Abstract
The genus Eustrongylides includes nematodes that infect fish species and fish-eating birds inhabiting freshwater ecosystems. Nematodes belonging to the genus Eustrongylides are potentially pathogenic for humans; infection occurs after the consumption of raw or undercooked fish. In the two-year period 2019–2020, a total of 292 fish belonging to eight species were examined for the occurrence of Eustrongylides spp. from Lake San Michele, a small subalpine lake in northwest Italy. The prevalence of infestation was 18.3% in Lepomis gibbosus, 16.7% in Micropterus salmoides, and 10% in Perca fluviatilis. The other five fish species (Ameiurus melas, Ictalurus punctatus, Squalius cephalus, Carassius carassius, and Scardinius erythrophthalmus) were all negative for parasite presence. There were no significant differences in prevalence between the three fish species (Fisher’s exact test; p = 0.744). The mean intensity of infestation ranged from 1 (M. salmoides and P. fluviatilis) to 1.15 (L. gibbosus), and the mean abundance ranged from 0.1 (P. fluviatilis) to 0.28 (L. gibbosus). There were significant differences in the infestation site between the four muscle quadrants (anterior ventral, anterior dorsal, posterior ventral, and posterior dorsal) and the visceral cavity (Kruskal–Wallis test; p = 0.0008). The study findings advance our knowledge about the distribution and host range of this parasite in Italy.
Keywords: public health, zoonoses, epidemiology, fishbone zoonotic parasite
1. Introduction
The genus Eustrongylides Jägerskiöld, 1909 (Family Dioctophymatidae) includes nematodes that infect numerous fish species and fish-eating birds inhabiting freshwater ecosystems. Eustrongylides species have a heteroxenous life cycle. Among fish-eating birds, the great cormorant (Phalacrocorax carbo Linnaeus, 1758) has been frequently reported to be a definitive host [1,2]. Aquatic oligochaetes (i.e., Lumbriculus variegatus, Tubifex tubifex, and Limnodrilus spp.) are the first intermediate hosts, while fish, amphibians, and/or reptiles are the second intermediate hosts. Furthermore, predatory fish, amphibians, and reptiles may serve in the life cycle as paratenic hosts [3,4,5]. Though the number of recorded cases is low, humans can enter the cycle as accidental hosts [6].
The geographical distribution of the genus Eustrongylides is vast (Asia, Africa, and North and South America) [4,7,8,9,10,11,12,13,14,15] probably due to live fish translocation and broad migratory routes of definitive hosts [16,17]. In Italy, Eustrongylides nematodes were first reported in Perca fluviatilis, Micropterus salmoides, and Atherina boyeri from Lake Trasimeno [15,18,19]. Furthermore, Mazzone et al. [20] first described E. excisus adults by morphological and molecular analyses.
Species belonging to the genus Eustrongylides are pathogenic for birds, fish, and humans [18,21,22]. In birds, the parasite is usually isolated in the wall of the proventriculus, ventriculus, and intestine, where it forms tunnels and provokes granulomatous inflammatory reaction [23]. In fish, infection may result in massive disease and an evident inflammatory response [18,24]. Larvae are found encapsulated in the muscles or visceral organs or free in the body cavity of fish hosts [25]. Death is relatively rare and generally from secondary infections consequent to a weakened immune system [5]. In humans, eustrongylidosis is a parasitic disease contractable after the consumption of raw/undercooked fish infected by the larval stages of the parasite [26]. Symptoms are generally gastrointestinal (e.g., gastritis or enteritis) [6,27,28] and may progress to intestinal perforation [21]. Eberhard and Ruiz-Tiben [29] described a cutaneous form of Eustrongylides in two people from South Sudan. The only effective treatment is surgical removal of larvae [21].
Strict application of inspection regulations of fish intended for human consumption and proper food preparation are the best ways to avoid foodborne parasitic zoonoses. Though no cases of human eustrongylidosis have been recorded in Italy, these nematodes merit attention because of their wide distribution and zoonotic potential. In response to reports from sports fishermen about the presence of “red worms” in fish caught from Lake San Michele (Piedmont, northwest Italy) during 2019 and 2020, a parasitological survey of several fish species was carried out. The main aim of this study was to report the presence of Eustrongylides nematodes in a subalpine area and to improve our knowledge about the distribution and host range of this parasite in Italy.
2. Materials and Methods
2.1. Study Area
Lake San Michele (241 m a.s.l.) is one out of a complex of five shallow lakes (Cinque Laghi di Ivrea) in Piedmont (Province of Turin, northwest Italy) (Figure 1).
Figure 1.
Sampling site: (a) Italy with insert, (b) Piedmont (outlined in red), and the location of (c) Lake San Michele (outlined in red; 45°28′39.1″ N 7°53′18.2″ E).
The lake has a surface area of 0.07 km2 and a maximum depth of 19 m [30]. The lake’s biodiversity of fish species and proximity to inhabited centers make it an attractive location for recreational fishing.
2.2. Fish Collection and Anatomopathological and Parasitological Examination
This study was carried out from September 2019 to February 2020. Fish were sampled using multimesh benthic and pelagic gillnets (mesh size, 10 to 55 mm) placed according to the lake’s bathymetry profile, following the standardized method for fish sampling in European lakes [31]. Permission for fish sampling was obtained from the Città Metropolitana di Torino (authorization no. 45-28591/18), as required by local laws. The fish species were black bullhead (Ameiurus melas), channel catfish (Ictalurus punctatus), European chub (Squalius cephalus), crucian carp (Carassius carassius), pumpkinseed (Lepomis gibbosus), largemouth bass (Micropterus salmoides), European perch (Perca fluviatilis), and rudd (Scardinius erythrophthalmus). A sample from each species was retained for parasitological analysis. Fish were transported in cold boxes kept at 4 °C to the Fish Diseases Laboratory of the Veterinary Medical Research Institute for Piemonte, Liguria and Valle d’Aosta (Turin, Italy) for analysis. Each fish was weighed (g), measured for total length (cm), and subjected to anatomopathological examination. Skeletal muscle and internal organs were examined for zoonotic helminths. Internal organs were removed from the body cavity and placed in Petri dishes containing saline solution and inspected. Skeletal musculature was fileted in 2–3 mm slices and observed by transillumination (UVP White Light Transilluminators, TW-43, Analytik Jena, Jena, Germany) to detect encysted nematodes. Isolated nematodes were excysted with a fine needle, rinsed in deionized water, and fixed in 96% molecular grade ethanol for molecular analysis. Isolated nematodes were classified at the genus level by morphological examination according to keys provided by Moravec [24]. Nematode location in the skeletal muscle was recorded: anterior ventral (AV), which is the belly flap; anterior dorsal (AD); posterior ventral (PV); and posterior dorsal (PD).
2.3. Molecular Analysis
DNA extraction was performed on the entire larvae using a commercial kit (Extractme Genomic DNA kit, Blirt S.A., Gdańsk, Poland). Total DNA was extracted following the manufacturer’s instructions. According to Gustinelli et al. [32], species identification was performed by sequencing the internal transcribed spacer (ITS) that is the conserved regions in samples belonging to the same species. Both strands were sequenced, and they were aligned using Seqman Software (Lasergene). The ITS region was sequenced and compared with similar sequences on the GenBank database using BLASTn. An identity of 98% was considered a cutoff to assign the species.
2.4. Statistical Analysis
The Shapiro–Wilk test was used to verify normality of data distribution. The prevalence of infestation was calculated for each species. Differences in the prevalence of infestation between the fish species were tested using the Fisher’s exact test. Mean intensity and mean abundance of infestation were calculated according to Bush [33].
Whereas the Shapiro–Wilk test showed that the data deviated significantly from a normal distribution, the nonparametric Kruskal–Wallis test was used to determine differences in the infestation sites in the musculature (AD, AV, PD, and PV) and the visceral cavity (VC). Dunn’s post hoc test was used for multiple comparisons. Significance was set at 0.05 %. Statistical analyses were performed using open source data analysis software RStudio® version 1.1.463 (RStudio, Inc., Boston, MA, USA).
3. Results
A total of 292 fish were examined (Table 1) for the presence of nematodes. All isolated nematodes were morphologically attributable with the species to the genus Eustrongylides (Figure 2). Two of them were subjected to molecular analysis, and both showed an identity > 99% with the genus Eustrongylides. It was no possible to assign the specimens to the species level because the samples unveiled an identity > 99% with ITS sequences of Eustrongylides ignotus, Eustrongylides excisus, and Eustrongylides sp. deposited in Genbank.
Table 1.
Total length (cm) and total weight (g) of fish (N) from Lake San Michele. Plus-minus values are the mean ± standard deviation (SD).
| Fish Species | Captured Fish No. |
Total Length (cm) |
Total Weight (g) |
|---|---|---|---|
| Ameiurus melas | 30 | 20.1 ± 2.1 | 102.3 ± 35.3 |
| Ictalurus punctatus | 30 | 26.8 ± 15.1 | 58.2 ± 28.1 |
| Squalius cephalus | 30 | 37.7 ± 5.8 | 876.9 ± 352.1 |
| Carassius carassius | 30 | 34.8 ± 6.2 | 985 ± 623.7 |
| Lepomis gibbosus | 82 | 10.9 ± 2.4 | 32.6 ± 18.3 |
| Micropterus salmoides | 30 | 15.6 ± 3.7 | 62.2 ± 56.4 |
| Scardinius erythrophthalmus | 30 | 21.3 ± 2.1 | 165 ± 4.5 |
| Perca fluviatilis | 30 | 14.3 ± 2.1 | 42.4 ± 41.4 |
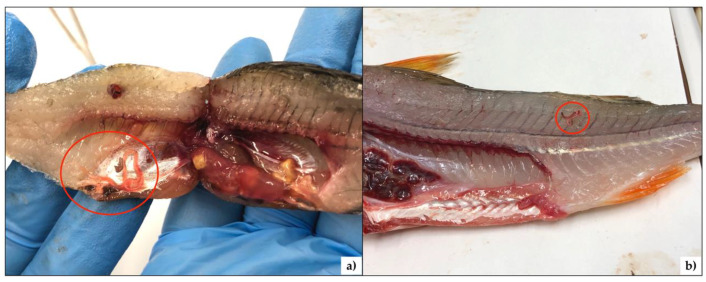
Figure 2.
Larvae of Eustrongylides spp. (red circles) in (a) the visceral cavity of Lepomis gibbosus and (b) in the posterior dorsal portion of musculature in Perca fluviatilis.
No visible lesions in external and internal organs were observed. Larvae were found only in the following species: Lepomis gibbosus, Micropterus salmoides, and Perca fluviatilis. All other species tested negative for nematode larvae. The prevalence was 18.3% (15/82) (95% confidence interval (CI) 11–28) in L. gibbosus; 16.7% (5/30) (95% CI 6–35) in M. salmoides; and 10% (3/30) (95% CI 2–27) in P. fluviatilis. No significant differences in prevalence between the three fish species were found (Fisher’s exact test; p = 0.744). Table 2 presents the number, location (AV, AD, PV, PD, and VC) of nematode larvae, mean intensity, and mean abundance of infestation. There was a significant difference in larvae localization between muscle quadrants and body cavity (AD = 2; PD = 2; AV = 3; PV = 5; and VC = 19) (Kruskal–Wallis test; p = 0.0008), with significant differences between AD and VC (Dunn test; p = 0.003), PD and VC (Dunn test; p = 0.003), AV and VC (Dunn test; p = 0.01), and PV and VC (Dunn test; p = 0.03).
Table 2.
Mean intensity and mean abundance of infestation and number and location of larvae in the musculature (anterior ventral (AV), anterior dorsal (AD), posterior ventral (PV), and posterior dorsal (PD)) and in the visceral cavity (VC).
| Lepomis Gibbosus | Micropterus Salmoides | Perca Fluviatilis | |
|---|---|---|---|
| Mean intensity | 1.15 | 1 | 1 |
| Mean abundance | 0.28 | 0.16 | 0.1 |
| Number of larvae | 23 | 5 | 3 |
| Location of larvae | 2 PD; 5 PV; 16 VC | 3 AV; 2 VC | 2 AD; 1 VC |
4. Discussion
Humans are at risk of exposure to parasitic foodborne zoonoses after the consumption of raw or improperly processed fish. The increasing popularity of raw fish consumption has led to a rise in the incidence of parasitic infections [34]. For example, P. fluviatilis is an invaluable resource for local fishers. It is largely used in preparing raw and cooked dishes by local people and restaurants. Thus, the presence of Eustrongylides nematodes in fish intended for human consumption is potentially harmful for human health.
Eustrongylides spp. was previously isolated in three fish species (Perca fluviatilis, Atherina boyeri, and Micropterus salmoides) from Central Italy [15,18,19]. Our study is the first to report Eustrongylides spp. in fish from a subalpine lake (northwest Italy). Also, we found that Lepomis gibbosus is a new host for Eustrongylides spp. in Italy.
Our morphological and molecular results confirm the specimens as species of the genus Eustronglyides. The presence of numerous sequences deposited in GenBank as Eustrongylides sp. could be misleading for further genetic studies, i.e., the construction of phylogenetic tree. This is due to the same similarity value of the ITS sequences between our samples and Eustrongylides sp., E. excisus and E. ignotus present in GenBank, making difficult the assignment of a nematode to one species rather than another. For this reason, further studies could be focused on other conserved regions.
The prevalence we recorded for P. fluviatilis was higher than that reported by fish from central Italy [15,18] (6.84% and 6%, respectively). Also, the prevalence in our M. salmoides was higher than the 1.89% reported by Branciari et al. [15]. Other published data on Eustrongylides infection in P. fluviatilis describes higher prevalence rates than our findings: Goncharov et al. [5] reported a prevalence from 63.6% to 100% (intensity of infestation: 1–13) for different sampling sites in the Ukraine. Sattari [35] reported a prevalence of 33% (mean intensity of infestation, 1.5) in P. fluviatilis from Iran. Coyner et al. [36] described the prevalence of E. ignotus in 39 fish species from Florida. Moreover, our findings for nematode infestation sites contrast with those reported by other authors [15,18] who found larvae only in skeletal muscle but not in the visceral cavity.
Eustrongylides eggs are released by the final hosts throughout the year and may hatch at any time [25]. The eggs remain viable and infective for up to 2 years, and the larvae can survive in the intermediate host for more than 1 year [25]. Piscivorous birds, being a definitive host for numerous parasite species, contribute to their spread [16,37]. The cormorant population has increased in number and area in recent decades in northern Italy [38], where it is concentrated around large lakes and other pre-alpine water basins [38]. Several bird species are well-documented as examples of shifts in geographic distribution related to climate change [39,40]. For parasitic zoonoses, climate change has the potential to shift the boundaries of spatial distribution, host–parasite assemblages, life-cycle phenology, and associations within ecosystems [41]. The microclimate surrounding Lake San Michele and the possibility to nest in protected places such as reed beds make these lentic water bodies favorable for avifauna and for many fish species alike [30].
Lake San Michele has a high concentration of nutrients, typical of eutrophic basins [30]. This may facilitate the growth of oligochaete populations, the intermediate hosts of Eustrongylides [36]. Eustrongylides larvae have been reported in 14 orders of fish worldwide; the host range is restricted neither to a specific taxonomic group of fish nor to fish with specific feeding habits [42]. A look inside the feeding habits of fish can help us better understand the presence of Eustrongylides spp. Largemouth bass, pumpkinseed, and European perch generally inhabit the littoral zone of lakes [43,44,45]. The dimensional and structural attributes of aquatic ecosystems are important determinants of community structure and feeding ecology of fish populations [46]. For example, European perch juveniles feed on zooplankton, bottom invertebrate fauna (i.e., oligochaetes), and other perch fry, while adults feed on both macroinvertebrates and fish [47]. Young largemouth bass also prey mainly on zooplankton, shifting to macrobenthic invertebrates as they grow and then to fish very early [48].
Piscivorous fish may contract multiple infections by consuming infected prey and then host larvae that can survive and remain infective to piscivorous birds [36]. Pumpkinseed feed on zooplankton and benthic invertebrates. Shifts in its diet include replacement of planktonic prey by greater consumption of macroinvertebrates [49]. Frequency of fish consumption by pumpkinseed is reportedly low; thus, it probably acquires Eustrongylides by feeding on infected oligochaetes [42]. Each component in the host–parasite relationship is fundamental and determines the dynamics and the outcome of disease transmission and control [50]. Hypothetically, disruption of a parasite’s life cycle may be an effective solution to prevent its spread, but this goal is basically impossible to achieve in natural environments. Further studies are needed to better clarify the link between water quality (i.e., trophic status) and the occurrence of the main oligochaete species involved in the life cycle of nematodes belonging to the Eustrongylides genus. Parasite life-cycle stages are distributed across aquatic ecosystem components, and this distribution can vary with place and time. A holistic approach to the management of fishborne parasitic zoonoses entails a large amount of high-quality data and collaboration between the scientific community and public health authorities.
5. Conclusions
Our study reports the prevalence of Eustrongylides spp. in a subalpine lake in Italy and improves our knowledge about the distribution and host range of this zoonotic parasite in freshwater environments. P. fluviatilis and M. salmoides are two fish species of commercial interest and importance for recreational fishing in European lake systems, Italy included [51]. Lake San Michele is popular among sports fishermen but not professional fishermen because of its small size. The presence of these nematodes should be investigated in larger subalpine lakes (i.e., Garda, Como, Maggiore, and Iseo) where commercial fishing is present. While no human infections have been reported in Italy so far, the prevalence of this parasite is possibly underestimated and understudied. Knowledge of numerous aspects of the biology, epidemiology, and control of Eustrongylides species is scarce. It is hoped that greater awareness of the zoonotic risk due to changes in dietary habits, globalization through fish product trade, and climatic change will help to inform effective control programs also for fishborne zoonotic parasites.
Acknowledgments
The authors express their special thanks to Pietro Volta (CNR-ISE) and Alessandra Pucci (Città Metropolitana di Torino) for fish sampling and to Giovanni Pistis for GIS analysis.
Author Contributions
Conceptualization, M.P.; data curation, V.M., P.P. and M.C.B.; funding acquisition, M.P.; investigation, V.M., M.V.R., P.P., D.M., S.C., E.P., M.C.B., A.D. and P.L.A.; methodology, V.M., M.V.R. and D.M.; supervision, M.P.; writing—original draft, V.M.; writing—review and editing, M.V.R., P.P. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, project IZS PLV 18/14 RC.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.El-Dakhly K.M., El-Nahass E., Uni S., Tuji H., Sakai H., Yanai T. Levels of infection of gastric nematodes in a flock of great cormorants (Phalacrocorax carbo) from Lake Biwa, Japan. J. Helminthol. 2012;86:54–63. doi: 10.1017/S0022149X11000046. [DOI] [PubMed] [Google Scholar]
- 2.Zhokhov A.E., Pugacheva M.N. First Record of Eustrongylides excisus (Dorylaimea: Dioctophymatidae) in Fish of the Rybinsk Reservoir. Russ. J. Biol. Invasions. 2019;10:325–327. doi: 10.1134/S207511171904012X. [DOI] [Google Scholar]
- 3.Measures L.N. Revision of the genus Eustrongylides Jagerskiold, 1909 (Nematoda: Dioctophymatoidea) of piscivorous birds. Can. J. Zool. 1988;66:885–895. doi: 10.1139/z88-131. [DOI] [Google Scholar]
- 4.Novakov N., Bjelic-Cabrilo O., Circovic M., Jubojevik D., Lujic J. Eustrongylidosis of European Catfish (Siluris glaris) Bulg. J. Agric. Sci. 2013;1:72–76. [Google Scholar]
- 5.Goncharov S.L., Soroka N.M., Pashkevich I.Y., Dubovy A.I., Bondar A.O. Infection of predatory fish with larvae of Eustrongylides excisus (Nematoda, Dioctophymatidae) in the Delta of the Dnipro River and the Dnipro-Buh Estuary in Southern Ukraine. Vestn. Zool. 2018;52:137–144. doi: 10.2478/vzoo-2018-0015. [DOI] [Google Scholar]
- 6.Guerin P.F., Marapendi S., MC Grail L. Intestinal perforation caused by larval Eustrongylides. Morb. Mort. Week Rep. 1982;31:383–389. [PubMed] [Google Scholar]
- 7.Paperna I. Hosts distribution and pathology of infections with larvae of Eustrongylides (Dioctophymidae, Nematoda) in fish from East African lakes. J. Fish. Biol. 1974;6:67–76. doi: 10.1111/j.1095-8649.1974.tb04523.x. [DOI] [Google Scholar]
- 8.Lichtenfels J.R., Stroup C.F. Eustrongylides ssp. (Nematoda: Dioctophymatoidea): First Report of an Invertebrate Host (Oligochaeta: Tubificidae) in North America. Proc. Helminthol. Soc. Wash. 1985;52:320–323. [Google Scholar]
- 9.Pazouki J., Masoumian M., Yahyazadeh M., Abbasi J. Metazoan Parasites from Freshwater Fishes of Northwest Iran. J. Agric. Sci. Technol. 2007;9:25–33. [Google Scholar]
- 10.Soylu E. Metazoan Parasites of Perch Perca fluviatilis L. from Lake Sığırcı, Ipsala, Turkey. Pak. J. Zool. 2013;45:47–52. [Google Scholar]
- 11.Yesipova N.B. The Spread of Parasitic Nematodes in Fish Eustrongylides Excisus Zaporozhye (Dnipro) Reservoir. [(accessed on 9 May 2020)];2013 :86–88. Available online: https://www.degruyter.com/viewarticle/j$002fvzoo.2018.52.issue-2$002fvzoo-2018-0015$002fvzoo-2018-0015.xml. (In Russian)
- 12.Fedorov N.M., Firsov N.F., Soloviev N.A. Veterinary and sanitary examination in river perch with Eustrongylidosis. Vet. Pathol. 2014;3:68–73. [Google Scholar]
- 13.Melo F.T., Melo C.S., Nascimento L.C. Morphological characterization of Eustrongylides ssp. Larvae (Nematoda, Dioctophymatoidea) parasite of Rhinella marina (Amphibia: Bufonidae) from Eastern Amazonia. Braz. J. Vet. Parasitol. Jaboticabal. 2015:7–12. doi: 10.1590/S1984-29612016024. [DOI] [PubMed] [Google Scholar]
- 14.Noei M.R., Ibrahimov S., Sattari M. Parasitic worms of the Persian sturgeon, Acipenser persicus Borodin, 1897 from the southwestern shores of the Caspian Sea. Iran. J. Ichthyol. 2015;2:287–295. doi: 10.22034/iji.v2i4. [DOI] [Google Scholar]
- 15.Branciari R., Ranucci D., Miraglia D., Valiani A., Veronesi F., Urbani E., Lo Vaglio G., Pascucci L., Franceschini R. Occurrence of parasites of the genus Eustrongylides spp. (Nematoda: Dioctophymatidae) in fish caught in Trasimeno lake, Italy. Ital. J. Food Saf. 2016;5:6130. doi: 10.4081/ijfs.2016.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Švažas S., Chukalova N., Grishanov G., Pūtys Ž., Sruoga A., Butkauskas D., Raudonikis L., Prakas P. The role of great cormorant (Phalacrocorax carbo sinensis) for fish stock and dispersal of helminthes parasites in the Curonian lagoon area. Vet. Med. Zoot. 2011;55:79–85. [Google Scholar]
- 17.Menconi V., Pastorino P., Cavazza G., Santi M., Mugetti D., Zuccaro G., Prearo M. The role of live fish trade in the translocation of parasites: The case of Cystidicola farionis in farmed rainbow trout (Oncorhynchus mykiss) Aquac. Int. 2019;27:1667–1671. doi: 10.1007/s10499-019-00422-1. [DOI] [Google Scholar]
- 18.Dezfuli B.S., Manera M., Lorenzoni M., Pironi F., Shinn A.P., Giari L. Histopathology and the inflammatory response of European perch, Perca fluviatilis muscle infected with Eustrongylides sp. (Nematoda) Parasit. Vectors. 2015;8:227. doi: 10.1186/s13071-015-0838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agnetti F., Sensidoni L., Di Raimo Marrocchi E., Vaglio G., Sgariglia E., Valentini A., Ghittino C., Caffara M., Gustinelli A., Fioravanti M.L. Presence of Eustrongylides sp. (Nematoda: Dioctophymatidae) in fish species of the Trasimeno lake (Umbria): Preliminary data; Proceedings of the XVII Congresso Nazionale, S.I.Di.L.V. Società Italiana di Diagnostica di Laboratorio Veterinaria: Pacengo di Lazise (VR); Lazise, Italy. 28–30 September 2016; pp. 253–254. [Google Scholar]
- 20.Mazzone A., Caffara M., Gustinelli A., Agnetti F., Sgariglia E., Lo Vaglio G., Quaglio F., Fioravanti M.L. Morphological and molecular characterization of larval and adult stages of Eustrongylides excisus (Nematoda: Dioctophymatoidea) with histopathological observations. J. Parasitol. 2019;105:882–889. doi: 10.1645/19-44. [DOI] [PubMed] [Google Scholar]
- 21.Eberhard M.L., Hurwitz H., Sun A.M., Coletta D. Intestinal perforation caused by larval Eustrongylides (Nematoda: Dioctophymatoidae) in New Jersey. Am. J. Trop. Med. Hyg. 1989;40:648–650. doi: 10.4269/ajtmh.1989.40.648. [DOI] [PubMed] [Google Scholar]
- 22.Caudill G., Wolf D., Caudill D., Brown J., Shearn-Bochsler V.A. Juvenile wading-bird mortality event in urban Jacksonville, Florida, associated with the parasite Eustrongylides. Florida Field Nat. 2014;42:108–113. [Google Scholar]
- 23.Cole R.A. Eustrongylidosis. In: Friend M., Franson J.C., editors. Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds. U.S. Department of the Interior Fish and Wildlife Service; Washington, DC, USA: 1999. pp. 223–228. [Google Scholar]
- 24.Moravec F. Nematodes parasitic in fishes as larvae. In: Moravec F., editor. Parasitic Nematodes of Freshwater Fishes of Europe. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1994. pp. 374–421. [Google Scholar]
- 25.Bjeli-Cabrilo O., Novakov N., Irkovi M., Kosti D., Popovi E., Aleksi N., Luji J. The first determination of Eustrongylides excisus Jägerskiöld, 1909: Larvae (Nematoda: Dioctophymatidae) in the pike-perch Sander lucioperca in Vojvodina (Serbia) Helminthologia. 2013;50:291–294. doi: 10.2478/s11687-013-0143-1. [DOI] [Google Scholar]
- 26.Wittner M., Turner J.W., Jacquette G., Ash L.R., Salgo M.P., Tanowitz H.B. Eustrongylidiasis: A parasitic infection acquired by eating sushi. N. Engl. J. Med. 1989;320:1124–1126. doi: 10.1056/NEJM198904273201706. [DOI] [PubMed] [Google Scholar]
- 27.Gunby P. One worm in the minnow equals too many in the gut. JAMA. 1982;248:163. doi: 10.1001/jama.1982.03330020011004. [DOI] [PubMed] [Google Scholar]
- 28.Narr L.L., O’Donnell J.G., Libster B., Alessi P., Abraham D. Eustrongylidiasis a parasitic infection acquired by eating live minnow. J. Am. Osteopath. Assoc. 1996;96:400–402. doi: 10.7556/jaoa.1996.96.7.400. [DOI] [PubMed] [Google Scholar]
- 29.Eberhard M.L., Ruiz-Tiben E. Cutaneous emergence of Eustrongylides in two persons from South Sudan. Am. J. Trop. Med. Hyg. 2014;90:315–317. doi: 10.4269/ajtmh.13-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minuzzo C., Tisi A., Caramiello R., Siniscalco C. Flora acquatica e palustre della zona dei “Cinque Laghi” di Ivrea. Riv. Piem. St. Nat. 2005;26:41–71. [Google Scholar]
- 31.CEN . Water Quality-Sampling of Fish with Multi-Mesh Gillnets (EN 14757: 2005) CEN; Brussels, Belgium: 2005. [Google Scholar]
- 32.Gustinelli A., Caffara M., Florio D., Elick O.O., Euty M.W., Fioravanti M.L. First description of the adult stage of Clinostomum cutaneum Paperna, 1964 (Digenea: Clinostomidae) from grey herons Ardea cinerea L. and a redescription of the metacercaria from the Nile tilapia Oreochromis niloticus niloticus (L.) in Kenya. Syst. Parasitol. 2010;76:39–51. doi: 10.1007/s11230-010-9231-5. [DOI] [PubMed] [Google Scholar]
- 33.Bush A.O., Lafferty K.D., Lotz M., Shostak A.W. Parasitology meets ecology. J. Parasitol. 1997;83:575–583. doi: 10.2307/3284227. [DOI] [PubMed] [Google Scholar]
- 34.Chai J.Y., Murrell K.D., Lymbery A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Sattari M. The occurrence and intensity of Eustrongylides excisus (Nematoda: Dioctophymidae) in some bony fish species of Caspian Sea and its basin. Casp. J. Environ. Sci. 2004;2:9–12. [Google Scholar]
- 36.Coyner D.F., Spalding M.G., Forrester D.J. Epizootiology of Eustrongylides ignotus in Florida: Distribution, density, and natural infections in intermediate hosts. J. Wildl. Dis. 2002;38:483–499. doi: 10.7589/0090-3558-38.3.483. [DOI] [PubMed] [Google Scholar]
- 37.Menconi V., Manfrin C., Pastorino P., Mugetti D., Cortinovis L., Pizzul E., Pallavicini A., Prearo M. First Report of Clinostomum complanatum (Trematoda: Digenea) in European Perch (Perca fluviatilis) from an Italian Subalpine Lake: A Risk for Public Health? Int. J. Environ. Res. Public Health. 2020;17:1389. doi: 10.3390/ijerph17041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagliardi A., Preatoni D., Wauters L., Martinoli A. Selective predators or choosy fishermen? Relation between fish harvest, prey availability and great cormorant (Phalacrocorax carbo sinensis) diet. Ital. J. Zool. 2015;82:544–555. doi: 10.1080/11250003.2015.1093661. [DOI] [Google Scholar]
- 39.Root T.L., Price J.T., Hall K.R., Schneider S.H., Rosenzweig C., Pounds J.A. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 40.Tottrup A.P., Thorup K., Rainio K., Yosef R., Lehikoinen E., Rahbek C. Avian migrants adjust migration inresponse to environmental conditions en route. Biol. Lett. 2008;4:685–688. doi: 10.1098/rsbl.2008.0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polley L., Thompson R.C.A. Parasite zoonoses and climate change: Molecular tools for tracking shifting boundaries. Trends Parasitol. 2009;25:285–291. doi: 10.1016/j.pt.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Measures L.N. Epizootiology, pathology, and description of Eustrongylides tubifex (Nematoda: Dioctophymatoidea) in fish. Can. J. Zool. 1988;66:2212–2222. doi: 10.1139/z88-329. [DOI] [PubMed] [Google Scholar]
- 43.Persson L., Diehl S., Johansson L., Andersson G., Hamrin S.F. Shifts in fish communities along the productivity gradient of temperate lakes—patterns and the importance of size-structured interactions. J. Fish Biol. 1991;38:281–293. doi: 10.1111/j.1095-8649.1991.tb03114.x. [DOI] [Google Scholar]
- 44.Hatzenbeler G.R., Bozek M.A., Jennings M.J., Emmons E.E. Seasonal variation in fish assemblage structure and habitat structure in the nearshore littoral zone of Wisconsin lakes. N. Am. J. Fish. Manag. 2000;20:360–368. doi: 10.1577/1548-8675(2000)020<0360:SVIFAS>2.3.CO;2. [DOI] [Google Scholar]
- 45.Long J.M., Fisher W.L. Inter-annual and size-related differences in the diets of three sympatric black bass in an Oklahoma reservoir. J. Freshw. Ecol. 2000;15:465–474. doi: 10.1080/02705060.2000.9663768. [DOI] [Google Scholar]
- 46.Scheuerell M.D., Schindler D.E. Changes in the spatial distribution of fishes in lakes along a residential development gradient. Ecosystems. 2004;7:98–106. doi: 10.1007/s10021-003-0214-0. [DOI] [Google Scholar]
- 47.Persson L. Asymmetries in competitive and predatory interactions in fish populations. In: Ebenman B., Persson L., editors. Size-Structured Populations-Ecology and Evolution. Springer; Berlin, Germany: 1988. pp. 203–218. [Google Scholar]
- 48.García--Berthou E. Ontogenetic diet shifts and interrupted piscivoryin introduced largemouth bass (Micropterus salmoides) Internat. Rev. Hydrobiol. 2002;87:353–363. doi: 10.1002/1522-2632(200207)87:4<353::AID-IROH353>3.0.CO;2-N. [DOI] [Google Scholar]
- 49.Wainwright P.C., Osenberg C.W., Mittelbach G.G. Trophic polymorphism in the pumpkinseed sunfish (Lepomis gibbosus Linnaeus): Effects of environment on ontogeny. Funct. Ecol. 1991;5:40–55. doi: 10.2307/2389554. [DOI] [Google Scholar]
- 50.Azim S., Dojki F., Ahmad S.S., Beg A.M. Role of Human Behaviour and Parasitic Diseases. IFDJP. 2008;17:128–134. [Google Scholar]
- 51.Volta P., Jeppesen E., Sala P., Galafassi S., Foglini C., Puzzi C., Winfield I.J. Fish assemblages in deep talian subalpine lakes: History and present status with an emphasis on non-native species. Hydrobiologia. 2018;824:255–270. doi: 10.1007/s10750-018-3621-0. [DOI] [Google Scholar]