Abstract
The obligate biotroph Plasmodiophora brassicae causes clubroot disease in oilseeds and vegetables of the Brassicaceae family, and cytokinins play a vital role in clubroot formation. In this study, we examined the expression patterns of 17 cytokinin-related genes involved in the biosynthesis, signaling, and degradation in Chinese cabbage inoculated with the Korean pathotype group 4 isolate of P. brassicae, Seosan. This isolate produced the most severe clubroot symptoms in Chinese cabbage cultivar “Bullam-3-ho” compared to three other Korean geographical isolates investigated. BrIPT1, a cytokinin biosynthesis gene, was induced on Day 1 and Day 28 in infected root tissues and the upregulation of this biosynthetic gene coincided with the higher expression of the response regulators BrRR1, on both Days and BrRR6 on Day 1 and 3. BrRR3 and 4 genes were also induced during gall enlargement on Day 35 in leaf tissues. The BrRR4 gene, which positively interact with phytochrome B, was consistently induced in leaf tissues on Day 1, 3, and 14 in the inoculated plants. The cytokinin degrading gene BrCKX3-6 were induced on Day 14, before gall initiation. BrCKX2,3,6 were induced until Day 28 and their expression was downregulated on Day 35. This insight improves our current understanding of the role of cytokinin signaling genes in clubroot disease development.
Keywords: Chinese cabbage, cytokinin, clubroot, Plasmodiophora brassicae, expression analysis
1. Introduction
Clubroot is a devastating disease of Brassica vegetables and oilseed crops. This infectious disease is caused by the obligate parasite, Plasmodiophora brassicae Woronin. This pathogen is a Cercozoan biotrophic protist of Plasmodiophoraceae family. Clubroot disease is a serious problem of oilseed rape growers throughout the world [1]. The pathogen is variable among geographic regions and therefore regional difference in severity of this disease is reported [2]. According to pathogenic reaction of 12 geographical isolates and the reactions of those isolate to Williams’ differential set the Korean P. brassicae isolates were divided into four pathotypes [2,3]. In addition, the intron 1 of 18S smaller subunit of those isolates were found variable after ribosomal DNA sequencing [4].
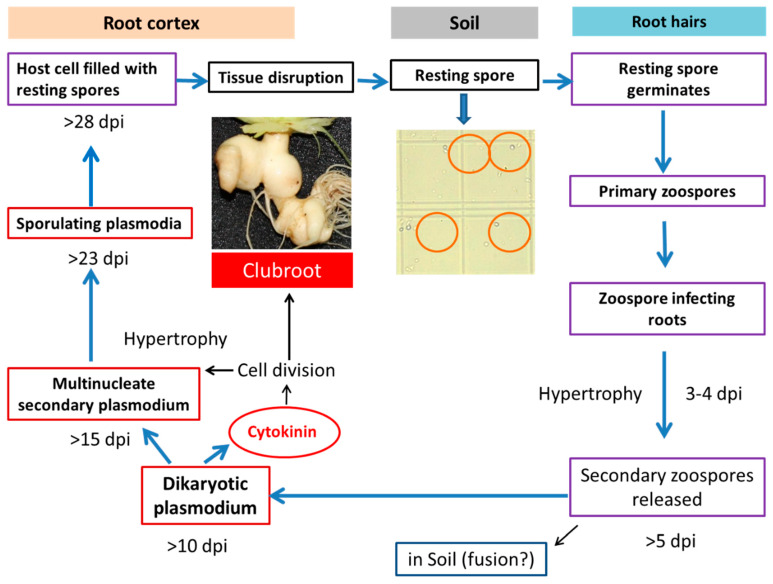
The life cycle of P. brassicae is complex (Figure 1) [5,6]. This pathogen is difficult to control because of the durability of spores and lack of suitable chemical control [1,7,8]. Despite the complex life cycle, two distinct phases of infection to its Brassica hosts are noted, primary and secondary phases of infection [9,10]. The way of infection and types of roots that are infected differ between primary and secondary phases of infection [11,12]. Primary infection starts from the root hairs and this phase usually lasts first three days (Figure 1). In this phase, the resting spores residing in soil germinates, infects root hairs and releases secondary zoospores. In the secondary phase of infection, the pathogen enters into the cortex of the roots and steles of roots and hypocotyls of the infected plants. When the secondary infection process continues root tissues divide abnormally and that results in rapid swelling in the root zone which is termed as “galls” or “clubs” (Figure 1) [9,11].
Figure 1.
Involvement of cytokinin in clubroot formation during the life cycle of Plasmodiophora brassicae (Robin et al. [13] after Dekhuijzen [14], Müller and Hilgenberg [15]). dpi, days post inoculation.
It is generally believed that an alteration in the usual hormonal balance has a vital role in the clubroot formation [13,16,17]. The contribution of hormones in club formation in the infected and hypertrophied roots during the invasion of P. brassicae was of an area of interest of the many clubroot researchers over the past several decades [14,16,17,18]. Both auxins and cytokinins have key regulatory role at the beginning of hypertrophy in the infected root tissues at the early secondary phase of infection [16,17,19,20,21].
Hormonal homeostasis of auxin and cytokinin is pivotal in the formation of clubroot [21,22,23,24,25]. Stimulation of the cell division which is associated with club formation in the infected roots requires the activity of cytokinin and auxin. In Arabidopsis thaliana and Brassica spp. an increased level of cytokinin and faster cell division were observed [16,17,26]. Further, cytokinin increases availability of nutrients including carbohydrates, amino acids, and lipids which are essential for the multiplication of pathogen during gall formation [22,26]. At the later stage of club development, a decreasing level of cytokinin primarily reflected a repression of cytokinin biosynthesis genes although few response regulator and receptor genes were induced [16,26,27,28].
Despite the well-recognized importance of auxins and cytokinins in gall formation, the fluctuation in plant hormone contents does not correspond to the severity of infection in B. rapa roots [23]. Transcriptome analysis revealed a wide range of variation in the expression of cytokinin biosynthesis-related genes between infected and non-infected tissues. Laser micro-dissection and pressure catapulting (LMPC) detected up-regulated genes associated with cytokinin metabolism in A. thaliana during gall formation [29]. Microarray analysis during early phase of root infection at 4, 7, and 10 days revealed significant alteration in expression of a relatively lower number of genes in A. thaliana [30]. A few of those genes were found to change the level of expression during infection of the cortex from day 4 to day 10 [30]. By contrast, transcriptomes at two time-points, 10 and 23 days after inoculation, in the root cortex revealed that more than 1000 genes were differentially expressed between infected and control roots [28]. RNAseq data reported 2089 differentially expressed genes in resistant Chinese cabbage line at 30 days post inoculation compared to susceptible line where 188 and 138 genes were associated with plant-pathogen interaction and hormone signal transduction, respectively [24]. In another study, isobaric tags for relative and absolute quantitation (iTRAQ) analysis detected a total of 5003 differentially expressed proteins in resistant vs. susceptible line in secondary phase of disease infection [31].
Malinowski et al. [16] reported that the genes associated with cytokinin biosynthesis, signaling, and degradation show a remarkable repression in host during gall initiation at the secondary phase of infection in A. thaliana at 16 and 26 days post inoculation. However, to date, little is known about the behavior of cytokinin regulating, synthesizing, and degrading genes in plants during both primary and secondary phase of clubroot infection and gall expansion in Chinese cabbage. In the current study, we have characterized cytokinin regulating, synthesizing, and degrading genes from seven gene families in silico. We, then, analyzed the expression of 17 cytokinin-related genes, selected based on Schuller et al. [29] in Arabidopsis thaliana, in a Chinese cabbage cultivar inoculated with a highly virulent Korean P. brassicae isolate during both primary and secondary phase of clubroot infection. However, the specific role of BrCRR1, involved in protein phosphorylation, and BrPYL1, a receptor of abscisic acid (ABA), in cytokinin accumulation is not obvious. The pattern of expression of cytokinin-related genes was discussed to explain their role during primary infection, gall initiation, and gall expansion. We have also explored an association between increased expression of cytokinin biosynthesis genes and increase/decrease of cytokinin contents (in the published data) at the adjacent time-points in the clubroot infected root tissues to discern the role of cytokinin-related genes. The results of this study shed light on the changes in transcript levels for cytokinin-related genes, both in primary and secondary phases of clubroot infection, involved in metabolism, transport, and signaling in the host, during infection by this important pathogen in Chinese cabbage.
2. Results
2.1. Disease Severity Index of Four Korean Field Isolates
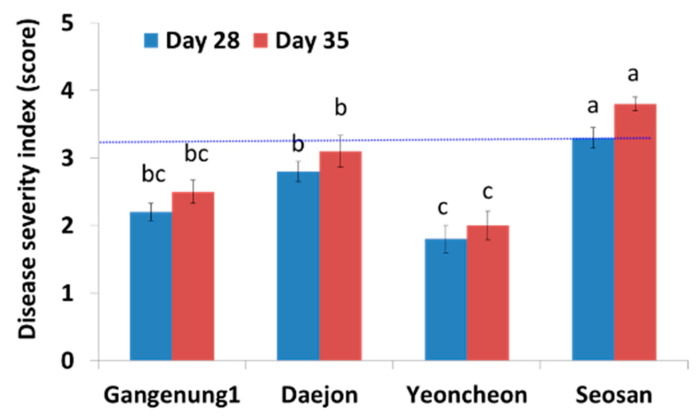
A small but visible gall appeared at 21 DAI onwards. The isolate Seosan produced the largest galls at both 28 and 35 DAI, followed by Daejon, Gangneung1, and Yeoncheon (Figure 2). Disease severity index (DSI), significantly varied among the four isolates (Figure 2).
Figure 2.
Mean score indicating the severity of clubroot infection in Chinese cabbage cultivar “Bullam-3-ho” at 28 and 35 days after inoculation (DAI) in response to infection with four different Korean P. brassicae isolates. Data represent the average disease scores of 10 plants ± standard error. Values with different letters are significantly different at 5% level of significance according to Tukey’s pairwise comparisons.
2.2. Properties of Cytokinin-Related Proteins
The 17 selected cytokinin-related genes were from seven different families: cytokinin oxidase, response regulator, histidine kinase, protein kinase, purine permease, transcriptional adaptor, isopentenyl transferase, and cytokinin-specific binding protein. The isoelectric point of purine permease, transcriptional adaptor, and isopentenyl transferase proteins are >7.0, whereas those of the other protein families are generally <7.0 (Table 1). The molecular weights of these proteins range from 18.37 to 115 kD. Histidine kinase has the highest molecular weight (115 kD). All proteins except isopentenyl transferase, kinase, and cytokinin oxidase were predicted to localize to the nucleus.
Table 1.
Properties of genes involved in cytokinin metabolism, signaling, and transport in Brassica rapa. aa, amino acid; Pi, isoelectric point; MW, molecular weight; ER, endoplasmic reticulum. BrCRR1 is involved in protein phosphorylation and BrPYL1 is receptor of ABA.
| Gene Name | BRAD ID | Chromosomal Location | Start Codon | Stop Codon | Strand | Gene Identity | (aa) | Pi | MW (kDa) | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|---|
| BrRR1 | Bra023972 | A03 | 28566478 | 28568797 | − | Two-component response regulator | 518 | 5.92 | 57.96 | Nuclear |
| BrHK1 | Bra024849 | A06 | 22745808 | 22750254 | + | Histidine kinase (CRE1/AHK4) | 1040 | 6.21 | 115.0 | Endoplasmic reticulum |
| BrRR2 | Bra018084 | A06 | 9832893 | 9834443 | + | Response regulator 5 (ARR5) | 179 | 6.18 | 20.30 | Nuclear |
| BrRR2.1 | Bra033773 | A01 | 13811201 | 13812750 | − | 180 | 5.54 | 20.404 | Nuclear | |
| BrRR3 | Bra036963 | Scaffold000123 | 487890 | 489100 | − | Response regulator 4 (ARR4) | 261 | 4.62 | 28.525 | Nuclear |
| BrRR3.1 | Bra031714 | A09 | 36858902 | 36860112 | + | 261 | 4.62 | 28.525 | Nuclear | |
| BrRR3.2 | Bra019932 | A06 | 3644746 | 3645865 | − | 253 | 4.63 | 27.682 | Nuclear | |
| BrRR3.3 | Bra018439 | A05 | 8234767 | 8235847 | + | 251 | 4.55 | 27.317 | Nuclear | |
| BrRR4 | Bra015885 | A07 | 23646646 | 23647740 | − | Response regulator 15 (ARR15) | 185 | 5.94 | 20.55 | Nuclear |
| BrRR4.1 | Bra003782 | A07 | 18316602 | 18317690 | + | 197 | 5.06 | 21.850 | Nuclear | |
| BrRR4.2 | Bra025708 | A06 | 7115317 | 7116482 | − | 213 | 5.54 | 23.480 | Nuclear | |
| BrPUP1 | Bra010890 | A08 | 16197869 | 16199402 | + | Purine permease (PUP1) | 302 | 8.72 | 38.77 | Nuclear |
| BrADA1 | Bra040578 | Scaffold000219 | 46992 | 49506 | − | Transcriptional adaptor (ADA2b) | 486 | 6.51 | 56.07 | Nuclear |
| BrADA1.1 | Bra012721 | A03 | 22587864 | 22590255 | − | 469 | 7.53 | 54.33 | Nuclear | |
| BrIPT1 | Bra001737 | A03 | 18198110 | 18199443 | − | Isopentenyl transferase (IPT8) | 327 | 9.26 | 37.03 | Chloroplast |
| BrRR5 | Bra003265 | A07 | 15543847 | 15545066 | + | Response regulator 9 (ARR9) | 240 | 5.07 | 26.847 | Nuclear |
| BrRR5.1 | Bra014649 | A04 | 2014110 | 2015202 | − | 234 | 5.20 | 25.932 | Nuclear | |
| BrRR6 | Bra016526 | A08 | 18109570 | 18110672 | + | Response regulator 7 (ARR7) | 207 | 5.62 | 23.04 | Nuclear |
| BrRR6.1 | Bra025708 | A06 | 7115317 | 7116482 | − | 213 | 5.54 | 23.48 | Plasma membrane | |
| BrCKX2 | Bra036719 | A09 | 5855383 | 5858908 | − | Cytokinin oxidase | 502 | 5.96 | 55.48 | ER |
| BrCKX2.1 | Bra040677 | Scaffold000232 | 36707 | 41121 | − | 505 | 5.62 | 55.60 | ER | |
| BrCKX3 | Bra035640 | A02 | 6020617 | 6023613 | + | 518 | 5.62 | 58.89 | Vacuole/ER | |
| BrCKX3.1 | Bra002777 | A10 | 7709311 | 7712297 | − | 366 | 5.63 | 40.97 | Vacuole/ER | |
| BrCKX4 | Bra024135 | A03 | 27423204 | 27426573 | + | 524 | 5.71 | 57.99 | Extracellular | |
| BrCKX5 | Bra015842 | A07 | 23842777 | 23845621 | − | 524 | 5.62 | 58.83 | Extracellular | |
| BrCKX6 | Bra007743 | A09 | 32193279 | 32195072 | + | 449 | 7.30 | 50.498 | Extracellular | |
| BrCRR1 | Bra007202 | A09 | 29452438 | 29453460 | − | Protein kinase family | 340 | 5.71 | 37.562 | Plasma membrane |
| BrPYL1 | Bra009113 | A10 | 15189844 | 15190458 | − | ABA binding protein | 204 | 6.02 | 22.777 | Peroxisomal |
| BrPYL1.1 | Bra005853 | A03 | 930207 | 930818 | + | 203 | 5.72 | 22.72 | Peroxisomal | |
| BrPYL11.2 | Bra028758 | A02 | 798022 | 798519 | + | 165 | 5.52 | 18.37 | Peroxisomal |
2.3. Expression Level Difference of Cytokinin-Related Genes in Leaf vs. Root
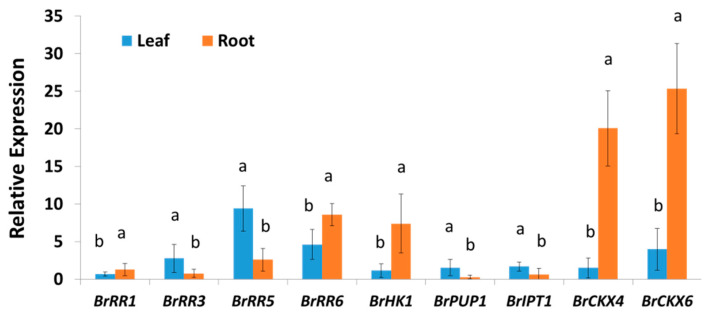
Nine cytokinin regulating, synthesizing, and degrading genes exhibited significant variation in their relative expression levels between leaf and root samples as the average of both inoculated and mock-treated samples at five different time-points (Figure 3). Another eight genes showed non-significant variation between leaves and roots (Table S1). Two cytokinin degrading genes, BrCKX4 and BrCKX6 showed 13.3- and 6.4-folds higher expression, respectively, in root tissues compared to leaf tissues (Figure 3). Both of the two response regulating genes, BrRR1 and BrRR6 accounted for 1.9-fold higher expression in root tissues compared to leaf tissues (Figure 3). By contrast, both BrRR3 and BrRR5 genes showed 3.6-fold lower expression in root tissues compared to leaf tissues (Figure 3). Similarly, BrPUP1 and BrIPT1 exhibited 5.5- and 2.7-folds lower expression, respectively, in root tissues compared to leaf tissues (Figure 3). But the BrHK1 gene showed 6.5-fold higher expression in root tissues compared to leaf tissues (Figure 3).
Figure 3.
Variation in relative expression of cytokinin regulating, synthesizing, and degrading genes in root and leaf samples. Each data is the average of both inoculated and mock-treated samples at five different time-points (average of 30 samples). Vertical bars represent standard deviations. Letters a and b indicate statistically significant difference between leaf and root samples at 5% level of significance. Gene expression levels in leaves from non-treated plants on Day 0 were set to 1.
2.4. Expression Profiles of Response Regulator Genes
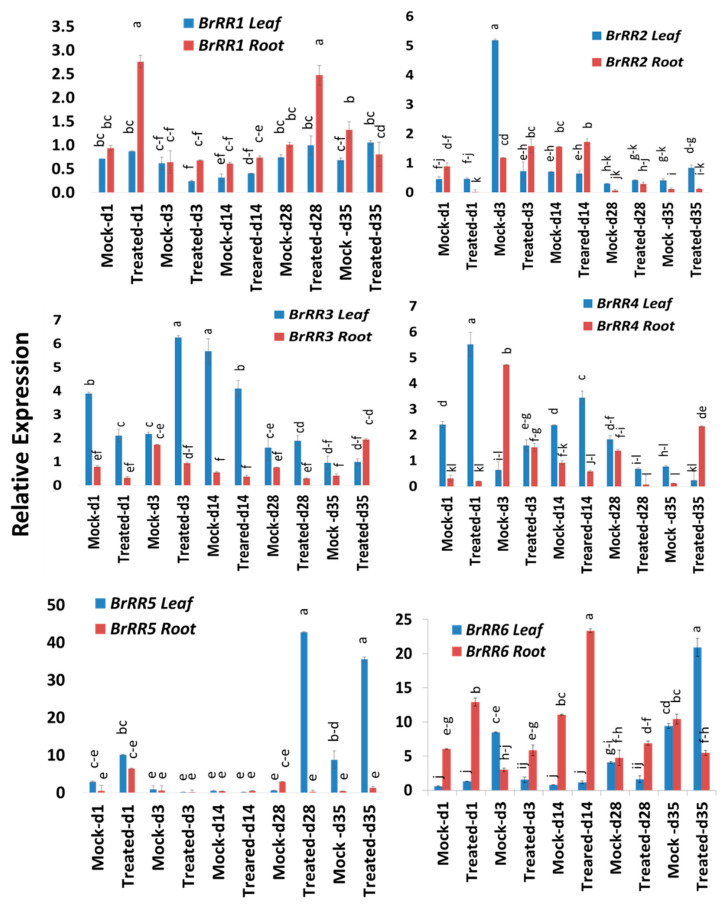
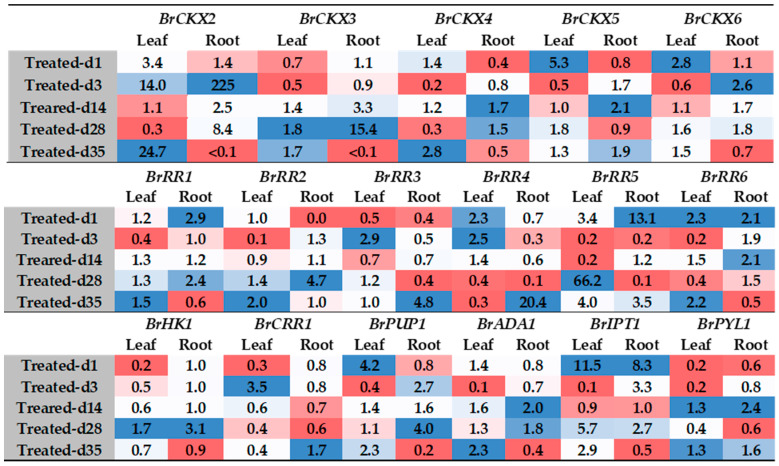
Six response regulator genes, BrRR1-6, showed significant variations in time-points, treatment within time-points and sample types within treatment and time-points (Table 2). These response regulators, except BrRR3 and BrRR4, also exhibited significant treatment difference (Table 2). Among the six response regulator genes, BrRR1 gene exhibited a 2.9- and 2.4-fold increase in expression on Day 1 and Day 28, respectively, in roots compared to mock-treated root samples at the same time-point (Figure 4 and Figure 5; Table 2). BrRR2 was upregulated 4.7-fold in root tissues on Day 28 in Seosan-inoculated plants compared to mock-treated roots (Figure 4). BrRR3 gene exhibited a 2.9-fold increase in expression in the leaves of Seosan-inoculated plants compared to mock-treated leaves at the same time-point (Figure 4 and Figure 5). In Seosan-inoculated plants, BrRR4 expression increased 2.3-fold in leaves on Day 1 and 20.4-fold in roots on Day 35 compared to mock-treated samples (Figure 4). BrRR5 gene was upregulated 66.2- and 4.0-fold in the leaves of Seosan-inoculated plants on Day 28 and Day 35, respectively, compared to mock-treated leaf samples at the same time-point (Figure 4). BrRR6 showed a 2.1-, 1.9-, and 2.1-fold increase in expression in Seosan-inoculated root tissues on Day 1, Day 3, and Day 14, respectively, compared to mock-treated root samples at the respective time-points (Figure 5). This gene also showed 2.2-fold higher expression in Seosan-inoculated leaf samples compared to mock-treated samples on Day 35 (Figure 5).
Table 2.
Sources of variation, degrees of freedom (df), mean squares (MS), F statistic, p value for relative expression levels of 17 cytokinin-biosynthesis related genes in leaf and root samples at five different time-points (1, 3, 14, 28, and 35 days after inoculation) under two different treatments (mock and inoculation with Seosan-isolate of P. brassicae) in Chinese cabbage cultivar “Bullam-3-ho.”
| Sources of Variation | df | Genes | MS | F Statistic | p Value | Genes | MS | F Statistic | p Value |
|---|---|---|---|---|---|---|---|---|---|
| Time-point (Tm) | 4 | BrRR1 | 1.84 | 253.7 | <0.01 | BrADA1 | 1.05 | 62.5 | <0.01 |
| Treatment (Tr) | 1 | 1.74 | 239.4 | <0.01 | 0.0003 | 0.02 | 0.891 | ||
| Tm x Tr | 4 | 0.88 | 121.1 | <0.01 | 0.559 | 33.06 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 1.01 | 139 | <0.01 | 0.56 | 33.13 | <0.01 | ||
| Error | 40 | 0.033 | 0.016 | ||||||
| Time-point | 4 | BrRR2 | 7.64 | 1305.2 | <0.01 | BrIPT1 | 7.52 | 671.2 | <0.01 |
| Treatment | 1 | 2.46 | 420.9 | <0.01 | 12.2 | 1089.8 | <0.01 | ||
| Tm x Tr | 4 | 2.67 | 457.9 | <0.01 | 5.58 | 498.3 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 2.97 | 507.1 | <0.01 | 5.63 | 502.3 | <0.01 | ||
| Error | 40 | 0.055 | 0.0112 | ||||||
| Time-point | 4 | BrRR3 | 7.98 | 241.5 | <0.01 | BrPYL1 | 85.1 | 642.1 | <0.01 |
| Treatment | 1 | 0.068 | 2.07 | 0.158 | 79.3 | 598.1 | <0.01 | ||
| Tm x Tr | 4 | 4.04 | 122.4 | <0.01 | 106.4 | 802.7 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 12.8 | 389.9 | <0.01 | 115.9 | 873.8 | <0.01 | ||
| Error | 40 | 0.033 | 0.133 | ||||||
| Time-point | 4 | BrRR4 | 4.48 | 242.5 | <0.01 | BrCKX2 | 114.1 | 562.9 | <0.01 |
| Treatment | 1 | 0.073 | 3.95 | 0.054 | 41.4 | 204.5 | <0.01 | ||
| Tm x Tr | 4 | 4.41 | 238.6 | <0.01 | 32.8 | 161.9 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 9.74 | 526.8 | <0.01 | 53.8 | 265.8 | <0.01 | ||
| Error | 40 | 0.018 | 0.203 | ||||||
| Time-point | 4 | BrRR5 | 376.1 | 191.9 | <0.01 | BrCKX3 | 131.6 | 586.8 | <0.01 |
| Treatment | 1 | 925.2 | 472.2 | <0.01 | 53.9 | 240.4 | <0.01 | ||
| Tm x Tr | 4 | 235.2 | 120.1 | <0.01 | 139.8 | 623.2 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 461.6 | 235.6 | <0.01 | 135.2 | 602.5 | <0.01 | ||
| Error | 40 | 1.959 | 0.224 | ||||||
| Time-point | 4 | BrRR6 | 121.8 | 479.8 | <0.01 | BrCKX4 | 1248.8 | 2545.6 | <0.01 |
| Treatment | 1 | 75.4 | 297.0 | <0.01 | 70.6 | 144.1 | <0.01 | ||
| Tm x Tr | 4 | 33.4 | 131.5 | <0.01 | 78.8 | 160.6 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 161.9 | 637.8 | <0.01 | 1074.3 | 2189.9 | <0.01 | ||
| Error | 40 | 0.254 | 0.49 | ||||||
| Time-point | 4 | BrHK1 | 32.04 | 351.1 | <0.01 | BrCKX5 | 112,4 | 2488.7 | <0.01 |
| Treatment | 1 | 0.05 | 0.56 | 0.458 | 75.6 | 1675.7 | <0.01 | ||
| Tm x Tr | 4 | 12.55 | 137.6 | <0.01 | 4.89 | 108.4 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 72.8 | 798.7 | <0.01 | 87.3 | 1933.2 | <0.01 | ||
| Error | 40 | 0.09 | 0.045 | ||||||
| Time-point | 4 | BrCRR1 | 3.19 | 968.4 | <0.01 | BrCKX6 | 1618.7 | 2676.6 | <0.01 |
| Treatment | 1 | 0.47 | 145.3 | <0.01 | 431.9 | 714.2 | <0.01 | ||
| Tm x Tr | 4 | 1.11 | 336.8 | <0.01 | 157.1 | 259.8 | <0.01 | ||
| Leaf vs. Root (Tm Tr) | 10 | 1.74 | 530.6 | <0.01 | 1558.9 | 2577.8 | <0.01 | ||
| Error | 40 | 0.003 | 0.60 | ||||||
| Time-point | 4 | BrPUP1 | 3.43 | 354.4 | <0.01 | ||||
| Treatment | 1 | 2.21 | 227.4 | <0.01 | |||||
| Tm x Tr | 4 | 0.769 | 79.2 | <0.01 | |||||
| Leaf vs. Root (Tm Tr) | 10 | 4.38 | 451.8 | <0.01 | |||||
| Error | 40 | 0.009 |
Figure 4.
Variation in relative expression levels of BrRR cytokinin-related genes in leaf and root samples under different treatment × time-point combinations. Vertical bars compare relative expression levels among leaf vs. root, mock vs. treated samples at five time-points. Each data point represents the average of three samples. Vertical bars represent standard deviation. Values with different letters are significantly different at 5% level of significance according to Tukey’s pairwise comparisons. Gene expression levels in leaves from non-treated plants on Day 0 were set to 1.
Figure 5.
Fold changes in relative expression levels of cytokinin-related genes in Seosan-inoculated plants compared to mock-treated leaf samples at the same time-point. Blue and red colors represent upregulation and downregulation of genes, respectively. BrCRR1 is involved in protein phosphorylation and BrPYL1 is a receptor of ABA.
2.5. Expression Profiles of Other Cytokinin-Related Genes
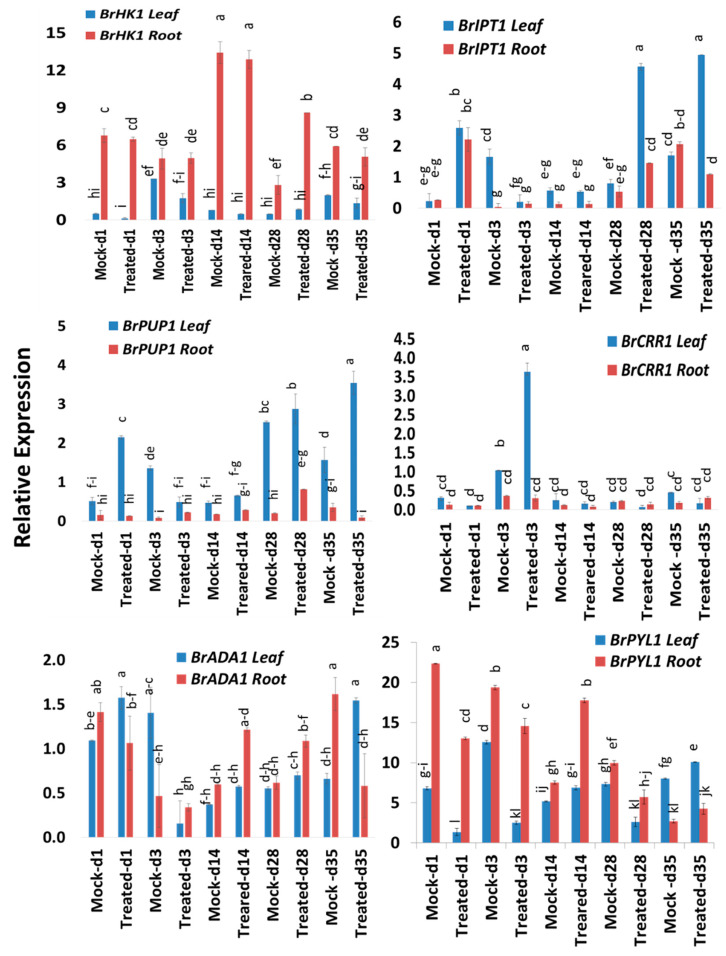
In addition to investigating the expression patterns of response regulator and cytokinin oxidase genes, we analyzed the expression of five other genes, including histidine kinase, protein kinase, purine permease, transcriptional adaptor, and isopentenyl-transferase genes. At most time-points, the histidine kinase gene BrHK1 was more highly expressed in root vs. leaf tissue (Figure 6). BrHK1 gene was expressed at 3.1-fold higher levels in Seosan-inoculated roots on Day 28 compared to mock-treated roots at the same time-point (Figure 5). The protein kinase gene BrCRR1 was upregulated 3.5-fold in the leaves of Seosan-inoculated plants on Day 3 compared to mock-treated leaf samples (Figure 6).
Figure 6.
Variation in relative expression levels of cytokinin-related genes BrHK, BrCRR, BrPUP, BrADA, and BrIPT in leaf and root samples under different treatment × time-point combinations. Vertical bars compare relative expression levels among leaf vs. root, mock vs. treated samples at five time-points. Each data point represents the average of three samples. Vertical bars represent standard deviation. Values with different letters are significantly different at 5% level of significance according to Tukey’s pairwise comparisons. Gene expression levels in leaves from non-treated plants on Day 0 were set to 1.
The purine permease gene BrPUP1 exhibited a 4.2-fold and 2.3-fold increase in expression on Day 1 and Day 35, respectively, in the leaves of Seosan-inoculated plants compared to mock-treated leaves at the same time-point (Figure 5). The isopentenyl-transferase gene BrIPT1 exhibited an 11.5-, 5.7-, and 2.9-fold increase in expression on Day 1, Day 28, and Day 35, respectively, in leaf samples of Seosan-inoculated plants compared to mock-treated leaf samples at the same time-point (Figure 5). BrIPT1 also showed an 8.3- and 2.7-fold increase in expression in Seosan-inoculated root samples on Day 1 and Day 28, respectively, compared to the mock-treated root samples at the same time-point (Figure 5).
The expression levels of transcriptional adapter gene BrADA1 were quite consistent in all sample types across all time-points. BrADA1 gene showed 2.3-fold higher expression in leaf samples of Seosan-inoculated plants compared to root samples of mock-treated plants on Day 35 (Figure 6). Finally, BrPYL1 gene showed a 2.4-fold increase in expression in root tissues on Day 14 in Seosan-inoculated plants compared to the root samples of mock-treated plants (Figure 6).
2.6. Expression Profiles of Cytokinin Oxidase Genes
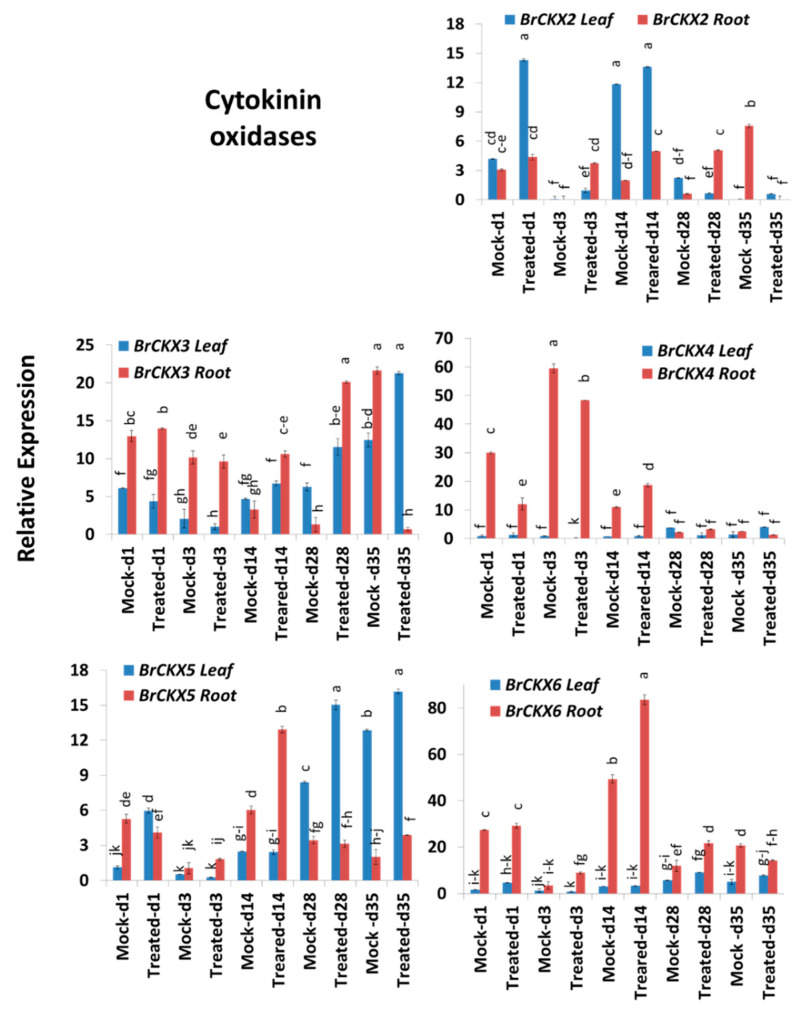
Cytokinin oxidase genes, BrCKX2-6, exhibited significant variations for time-points, treatment, time-points x treatment and sample types within treatment and time-points (Table 2). BrCKX2 was highly expressed on Day 1, with 3.4-fold higher expression in leaves of Seosan-inoculated plants compared to mock-treated leaves at the same time-point (Figure 5 and Figure 7; Table 2). This gene also showed 225-, 2.5-, and 8.4-fold higher expression in roots on Day 3, Day 14, and Day 27, respectively, compared to mock-treated roots at the same time-point (Figure 5 and Figure 7). BrCKX3 gene was expressed at 3.3- and 15.4-fold higher levels in Seosan-inoculated roots on Day 14 and Day 28, respectively, compared to mock-treated roots at the same time-point (Figure 5). In general, BrCKX4 was expressed at higher levels in roots vs. leaves in control plants and in both mock- and Seosan-inoculated plants up to Day 14 compared to leaves (Figure 7). The expression of BrCKX4 increased 1.7-fold in Seosan-inoculated roots on Day 14 compared to mock-treated roots at the same time-point (Figure 5 and Figure 7). BrCKX5 was upregulated 5.3-, 1.8-, and 1.3-fold in leaves of Seosan-inoculated plants on Day 1, Day 14, and Day 28 compared to mock-treated leaves at the same time-point (Figure 5 and Figure 7). This gene also showed 2.1-fold increase in expression in Seosan-inoculated roots on Day 14 compared to mock-treated roots at the same time-point (Figure 5). This gene was expressed at significantly (1.7-fold) higher levels in Seosan-inoculated roots on Day 14 compared to mock-treated roots at the same time-point (Figure 7).
Figure 7.
Variation in the relative expression levels of BrCKX cytokinin-related genes in leaf and root samples under different treatment × time-point combinations. Vertical bars compare relative expression levels among leaf vs. root, mock vs. treated samples at five time-points. Each data point represents the average of three samples. Vertical bars represent standard deviation. Values with different letters are significantly different at 5% level of significance according to Tukey’s pairwise comparisons. Gene expression levels in leaves from non-treated plants on Day 0 were set to 1.
3. Discussion
The main objective of this study was to assess the transcript levels of cytokinin-related genes in Chinese cabbage inoculated with P. brassicae isolate “Seosan” at five different time-points. Cytokinins are one of the major hormone group present in plants those regulated numerous physiological and biochemical processes in plants including growth, development, and metabolism [32,33,34]. We selected “Seosan” isolate because of its high virulence compared to the three other Korean isolates examined.
3.1. Expression Level Difference in Leaf vs. Root Tissue
Cytokinins play a crucial role in maintaining root-shoot homeostasis in plants through transduction of nutritional signals [35,36]. In this study, relative expression of several genes including BrHK1 and BrIPT1 dramatically changed in root and leaf tissues of both mock and Seosan-inoculated plants at different time-points indicating that hormonal homeostasis within plant is dynamic and that may be changed over time between organs (Figure 3 and Figure 6).
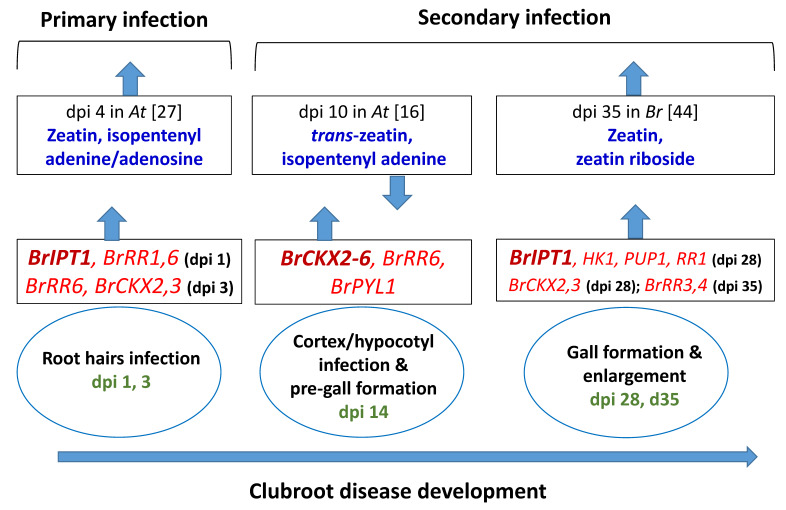
We found that several genes, such as BrRR1, BrRR6, BrHK1, BrCKX4, and BrCKX6 were expressed at higher levels in root tissues compared to leaf tissues (Figure 3). By contrast, the cytokinin biosynthesis gene BrIPT1 and response regulators BrRR3, BrRR5, and BrPUP1 had lower expression in root tissues indicating that cytokinin levels differ in root vs. leaf tissues considering that expression level of regulating and biosynthesis genes is positively and that of degrading genes is negatively associated with cytokinin biosynthesis (Figure 8, [37]).
Figure 8.
Association between relative expressions of cytokinin biosynthesis, response regulating and degrading genes in this study (red color) and published cytokinin contents at the closer time-points in Arabidopsis thaliana and Brassica rapa (blue color). dpi, days post inoculation. Green color represents dpi at which samples were collected. Upwards- and downwards-pointing arrows represent upregulation/increase and downregulation/decrease of genes/cytokinin contents, respectively, in clubroot infected plants compared to non-infected plants.
3.2. Expression Level Difference of BrIPT1 Gene
Isopentenyl-transferase (IPT) is a critically important enzyme for the biosynthesis of cytokinin [38,39,40,41]. This enzyme is vital for cytokinin homeostasis under the infection of P. brassicae [42]. In B. rapa, Ando et al. [42] found that several BrIPT genes, including BrIPT1, were induced in the inoculated plants with P. brassicae at the first appearance of gall after 20 dpi. In this study a high level of expression of BrIPT1 gene by 11.5- and 8.3-fold in leaf and root tissues, respectively, on Day 1 after inoculation and a marginal upregulation in inoculated root tissues by 3.3-fold on Day 3 (Figure 5) indicated that primary infection of P. brassicae in the root tissues transiently stimulated the expression of this gene. Expression of this gene was repressed on Day 3 and Day 14, during gall initiation (Figure 6). Malinowski et al. [16] reported a comparatively lower expression of BrIPT1 gene on Day 16 compared to the expression level of BrIPT3 and BrIPT5. A quadruple mutant of IPT- ipt1 ipt3 ipt5 ipt7, measured a very low content of cytokinin in a previous study in A. thaliana indicating the importance of IPT1 gene in cytokinin biosynthesis [43]. This gene again showed 5.7- and 2.7-fold higher expression in leaves and roots of the treated plants, respectively, on Day 28 after inoculation, during gall enlargement, in infected plants indicating its role in gall expansion through induction of cell division (Figure 6). Devos et al. [27] in Arabidopsis and Dekhuijzen [44] in B. rapa measured significantly higher contents of cytokinin at 4 dpi and 35 dpi, respectively (Figure 8). Increased expression levels of BrIPT1 gene, in this study, during primary infection of root hairs (on Day 1 and 3) and during gall formation (on Day 28) could be associated with higher biosynthesis of zeatin, isopentenyl adenine, or zeatin riboside in the infected root tissues compared to non-inoculated plants at the adjacent dpi (Figure 8).
3.3. Expression Level Difference of BrRR Genes
The BrRR proteins with two receptor (signal transduction) components have a specific histidine kinase to sense environmental changes to mediate response of a cell to environmental homeostasis [45]. The BrRR1–BrRR6 response regulator genes are the Type-A members bearing a short C-terminal extension in addition to the conserved receiver domain [45,46,47]. Exogenous application of trans-zeatin (tZ) transcriptionally upregulated the Type-A gene members [45]. A higher expression level of BrRR genes in Chinese cabbage might negatively regulate the expression of BrHK and BrHP genes instantly [45,48]. Induced expression of BrRR1 and BrRR6 during primary infection in roots, coincided with the expression of BrIPT1 gene. A 20.4-fold higher expression of BrRR4 gene on Day 35 indicated its vital role during gall enlargement in the infected root tissues as Type-A RR genes switch of cytokinin signal (Figure 5 and Figure 8) [16,17,21,26]. In this study only BrRR4 gene was induced in leaf samples on Day 1, 3, and 14 (Figure 6). The unregulated BrRR4 gene in leaves may enhance photosynthesis by interacting with phytochrome B after the infection of P. brassicae [49].
3.4. Expression Level of CKX Genes
The cytokinin oxidase (CKX) enzymes have proven role in irreversible degradation of cytokinins cleaving side-chain in roots and leaves of higher plants [50]. The CKX genes in both A. thaliana and B. rapa differ in subcellular localizations, biochemical properties, and level of expression from spatial and temporal perspectives (Table 1) [51,52]. In transgenic A. thaliana, overexpressed plants with six different CKX genes showed repressed accumulation of cytokinin that affected root and shoot growth [35]. Exogenous treatment of cytokinin was found to alter the responses of CKX genes in B. napus [53].
In this study, we analyzed the expression patterns of cytokinin-related genes in leaf and root tissues at different time-points in both non-inoculated and Seosan-inoculated plants. In a previous study, in transgenic Arabidopsis plants, the CKX1- and CKX3-overexpressing plants exhibited increased resistance to P. brassicae [26]. We detected notably higher expression of BrCKX2, BrCKX3, BrCKX4, BrCKX5, and BrCKX6 on Day 14 (before gall formation) in Seosan-treated roots compared to mock-treated root indicating that cytokinin biosynthesis and signaling was repressed on Day 14 (Figure 8) [16,27,28]. Our prediction is supported by the significantly lower accumulation of zeatin and isopentenyl adenine in the clubroot infected root tissues of Arabidopsis at 10 dpi in Malinowski et al. [16]. BrCKX2 and BrCKX3 were highly induced by 8.4- and 15.4-folds, respectively, on Day 28 when gall appeared but both of these two genes were repressed on Day 35 indicating that cytokinin signaling was enhanced during rapid gall enlargement phase (Figure 5). Previous studies reported that down-regulation of CKX genes in A. thaliana caused biosynthesis of higher levels of cytokinin upon the infection of P. brassicae [22,28].
4. Materials and Methods
4.1. Preparation of Plant Materials
Seeds of Chinese cabbage (Brassica rapa var. pekinensis) cultivar “Bullam-3-ho” were obtained from Woori Seeds, Gwacheon, Gyeonggi, South Korea. The seeds were sown in 50-cell trays containing an artificial soil mixture and placed in a controlled plant culture room (temperature 25 ± 1 °C, relative humidity 65–70%, light intensity 230–250 µmol m−2 s−1, 16:8 h light-dark cycle) for germination and seedling establishment.
4.2. Gall Sample Collection
Four isolates from four different pathotype groups obtained from infected seedlings were previously collected from four different regions of South Korea by Jo et al. [54]. These isolates included Gangneung 1, Daejon, Yeoncheon, and Seosan, belonging to pathotype group 1, 2, 3, and 4, respectively [2].
4.3. Spore Preparation from Galls and Infection of Plant Materials
P. brassicae spores were extracted from each isolate collected from four different regions of South Korea according to Feng et al. [9] and Laila et al. [4]. The concentration of spores was adjusted to 1 × 107 spores per mL after counting the number of spores in a hemocytometer. Two-week-old plants were inoculated with the four selected isolates via the root cutting method, followed by culture in a controlled environment. Specifically, the plants were inoculated with 6 mL spore suspension (107 spores mL−1) for 10‒15 min after cutting the root branches with scissors. The plants were transferred to a 200 cm3 pot containing artificial soil in a controlled growth chamber. Mock treatment was performed using 6 mL water.
4.4. Disease Scoring Criteria
Inoculated plants were inspected weekly to observe the severity of infection (Figure 9). Each sample was washed with running tap water. The disease severity of clubroot infection was rated on a scale of 0–4 as described by Laila et al. [55] (Figure 9). Disease severity index (DSI) was calculated as the average disease scores of 10 plants (replicates) under each treatment.
Figure 9.
Scoring criteria for clubroot development in Chinese cabbage cultivar “Bullam-3-ho.” Scale of 0–4: 0 = root system without clubs, 1 = one or a few small clubs <5 mm in diameter on lateral roots, 2 = a few medium-sized, separate globular clubs >5 mm in diameter on lateral roots, 3 = medium-sized clubs 5–10 mm in diameter on the main roots, 4 = severe clubbing, with clubs >10 mm in diameter on the lateral and main roots [55].
4.5. Collection of Leaf and Root Samples from Plants Inoculated with Seosan Isolate
For qRT-PCR expression analysis of cytokinin-related genes, 14-day-old seedlings of Chinese cabbage cultivar “Bullam-3-ho” were inoculated with spores of the Seosan isolate (107 spores mL−1). Root and leaf samples were harvested from mock and inoculated plants for qRT-PCR analysis at 1, 3, 14, 28 and 35 days after inoculation (DAI). A list of sampling combinations is given in Table 3. Three biological replicates were collected for each sample. During sample collection, any adhering soil residue was carefully removed from the roots using running tap water. The excess water from the roots were then dried between two pieces of filter paper and immediately frozen in liquid nitrogen. The leaf and root samples were stored at −80 °C.
Table 3.
Twenty samples collected from Seosan-inoculated and non-inoculated plants at five different time-points under two different treatments.
| Sample ID | Treatment | Time-Point | Sample Type |
|---|---|---|---|
| 1 | Mock | Day 1 | Leaf |
| 2 | Mock | Day 1 | Root |
| 3 | Seosan-inoculated | Day 1 | Leaf |
| 4 | Seosan-inoculated | Day 1 | Root |
| 5 | Mock | Day 3 | Leaf |
| 6 | Mock | Day 3 | Root |
| 7 | Seosan-inoculated | Day 3 | Leaf |
| 8 | Seosan-inoculated | Day 3 | Root |
| 9 | Mock | Day 14 | Leaf |
| 10 | Mock | Day 14 | Root |
| 11 | Seosan-inoculated | Day 14 | Leaf |
| 12 | Seosan-inoculated | Day 14 | Root |
| 13 | Mock | Day 28 | Leaf |
| 14 | Mock | Day 28 | Root |
| 15 | Seosan-inoculated | Day 28 | Leaf |
| 16 | Seosan-inoculated | Day 28 | Root |
| 17 | Mock | Day 35 | Leaf |
| 18 | Mock | Day 35 | Root |
| 19 | Seosan-inoculated | Day 35 | Leaf |
| 20 | Seosan-inoculated | Day 35 | Root |
4.6. RNA Extraction and cDNA Synthesis
Approximately 100 mg of leaf or root tissue was ground to a powder in liquid nitrogen. Total RNA was extracted from all 22 types of samples using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). RNase-free DNase (Qiagen, Hilden, Germany) treatment was performed to remove DNA contamination. The purity of the extracted RNA was determined at a 260/280 nm ratio using a NanoDrop® ND-1000 (Thermo Scientific, Hudson, NH, USA). The cDNA was synthesized from RNA following the manufacturer’s instructions using a Superscript® III First-Strand Synthesis Kit (Invitrogen, CA, USA).
4.7. Identification of Cytokinin-Related Genes
Schuller et al. [29] published a list of genes involved in cytokinin metabolism, signaling, and transport in Arabidopsis thaliana. In this study, genes were selected based on their high expression level in Arabidopsis thaliana. The corresponding Brassica rapa orthologs were retrieved from the BRAD database (http://brassicadb.org/brad/; Cheng et al. [56] against each cytokinin-related gene (Table 1, Figure S1). The subcellular localization of each corresponding cytokinin biosynthetic protein was predicted using both UniProt (https://www.uniprot.org/uniprot/) and ProtComp 9.0 from Softberry (http://linux1.softberry.com/berry.phtml). A phylogenetic tree was constructed using Neighbor-joining method in MEGA7.0 software [57]. ProtParam tool [58] was used to determine the properties of the proteins. However, a database research in The Arabidopsis Information Resource (TAIR) (https://www.arabidopsis.org/) identified that At3g55950 (BrCRR1, Bra007202) is a protein kinase that is involved in protein phosphorylation. In addition, At5g05440 gene (BrPYL1, Bra009113) is an abscisic acid (ABA) binding protein that acts as a receptor of ABA.
4.8. qRT-PCR Expression Analysis
We performed qRT-PCR to investigate the relative expression patterns of cytokinin-related genes in 20 samples representing five different time-points, two treatments, and two sample types: leaves and roots. Gene expression levels in leaves from non-treated plants on Day 0 were set to 1. There were two technical replicates for each sample. Cytokinin-related genes-specific primers were used to analyze the gene expression in Seosan-inoculated leaf and root samples via quantitative reverse-transcription PCR (qRT-PCR) (Table 4). The primers were designed based on the coding sequence of each gene using Primer3 software (http://frodo.wi.mit.edu/primer3). The specificity of the primers was checked following Robin et al. [59]. Two different ACTIN genes (GenBank Accession Nos. XM_009147610.2 and FJ969844.1, housekeeping genes) from B. rapa were used to normalize the expression levels of cytokinin-related genes. Each 20 μL PCR reaction mixture contained 1 μL template cDNA at 50 ng µL−1 concentration, 1 μL each of forward and reverse primers at 10 pmol concentration, 10 μL qPCR BIOSyGreen Mix Lo-ROX (PCR Biosystems, London, UK), and 7 μL ultra-pure double distilled water. The qRT-PCR conditions were as follows: denaturation for 10 min at 95 °C, followed by 40 cycles of amplification at 95 °C for 20 s, 58 °C for 20 s, and 72 °C for 25 s. The quantification cycle value (a measure of fluorescence) was recorded at the end of each cycle. The amplified products were detected and the data were analyzed using a LightCycler96 system (Roche, Mannheim, Germany). The 2−ΔΔCT method was used to calculate the relative expression levels of cytokinin-related genes because equal primer efficiency of the tested genes was used, two stable housekeeping genes were used for standardization, and the selected genes were tested at both control and experimental conditions [60].
Table 4.
List of primer sequences used for qRT-PCR of cytokinin-related genes. BrCRR1 is involved in protein phosphorylation and BrPYL1 is a receptor of ABA.
| Arabidopsis Homolog (Accession Number) | Gene Name | BRAD ID | cDNA Size (bp) | Primer Forward (F) and Reverse(R) | Product Size (bp) |
|---|---|---|---|---|---|
| At4g31920 | BrRR1 | Bra023972 | 1557 | F:AGCTCAAGAACATATGGCAA | 191 |
| R:TGGATCATCGTTCTCATTCC | |||||
| At2g01830 | BrHK1 | Bra024849 | 3123 | F:AGCTCTGAAGAAGTTTGGAG | 200 |
| R:GTAAATGCCATTCCAGCTTC | |||||
| At3g48100 | BrRR2 | Bra018084 | 540 | F:TACTCAGAGTCTCTTCGTGT | 171 |
| R:CTTCTTGAGTAGTTCATATC | |||||
| At1g10470 | BrRR3 | Bra036963 | 786 | F:GTTCAGAGATGATGAGGGTC | 208 |
| R:GCAGATGCTTTCTCGTTGTC | |||||
| At1g74890 | BrRR4 | Bra015885 | 558 | F:TCTACCTCGGAGTTACATGT | 161 |
| R:CCAGAAGATCCTTTGTCTCC | |||||
| At3g55950 | BrCRR1 | Bra007202 | 1023 | F:GATTACTATGGGTGAGCTGG | 167 |
| R:CTCTCTCCAAATTTCCGACA | |||||
| At1g28230 | BrPUP1 | Bra010890 | 1059 | F:TAGCTTTCAACGCTCTCTTT | 159 |
| R:CAACCACATACTCCTTGTGA | |||||
| At4g16420 | BrADA1 | Bra040578 | 1461 | F:ATGAAATGCTTCTCCTGGAG | 178 |
| R:TTCTTGTTCTTCCCTGCTAC | |||||
| At3g19160 | BrIPT1 | Bra001737 | 984 | F:TTCTGAGCTGAGGTACGATT | 194 |
| R:ACTCCGGTACTCCTATAGCC | |||||
| At3g57040 | BrRR5 | Bra003265 | 723 | F:GGCATGACTGGTTATGATTT | 237 |
| R:AAGAGATTGAAGCACCTTCA | |||||
| At1g19050 | BrRR6 | Bra016526 | 624 | F:TTAGTTCGCCTGACCTACAT | 163 |
| R:TCAGAATCTCCTGTGTTTCC | |||||
| At5g05440 | BrPYL1 | Bra009113 | 615 | F:GAGAGGCTCGAGATCCTGGAC | 237 |
| R:ACAGCTTCTAGCCAGAGACTG | |||||
| At2g19500 | BrCKX2 | Bra036719 | 1509 | F:TTAGTGAAGAGCCACGGTAT | 210 |
| R:CCATAAACCCAAAGATCTGA | |||||
| At5g56970 | BrCKX3 | Bra035640 | 1557 | F:GGGAGAGCTAAACCTGAAAT | 238 |
| R:TAAGAACCCTACCGCATAAA | |||||
| At4g29740 | BrCKX4 | Bra024135 | 1575 | F:GCTGGTGGATCACATAACTT | 170 |
| R:ATTTTCCTCCTCGAGAAATC | |||||
| At1g75450 | BrCKX5 | Bra015842 | 1575 | F:GTATCTTTCCGTTGGTGGTA | 200 |
| R:CTCGTGTAATGATCCCAAAT | |||||
| At3g63440 | BrCKX6 | Bra007743 | 1350 | F:GGTTATCCTTCACACCAGAA | 225 |
| R:AGACATGTACCCTGTCCAAG | |||||
| XM_009147610.2 | ACTIN1 | 1371 | F: CAACCAATCGTCTGTGACAA | 105 | |
| R: ATGTCTTGGCCTACCAACAA | |||||
| FJ969844.1 | ACTIN2 | 491 | F: AATGGTACCGGAATGGTCAA | 119 | |
| R: TCCTTCTGGTTCATCCCAAC |
4.9. Statistical Analysis
Analysis of variance (ANOVA) of relative expression levels of five time-points (dpi), two treatments (mock and Seosan-inoculated), and two sample types (leaf vs. root) was conducted following a nested-ANOVA under general linear model using MINITAB 17 Statistical Software (Minitab Inc., State College, PA, USA) to assess the variation among time-points, treatments, time-point x treatment, and sample type within time-point and treatment. Separate statistical analyses were conducted for disease severity scores and relative expression levels of cytokinin-related genes at two different sample types (leaf vs. root) via one-way ANOVA using MINITAB 17 Statistical Software (Minitab Inc., State College, PA, USA). For pairwise comparisons of means of disease severity scores or relative expression values, Tukey’s pairwise comparisons were performed.
5. Conclusions
In this study, the Korean pathotype group 4 isolate Seosan was found to be more virulent than three other isolates, Gangneung1, Daejon, and Yeoncheon, with respect to DSI. The expression patterns of cytokinin-related genes involved in response regulation, biosynthesis, and degradation differed greatly between leaf and root tissues, pointing to their organ-specific expression. Cytokinin biosynthetic gene BrIPT1 and response regulator genes BrRR1, BrRR4, and BrRR6 showed a pattern of upregulation at the beginning of primary infection and during gall expansion. The induced expression of BrCKX genes before gall formation indicated their possible role in repression of gall formation. Altered cytokinin contents in root tissues, in the published data, upon the infection of P. brassicae temporally coincided with altered expression levels of biosynthetic and catalytic genes of this study. We conclude that the genes BrIPT1, BrRR1, BrRR6, and BrCKXs have vital role in clubroot disease development. Multi-genic manipulation of susceptible Chinese cabbage plants with BrCKX2-6 genes could be screened for clubroot disease resistance in future. The results of this study improve our current understanding of the possible role of relevant genes in cytokinin signaling during clubroot formation.
Acknowledgments
We thank the Woori Seeds, Republic of Korea for providing the seeds. The authors thank G.J.C. for providing clubroot-infected isolates collected from nine regions of Korea.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/11/3896/s1, Figure S1. Phylogenetic tree showing association between cytokinin biosynthesis genes (black) in Arabidopsis thaliana and orthologues in Brassica rapa. BrCRR1 is involved in protein phosphorylation and BrPYL1 is a receptor of ABA. Table S1. Variation in relative expression of cytokinin regulating, synthesizing and degrading genes in root and leaf samples showing non-significant variation between leaves and roots. Each data is the average (±sd) of both inoculated and non-inoculated samples at five different time points. BrCRR1 is involved in protein phosphorylation and BrPYL1 is a receptor of ABA.
Author Contributions
I.-S.N., J.-I.P., A.H.K.R., and H.-T.K. conceived and designed the study. R.L., G.S., and M.A.K. conducted in silico analysis. G.S. designed primers. R.L. and A.H.K.R. managed the experimental plants and collected samples. R.L. prepared cDNA and conducted qRT-PCR analysis. A.H.K.R. and R.L. analyzed the data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Center for Horticultural Seed Development (Golden Seed Project No. 213007-05-4-CG100) of the Ministry of Agriculture, Food and Rural Affairs in the Republic of Korea (MAFRA).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dixon G.R. The occurrence and economic impact of Plasmodiophora brassicae and clubroot disease. J. Plant Growth Regul. 2009;28:194–202. doi: 10.1007/s00344-009-9090-y. [DOI] [Google Scholar]
- 2.Kim H., Jo E.J., Choi Y.H., Jang K.S., Choi G.J. Pathotype Classification of Plasmodiophora brassicae Isolates Using Clubroot-Resistant Cultivars of Chinese Cabbage. Plant Pathol. J. 2016;32:423. doi: 10.5423/PPJ.OA.04.2016.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams P.H. A system for the determination of races of Plasmodiophora brassicae that infect cabbage and rutabaga. Phytopathology. 1966;56:624–626. [Google Scholar]
- 4.Laila R., Robin A.H.K., Yang K., Choi G.J., Park J.I., Nou I.S. Detection of Ribosomal DNA sequence polymorphisms in the protist Plasmodiophora brassicae for the identification of geographical isolates. Int. J. Mol. Sci. 2017;18:84. doi: 10.3390/ijms18010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kageyama K., Asano T. Life cycle of Plasmodiophora brassicae. J. Plant Growth Regul. 2009;28:203. doi: 10.1007/s00344-009-9101-z. [DOI] [Google Scholar]
- 6.Malinowski R., Smith J.A., Fleming A.J., Scholes J.D., Rolfe S.A. Gall formation in clubroot-infected Arabidopsis results from an increase in existing meristematic activities of the host but is not essential for the completion of the pathogen life cycle. Plant J. 2012;71:226–238. doi: 10.1111/j.1365-313X.2012.04983.x. [DOI] [PubMed] [Google Scholar]
- 7.Donald C., Porter I. Integrated control of clubroot. J. Plant Growth Regul. 2009;28:289. doi: 10.1007/s00344-009-9094-7. [DOI] [Google Scholar]
- 8.Dixon G.R. (2014). Clubroot (Plasmodiophora brassicae Woronin)–an agricultural and biological challenge worldwide. Can. J. Plant Path. 2009;36(Suppl. 1):5–18. doi: 10.1080/07060661.2013.875487. [DOI] [Google Scholar]
- 9.Feng J., Hwang S.F., Strelkov S.E. Studies into primary and secondary infection processes by Plasmodiophora brassicae on canola. Plant Path. 2013;62:177–183. doi: 10.1111/j.1365-3059.2012.02612.x. [DOI] [Google Scholar]
- 10.McDonald M.R., Sharma K., Gossen B.D., Deora A., Feng J., Hwang S.F. The role of primary and secondary infection in host response to Plasmodiophora brassicae. Phytopathology. 2014;104:1078–1087. doi: 10.1094/PHYTO-07-13-0189-R. [DOI] [PubMed] [Google Scholar]
- 11.Hwang S.F., Strelkov S.E., Feng J.I.E., Gossen B.D., Howard R.J. Plasmodiophora brassicae: A review of an emerging pathogen of the Canadian canola (Brassica napus) crop. Mol. Plant Path. 2012;13:105–113. doi: 10.1111/j.1364-3703.2011.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deora A., Gossen B.D., McDonald M.R. Cytology of infection, development and expression of resistance to Plasmodiophora brassicae in canola. Ann. Appl. Biol. 2013;163:56–71. doi: 10.1111/aab.12033. [DOI] [Google Scholar]
- 13.Robin A.H.K., Hossain M.R., Kim H.T., Nou I.S., Park J.I. Role of Cytokinins in Clubroot Disease Development. Plant Breed. Biotech. 2019;7:73–82. doi: 10.9787/PBB.2019.7.2.73. [DOI] [Google Scholar]
- 14.Müller P., Hilgenberg W. Isomers of zeatin and zeatin riboside in clubroot tissue: Evidence for trans-zeatin biosynthesis by Plasmodiophora brassicae. Physiologia Plantarum. 1986;66:245–250. doi: 10.1111/j.1399-3054.1986.tb02415.x. [DOI] [Google Scholar]
- 15.Dekhuijzen H.M. The occurrence of free and bound cytokinins in plasmodia of Plasmodiophora brassicae isolated from tissue cultures of clubroots. Plant Cell Rep. 1981;1:18–20. doi: 10.1007/BF00267649. [DOI] [PubMed] [Google Scholar]
- 16.Malinowski R., Novák O., Borhan M.H., Spíchal L., Strnad M., Rolfe S.A. The role of cytokinins in clubroot disease. Eur. J. Plant Path. 2016;145:543–557. doi: 10.1007/s10658-015-0845-y. [DOI] [Google Scholar]
- 17.Prerostova S., Dobrev P.I., Konradyova V., Knirsch V., Gaudinova A., Kramna B., Kazda J., Ludwig-Müller J., Vankova R. Hormonal responses to Plasmodiophora brassicae infection in Brassica napus cultivars differing in their pathogen resistance. Int. J. Mol. Sci. 2018;19:4024. doi: 10.3390/ijms19124024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekhuijzen H.M., Overeem J.C. The role of cytokinins in clubroot formation. Physiol. Plant Path. 1971;1:151–161. doi: 10.1016/0048-4059(71)90024-5. [DOI] [Google Scholar]
- 19.Giron D., Kaiser W., Imbault N., Casas J. Cytokinin-mediated leaf manipulation by a leaf miner caterpillar. Biol Lett. 2007;3:340–343. doi: 10.1098/rsbl.2007.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boivin S., Fonouni-Farde C., Frugier F. How auxin and cytokinin phytohormones modulate root microbe interactions. Front. Plant Sci. 2016;7:1240. doi: 10.3389/fpls.2016.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludwig-Müller J., Auer S., Jülke S., Marschollek S. Auxins and Cytokinins in Plant Biology. Humana Press; New York, NY, USA: 2017. Manipulation of Auxin and Cytokinin Balance during the Plasmodiophora brassicae–Arabidopsis Thaliana Interaction; pp. 41–60. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig-Müller J., Rolfe S.A., Scholes J.D. Metabolism and plant hormone action during clubroot disease metabolism and plant hormone action during clubroot disease. J Plant Growth Regul. 2009;28:229. doi: 10.1007/s00344-009-9089-4. [DOI] [Google Scholar]
- 23.Schuller A., Ludwig-Müller J. A family of auxin conjugate hydrolases from Brassica rapa: Characterization and expression during clubroot disease. New Phytol. 2006;171:145–158. doi: 10.1111/j.1469-8137.2006.01727.x. [DOI] [PubMed] [Google Scholar]
- 24.Jia H., Wei X., Yang Y., Yuan Y., Wei F., Zhao Y., Yang S., Yao Q., Wang Z., Tian B., et al. Root RNA-seq analysis reveals a distinct transcriptome landscape between clubroot-susceptible and clubroot-resistant Chinese cabbage lines after Plasmodiophora brassicae infection. Plant Soil. 2017;421:93–105. doi: 10.1007/s11104-017-3432-5. [DOI] [Google Scholar]
- 25.Ciaghi S., Schwelm A., Neuhauser S. Transcriptomic response in symptomless roots of clubroot infected kohlrabi (Brassica oleracea var. gongylodes) mirrors resistant plants. BMC Plant Biol. 2019;19:288. doi: 10.1186/s12870-019-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siemens J., González M.C., Wolf S., Hofmann C., Greiner S., Du Y., Rausch T., Roitsch T., Ludwig-Müller J.U. Extracellular invertase is involved in the regulation of clubroot disease in Arabidopsis thaliana. Mol. Plant Path. 2011;12:247–262. doi: 10.1111/j.1364-3703.2010.00667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devos S., Laukens K., Deckers P., Van Der Straeten D., Beeckman T., Inzé D., Van Onckelen H., Witters E., Prinsen E. A hormone and proteome approach to picturing the initial metabolic events during Plasmodiophora brassicae infection on Arabidopsis. Mol Plant Microbe Interact. 2006;19:1431–1443. doi: 10.1094/MPMI-19-1431. [DOI] [PubMed] [Google Scholar]
- 28.Siemens J., Keller I., Sarx J., Kunz S., Schuller A., Nagel W., Schmülling T., Parniske M., Ludwig-Müller J. Transcriptome analysis of Arabidopsis clubroots indicate a key role for cytokinins in disease development. Mol. Plant Microbe Interact. 2006;19:480–494. doi: 10.1094/MPMI-19-0480. [DOI] [PubMed] [Google Scholar]
- 29.Schuller A., Kehr J., Ludwig-Müller J. Laser microdissection coupled to transcriptional profiling of Arabidopsis roots inoculated by Plasmodiophora brassicae indicates a role for brassinosteroids in clubroot formation. Plant Cell Physiol. 2013;55:392–411. doi: 10.1093/pcp/pct174. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal A., Kaul V., Faggian R., Rookes J.E., Ludwig-Müller J., Cahill D.M. Analysis of global host gene expression during the primary phase of the Arabidopsis thaliana–Plasmodiophora brassicae interaction. Functional Plant Biol. 2011;38:462–478. doi: 10.1071/FP11026. [DOI] [PubMed] [Google Scholar]
- 31.Lan M., Li G., Hu J., Yang H., Zhang L., Xu X., Liu J., He J., Sun R. iTRAQ-based quantitative analysis reveals proteomic changes in Chinese cabbage (Brassica rapa L.) in response to Plasmodiophora brassicae infection. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-48608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan S., Amasino R.M. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- 33.Mok D.W., Mok M.C. Cytokinin metabolism and action. Annu. Rev. Plant Biol. 2001;52:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- 34.Sakakibara H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006;57:431–449. doi: 10.1146/annurev.arplant.57.032905.105231. [DOI] [PubMed] [Google Scholar]
- 35.Werner T., Motyka V., Laucou V., Smets R., Van Onckelen H., Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werner T., Motyka V., Strnad M., Schmülling T. Regulation of plant growth by cytokinin. Proc. Natl. Acad. Sci. USA. 2001;98:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanov G.A., Kieber J.J., Schmülling T. A rapid cytokinin response assay in Arabidopsis indicates a role for phospholipase D in cytokinin signalling. FEBS Lett. 2002;515:39–43. doi: 10.1016/S0014-5793(02)02415-8. [DOI] [PubMed] [Google Scholar]
- 38.El-Showk S., Ruonala R., Helariutta Y. Crossing paths: Cytokinin signalling and crosstalk. Development. 2013;140:1373–1383. doi: 10.1242/dev.086371. [DOI] [PubMed] [Google Scholar]
- 39.Rivero R.M., Shulaev V., Blumwald E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009;150:1530–1540. doi: 10.1104/pp.109.139378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qin H., Gu Q., Zhang J., Sun L., Kuppu S., Zhang Y., Burow M., Payton P., Blumwald E., Zhang H. Regulated expression of an isopentenyltransferase gene (IPT) in peanut significantly improves drought tolerance and increases yield under field conditions. Plant Cell Physiol. 2011;52:1904–1914. doi: 10.1093/pcp/pcr125. [DOI] [PubMed] [Google Scholar]
- 41.Liu Z., Lv Y., Zhang M., Liu Y., Kong L., Zou M., Lu G., Cao J., Yu X. Identification, expression, and comparative genomic analysis of the IPT and CKX gene families in Chinese cabbage (Brassica rapa ssp. pekinensis) Bmc Genom. 2013;14:594. doi: 10.1186/1471-2164-14-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ando S., Asano T., Tsushima S., Kamachi S., Hagio T., Tabei Y. Changes in gene expression of putative isopentenyltransferase during clubroot development in Chinese cabbage (Brassica rapa L.) Physiol. Mol. Plant Pathol. 2005;67:59–67. doi: 10.1016/j.pmpp.2005.09.005. [DOI] [Google Scholar]
- 43.Miyawaki K., Tarkowski P., Matsumoto-Kitano M., Kato T., Sato S., Tarkowska D., Tabata S., Sandberg G., Kakimoto T. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Nat. Acad. Sci. USA. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dekhuijzen H.M. The occurrence of free and bound cytokinins in clubroots and Plasmodiophora brassicae infected turnip tissue cultures. Physiol. Plant. 1980;49:169–176. doi: 10.1111/j.1399-3054.1980.tb02647.x. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z., Zhang M., Kong L., Lv Y., Zou M., Lu G., Cao J., Yu X. Genome-wide identification, phylogeny, duplication, and expression analyses of two-component system genes in Chinese cabbage (Brassica rapa ssp. pekinensis) DNA Res. 2014;21:379–396. doi: 10.1093/dnares/dsu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaller G.E., Kieber J.J., Shiu S.H. Two-component signaling elements and histidyl-aspartyl phosphorelays. Arabidopsis Book. 2008;6:e0112. doi: 10.1199/tab.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zubo Y.O., Schaller G.E. Role of the Cytokinin-Activated Type-B Response Regulators in Hormone Crosstalk. Plants. 2020;9:166. doi: 10.3390/plants9020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang I., Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature. 2001;413:383–389. doi: 10.1038/35096500. [DOI] [PubMed] [Google Scholar]
- 49.Sweere U., Eichenberg K., Lohrmann J., Mira-Rodado V., Bäurle I., Kudla J., Nagy F., Schäfer E., Harter K. Interaction of the response regulator ARR4 with phytochrome B in modulating red light signaling. Science. 2001;294:1108–1111. doi: 10.1126/science.1065022. [DOI] [PubMed] [Google Scholar]
- 50.Mok M.C., Martin R.C., Mok D.W. Cytokinins: Biosynthesis metabolism and perception. In Vitro Cell. Dev. Biol. Plant. 2000;36:102–107. doi: 10.1007/s11627-000-0021-7. [DOI] [Google Scholar]
- 51.Werner T., Schmülling T. Cytokinin action in plant development. Curr. Opin. Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Mrízová K., Jiskrová E., Vyroubalová Š., Novák O., Ohnoutková L., Pospíšilová H., Frébort I., Harwood W.A., Galuszka P. Overexpression of cytokinin dehydrogenase genes in barley (Hordeum vulgare cv. Golden Promise) fundamentally affects morphology and fertility. PLoS ONE. 2013;8:e79029. doi: 10.1371/journal.pone.0079029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu P., Zhang C., Ma J.Q., Zhang L.Y., Yang B., Tang X.Y., Huang L., Zhou X.T., Lu K., Li J.N. Genome-wide identification and expression profiling of cytokinin oxidase/dehydrogenase (CKX) genes reveal likely roles in pod development and stress responses in oilseed rape (Brassica napus L.) Genes. 2018;9:168. doi: 10.3390/genes9030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jo E.J., Jang K.S., Choi Y.H., Ahn K.G., Choi G.J. Resistance of Cabbage Plants to Isolates of Plasmodiophora brassicae. Korean J. Hortic. Sci. Technol. 2016;34:442–452. [Google Scholar]
- 55.Laila R., Park J.I., Robin A.H., Natarajan S., Vijayakumar H., Shirasawa K., Isobe S., Kim H.T., Nou I.S. Mapping of a novel clubroot resistance QTL using ddRAD-seq in Chinese cabbage (Brassica rapa L.) BMC Plant Biol. 2019;19:13. doi: 10.1186/s12870-018-1615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng F., Liu S., Wu J., Fang L., Sun S., Liu B., Li P., Hua W., Wang X. BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biol. 2011;11:136. doi: 10.1186/1471-2229-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 58.Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R.D., Bairoch A. ExPASy—the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robin A.H., Yi G.E., Laila R., Yang K., Park J.I., Kim H.R., Nou I.S. Expression profiling of glucosinolate biosynthetic genes in Brassica oleracea L. var. capitata inbred lines reveals their association with glucosinolate content. Molecules. 2016;21:787. doi: 10.3390/molecules21060787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.