have failed to downstage breast cancer nationally. The reasons for these increases in advanced-stage breast cancer incidence in NSW, Victoria, and Australia are not known; however, Australian state and national all-stage incidences are increasing, and Australia now has the third highest age-standardized incidence rate in the world after Europe and North America (Figure 1).29 These increases are cause for global research efforts.29
In 1999, 69% of all Victorian women with EBC, 64% of women with EBC less than 1 cm in diameter, and more than 80% of women with EBC of 1 cm or more in diameter received postoperative external beam radiotherapy (EBRT).12 The potentially fatal cardiac adverse effects of EBRT to the breast have been recognized for decades,27,33 so these women were at risk of premature death without any possible benefit of reduced mortality.
The international, multicenter EBC TARGIT-A randomized clinical trial,33 in which the Western Australian female population participated, compared single-dose targeted intraoperative radiotherapy with fractionated daily EBRT after curative surgery. Overall breast cancer mortality was similar between the 2 groups after 5 years of follow-up, but there were significantly fewer deaths related to non–breast cancer causes with intraoperative radiotherapy (1.4% [95% CI, 0.8%-2.5%] vs 3.5% [95% CI, 2.3%-5.2%]; P = .009), attributable to fewer deaths due to cardiovascular causes and other cancers.33 When intraoperative radiotherapy was given concurrently with lumpectomy, compared with women receiving EBRT, there was a potential 2.3% decrease in overall mortality at 5 years.33 In 1999 in Victoria, 69% of the 1342 women with EBC received postoperative EBRT,12 so there could have been an extra 54 deaths in 2004, increasing to 78 deaths 5 years after 2013 if the proportions of women receiving EBRT had remained the same after 1999. Using data of 30% overdiagnosis of women aged 50 to 69 years in the NSW BreastScreen program,35 in 2012, we calculated an Australian ratio of harm of overdiagnosis to benefit (breast cancer deaths avoided) of 15:1 and recommended stopping the invitation to screening.34
Limitations
This secondary analysis of cross-sectional observational data examining time trends across the study period can only show associations among Victorian breast cancer mortality, mammographic screening participation, and adjuvant therapy uptake. We can only infer causality by showing that these associations are unlikely to be compatible with a causal relationship between screening and a fall in mortality and are likely to be compatible with the uptake of adjuvant therapy after curative surgery for EBC.
Conclusions
This analysis of cross-sectional studies showed no downstaging of breast cancer by mammographic screening, which suggests that persistence with BreastScreen Victoria may continue to expose Victorian women to unnecessary morbidity and mortality. We found that adjuvant therapy accounted for the observed 30% mortality decline; given this finding, we propose that BreastScreen should be terminated. Continuous measurement of breast cancer stages at diagnosis, all-cause and breast cancer–specific mortality, and adjuvant therapy uptake should be mandatory in monitoring and evaluating mammographic screening programs.
eMethods 1. Data Collection
eMethods 2. Calculations
eTable. Data Provided by Vicky Thursfield From VCR
eReferences.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomized trials among 28 896 women. N Engl J Med. 1988;319(26):1681-1692. doi: 10.1056/NEJM198812293192601 [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717. doi: 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 3.Bleyer A, Welch GH Effect of three decades of mammographic screening on breast cancer-incidence. N Engl J Med. 2012;367(21):1998-2005. doi: 10.1056/NEJMoa1206809 [DOI] [PubMed] [Google Scholar]
- 4.Hill DJ, Jamrozik K, White VM, et al. . Surgical Management of Breast Cancer in Australia in 1995. NHMRC National Breast Cancer Centre, Kings Cross NSW. [Google Scholar]
- 5.Jacklyn G, McGeechan K, Irwig L, et al. . Trends in stage-specific breast cancer incidence in New South Wales, Australia: insights into the effects of 25 years of screening mammography. Breast Cancer Res Treat. 2017;166(3):843-854. doi: 10.1007/s10549-017-4443-x [DOI] [PubMed] [Google Scholar]
- 6.Lousdal ML, Kristiansen IS, Møller B, Støvring H. Effect of organised mammography screening on stage-specific incidence in Norway: population study. Br J Cancer. 2016;114(5):590-596. doi: 10.1038/bjc.2016.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autier P, Boniol M, Koechlin A, Pizot C, Boniol M. Effectiveness of and overdiagnosis from mammography screening in the Netherlands: population based study. BMJ. 2017;359:j5224. doi: 10.1136/bmj.j5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton RC, Bell RJ, Thiagarajah G, Stevenson C. Adjuvant therapy, not mammographic screening, accounts for most of the observed breast cancer specific mortality reductions in Australian women since the national screening program began in 1991. Breast Cancer Res Treat. 2012;131(3):949-955. doi: 10.1007/s10549-011-1794-6 [DOI] [PubMed] [Google Scholar]
- 9.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 10.Hill DJ, Giles GG, Russell IS, Collins JP, Mapperson KJ. Management of primary, operable breast cancer in Victoria. Med J Aust. 1990;152(2):67-72. doi: 10.5694/j.1326-5377.1990.tb124457.x [DOI] [PubMed] [Google Scholar]
- 11.Hill DJ, White VM, Giles GG, Collins JP, Kitchen PR. Changes in the investigation and management of primary operable breast cancer in Victoria. Med J Aust. 1994;161(2):110-111, 114, 118 passim. doi: 10.5694/j.1326-5377.1994.tb127341.x [DOI] [PubMed] [Google Scholar]
- 12.White V, Pruden M, Giles G, et al. . The management of early breast carcinoma before and after the introduction of clinical practice guidelines. Cancer. 2004;101(3):476-485. doi: 10.1002/cncr.20401 [DOI] [PubMed] [Google Scholar]
- 13.Cancer Australia; National Breast and Ovarian Cancer Centre Breast cancer staging and treatment: data linkage report. Published 2010. Accessed March 2020. https://canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/breast-cancer-staging-and-treatment
- 14.Thursfield V, Giles G, Farrugia H Cancer in Victoria: statistics and trends 2013. Cancer Council Victoria, Melbourne. Published October 26, 2014. Accessed August 26, 2019. https://www.cancervic.org.au/downloads/cec/cancer-in-vic/ccv-statistics-trends-2013.pdf
- 15.Thursfield V, Farrugia H Cancer in Victoria: statistics and trends 2010. Published November 2012. Accessed August 26, 2019. https://www.cancervic.org.au/downloads/cec/cancer-in-vic/CCV-Statistics-trends.pdf
- 16.Australian Bureau of Statistics. 3105.0.65.001—Australian historical population statistics, 2014. Updated April 17, 2019. Accessed March 2020. https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3105.0.65.0012014
- 17.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 18.A Decade of Achievement: BreastScreen Victoria 1992-2002. BreastScreen Victoria, Inc, Coordination Centre; 2003. [Google Scholar]
- 19.Australian Institute of Health and Welfare BreastScreen Australia Monitoring Report 2011-2012 Published October 1, 2014. Accessed March 2020. https://www.aihw.gov.au/reports/cancer-screening/breastscreen-australia-monitoring-2011-2012/contents/summary
- 20.Australian Institute of Health and Welfare BreastScreen Australia Monitoring Report 2005-2006 Published August 26, 2009. Accessed March 2020. https://www.aihw.gov.au/reports/cancer/breastscreen-australia-monitoring-report-2005-2006/contents/summary
- 21.National Cancer Institute; Division of Cancer Control & Population Sciences. Joinpoint trend analysis software: Joinpoint Regression Program, version 4.8.0.1. Updated April 21, 2020. Accessed May 13, 2020. https://surveillance.cancer.gov/joinpoint/
- 22.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335-351. doi: [DOI] [PubMed] [Google Scholar]
- 23.Peto R, Davies C, Godwin J, et al. ; Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) . Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432-444. doi: 10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell RJ, Schwarz M, Fradkin P, Robinson PJ, Davis SR. Patterns of use of oral adjuvant endocrine therapy in Australian breast cancer survivors 5 years from diagnosis. Menopause. 2013;20(7):721-726. doi: 10.1097/GME.0b013e31827ce094 [DOI] [PubMed] [Google Scholar]
- 25.Cancer Council Western Australia. Western Australia cancer statistics, 2014: breast cancer. Posted May 18, 2016. Accessed August 26, 2019. https://web.archive.org/web/20170301071500/https://www.cancerwa.asn.au/resources/2016-05-18-Breast-cancer-2014-FINAL.pdf
- 26.McEvoy SP, Ingram DM, Byrne MJ, et al. . Breast cancer in Western Australia: clinical practice and clinical guidelines. Med J Aust. 2004;181(6):305-309. doi: 10.5694/j.1326-5377.2004.tb06294.x [DOI] [PubMed] [Google Scholar]
- 27.Baum M. Harms from breast cancer screening outweigh benefits if death caused by treatment is included. BMJ. 2013;346:f385. doi: 10.1136/bmj.f385 [DOI] [PubMed] [Google Scholar]
- 28.Miller AB. The role of screening mammography in the era of modern breast cancer treatment. Climacteric. 2018;21(3):204-208. doi: 10.1080/13697137.2017.1392503 [DOI] [PubMed] [Google Scholar]
- 29.IARC Working Group on the Evaluation of Cancer-Preventive Interventions. Breast Cancer Screening. International Agency for Research on Cancer; 2016:174-217. [PubMed] [Google Scholar]
- 30.Broeders MJM, Allgood P, Duffy SW, et al. . The impact of mammography screening programmes on incidence of advanced breast cancer in Europe: a literature review. BMC Cancer. 2018;18(1):860. doi: 10.1186/s12885-018-4666-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tabár L, Yen AM-F, Wu WY-Y, et al. . Insights from the breast cancer screening trials: how screening affects the natural history of breast cancer and implications for evaluating service screening programs. Breast J. 2015;21(1):13-20. doi: 10.1111/tbj.12354 [DOI] [PubMed] [Google Scholar]
- 32.Cancer Australia. National cancer control indicators: distribution of cancer stage. Published April 26, 2018. Accessed November 3, 2019. https://ncci.canceraustralia.gov.au/diagnosis/distribution-cancer-stage/distribution-cancer-stage
- 33.Vaidya JS, Wenz F, Bulsara M, et al. ; TARGIT Trialists’ Group . Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT: a randomised trial. Lancet. 2014;383(9917):603-613. doi: 10.1016/S0140-6736(13)61950-9 [DOI] [PubMed] [Google Scholar]
- 34.Bell R, Loff B, Baum M, Burton R Is routine breast cancer screening doing more harm than good? Published November 18, 2012. Accessed April 1, 2020. https://theconversation.com/is-routine-breast-cancer-screening-doing-more-harm-than-good-10531
- 35.Morrell S, Barratt A, Irwig L, Howard K, Biesheuvel C, Armstrong B. Estimates of overdiagnosis of invasive breast cancer associated with screening mammography. Cancer Causes Control. 2010;21(2):275-282. doi: 10.1007/s10552-009-9459-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Data Collection
eMethods 2. Calculations
eTable. Data Provided by Vicky Thursfield From VCR
eReferences.