Abstract
Introduction
Gastro-oesophageal variceal bleeding is one of the most common and severe complications with high mortality in cirrhotic patients who developed portal hypertension. Hepatic venous pressure gradient (HVPG) is a globally recommended golden standard for the portal pressure assessment and an HVPG ≥16 mm Hg indicates a higher risk of death and rebleeding. This study aims to compare the effectiveness and safety of splenectomy and pericardial devascularisation (laparoscopic therapy) plus propranolol and endoscopic therapy plus propranolol for variceal rebleeding in cirrhotic patients with HVPG between 16 and 20 mm Hg.
Methods and analysis
This is a multicenter, randomised, controlled clinical trial. Participants will be 1:1 assigned randomly into either laparoscopic or endoscopic groups. Forty participants whose transjugular HVPG lies between 16 and 20 mm Hg with a history of gastro-oesophageal variceal bleeding will be recruited from three sites in China. Participants will receive either endoscopic therapy plus propranolol or laparoscopic therapy plus propranolol. The primary outcome measure will be the occurrence of gastro-oesophageal variceal rebleeding. Secondary outcome measures will include overall survival, occurrence of hepatocellular carcinoma, the occurrence of venous thrombosis, the occurrence of adverse events, quality of life and tolerability of treatment. Outcome measures will be evaluated at baseline, 12 weeks, 24 weeks, 36 weeks, 48 weeks and 60 weeks. Multivariate COX regression model will be introduced for analyses of occurrence data and Kaplan-Meier analysis with the log-rank test for intergroup comparison.
Ethics and dissemination
Ethical approval was obtained from all three participating sites. Primary and secondary outcome data will be submitted for publication in peer-reviewed journals and widely disseminated.
Trial registration number
NCT03783065; Pre-results.
Trial status
Recruitment for this study started in December 2018 while the first participant was randomised in January 2019. Recruitment is estimated to stop in October 2019.
Keywords: hepatology, hepatobiliary surgery, endoscopy, hepatobiliary disease
Strengths and limitations of this study.
This study is the first trial that concentrates on the best management on prevention of rebleeding for cirrhotic patients with hepatic venous pressure gradient between 16 and 20 mm Hg.
This trial is the first one to compare the effectiveness of laparoscopic therapy plus propranolol to endoscopic therapy plus propranolol recommended by international guidelines.
The surgical procedure involved in this study employs minimally invasive laparoscopy instead of a conventional operation, minimising trauma and complications.
Limitations of this trial include the lack of accessible data for sample size estimation, potential influence in applicability in other countries due to aetiological differences and the relatively short follow-up period.
Introduction
Cirrhosis is the result of multiple liver diseases and is accounted as a dynamic process.1 Portal hypertension is a vital event in the natural progression of cirrhosis that is responsible for decompensating events, such as gastro-oesophageal variceal bleeding, ascites and hepatic encephalopathy. Gastro-oesophageal varices could be seen in about 50% of cirrhotic patients and those who developed variceal bleeding face a mortality of 5%–20%.2 3 Thus, the stratification and applicable secondary prevention for patients with high risk is of great clinical significance.
Hepatic venous pressure gradient (HVPG) is the difference between the wedged hepatic venous pressure and free hepatic venous pressure.4 Eliminating the influence of abdominal pressure, HVPG is currently the most widely accepted reflection of portal pressure and has been demonstrated to have good performances in risk stratification3 5 and predicting the response to treatments.6 7 An HVPG over 12 mm Hg suggests the occurrence of gastro-oesophageal variceal bleeding.8 Patients with HVPG over 16 mm Hg face a higher risk of death8–11 and rebleeding,6 while an HVPG over 20 mm Hg predicts failure to control bleeding, early rebleeding and death due to acute variceal haemorrhage.12 13 Currently, international guidelines recommend endoscopic therapy combined with non-selective beta-blockers to be the first-line therapy of secondary prevention for cirrhotic patients with gastro-oesophageal variceal bleeding.3 5 Nevertheless, patients with high HVPG still suffer from the risk of treatment failure. Recent years, early transjugular intrahepatic portal-systemic shunts are recommended as a better choice for patients with HVPG ≥20 mm Hg,14 15 while there still lacks a strong evidence to determine the best method for patients with HVPG between 16 and 20 mm Hg.
Splenectomy and pericardial devascularisation, first performed by Hassab,16 17 is a promising surgical procedure for cirrhotic patients with gastro-oesophageal variceal bleeding, especially for those with hypersplenism. With the rapid advance of laparoscopic techniques, since the first laparoscopic splenectomy was reported in 1991,18 postoperational complications which used to be a major concern of Hassab’s operation have been cut down to a great extent due to less invasive procedures.19 Laparoscopic splenectomy and pericardial devascularisation (laparoscopic therapy) have been widely accepted for variceal bleeding in Asia-Pacific countries, where the predominant aetiology of cirrhosis is hepatitis B virus infection20 combined with very high occurrence of hypersplenism.21 However, there haven’t been any prospective trials comparing the effectiveness of laparoscopic therapy plus propranolol to the internationally recommended first-line therapy. Also, the precise indication to perform laparoscopic therapy is still unclear.
In this study, the outcomes of recruited patients whose HVPG lies within 16–20 mm Hg will be compared to explore the optimised management. Taking into consideration the preferred performance of HVPG in risk stratification and the lack of prospective study in the long-term performance of laparoscopic therapy, this trial will be meaningful for both the extension of HVPG risk stratification and the clarification of laparoscopic therapy indication.
Objectives
The aim of this trial is to assess the effectiveness and safety of laparoscopic therapy plus propranolol as first-line therapy of variceal rebleeding prevention for cirrhotic patients whose transjugular HVPG lies between 16 and 20 mm Hg with gastro-oesophageal variceal bleeding compared with endoscopic therapy plus propranolol. The primary outcome will be variceal rebleeding. Secondary outcomes include death, hepatocellular carcinoma, venous thrombosis, adverse events, quality of life and tolerability of treatment. We hypothesise that:
Compared with endoscopic therapy plus propranolol, laparoscopic therapy plus propranolol is more effective in reducing variceal rebleeding.
Participants receiving laparoscopic therapy plus propranolol show non-inferior overall survival over those receiving endoscopic therapy plus propranolol.
Participants receiving laparoscopic therapy plus propranolol show the lower occurrence of hepatocellular carcinoma over those receiving endoscopic therapy plus propranolol.
Methods and analysis
Study design
This study is a multicenter, prospective, randomised controlled clinical trial. The overview of the study process is illustrated in figure 1. After being screened for eligibility and measurement of HVPG, the participants will be randomly allocated to the laparoscopic group or endoscopic group. After the operative intervention, there will be a 60-week follow-up period. All tests and interventions will be performed at three involved centres in China: (1) Shunde Hospital, Southern Medical University, (2) Xingtai People’s Hospital and (3) The First Hospital of Lanzhou University.
Figure 1.
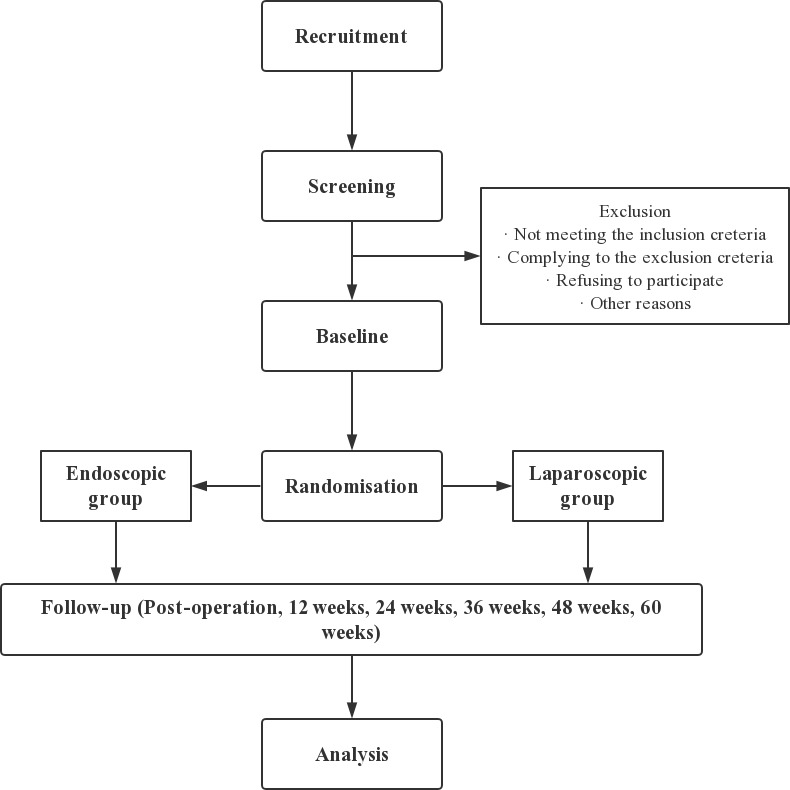
Flowchart for study design.
Eligibility criteria
Inclusion criteria
Eligible participants should be (a) aged between 18 and 75 years, (b) clinically and/or pathologically diagnosed cirrhosis with portal hypertension, (c) with a history of gastro-oesophageal variceal bleeding (melena, hematemesis, etc), without receiving splenectomy or any secondary prevention, (d) screened with transjugular HVPG between 16 and 20 mm Hg after hospitalisation, (e) with Eastern Cooperative Oncology Group score ≤2 and Karnofsky Performance Status (KPS) score ≥60 during screening, (f) assessed to be Child-Pugh class A or B and (g) voluntarily participate in the study and able to provide written informed consent and able to understand and willing to comply with the requirements of the study.
Exclusion criteria
Those who conforms to any of the following would be excluded: (a) pregnant or breastfeeding women, (b) with prior known or suspected malignancy (hepatocellular carcinoma, cholangiocarcinoma, etc), (c) with limited coagulation situation (Quick <50%, partial thromboplastin time >50 s, platelet count <5 × 109 or qualitative platelet dysfunction that affects conglutination function of congenital (Bernard-Soulier syndrome, Glanzmann thrombasthenia, storage pool disease, aspirin-like defects, platelet-type von Willebrand disease, etc) or acquired (medication or other systemic diseases) causes), (d) with massive ascites, (e) assessed to be Child-Pugh class C, (f) refusing or inadequate for transjugular HVPG measurement, (g) with active bleeding on screening, (h) patients with recurrent bleeding and (i) with other situations whose existence judged inadequate for participation by the investigators.
Recruitment
Recruitment has started in December 2018 and will continue until the intent sample size has been reached. Participants (n=40) from China are recruited in three sites through (1) posters, which show the condition of the trial, (2) social media (ie, websites and WeChat) and (3) the advice of the doctors.
Patient and public involvement
Patients and public were not involved in the design and development of the study.
Randomisation
Eligible patients will be randomly allocated (1:1) to either the laparoscopic group or the endoscopic group after signing on an informed consent, before which the patients will be informed about the trial in detail. The groups will be stratified by Child-Pugh class, age (≤60 years or >60 years) and gender. For the randomisation, the randPack package of R software (R Project for Statistical Computing, Vienna, Austria) will be introduced. The randomisatio will be generated by a statistician independent of the study.
HVPG measurement
Transjugular HVPG measurement will be performed for all participants when screening for eligibility by experienced interventional radiologists. The procedure will be performed using a balloon catheter with a pressure transducer at the tip (Edwards Lifesciences, Irvine, California). At first, a zero measurement will be made with the transducer open to the air. After transjugular catheterisation, free hepatic venous pressure will be measured in the right hepatic vein at about 1–3 cm from the inferior vena cava. Then, the right hepatic vein will be occluded completely by the inflated balloon, after which will the wedged hepatic venous pressure be measured. The measurement will be continued until the pressure reaches a plateau. Measurements will be performed in at least triplicate, and the average value will be used. HVPG is the difference between wedged hepatic venous pressure and free hepatic venous pressure.
Operative interventions
All operative interventions will be performed by trained and experienced specialists affiliated to the university centres. Doppler ultrasonography, CT, ECG and esophagogastroduodenoscopy will be performed routinely preoperation.
Participants assigned to the laparoscopic group will undergo laparoscopic therapy within 48 hours after randomisation. Laparoscopic therapy will be performed as previously described.20 General anaesthesia will be applied to all participants. Major procedures of the operation include splenectomy and the dissection and ligation of short gastric vessels, posterior gastric vessels and all branches of proximal lesser curvature, cardia and lower 6–8 cm part of abdominal oesophagus from the stomach coronary vein. During the process of devascularisation, the high oesophageal branches and heterotopic high oesophageal branches will be carefully screened.
Participants assigned to the endoscopic group will undergo initial endoscopic therapy within 48 hours after randomisation. Candidate procedures for endoscopic therapy include endoscopic variceal ligation (EVL), cyanoacrylate glue injection and sclerotherapy. The decision of which procedure to adopt will be made by the experienced specialist according to the condition of the participant, while EVL will be considered the first option as recommended by guidelines.3 5 Treatments will be performed again every 1–2 week until completely eradication of varices.
Propranolol oral administration
Participants assigned to both groups will begin to receive propranolol after the randomisation. Propranolol shall be administrated orally while monitoring the heart rate and blood pressure daily, starting from 20 to 40 mg two times per day and adjusting every 2 or 3 days (maximum dose: 320 mg/day for participants without ascites, 160 mg/day for participants with ascites) to achieve a resting heart rate of 55–60 beats/min while the systolic blood pressure maintains >90 mm Hg.5 The dose can always be adjusted according to the response on participants.
Outcomes and assessments
Primary outcome
In order to compare the effectiveness of laparoscopic group with the endoscopic group, the primary outcome of the study is set to be variceal rebleeding. Endpoints will be 1-year rebleeding rate and rebleeding time.
Secondary outcomes
Secondary outcomes include death, hepatocellular carcinoma, venous thrombosis, adverse events, quality of life and tolerability of treatment. Following endpoints will be applied, respectively: (1) overall survival, (2) the occurrence of hepatocellular carcinoma, (3) the occurrence of venous thrombosis, (4) the occurrence of adverse events, (5) quality of life (QOL) score and (6) KPS score. The occurrence of adverse events, QOL score and KPS score are treated as the reflection of safety and tolerability of treatment. Length stay and intrahospital mortality will be recorded also to this end and treated as outcome candidates. Also, serum markers will be introduced for monitor of change on liver functions and compared between two groups.
Assessments
Time points of involved assessments to be performed are outlined in table 1. For participants allocated to either the groups, the following assessments will be performed and corresponding data will be collected:
Table 1.
Assessments and time points
| Assessment | Time points | ||||||
| Preoperation | Postoperation | 12 weeks | 24 weeks | 36 weeks | 48 weeks | 60 weeks | |
| HVPG measurement | x | ||||||
| Laboratory tests | x | x | x | x | x | x | |
| Colour Doppler ultrasound | x | ||||||
| Liver stiffness | x | ||||||
| CT | x | ||||||
| Esophagogastroduodenoscopy | x | x | |||||
| ECG | x | ||||||
| QOL | x | x | x | x | x | ||
| KPS | x | x | x | x | x | ||
HVPG, hepatic venous pressure gradient; KPS, Karnofsky Performance Status; QOL, quality of life.
Demographic characteristics including gender, height, weight, date of birth and ethnic.
Transjugular measurement of HVPG.
Disease history with a clear record about the number of occurrence of gastro-oesophageal variceal bleeding and other complexes including ascites, spontaneous bacterial peritonitis, hepatic encephalopathy, electrolyte imbalance, portal venous thrombosis, hepatorenal syndrome, hepatopulmonary syndrome and so on.
Clinical diagnosis and aetiology for cirrhosis.
Laboratory test results including red blood cells, white cell count, haemoglobin, blood ammonia, platelet count, prothrombin time, activated partial thromboplastin time, international normalised ratio, total bilirubin, direct and indirect bilirubin, glutamine transferase, alanine aminotransferase, aspartate aminotransferase, albumin and serum creatinine.
Colour Doppler ultrasound results including the general condition of spleen and liver, spleen diameter, portal vein diameter, portal vein velocity, splenic vein velocity, splenic venous reflux, cardiac output, left ventricular ejection fraction and heart output.
Liver stiffness and spleen stiffness assessed by FibroTouch or FibroScan.
Abdominal CT scans.
Esophagogastroduodenoscopy results including the location, classification, diameter of varices and red signs.
ECG results.
Child-Pugh score and classes.
Model for end-stage liver disease (MELD) score.
QOL score.
KPS score.
Adjustment records of dosage of propranolol.
Adverse events and severe adverse events of any cause.
Length stay.
On occurrence of the first variceal rebleeding after the operative intervention, the following data will be additionally collected:
Cause of rebleeding.
Time of rebleeding since enrolment.
Treatment and outcome of the rebleeding.
On death of a participant, the following data will be additionally collected:
Time of death since enrolment.
Cause of death.
Sample size estimation
No study has yet compared the outcome between cirrhotic patients with gastro-oesophageal variceal bleeding receiving either laparoscopic therapy or endoscopic therapy. Also, because of the lack of study restricting HVPG baseline level and studies about laparoscopic therapy plus propranolol oral administration, the sample size is determined based on pooled data of variceal rebleeding rate of several studies including endoscopic therapy plus propranolol oral administration or laparoscopic therapy. The variceal rebleeding rate of endoscopic therapy plus propranolol oral administration is estimated by six randomised controlled trials (table 2).22–27 The variceal rebleeding rate of laparoscopic therapy is estimated by six retrospective studies (table 2).20 28–32 Pooled rates of variceal rebleeding for the endoscopic group and laparoscopic group are 44% and 6%, respectively. Considering a type I error rate (α) of 5% and a type II error rate (1−β) of 20% and a dropout rate of 10%, the calculated sample size for this trial is 40.
Table 2.
Variceal rebleeding rates in cirrhotic patients with portal hypertension bleeding treated by endoscopic therapy plus propranolol or laparoscopic therapy: a review of 12 studies
| Laparoscopic therapy | Endoscopic therapy plus propranolol | ||||
| First author, year | Patients (n) | Rebleeding, n (%) | First author, year | Patients (n) | Rebleeding, n (%) |
| Zheng, 201829 | 250 | 9 (3.6) | Lv, 201822 | 25 | 13 (52) |
| Bai, 201728 | 40 | 2 (5) | Holster, 201623 | 35 | 10 (28.6) |
| Bao, 201730 | 76 | 19 (25) | Luo, 201524 | 36 | 21 (58.3) |
| Cheng, 201420 | 204 | 7 (3.4) | Hung, 201225 | 47 | 22 (46.8) |
| Jiang, 200931 | 26 | 0 (0) | Sauer, 200226 | 40 | 12 (30) |
| Wang, 200832 | 22 | 0 (0%) | Rössle, 199727 | 62 | 29 (46.8) |
Safety
Laparoscopic therapy is accepted and performed in Asia-Pacific countries, while endoscopic therapy is generally implemented worldwide. Both surgical interventions showed low risks of severe adverse events. Possible risks related to interventions include adverse events related to HVPG measurement (including but not limited to arrhythmia, allergy, intraoperative haemorrhage and ecchymoma), endoscopic therapy (including but not limited to nausea, vomit, fervescence, esophagostenosis, oesophageal ulcer, dysphagia and early rebleeding), laparoscopic therapy (including but not limited to intraoperative haemorrhage, portal vein thrombosis, subphrenic infection, pancreatic fistula and early rebleeding) and side-effects of propranolol. Participants and whose relatives will be able to contact the study team when any severe adverse event or disease complication occurs. The participants who rebled will receive proper treatments according to the recommendations of the Baveno VI guideline3 as soon as feasible. Liver transplantation will be performed in an expedite manner in cases it is needed.
The following data will be recorded when an adverse event occurs:
The exact kind of adverse event.
The starting, ending and reporting time of the adverse event.
The severity of the adverse event.
Treatment and outcome of the adverse event.
Adverse events will be documented and reported to the investigators and ethics board of the involved centre in 48 hours. Severe adverse events will be documented and reported to the investigators and ethics board of the involved centre, principle investigator and supervision departments required by good clinical practice (GCP) immediately.
Data management
For imaging data, the electronic form images will be collected. Other raw data will be recorded in the written form case report form when collecting, and an electronic form copy will be saved afterwards. All electronic data will be kept by a member of the study team without direct clinical contact with any of the centres. All written form data will be stored in cabinets with lock permitting access for only investigators. All data will be kept for 25 years after publication and destroyed after then.
Statistical analyses
Statistical analyses will be performed in intention-to-treat cases. A case will be censored when the participant received liver transplantation. Subgroup analysis will be performed for Child-Pugh class A and class B patients. Continuous variables will be shown as mean (±SE) or median (range). No interim analyses will be conducted on the primary outcome. Multivariate COX regression model including age, sex, platelet, HVPG, aspartate aminotransferase, alanine aminotransferase, albumin, total bilirubin, MELD score and Child-Pugh score as confounders will be introduced for analyses of variceal rebleeding and survival, while applying Kaplan-Meier analysis with the log-rank test for intergroup comparison. Occurrence data including variceal rebleeding, overall survival, hepatocellular carcinoma and portal venous thrombosis will be compared using the χ2 test. The occurrence of all adverse events will also be collected, described and compared using the χ2 test overall and specifically. For data with repeated measurements including QOL score, KPS score and laboratory tests results, repeated measures Analysis of variance will be applied. Student’s t-test or Wilcoxon rank-sum test (for continuous data) and χ2 test or Fisher’s exact test (for discrete variable) will be applied for analyses of other unmentioned outcomes. All results will be presented with 95% CIs.
Discussion
To the best of our knowledge, this study is the first to compare the effectiveness and safety of laparoscopic therapy plus propranolol with endoscopic therapy plus propranolol, the first-line therapy recommended by international guidelines,3 5 under an HVPG-guided manner. The risk stratification performance of HVPG has been receiving more concentration and several attempts have been made on HVPG-guided therapy.33 34 By introducing HVPG restriction as an eligibility criterion, this study targets the population that faces a high risk of variceal rebleeding and death6 8–11 better, enabling exploration of better management for these patients as well as the extension of clinical performance of HVPG.
The first splenectomy and pericardial devascularisation were performed by Hassab in 196435 and modified by Qiu Fazu in 1981.36 Benefitted from the rapid development of laparoscopic equipment and techniques, surgical procedures for variceal bleeding are becoming decreasingly invasive and also with much lower occurrence of adverse events.20 29 37–39 Such laparoscopic therapy has been accepted as one of the most commonly used methods for variceal bleeding in Asia-Pacific countries. Pericardial devascularisation and splenectomy increase the blood flow of the hepatic artery while lower portal pressure and ameliorate leucopenia and thrombocytopenia. Thus, laparoscopic therapy is considered an effective method for variceal bleeding while benefitting liver function with satisfying performance on long-term survival and the general condition of patients. However, it is also reported to be correlated with high occurrence of portal venous thrombosis40 and exacerbation of portal hypertensive gastropathy.41 Therefore, a multicenter prospective study about laparoscopic therapy will provide valuable information for the clarification of its influence on the overall outcome.
Endoscopic therapy plus non-selective beta-blockers is recommended as the first-line therapy and widely served as control groups in many studies.22–27 42 On the contrary, studies about laparoscopic therapy are mainly single-arm20 32 43 or compared with variants and other surgeries.28–31 40 44 45 To the best of our knowledge, there haven’t been any trials about laparoscopic therapy using endoscopic therapy plus non-selective beta-blockers as controls. Thus, this study will also provide data with better comparability to other commonly used therapies.
Still, this study has several limitations. First, this is the first prospective study investigating the HVPG-guided therapeutic effect of laparoscopic therapy plus propranolol and endoscopic therapy plus propranolol. The lack of enough previous studies may lead to deviations in sample size estimation. Second, the major cause of cirrhosis of the target population of this study is the hepatitis B virus infection while it is more complex in American and European countries. Differences in aetiology may bring problems in applicability. Third, the period of follow-up in this study is set to be about 1 year, which may not be enough to thoroughly unfold the long-term effect of laparoscopic therapy, the occurrence of hepatocellular carcinoma, especially. Patients with HVPG higher than 10 mm Hg suffer from a sixfold-increased incidence of hepatocellular carcinoma.46 Due to the effect of lower portal pressure of laparoscopic therapy, we expect a decrease in the incidence of hepatocellular carcinoma. We may not be likely to observe this difference owing to the short designed follow-up duration. However, follow-up will still be continued for the participants after the end of this study, somehow making up for this drawback.
Ethics and dissemination
Ethical approval was obtained from the ethics committee of Shunde Hospital, Southern Medical University, the ethics committee of Xingtai People’s Hospital, and the ethics committee of The First Hospital of Lanzhou University. Any modifications in protocol will be done under the premise of adequate communication and approval. All interventions and assessments included in this trial will be in full compliance with GCP.
Before the allocation, all participant candidates will be fully informed about the purpose, process and possible consequences of the trial. Before any treatment, the participants will be informed about the interventions they will undergo and the interventions will not be applied before a written informed consent signed by the participants themselves is provided.
The result of this trial (CHESS1803) will be presented at national and international conferences and published in peer-reviewed journals.
Supplementary Material
Footnotes
RS, ZL and JW contributed equally.
Contributors: XQ, WW, CZ, ZL and XL conceived and designed the study. QL, WZ, XM, XS, JW, LL and CL are responsible of the data collecting and management. RS and YL drafted this manuscript. RQ, XZ and XQ critically revised the manuscript. RS, ZL and JW contributed equally. The final version of the manuscript was reviewed and approved by all authors.
Funding: This work is funded by the grants from National Natural Science Foundation of China (81600510); Guangdong Science Fund for Distinguished Young Scholars (2018B030306019); Guangzhou Industry-Academia-Research Collaborative Innovation Major Project (201704020015).
Disclaimer: The funders do not participate in the design, recruitment, intervention, data collection, data management and analysis of the study and the preparation and revision of this protocol.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749–61. 10.1016/S0140-6736(14)60121-5 [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823–32. 10.1056/NEJMra0901512 [DOI] [PubMed] [Google Scholar]
- 3.Baveno VI Faculty Expanding consensus in portal hypertension: report of the baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743–52. 10.1016/j.jhep.2015.05.022 [DOI] [PubMed] [Google Scholar]
- 4.Bosch J, Abraldes JG, Berzigotti A, et al. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol 2009;6:573–82. 10.1038/nrgastro.2009.149 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American association for the study of liver diseases. Hepatology 2017;65:310–35. 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 6.Li G-Q, Yang B, Liu J, et al. Hepatic venous pressure gradient is a useful predictor in guiding treatment on prevention of variceal rebleeding in cirrhosis. Int J Clin Exp Med 2015;8:19709–16. [PMC free article] [PubMed] [Google Scholar]
- 7.Qi X-S, Fan D-M. Hepatic venous pressure gradient measurement before tips for acute variceal bleeding. World J Gastroenterol 2014;20:7523–4. 10.3748/wjg.v20.i23.7523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Tsao G, Groszmann RJ, Fisher RL, et al. Portal pressure, presence of gastroesophageal varices and variceal bleeding. Hepatology 1985;5:419–24. 10.1002/hep.1840050313 [DOI] [PubMed] [Google Scholar]
- 9.Berzigotti A, Rossi V, Tiani C, et al. Prognostic value of a single HVPG measurement and Doppler-ultrasound evaluation in patients with cirrhosis and portal hypertension. J Gastroenterol 2011;46:687–95. 10.1007/s00535-010-0360-z [DOI] [PubMed] [Google Scholar]
- 10.Silva-Junior G, Baiges A, Turon F, et al. The prognostic value of hepatic venous pressure gradient in patients with cirrhosis is highly dependent on the accuracy of the technique. Hepatology 2015;62:1584–92. 10.1002/hep.28031 [DOI] [PubMed] [Google Scholar]
- 11.Merkel C, Bolognesi M, Bellon S, et al. Prognostic usefulness of hepatic vein catheterization in patients with cirrhosis and esophageal varices. Gastroenterology 1992;102:973–9. 10.1016/0016-5085(92)90185-2 [DOI] [PubMed] [Google Scholar]
- 12.Moitinho E, Escorsell A, Bandi JC, et al. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology 1999;117:626–31. 10.1016/S0016-5085(99)70455-5 [DOI] [PubMed] [Google Scholar]
- 13.Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, et al. Influence of portal hypertension and its early decompression by tips placement on the outcome of variceal bleeding. Hepatology 2004;40:793–801. 10.1002/hep.20386 [DOI] [PubMed] [Google Scholar]
- 14.García-Pagán JC, Caca K, Bureau C, et al. Early use of tips in patients with cirrhosis and variceal bleeding. N Engl J Med 2010;362:2370–9. 10.1056/NEJMoa0910102 [DOI] [PubMed] [Google Scholar]
- 15.Zhang M, Wang G, Zhao L, et al. Second prophylaxis of variceal bleeding in cirrhotic patients with a high HVPG. Scand J Gastroenterol 2016;51:1502–6. 10.1080/00365521.2016.1193218 [DOI] [PubMed] [Google Scholar]
- 16.Hassab MA. Gastroesophageal decongestion and splenectomy in the treatment of esophageal varices in bilharzial cirrhosis: further studies with a report on 355 operations. Surgery 1967;61:169–76. [PubMed] [Google Scholar]
- 17.Hassab MA, Younis MT, el-Kilany MS. Gastroesophageal decongestion and splenectomy in the treatment of esophageal varices secondary to bilharzial cirrhosis: anatomical and experimental studies. Surgery 1968;63:731–7. [PubMed] [Google Scholar]
- 18.Delaitre B, Maignien B. [Splenectomy by the laparoscopic approach. Report of a case]. Presse Med 1991;20:2263. [PubMed] [Google Scholar]
- 19.Wang Y, Zhan X, Zhu Y, et al. Laparoscopic splenectomy in portal hypertension: a single-surgeon 13-year experience. Surg Endosc 2010;24:1164–9. 10.1007/s00464-009-0744-4 [DOI] [PubMed] [Google Scholar]
- 20.Cheng Z, Li J-wei, Chen J, et al. Therapeutic effects of laparoscopic splenectomy and esophagogastric devascularization on liver cirrhosis and portal hypertension in 204 cases. J Laparoendosc Adv Surg Tech A 2014;24:612–6. 10.1089/lap.2014.0036 [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Lan X, Shao C, et al. Clinical features of idiopathic portal hypertension in China: a retrospective study of 338 patients and literature review. J Gastroenterol Hepatol 2019;34:1417–23. 10.1111/jgh.14552 [DOI] [PubMed] [Google Scholar]
- 22.Lv Y, Qi X, He C, et al. Covered tips versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut 2018;67:2156–68. 10.1136/gutjnl-2017-314634 [DOI] [PubMed] [Google Scholar]
- 23.Holster IL, Tjwa ETTL, Moelker A, et al. Covered transjugular intrahepatic portosystemic shunt versus endoscopic therapy + β-blocker for prevention of variceal rebleeding. Hepatology 2016;63:581–9. 10.1002/hep.28318 [DOI] [PubMed] [Google Scholar]
- 24.Luo X, Wang Z, Tsauo J, et al. Advanced cirrhosis combined with portal vein thrombosis: a randomized trial of tips versus endoscopic band ligation plus propranolol for the prevention of recurrent esophageal variceal bleeding. Radiology 2015;276:286–93. 10.1148/radiol.15141252 [DOI] [PubMed] [Google Scholar]
- 25.Hung H-H, Chang C-J, Hou M-C, et al. Efficacy of non-selective β-blockers as adjunct to endoscopic prophylactic treatment for gastric variceal bleeding: a randomized controlled trial. J Hepatol 2012;56:1025–32. 10.1016/j.jhep.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 26.Sauer P, Hansmann J, Richter GM, et al. Endoscopic variceal ligation plus propranolol vs. transjugular intrahepatic portosystemic stent shunt: a long-term randomized trial. Endoscopy 2002;34:690–7. 10.1055/s-2002-33565 [DOI] [PubMed] [Google Scholar]
- 27.Rössle M, Deibert P, Haag K, et al. Randomised trial of transjugular-intrahepatic-portosystemic shunt versus endoscopy plus propranolol for prevention of variceal rebleeding. Lancet 1997;349:1043–9. 10.1016/S0140-6736(96)08189-5 [DOI] [PubMed] [Google Scholar]
- 28.Bai D-S, Chen P, Jin S-J, et al. Vagus nerve-preserving versus conventional laparoscopic splenectomy and azygoportal disconnection. Surg Endosc 2018;32:2696–703. 10.1007/s00464-017-5965-3 [DOI] [PubMed] [Google Scholar]
- 29.Zheng S, Sun P, Liu X, et al. Efficacy and safety of laparoscopic splenectomy and esophagogastric devascularization for portal hypertension: a single-center experience. Medicine 2018;97:e13703. 10.1097/MD.0000000000013703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao H, He Q, Dai N, et al. Retrospective study to compare selective decongestive devascularization and gastrosplenic shunt versus splenectomy with pericardial devascularization for the treatment of patients with esophagogastric varices due to cirrhotic portal hypertension. Med Sci Monit 2017;23:2788–95. 10.12659/MSM.904660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X-Z, Zhao S-Y, Luo H, et al. Laparoscopic and open splenectomy and azygoportal disconnection for portal hypertension. World J Gastroenterol 2009;15:3421. 10.3748/wjg.15.3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YD, Ye H, Ye ZY, et al. Laparoscopic splenectomy and azygoportal disconnection for bleeding varices with hypersplenism. J Laparoendosc Adv Surg Tech A 2008;18:37–41. 10.1089/lap.2007.0028 [DOI] [PubMed] [Google Scholar]
- 33.Villanueva C, Graupera I, Aracil C, et al. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology 2017;65:1693–707. 10.1002/hep.29056 [DOI] [PubMed] [Google Scholar]
- 34.Villanueva C, Aracil C, Colomo A, et al. Clinical trial: a randomized controlled study on prevention of variceal rebleeding comparing nadolol + ligation vs. hepatic venous pressure gradient-guided pharmacological therapy. Aliment Pharmacol Ther 2009;29:397–408. 10.1111/j.1365-2036.2008.03880.x [DOI] [PubMed] [Google Scholar]
- 35.Hassab MA. Gastroesophageal Decongestion and splenectomy. A method of prevention and treatment of bleeding from esophageal varices associated with bilharzial hepatic fibrosis: preliminary report. J Int Coll Surg 1964;41:232–48. [PubMed] [Google Scholar]
- 36.Qiu FZ. [Evaluation of the pericardial devascularization in portal hypertension]. Zhonghua Wai Ke Za Zhi 1983;21:275–7. [PubMed] [Google Scholar]
- 37.Zhan X-L, Ji Y, Wang Y-D. Laparoscopic splenectomy for hypersplenism secondary to liver cirrhosis and portal hypertension. World J Gastroenterol 2014;20:5794–800. 10.3748/wjg.v20.i19.5794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Guo S, Wang L, et al. Laparoscopic splenectomy and esophagogastric devascularization for liver cirrhosis and portal hypertension is a safe, effective, and minimally invasive operation. J Laparoendosc Adv Surg Tech A 2016;26:524–30. 10.1089/lap.2016.0032 [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Li Y, Ma J, et al. A modified Hassab's operation for portal hypertension: experience with 562 cases. J Surg Res 2013;185:463–8. 10.1016/j.jss.2013.05.046 [DOI] [PubMed] [Google Scholar]
- 40.Sun L, Zhou H, Gu L, et al. Effects of surgical procedures on the occurrence and development of postoperative portal vein thrombosis in patients with cirrhosis complicated by portal hypertension. Int J Surg 2015;16:31–5. 10.1016/j.ijsu.2015.02.005 [DOI] [PubMed] [Google Scholar]
- 41.Huang Y, Wang W, Wang J, et al. Surgical treatment of portal hypertension: 45 year experience. Zhonghua Wai Ke Za Zhi 2000;38:85–8. [PubMed] [Google Scholar]
- 42.Sarin SK, Wadhawan M, Agarwal SR, et al. Endoscopic variceal ligation plus propranolol versus endoscopic variceal ligation alone in primary prophylaxis of variceal bleeding. Am J Gastroenterol 2005;100:797–804. 10.1111/j.1572-0241.2005.40468.x [DOI] [PubMed] [Google Scholar]
- 43.Wu S-D, Fan Y, Kong J, et al. Transumbilical single-incision laparoscopic splenectomy plus pericardial devascularization using conventional instruments: initial experience of 5 cases. J Laparoendosc Adv Surg Tech A 2013;23:150–3. 10.1089/lap.2012.0337 [DOI] [PubMed] [Google Scholar]
- 44.Ando K, Kurokawa T, Nagata H, et al. Laparoscopic surgery in the management of hypersplenism and esophagogastric varices: our initial experiences. Surg Innov 2012;19:421–7. 10.1177/1553350611432724 [DOI] [PubMed] [Google Scholar]
- 45.Chen H, Yang F, Li T-T, et al. Comparison of efficacy of laparoscopic and open splenectomy combined with selective and nonselective pericardial devascularization in portal hypertension patients. Surg Laparosc Endosc Percutan Tech 2018;28:401–3. 10.1097/SLE.0000000000000581 [DOI] [PubMed] [Google Scholar]
- 46.Ripoll C, Groszmann RJ, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol 2009;50:923–8. 10.1016/j.jhep.2009.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


