Abstract
The excitability of spinal motoneurons is both fundamental for motor behavior and essential in diagnosis of neural disorders. There are two mechanisms for altering this excitability. The classic mechanism is mediated by synaptic inputs that depolarize or hyperpolarize motoneurons by generating postsynaptic potentials. This “ionotropic” mechanism works via neurotransmitters that open ion channels in the cell membrane. In the second mechanism, neurotransmitters bind to receptors that activate intracellular signaling pathways. These pathways modulate the properties of the voltage sensitive channels that determine the intrinsic input-output properties of motoneurons. This “neuromodulatory” mechanism usually does not directly activate motoneurons but instead dramatically alters the neuron’s response to ionotropic inputs. We present extensive evidence that neuromodulatory inputs exert a much more powerful effect on motoneuron excitability than ionotropic inputs. The most potent neuromodulators are probably serotonin and norepinephrine, which are released by axons originating in the brainstem and can increase motoneuron excitability 5-fold or more. Thus, the standard tests of motoneuron excitability (H-reflexes, tendon-taps, tendon vibration and stretch reflexes) are strongly influenced by the level of neuromodulatory input to motoneurons. This insight is likely to be profoundly important for clinical diagnosis and treatment.
Keywords: motor neuron, motoneuron, neuromodulation, serotonin, norepinephrine, reflex, reflex excitability
Introduction
Motoneurons are unique in the CNS, being the only cells whose firing patterns can be readily recorded in human subjects. These recordings are possible because motoneurons normally drive the muscle fibers that they innervate in a one to one fashion (Burke, 1981; Kernell, 2006). Thus the action potentials in the muscle fibers of the motor unit reveal the firing patterns of the motoneurons. The motoneuron produces its firing pattern by converting its synaptic input into action potentials. This conversion of input to output is determined by the intrinsic electrical properties of motoneurons, which are specified by the voltage sensitive conductances determining the cell’s threshold and firing behaviors (Kernell, 2006). If these intrinsic electrical properties were constant in all motor behaviors, then all variations in motor unit firing patterns could be ascribed to variations in synaptic input, i.e. to differences in synaptic connectivity. A primary goal of this review is to convince the reader that studies over the past 20 years in animal preparations force us to abandon this simplistic view and acknowledge that the intrinsic electrical properties of motoneurons, including their basic mechanisms of synaptic integration, may differ depending on the motor behavior. Initially, this type of variation may seem unlikely. It is easy to imagine that the synaptic inputs onto spinal motoneurons from the corticospinal system might differ from the synaptic inputs onto motoneurons from the vestibulospinal system and hence, that motor unit firing patterns during volition and posture might differ. How would the intrinsic electrical properties change in different motor behaviors? The classic view of motoneurons is that when the sum of synaptic inputs exceeds the motoneuron’s threshold for firing, action potential are generated at a rate in proportion to input amplitude. Unfortunately, this view ignores a whole class of inputs to motoneurons, the neuromodulatory system.
The neuromodulatory system can be best be appreciated by defining its fundamental differences from the classic input system, which is now often referred to as the “ionotropic” system because it operates by opening ion channels. A standard example of an ionotropic input is that evoked by the tendon tap, which is applied to the patellar tendon to briefly excite muscle spindle Ia afferents. These afferents then generate a transient release of the neurotransmitter glutamate at synapses on motoneurons, which bind to receptors that open ion channels and produce a brief but rapid depolarization in the motoneuron (the excitatory postsynaptic potential, or EPSP). In contrast, a neuromodulatory neurotransmitter binds to a different kind of receptor, one that does not open an ion channel but instead activates an intracellular signaling pathway involving multiple steps. Consider for example the neurotransmitter serotonin (5HT), a major focus of this review and a fundamentally important neuromodulator throughout the CNS. When 5HT binds to one of its receptors, a cascade of intracellular events is initiated. The end result of this cascade is often modification of the properties of voltage-sensitive ion channels and subsequent enhanced intrinsic electrical excitation of the target neuron. Note that the neuromodulatory input usually does not activate the neuron to produce an output - instead the neuromodulatory input changes the response of the neuron to subsequent ionotropic inputs.
Although an additional type of control system for motoneuron excitability might be viewed as an unnecessary complexity, it is possible that the goal of the neuromodulatory system is to “optimize” motoneuron properties to match different motor behaviors. Moreover, considerable data now emerging suggests that that the distortions in motor unit firing patterns in spinal injury and, perhaps, cerebral stroke are due to disruptions in the neuromodulatory control system (see below). It is also possible that disruptions in the excitability control system are important in the degeneration of motoneurons in amyotrophic lateral sclerosis (ALS). This insight is important from a clinical perspective, because the receptors that bind neuromodulators can be targeted by a wide range of pharmacological agents (Gawrylewski, 2008). The neuromodulatory systems thus potentially provide a basis for novel and highly effective therapeutic strategies for motor disorders.
A number of excellent reviews on neuromodulation of the spinal cord are available (Rekling et al., 2000; Schmidt and Jordan, 2000; Alaburda et al., 2002; Hornby et al., 2002; Heckman et al., 2003; Hultborn et al., 2004; Heckman et al., 2005; Toledo-Rodriguez et al., 2005; Lopez-Garcia, 2006; Heckman et al., 2008a; Heckman et al., 2008b). The monograph by Kernell (2006) provides an outstanding in depth consideration of motoneurons and muscle fibers.
Determinants of motoneuron excitability
Basic differences in ionotropic vs neuromodulatory effects
As noted in the Introduction, the excitability of a motoneuron was classically viewed as being determined by its relative level of ionotropic input from supraspinal centers or sensory receptors (Burke, 1981; Henneman and Mendell, 1981). Ionotropic inputs generate synaptic currents by releasing neurotransmitters that bind to postsynaptic ligand-gated channels that open pores to allow ions to enter or exit the cell. Depending on the neurotransmitter, the result can be either depolarization or hyperpolarization of the cells - i.e. ionotropic inputs generate the well-known excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) (Powers and Binder 2001). The classic neurotransmitters are glutamate (excitatory), glycine (inhibitory) and GABA (inhibitory).
Yet motoneurons are also densely innervated by neuromodulatory inputs. Much of our initial understanding of neuromodulatory effects came from studies in invertebrates (Nusbaum and Beenhakker, 2002; Marder and Bucher, 2007). Neuromodulatory receptors are not coupled directly to ion channels but instead to receptors linked to G-proteins, which then initiate intracellular signaling cascades (Hille 2001). (We focus here on neuromodulatory effects on voltage-sensitive channels. Neuromodulators can also alter the behavior of the ionotropic receptors; this effect has yet to be detected in motoneurons but requires further study). Various terms have been used for these types of inputs: neuromodulatory (because neuron properties are altered or “modulated”), G-protein coupled (because virtually all neuromodulatory inputs act via G-protein coupled receptors), metabotropic (because the intracellular cascades can be considered part of the neurons “metabolism”), and slow synaptic transmission (because the intracellular cascades are slower than opening of ion channels, which are called “fast synaptic transmission”). Essentially all these terms are equivalent and in this review we will use the term “neuromodulation”. One potentially confusing point is that almost all neurotransmitters can have both ionotropic and neuromodulatory effects because they influence neurons via more than one subtype of receptor (more on this below). For example, 5HT acts via 7 different receptor subclasses, 6 of which are neuromodulatory and 1 ionotropic (Nichols and Nichols, 2008). Glutamate, the transmitter for the classic EPSP, works via 6 basic types of receptor subtypes, of which only 3 are ionotropic and 3 neuromodulatory (Rekling et al., 2000). Motoneurons have receptors for 5HT and norepinephrine (NE) that tend to cause increases in intrinsic excitability, whereas interneurons in the dorsal horn have 5HT and NE receptors that tend to decrease intrinsic excitability. It should also be emphasized that neuromodulators can have presynaptic inhibitory effects.
Neuromodulation is but one example of a host of effects of G-protein coupled receptors throughout the body. The importance of this system for physiology can hardly be overstated: basic functions controlled by G-protein coupled receptors include many hormonally controlled processes, including cardiac function, glucose metabolism, pituitary function, steroid production, and embryonic development (Neves et al., 2002).The genes encoding G-protein coupled receptors constitute the largest single family in the human genome and it has been estimated that nearly half of the drugs currently in therapeutic use target these receptors (Gawrylewski, 2008). In the following paragraphs, we consider how neuromodulatory and ionotropic inputs compare in altering the excitability of motoneurons.
Basic parameters that control excitability of a motoneuron pool
Consider the two fundamental parameters of motor output, recruitment and rate modulation. A motoneuron’s recruitment threshold is assessed in comparison to other cells in the same motor pool (defined as the set of motoneurons innervating a single muscle). Recruitment threshold of a motoneuron is defined by two factors: 1) its relative share of the synaptic input (a motoneuron that receives a larger synaptic input will tend to be recruited before cells with smaller inputs) and 2) its intrinsic threshold (cells that have lower thresholds require less input to generate action potentials and be recruited) (Heckman and Binder, 1993b). The range of intrinsic thresholds is large, about 10-fold. Using the example of cat medial gastrocnemius motoneurons, the threshold range is from about 3 nA to more than 30 nA (Heckman and Binder, 1991). Most of this 10-fold range is due to differences in input resistance, as action potential voltage thresholds are similar across the pool (Gustafsson and Pinter, 1984). These threshold differences constitute the heart of Henneman’s size principle, because low threshold motoneurons tend to innervate slow twitch, low force but highly fatigue resistant muscle units (Henneman and Mendell, 1981). Progressively higher threshold units innervate progressively faster, stronger and more fatigable muscle units. In this review, we use Burke’s terminology for the resulting differences in motor unit type: S (slow), FR (fast, fatigue resistant) and FF (fast, fatigable) (Burke, 1981). The wide range of intrinsic threshold dominates the recruitment process, as it is unlikely that the summed ionotropic synaptic input to the pool generates 10-fold larger currents in high threshold units than low threshold units (Heckman and Binder, 1993b; Powers and Binder, 2001). Neuromodulatory inputs such can markedly alter motoneuron intrinsic thresholds. These threshold effects of neuromodulatory inputs on recruitment has not yet been carefully studied, but appear to be about equal on the electrical properties of S, FR and FF motoneurons (Lee and Heckman 1998 a,b and unpublished data). Thus, Henneman’s size principle (S before FR before FF) prevails in a wide range of movements.
Rate modulation, at first glance, may seem to be simpler than recruitment. Once a motoneuron reaches threshold, it generates action potentials at a rate roughly proportional to input amplitude (Kernell, 2006), as illustrated in Figure 1. This current-to-frequency gain is much more uniform across motoneurons in a pool, exhibiting not more that a 2-fold difference (Kernell, 2006). Yet, when considering motor unit firing patterns, the relative slopes of plots of motor unit firing rates versus time, force or each other (rate-rate plots) depend on their relative distribution of input across the pool, just as do recruitment thresholds. Motoneurons receiving a higher proportion of the synaptic input would not only tend to be recruited earlier but also tend to have high slopes in rate versus time or force and in rate-rate plots (Heckman and Binder, 1993a, b). The effects of neuromodulation on rate modulation are very strong because it impacts the very voltage sensitive conductances that generate spikes and acts to boost synaptic input (see below).
Figure 1:
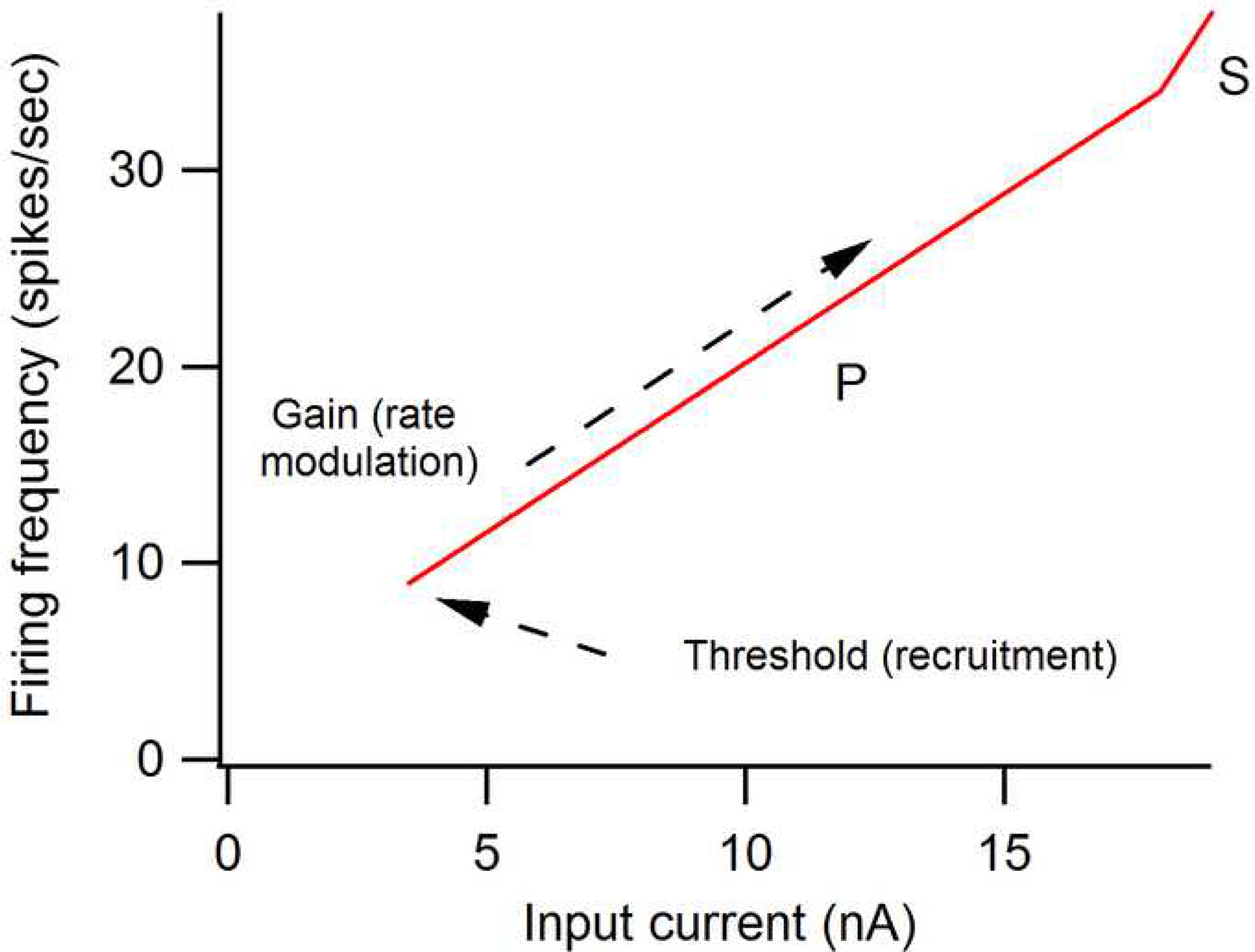
Simplified relation between current and frequency of firing of action potentials in a motoneuron, as in seen in an animal preparation where levels of neuromodulators are very low. These frequency-current (F-I) functions are usually generated using current injected via an intracellular microelectrode. Motoneurons always exhibit a relatively sharp threshold for initiation of firing, followed by a more or less linear conversion of increasing current amplitudes to progressively higher firing rates. Thus, the F-I function provides the motoneuron with a threshold and a gain (= slope of the function). The initial portion of the function is called the primary range (P) (Kernell, 2006). At higher levels, a higher gain region known as the secondary range (S) occurs. This is the “base” form of the F-I function; neuromodulators can greatly alter this form (see text and Fig. 6).
Ionotropic control of excitability of the motoneuron and the motor pool
Ionotropic descending and reflex inputs
Consider the net excitability of an entire motor pool. Ionotropic inputs have two interrelated effects on excitability, based on bringing the pool closer to threshold for recruitment and on the relative distribution of synaptic input between S, FR and FF motor units (Heckman, 1994). If the pool is quiescent, then steady activation bias that just brings the lowest threshold motoneurons right up to threshold will increase the effect of an additional input (e.g. suppose a steady vestibulospinal input is active, bringing the S units just up to threshold; a subsequent tendon tap will generate a larger reflex than when there is no vestibulospinal bias). This bias action continues to be effective at higher input levels. For example, with a bias sufficient to recruit a substantial portion of the pool, the recruitment of progressively larger motor units due to Henneman’s size principle gives a larger reflex force increment for a given input amplitude. Both modeling studies (Fuglevand et al., 1993; Heckman, 1994) and reflex experiments in human subjects (see Cathers et al., 2004 for a review) indicate that this “recruitment nonlinearity” mechanism is effective only up to about 30 to 50% of maximum voluntary force, as most of recruitment is complete by this level and those motor units still remaining un-recruited have progressively wider spacing between their thresholds.
Implicitly assumed in the previous paragraphs is that synaptic input to the pool is distributed uniformly among S, FR, and FF motoneurons. But non-uniform distributions of synaptic input between motor unit types provide the second potential effect of ionotropic input on excitability of the motor pool. As for the recruitment nonlinearity discussed above, this effect again depends on the size principle. If an input system tends lowers the threshold of the large FF units relative to the small S units, then this input will tend to generate more force for a given average activation level than an input which increases thresholds of FF units relative to S units.
Systematic studies of steady synaptic currents generated by a variety of sensory and descending systems by Binder, Powers and colleagues (Heckman and Binder, 1988; Powers and Binder, 2001) show that most inputs are in indeed non-uniform in their distribution among S, FR and FF motoneurons. In considering the relative strength of input on different motoneurons, these studies by Binder and colleagues relied on measurements of effective synaptic currents (IN’s) instead of EPSPs and IPSPs. IN is the net current generated at the soma by a synaptic input system. A steady EPSP is the product of IN times the input resistance of the cell. Because input resistance varies more than 10-fold in motoneurons, a system that generates equal IN in all cells would generate much larger EPSPs in FF than S motoneurons. IN thus more accurately reflects the organization of synaptic input. It should also be emphasized that equal distribution of IN strongly favors the normal, size principle recruitment sequence, because the amount of synaptic current required to reach spiking threshold in S units is much smaller than in FF units - largely because of the 10-fold range in input resistances (see Powers and Binder 2001 for a thorough review of these issues).
Rubrospinal and multisynaptic corticospinal inputs are much stronger in FF than S units, in fact tending to inhibit the S units. Vestibulospinal input is moderately larger in FF than S whereas Ia afferent input is moderately larger in S than FF. Synaptic currents from monosynaptic corticospinal inputs have not been measured yet, but measurements of EPSPs suggests an approximately equal distribution in S and FF motoneurons, so that effects of corticospinal input may be neutral with respect to recruitment (note: the EPSP is not a direct measure of current input because it is strongly influenced by input resistance, being proportional to current times resistance). Simulations based solely on recruitment at maximum rates suggest that inputs like the rubrospinal system could induce strong changes in gain (Kernell and Hultborn, 1990). Realistic consideration of rate modulation however suggests that gain increases of only about 20–40% are possible by these ionotropic mechanisms, assuming that recruitment of FF units is not so altered as to violate the size principle (Cope and Clark, 1991; Heckman and Binder, 1993b) (see Fig. 5 below). It is acknowledged that the relative potency of rubrospinal, corticospinal and vestibulospinal inputs on the different types of motor units described above are derived from data obtained using electrical stimulation. It is possible that these features change during the functional activation of these descending systems, that is, while the subject is performing a specific motor task, however, this issue is difficult to examine with presently available techniques.
Figure 5.
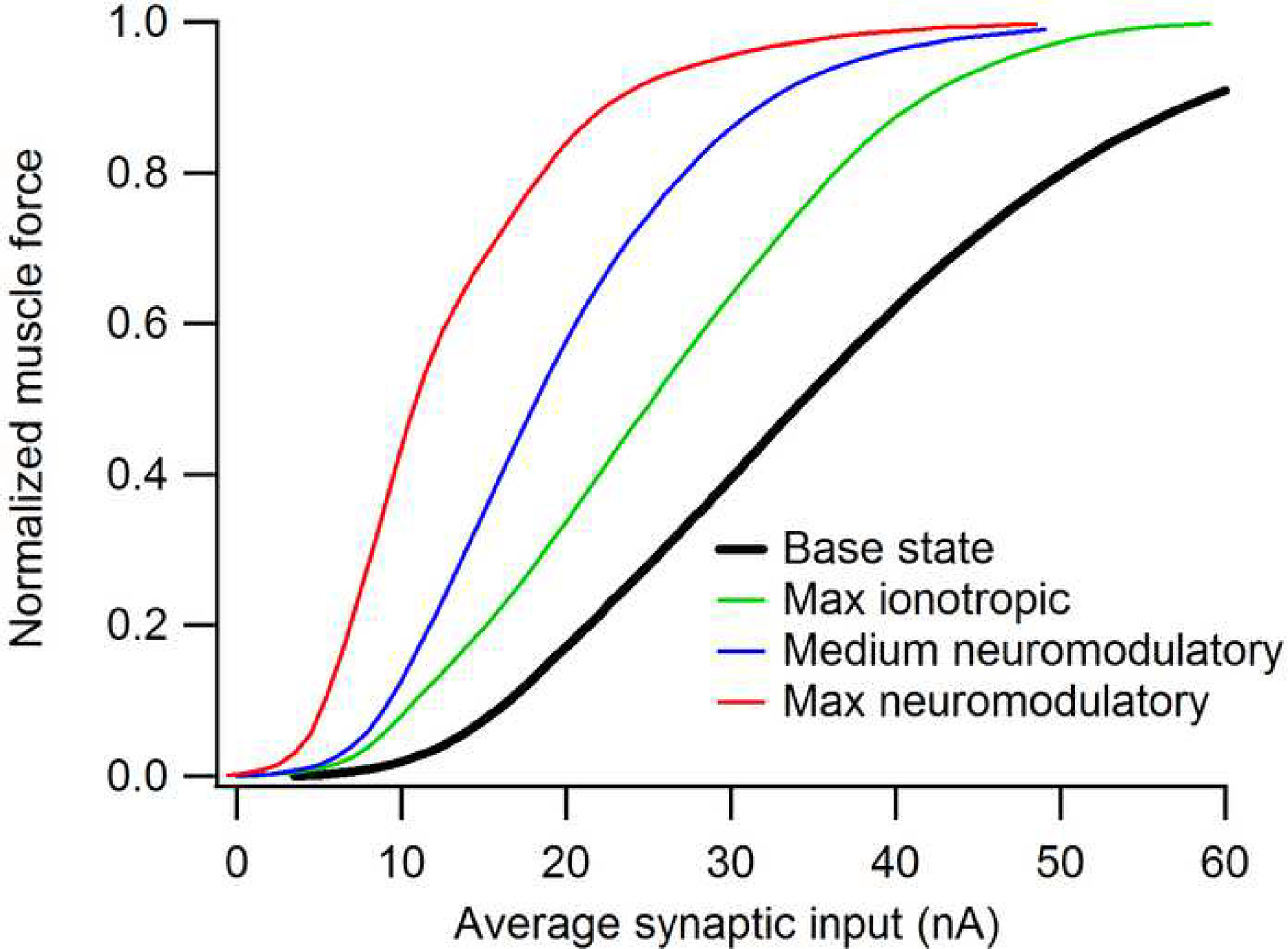
The effect of synaptic input on the input-output function of a motor pool and the muscle that it innervates, based on computer simulations of data for the feline medial gastrocnemius pool and muscle (Heckman and Binder, 1991, 1993a, b). The base state is simulated from data obtained in preparations where brainstem neuromodulatory input is suppressed and all motoneurons receive exactly equal proportions of synaptic input. The “Max ionotropic” effect is based the maximum sub-threshold depolarization that can be achieved without producing any motor unit recruitment and a distribution of input that favors high over low threshold units. This distribution was set as non-uniformly as possible with the the constraint that recruitment reversals should not exceed about 20% (Heckman and Binder, 1993b). Medium and Max neuromodulatory effects are based on studies where the brainstem is highly active and where this activity is supplemented by an exogenous monoaminergic agonist (Lee and Heckman, 1998a, b, 1999a).
Effects of presynaptic inhibition on motoneuron excitability
Presynaptic inhibition plays a major role in adjusting inputs among spinal circuits (Rudomin, 2002) and thus can also control motoneuron excitability. Presynaptic inhibition acts via synapses on presynaptic terminals (Rudomin, 2002) and can be mediated by either ionotropic or neuromodulatory actions on these terminals. Ionotropic effects are exerted by GABA but GABA can also have neuromodulatory presynaptic actions as can both 5HT and NE (Jankowska, 1992) (and see below). As yet presynaptic inhibition, whether mediated by ionotropic or neuromodulatory mechanisms, seems to be directly solely onto ionotropic inputs, though evidence of presynaptic inhibition of neuromodulatory inputs may emerge. Thus, for the present, the effects of presynaptic inhibition on motoneuron excitability should be considered an extension of ionotropic effects discussed above. It is important to note that the same last order GABAergic interneurons can make both pre- and postsynaptic contacts (Jankowska, 1992). Thus it may be difficult to separate pre- and postsynaptic actions within this system.
Effects of brainstem neuromodulatory systems on spinal neurons
Basic features of descending monoaminergic systems
Both 5HT and NE are monoamines and both have many similar effects on spinal neurons, so we discuss them together. The projection of the 5HT and NE systems to the spinal cord is diffuse, with individual axons giving off branches at multiple levels of the cord. There is substantial specificity in terms of effects dorsally versus ventrally: axons innervating the dorsal horn travel dorsolaterally in the superficial white matter while the ventral innervation to motoneurons is ventrolateral and ventromedial in the superficial white matter (Holstege and Kuypers, 1987). For specificity of action, the most important point is the existence of multiple receptor subtypes for both of these monoaminergic neuromodulatory systems (Bylund et al., 1994; Hoyer et al., 2002). For 5HT there are 7 subclasses and 14 basic subtypes. For NE there are two basic subclasses (alpha and beta) and several subtypes (Bylund et al., 1994). Generally speaking, the subtypes coupled to the Gq class of G-proteins tend to have facilitatory effects on their target neurons (e.g. the 5HT2 and NE alpha1 receptors on motoneurons), whereas those coupled to the Gi/o system exhibit inhibitory effects (i.e. the 5HT1 and NE alpha2 receptors in the dorsal horn of the spinal cord) (Hochman et al., 2001). It should also be emphasized that even within a receptor subclass, (e.g. 5HT2C receptors), post-transcriptional editing can take place to induce significant changes in receptor behavior (Nichols and Nichols, 2008). These effects are as yet far from fully understood yet may eventually have important implications for how receptor systems adapt to injury and disease. The most important point is that the effects of 5HT and NE on spinal neurons vary, depending on the receptor subtypes at the target neuron.
Differences in monoaminergic actions in dorsal, intermediate and ventral spinal cord
Speaking very broadly, there is an overall gradient of effects of the monoamines 5HT and NE from dorsal to ventral on spinal neurons. In the dorsal horn, inhibition of high threshold afferent input prevails (Garraway and Hochman, 2001b, a; Lu and Perl, 2007). This inhibition is probably presynaptic and is linked to suppression of pain pathways (Jankowska, 1992; Garraway and Hochman, 2001a). For the motor system, both 5HT and NE strongly suppress the flexion (withdrawal) reflex (Jankowska, 1992). In contrast, as described in this review, in the ventral horn the intrinsic properties of motoneurons are strongly facilitated by both 5HT and NE. In the intermediate portion of the cord, the interneurons that process low threshold afferent inputs, especially the proprioceptive inputs mediated by muscle spindle Ia and II afferents and Golgi tendon organ Ib afferents, are intermediate in terms of the effects of 5HT and NE. Group I pathways (both Ia and Ib) largely undergo facilitation (Jankowska et al., 2000; Hammar and Jankowska, 2003), but this facilitation does not appear to be as strong as in motoneurons. The excitatory effects of 5HT and NE on most ventral interneurons are modest (Theiss and Heckman, 2005). Moreover, after spinalization eliminates the brainstem 5HT and NE projections to the cord along with all other descending inputs, there is a profound drop in intrinsic excitability of motoneurons (Hounsgaard et al., 1988; Miller et al., 1996) but changes in reciprocal inhibition mediated by Ia inhibitory interneurons are small (Hyngstrom et al., 2008).Group II pathways are subject to mixed effects, with excitation for some components of these pathways and inhibition for others (Jankowska et al., 2002).Thus, overall effects of 5HT and NE progress along the dorsal-ventral gradient from strongly inhibitory to mixed to strongly excitatory.
There are exceptions to the generalizations described above. For example, neurons in the dorsal horn undergo an increase in intrinsic excitability while at the same time their afferent input undergoes presynaptic inhibition (Garraway and Hochman, 2001a). Nonetheless, from a motor perspective, the above dorsal-ventral generalization is a reasonable approximation. For motor reflexes, it is clear that the flexion reflex is inhibited by 5HT1 and NE alpha 2 receptors (Jankowska, 1992; Miller et al., 1995), that proprioceptive input from group I is slightly facilitated by 5H2 and NE alpha 1 receptors (Jankowska et al., 2000; Hammar and Jankowska, 2003), while motoneurons are profoundly excited by 5HT2 and NE alpha 1 receptors (see below). The interneurons involved in the genesis of locomotor patterns may be an exception to this generalization (see next section). These interneurons appear to be mainly facilitated by 5HT and NE, but their distribution along the dorsal-ventral gradient has not been fully investigated (next section).
Multiple neuromodulators affect motoneuron excitability but monoamines are the most powerful
As emphasized above, the neuromodulatory system that we focus on is the monoaminergic system, especially the axons releasing the monoamines 5-HT and NE. Admittedly, there are many other neuromodulators, many of which have not been systematically tested on motoneurons. Yet among those neuromodulators that have been tested, including peptides such as thyroid releasing hormone, substance P and amines such as acetylcholine and dopamine (Rekling et al., 2000; Powers and Binder, 2001), it appears that 5HT and NE have the most powerful effects on spinal motoneurons (this may not be true of phrenic and brainstem motoneurons; see (Rekling et al., 2000)). Axons that release 5HT or NE (the two systems are separate) originate in the brainstem (caudal raphe nucleus for 5HT and locus coeruleus and associated structures for NE) (Bowker et al., 1982; Holstege and Kuypers, 1987; Hochman et al., 2001). The detailed effects of brainstem monoaminergic systems on motoneurons are discussed in the next section, but first we briefly consider three other potential sources of neuromodulatory input to motoneurons.
Do sensory inputs have neuromodulatory effects?
The systematic studies of input onto motoneurons from Ia afferents show no sign of anything but ionotropic actions - for example, both transient and steady Ia EPSPs reduce in amplitude as membrane potential is depolarized when there is no significant output from brainstem neuromodulatory systems (e.g. Lee and Heckman, 1998a). A possible exception to lack of neuromodulatory input from sensory afferents is the excitation of flexor motoneurons by flexion reflex afferents, which exhibit voltage dependent amplification (Brownstone et al., 1994), perhaps due to activation of NMDA receptors (an ionotropic receptor that uniquely allows increased current with depolarization) or activation of as yet unknown neuromodulatory receptors.
Central pattern generators (CPGs)
From the initiation of locomotion in the mesencephalic locomotor region (MLR) to its execution by skeletal muscles driven by spinal locomotor central pattern generators (CPGs), locomotor activity depends on neuromodulatory systems for the appropriate excitation and patterning (Grillner et al., 2008). As for other types of movements, ionotropic actions are also necessary for locomotion (Jordan et al., 2008). Nonetheless it is clear that both 5HT and NE can initiate locomotion in vitro, where the cord is disconnected from descending and sensory inputs. Thus both systems can be considered to be strongly facilitory for the locomotor CPG (Schmidt and Jordan, 2000). The mechanism of these neuromodulatory actions is not yet clear and may perhaps be due to a generalized increase in excitability among the interneurons involved in the CPG. Nonetheless, as for motoneurons, the source of these monoaminergic effects is the brainstem and the parallel excitatory actions on the CPG and motoneurons undoubtedly increase net locomotor output. It should be emphasized however that the brainstem 5HT system is organized into multiple nuclei and that different nuclei probably mediate the effects on CPG interneurons and on motoneurons (Hochman et al., 2001).
The effects of monoamines on excitability of motoneurons and the motor pool
The effects of 5HT and NE on motoneuron excitability are multiple (Rekling et al., 2000; Krawitz et al., 2001; Powers and Binder, 2001), but their impact on PICs in motoneurons is especially potent and is the primary focus of this review. We do not however neglect other important actions: spike hyperpolarization, subthreshold depolarization and a reduction in the afterhyperpolarization (AHP) that follows each action potential.
Receptor subtypes for monoaminergic actions on motoneurons
As emphasized above, both 5HT and NE operate via multiple subtypes. Each type of neuron can have more than one subtype. For motoneurons, it is now clear that 5HT2 and NE alpha1 receptors facilitate PICs very strongly (Lee and Heckman, 1999a; Perrier and Hounsgaard, 2003; Harvey et al., 2006a)(see below for details). 5HT and NE also markedly depolarize the resting membrane potential but this is probably via a different receptor subtypes, perhaps 5HT6 or 7 (Harvey et al., 2006a). Motoneurons may also have 5HT1 receptors that have inhibitory actions but these are probably confined to the initial segment and may only be activated at high levels of 5HT input, perhaps serving as a brake for excessive excitability (Perrier and Cotel, 2008). Overall, the net impact of both brainstem 5HT and NE inputs on motoneuron is to greatly enhance their intrinsic excitability.
Properties of PICs
The classic studies of PICs focused on the striking phenomenon of “bistability”, in which a short pulse of excitatory input can produce self-sustained firing, which then can be terminated by a short pulse of inhibition (Schwindt and Crill, 1980b, a; Hounsgaard et al., 1988). Self-sustained firing is likely a fundamental component in the maintenance of posture (Hounsgaard et al., 1988; Lee and Heckman, 1998b). The dendrites of motoneurons are densely covered with 5HT-containing synaptic boutons (Alvarez et al., 1998) and studies both in vitro (Hounsgaard and Kiehn, 1993; Carlin et al., 2000) and in vivo (Lee and Heckman, 1996; Bennett et al., 1998) established that most of the PIC is of dendritic origin. The PIC in mammals is generated primarily by two types of channels: L-type calcium channels (CaV 1.3) and persistent Na channels (molecular subtype unknown) (Lee and Heckman, 1999b; Li et al., 2004a). Thus there is a CaPIC and an NaPIC. Figure 2 illustrates the basic nature of the CaPIC (the NaPIC is blocked): once turned on by the linearly rising input current, the CaPIC stays on steadily until input falls well below the initial onset level. This “hysteresis” is an inherent consequence of the persistence of these currents: once activated, the CaPIC adds a very large net current and thus requires a large decrease in input to deactivate it. In addition, the CaV 1.3 channels have an inherent, still poorly understood, tendency to remain open even when membrane potential is hyperpolarized to their activation threshold (Moritz et al., 2007). This resistance to deactivation is a major feature of the CaPIC. The NaPIC is more readily deactivated yet is still highly persistent as long as membrane potential exceeds its voltage threshold. For the first few seconds of a PIC, the NaPIC contributes about half of the total (Lee and Heckman, 1999b; Li et al., 2004a). After that point, the NaPIC tends to decay while the CaPIC can persist for many seconds (Lee and Heckman, 1999b; Li et al., 2007). An important related point is that the CaPIC is relatively slow in both its activation and deactivation (Elbasiouny et al., 2006; Harvey et al., 2006a; Li et al., 2007). In contrast, the NaPIC is very fast, as it is likely due to a subset of the channels producing the action potential. Thus, the NaPIC may serve to amplify and prolong transient synaptic inputs that would otherwise be too fast to activate the CaPIC (Jones and Lee, 2006).
Figure 2:
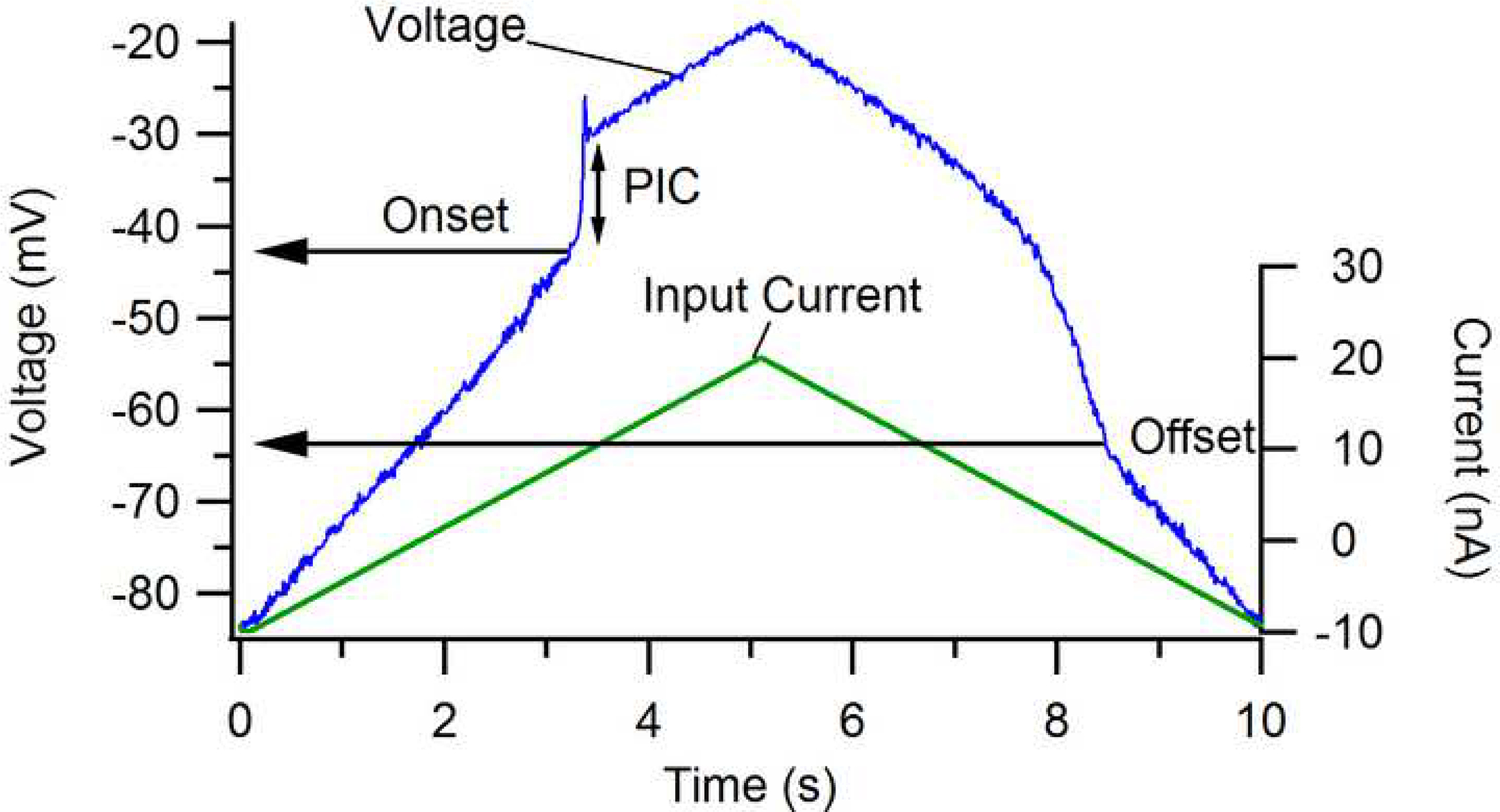
Effect of the persistent inward current (PIC) on motoneuron membrane potential when action potentials are blocked (via intracellular injection of QX314, a lidocaine derivative). The PIC is manifest as a strong depolarization (vertical arrow). Note that its onset is at a more depolarized level than its offset. This hysteresis is a fundamental behavior of the Ca channel that mediates about half of the total PIC. Blue trace: membrane potential. Green trace: current injected via the microelectrode. Data from Lee and Heckman (1999b).
PIC mediated amplification of synaptic input
The development of voltage clamp techniques in the decerebrate cat preparation (Lee and Heckman, 2000) focused attention on a new aspect of dendritic PICs: their extremely potent amplification of synaptic input (Lee and Heckman, 1996, 2000; Hultborn et al., 2003). Figure 3 illustrates the effects of the PIC on excitatory synaptic input generated by tendon vibration that selectively activate muscle spindle Ia afferents (data taken from a feline medial gastrocnemius motoneuron). This monosynaptic input produces a synaptic current with a crisp onset and offset - but only when the cell is clamped at a hyperpolarized potential (upper trace; in voltage clamp, excitatory inputs generate downward currents). When the cell is clamped at a depolarized potential, approximately equal to the level at which spiking would occur in the unclamped state, the very same input generates a much larger net synaptic current due to activation of the dendritic PIC (green vertical arrow; baseline currents removed to allow traces to be superimposed). Equally important, the PIC continues to be active once the excitatory synaptic reflex afferents, which exhibit voltage dependent amplification (Brownstone et al., 1994), perhaps due to activation of NMDA receptors (an ionotropic receptor that uniquely allows increased current with depolarization) or activation of as yet unknown neuromodulatory receptors.
Figure 3:
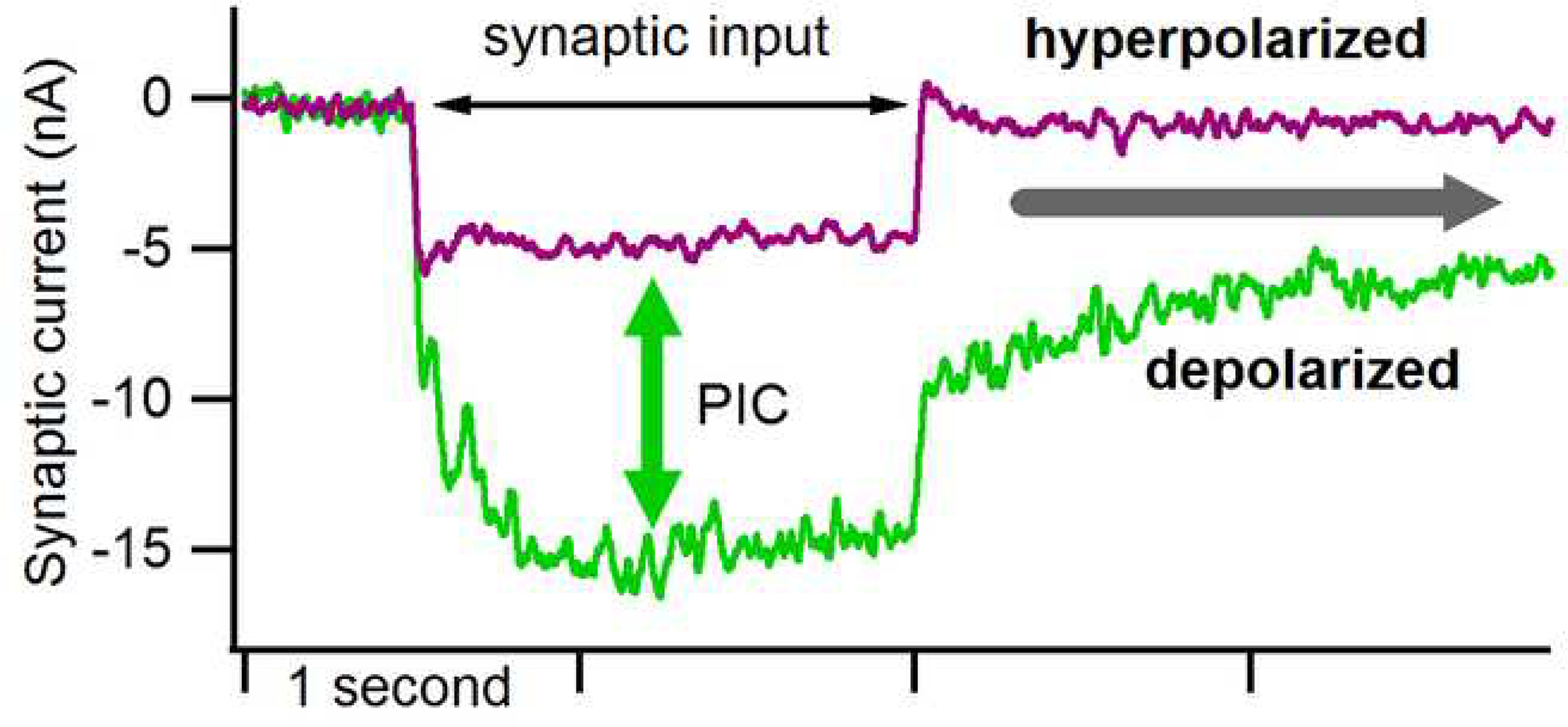
Effect of the PIC on excitatory synaptic input. The synaptic currents were measured during voltage clamp and thus, by convention, excitatory input is downwards. When the cell was clamped at a hyperpolarized level (about −90 mV in a cell with a resting potential of about −60 mV), steady activation of muscle spindle Ia afferents via tendon vibration generated a modest current with a crisp onset and offset (purple trace). In the same cell, shifting the voltage clamp to about −50 mV (the level at which firing of action potentials would occur if the cell was not voltage clamped), the very same input generated a much larger synaptic current (more than 2-fold) as well as a sustained current lasting long after the input ceased (gray arrow). Note that baseline currents have been removed to allow the traces to be superimposed. The difference between the two traces (green arrow) reflects the potent effect of the PIC. Data from Lee and Heckman (1996).
Central pattern generators (CPGs)
From the initiation of locomotion in the mesencephalic locomotor region (MLR) to its execution by skeletal muscles driven by spinal locomotor central pattern generators (CPGs), locomotor activity depends on neuromodulatory systems for the appropriate excitation and patterning (Grillner et al., 2008). As for other types of movements, ionotropic actions are also necessary for locomotion (Jordan et al., 2008). Nonetheless it is clear that both 5HT and NE can initiate locomotion in vitro, where the cord is disconnected from descending and sensory inputs. Thus both systems can be considered to be strongly facilitory for the locomotor CPG (Schmidt and Jordan, 2000). The mechanism of these neuromodulatory actions is not yet clear and may perhaps be due to a generalized increase in excitability among the interneurons involved in the CPG. Nonetheless, as for motoneurons, the source of these monoaminergic effects is the brainstem and the parallel excitatory actions on the CPG and motoneurons undoubtedly increase net locomotor output. It should be emphasized however that the brainstem 5HT system is organized into multiple nuclei and that different nuclei probably mediate the effects on CPG interneurons and on motoneurons (Hochman et al., 2001).
The effects of monoamines on excitability of motoneurons and the motor pool
The effects of 5HT and NE on motoneuron excitability are multiple (Rekling et al., 2000; Krawitz et al., 2001; Powers and Binder, 2001), but their impact on PICs in motoneurons is especially potent and is the primary focus of this review. We do not however neglect other important actions: spike hyperpolarization, subthreshold depolarization and a reduction in the afterhyperpolarization (AHP) that follows each action potential.
Receptor subtypes for monoaminergic actions on motoneurons
As emphasized above, both 5HT and NE operate via multiple subtypes. Each type of neuron can have more than one subtype. For motoneurons, it is now clear that 5HT2 and NE alpha1 receptors facilitate PICs very strongly (Lee and Heckman, 1999a; Perrier and Hounsgaard, 2003; Harvey et al., 2006a)(see below for details). 5HT and NE also markedly depolarize the resting membrane potential but this is probably via a different receptor subtypes, perhaps 5HT6 or 7 (Harvey et al., 2006a). Motoneurons may also have 5HT1 receptors that have inhibitory actions but these are probably confined to the initial segment and may only be activated at high levels of 5HT input, perhaps serving as a brake for excessive excitability (Perrier and Cotel, 2008). Overall, the net impact of both brainstem 5HT and NE inputs on motoneuron is to greatly enhance their intrinsic excitability.
Properties of PICs
The effects of the brainstem neuromodulatory systems on spinal circuits described in the previous section provide a basis for defining their overall function in comparison to ionotropic systems. A comparison of ionotropic Ia input from muscle spindles and neuromodulatory 5HT input from the brainstem provides a clear illustration of the differences between these two systems. Both inputs are monosynaptic. If the Ia input is activated when the 5HT input is absent, as in deeply anesthetized preparations or acute spinal injury, there is no activation of the PIC for the simple reason that the PIC channels are in a dis-facilitated unresponsive state (hence the lack of PIC amplification of Ia input in the “low” monoaminergic state in Fig. 4). At the other extreme, if there is substantial steady output from the 5HT brainstem system to activate the 5HT2 receptors that facilitate the PIC but no ionotropic input, there is also no PIC activation. The 5HT2 receptors just put the PIC channels in a facilitated state but provide no depolarization to cause the membrane potential to reach voltage threshold for these channels (Note: as noted above 5HT does depolarize the cell, but via a different set of receptors subtypes - perhaps 5HT6 or 7 (Harvey et al., 2006a). The 5HT2 receptors do not cause the PIC channels to open, only membrane voltage changes can do that). Thus, neuromodulatory inputs control the intrinsic excitability of the motoneuron; they are unlikely to be the source of motor commands to recruit and de-recruit motoneurons in the patterns needed for specific movements (Heckman et al., 2003).
Figure 4:
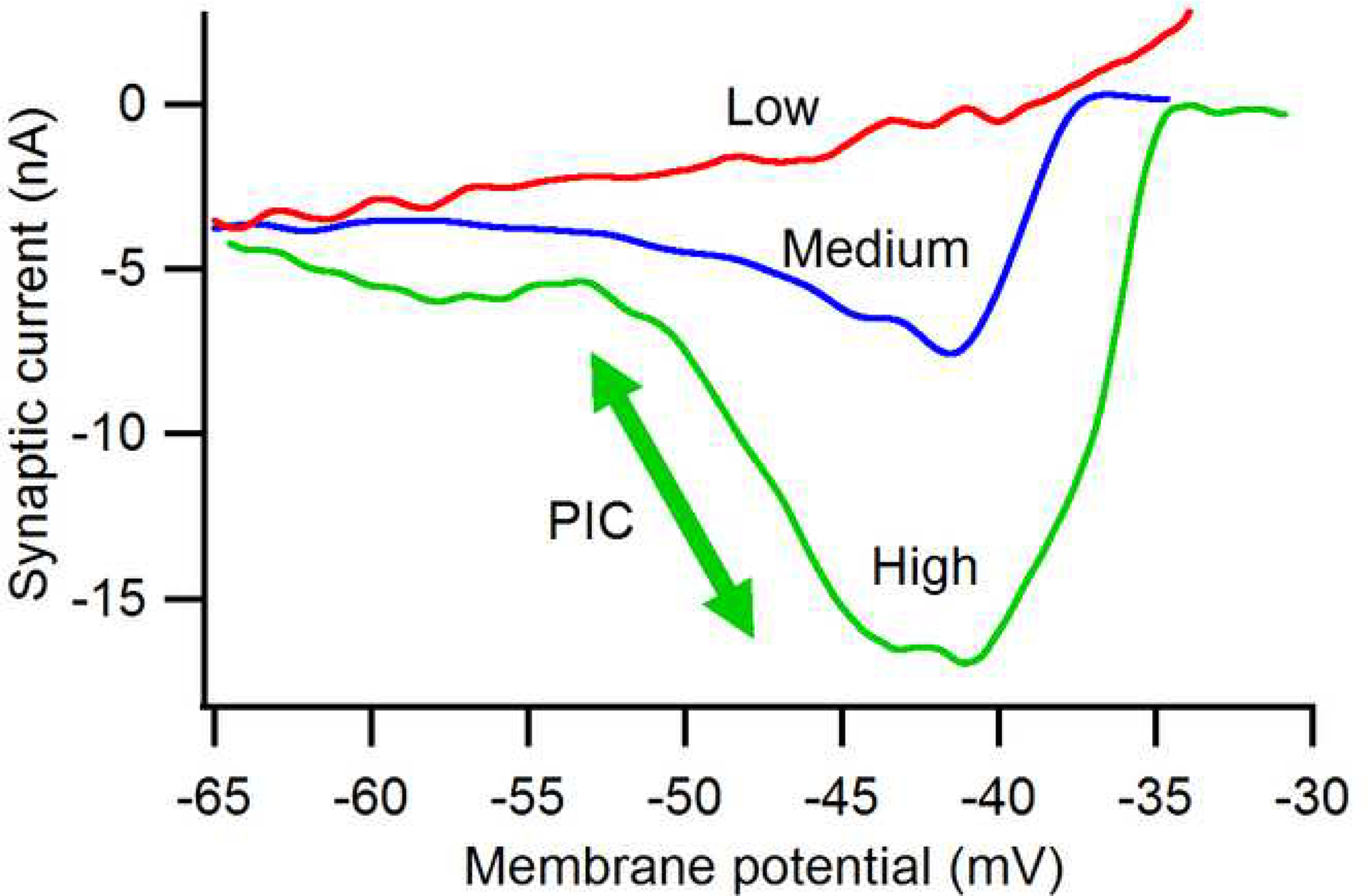
Typical amplification of synaptic currents generated by sustained activation of muscle spindle Ia afferents. As the background of neuromodulatory input from the brainstem is increased (low to medium to high), the peak amplitude of this ionotropic synaptic input increases 5-fold. The arrow indicates the effect of the PIC at the high level of neuromodulatory input. Data from Lee and Heckman (2000).
The sources of neuromodulatory input provide another key to understanding its functional role. Kuypers and colleagues, who played a major role in establishing the anatomy of these systems, emphasized the links between the 5HT and NE systems and the “emotional” systems of the brain (Bowker et al., 1982; Holstege and Kuypers, 1987).The locus coeruleus and associated nuclei, which are the source of the axons containing NE in the spinal cord and elsewhere in the brain, have long been known to play a fundamental role in attention and arousal (Aston-Jones et al., 2001; Aston-Jones and Cohen, 2005). The 5HT system is involved in many behaviors, including anxiety and appetite (Nichols and Nichols, 2008). Thus Kuypers and Holstedge emphasized that “… the emotional brain can exert a powerful influence on all regions of the spinal cord and may thus control both its sensory input and motor output.”(Holstege and Kuypers, 1987). In contrast, Jacobs and colleagues have emphasized the motor output effects of the 5HT system (Jacobs and Fornal, 1993; Jacobs et al., 2002). Their chronic recordings of putative serotonergic neurons in the caudal raphe nuclei, which project to the spinal cord, revealed that these cells varied their firing rates in proportion to speed of locomotion. A striking feature of these results is that most raphespinal neurons exhibit tonic firing during locomotion instead of modulating in phase with the locomotor cycle, suggesting a role in setting overall state instead of moment to moment variations. Jacobs and colleagues proposed the hypothesis that primary function of the spinal 5HT system is to suppress sensory input and facilitate motor output (Jacobs and Fornal, 1993). Because the raphespinal neurons increase activity whenever motor output increases, there is a substantial increase in 5HT in the cord during locomotion and this increase does not require a high state of fear or arousal. The primary link to the “emotional brain”, i.e. to arousal, is thus likely via the NE system. A final important point about both the 5HT and NE systems: activity in both is markedly reduced during sleep (Jacobs et al., 2002). For example, in the studies of Jacobs and colleagues, raphespinal neurons firing rate dropped dramatically during slow wave sleep and went to zero during rapid eye movement sleep. Suppression during sleep is entirely consistent with the proposed roles for the 5HT system in motor behavior and the NE system in arousal. In addition, the raphespinal neurons play a role in pain transmission and modulation (e.g. Hochman et al., 2001; Lu and Perl, 2007).
The question of just how much of normal motoneuron excitability is due to the brainstem neuromodulatory system is difficult to answer directly. Nonetheless, several lines of evidence indicate that this system is the dominant factor. As emphasized in the previous section, the effects of 5HT and NE on motoneurons are extremely potent, with increases in gain of up to 5 fold or more coupled to substantial decreases in threshold for activation. Moreover, the sum of maximal stimulation of multiple ionotropic inputs is rather modest: as emphasized by Binder and colleagues, maximal stimulation of the corticospinal, rubrospinal, vestibulospinal and Ia input systems would only generate about 20 to 30 nA of excitatory input (the inhibitory component in the rubro- and corticospinal components is ignored in this estimation) (Powers and Binder, 2001; Binder, 2002, 2003). This quantity is barely sufficient to bring an average motoneuron innervating fast twitch muscle units to recruitment threshold and would not produce significant rate modulation in these high threshold motoneurons. Or consider posture: together the Ia and vestibulospinal systems generate a maximum total of about 5–6 nA; this is sufficient to only generate about 1 to 3% of maximum muscle force (Heckman and Binder, 1991), whereas 5 to 10% is needed for posture (Walmsley et al., 1978).
An important additional point to consider is that application of both 5HT and NE result in depolarization of the resting membrane potential of motoneurons by closing a resting K channel and by depolarizing a mixed ion channel that generates the H current (Powers and Binder, 2001). This depolarization can be very strong, as much as 5 to 10 mV and, especially in high threshold motoneurons, may be important in lowering input levels needed for recruitment. Coupled with the hyperpolarization of the spike threshold (Krawitz et al., 2001; Fedirchuk and Dai, 2004; Gilmore and Fedirchuk, 2004), it is clear that the monoaminergic systems potently affect recruitment thresholds. The PIC greatly affects the pattern of rate modulation (see next section). In addition, both neuromodulators reduce the action potential AHP, which will also increase the gain of conversion of current to firing rate (Lindsay and Feldman, 1993; Lee and Heckman, 1999a) and this effect may be strong during locomotion (Brownstone et al., 1992).
Figure 5 summarizes the impact of ionotropic and monoaminergic actions on the net input-output function of a motor pool (the feline medial gastrocnemius). As technical limitations preclude direct measurements of this overall function in either human or animal subjects, this figure is based on computer simulations that synthesize the extensive data from animal preparations (Heckman and Binder, 1991, 1993b). Note that the overall form is sigmoidal (Heckman and Binder, 1991; Fuglevand et al., 1993), with the initial upwards curvature due to recruitment of larger units, the linear midrange largely being due to rate modulation and the slow approach to maximum due to the force limitations of the motor units. It is clear that moderate and strong levels of monoamines induce an enormous increase in net pool excitability (blue and green traces). As noted above, the maximum ionotropic effect that can be achieved without inducing more than 10 to 20% recruitment reversals (see Cope and Clark, 1991; Haftel et al., 2001) also increases gain (green trace), but not as much as the neuromodulatory effects. Note also that monoaminergic effects are unlikely to affect recruitment order, because they appear to be approximately equal in all motoneurons or, perhaps, favor recruitment of S units (Lee and Heckman, 1998a, b). Thus it is clear that the neuromodulatory effects of monoamines have a more potent effect on motoneuron excitability than ionotropic actions. One might however argue that large mononaminergic effects are confined to extreme states of intense motor output or extreme arousal (“fight or flight”). Yet the proportional changes in raphespinal output with locomotion (Jacobs et al., 2002) argue against this viewpoint, as does the need for monoaminergic input for even the low levels of motor output required for posture (see above).
Flexible control of motoneuron excitability: essential role for reciprocal inhibition
Given the potent effect of 5HT and NE on motoneuron gain and threshold and the clear variation in monoaminergic output with increasing motor output and increasing arousal, it is clear that this neuromodulatory system potentially provides the motor system with a highly flexible control of motoneuron excitability. The degree to which this system is used to fine tune motoneuron excitability remains an open question. It is possible that different types of motor behavior would be best matched by different levels of motoneuron excitability. For example, movements requiring a high degree of precision might benefit from low motoneuron gain, to minimize the effects of errors in descending motor command (Binder and Stuart, 1981). Yet it is difficult to reconcile fine tuning of motoneuron gain for different motor behaviors with the very diffuse nature of the descending 5HT and NE axons. It thus appears likely that specific increases in excitability to only the muscle used in a particular task are not possible with this system (Holstege and Kuypers, 1987). Perhaps more important, both agonist and antagonists will undergo increased excitability. Given the potent effects of both 5HT and NE on motoneurons, high levels of activity in the brainstem neuromodulatory system could force motor pools all over the body into highly excitable state, forcing widespread co-contraction and actually limiting movement capacity.
This diffuse yet potent system for control of excitability appears to require an opposing system - one that would readily deactivate PICs and be highly focused in its connections. Recent studies have shown that the classic Ia reciprocal inhibitory system is well suited to this role. It has long been known that synaptic inhibition, presumably of the ionotropic type, can deactivate the PIC (Hounsgaard et al., 1988). It now turns out that classic reciprocal Ia inhibition is especially effective for PIC deactivation (Heckman et al., 2008a). Because Ia afferents fire in response to joint rotations, the effect of reciprocal inhibition on the PIC potentially links motoneuron excitability to joint movements. In fact, we have shown that an ankle rotation of only 20 degrees can reduce PIC amplitude by an average of 50% (Hyngstrom et al., 2007). Overall, these results suggest that the control of motoneuron intrinsic excitability is dominated by two opposing systems: a diffuse, descending neuromodulatory input (i.e. 5HT, NE) and a local, focused inhibitory system (i.e. Ia reciprocal inhibition). Ia reciprocal inhibition is in large part mediated by the neurotransmitter glycine, which does not appear to act on G-protein coupled receptors and thus is likely to be purely ionotropic. The mechanism of the potent action of Ia reciprocal inhibition on the PIC is unclear - most of the PIC is dendritic, but it is generally thought that most of the Ia reciprocal inhibitory synapses are close to the soma (Fyffe, 1991; Fyffe, 2001). Further study is required, via both simulations (e.g. Bui et al., 2008a, b) and anatomical approaches.
Functionally however this interaction between a diffuse descending system and a specific local system provides a very effective control of motoneuron intrinsic excitability. Overall excitability of many muscles can be set to a high state and then reciprocal inhibition can be used to “sculpt” this overall state into a specific movement pattern. It should be emphasized that Ia inhibitory interneurons that mediate reciprocal inhibition receive strong inputs from descending systems, including cortico-, rubro- and vestibulospinal inputs (Jankowska, 1992). Thus descending motor commands adjust the pattern of motoneuron excitability as needed for any given movement. It is suggested below that disruption in this “voluntary” control of reciprocal inhibition may play a major role in the movement deficits that occur in stroke patients.
There are of course a number of other inhibitory systems in the spinal cord as well as presynaptic inhibition. These too are likely important in opposing monoaminergic facilitation of motoneuron excitability. Much further work on this issue is required. Perhaps the most important starting point is to reconsider the role of Renshaw cells and recurrent inhibition (Hultborn et al., 1979; Alvarez and Fyffe, 2007), which has recently been shown to provide potent deactivation of PICs (Hultborn et al., 2003; Bui et al., 2008a). Equally important is the potent inhibition generated by noiciceptive inputs - even relatively low threshold stimulation of a nerve with many flexion reflex afferents can completely suppress the PIC in an extensor motoneuron (Kuo et al., 2003). In addition, the question of the influence of GABAb receptors on motoneurons should be considered. These are inhibitory neuromodulatory receptors that are widespread in the CNS (Charles et al., 2003). Administration of baclofen, a GABAb agonist, has been shown to reduce PICs in vitro (Svirskis and Hounsgaard, 1998; Li et al., 2004c). This effect however occurs at substantially higher concentrations than its effect on EPSPs (Li et al., 2004c) and the relative roles of GABAb pre and postsynaptic remain uncertain.
The recent study by Berg et al (2007) has emphasized the possibility of mixing excitation and inhibition in activating motoneurons. If, as in the case of the scratch reflex in turtle motoneurons in the Berg et al work, the inhibitory component is large, than motoneurons can be activated while the PIC is suppressed. This type of combination of excitation and inhibition may contribute to force fluctuations in fatigue (Mottram et al., 2005). An alternative is to couple excitation and inhibition in a push-pull fashion, so that depolarization is mediated by a combination of excitation and disinhibition. Recent studies in our lab suggest this push-pull mode may provide a near ideal way of activating and de-activating PICs while preserving the ability of reciprocal inhibition to focus the effects of descending neuromodulation on the intended motor pool (Johnson and Heckman, unpublished studies).
Measuring PICs in humans
If PICs are a major component of normal motoneuron excitability, then motor unit firing patterns should exhibit behaviors consistent with PIC behaviors. Inferences about which features of the human motor unit firing patterns are due to specific intrinsic properties or synaptic inputs are necessarily indirect. Nonetheless, it is essential to be guided by the intracellular results from animal preparations. For example, it is impossible to measure the action potential afterhyperpolarization (AHP) directly in motor units - but no one doubts that the AHP is a major determinant of human motor unit firing rates. The AHP in fact provides a reasonable basis for comparison with the PIC: is there now sufficient evidence for the PIC’s role in normal motor unit firing patterns that it can be, like the AHP, generally accepted as a mechanism in human subjects? For the AHP, one particularly striking result is that motoneuron firing is a relatively non-variable process. A wide range of studies in humans and in animals show that spiking driven by synaptic input has a low co-efficient of variation (mean firing rate/standard deviation of firing rate) - 0.2 or less (Fetz et al., 2002). In animal preparations, injected current produces even more regular firing patterns (Kernell, 2006). Yet, when the AHP is reduced by a drug in animal preparations (Manuel et al., 2005, 2006), spiking to even current injection becomes highly irregular. An equally fundamental role can be ascribed to the NaPIC, which has been shown to be essential for generation of action potentials to slowly rising or steady inputs (Harvey et al., 2006b; Kuo et al., 2006). If NaPIC amplitude is too small, motoneurons can only generate action potentials to sharp transient inputs, but cannot produce prolonged output trains (the same is true of spinal interneurons (Theiss et al., 2007). The level of NaPIC needed for this basic action potential generating role is modest. In both acute spinal and deeply anesthetized preparations, where levels of 5HT and NE are low, motoneurons have significant NaPIC and usually exhibit reasonable repetitive firing.
As 5HT and NE levels increase to moderate to high levels, both the NaPIC and CaPIC become substantially larger and generate clear hallmarks in motoneuron firing patterns. Figure 6 (based on Bennett et al., 1998; Lee and Heckman, 1998b; Lee et al., 2003) shows that the firing pattern generated by a motoneuron with a strong PIC is very unlike the base state frequency-current function illustrated in Fig. 1 (repeated on the right in Fig. 6). As 5HT and NE input increases, the PIC becomes larger and more hyperpolarized. The PIC onset produces an acceleration in firing (which corresponds to the secondary range, labeled “S” in Fig. 6). As the PIC increases and its activation hyperpolarizes, this acceleration/secondary range increases and initiates closer to threshold. In cells with medium to high levels of monoaminergic input, the PIC generally activates at or before recruitment threshold (green trace in Fig. 6). Once the PIC is fully activated, the cell enters a “tertiary” range (“T” in Fig. 6) when it becomes less responsive to excitatory input. This tertiary range has also been called the “preferred firing range” (Hornby et al., 2002), as it appears that most of normal human motor unit rate modulation in fact takes place in this range (see below).
Figure 6:
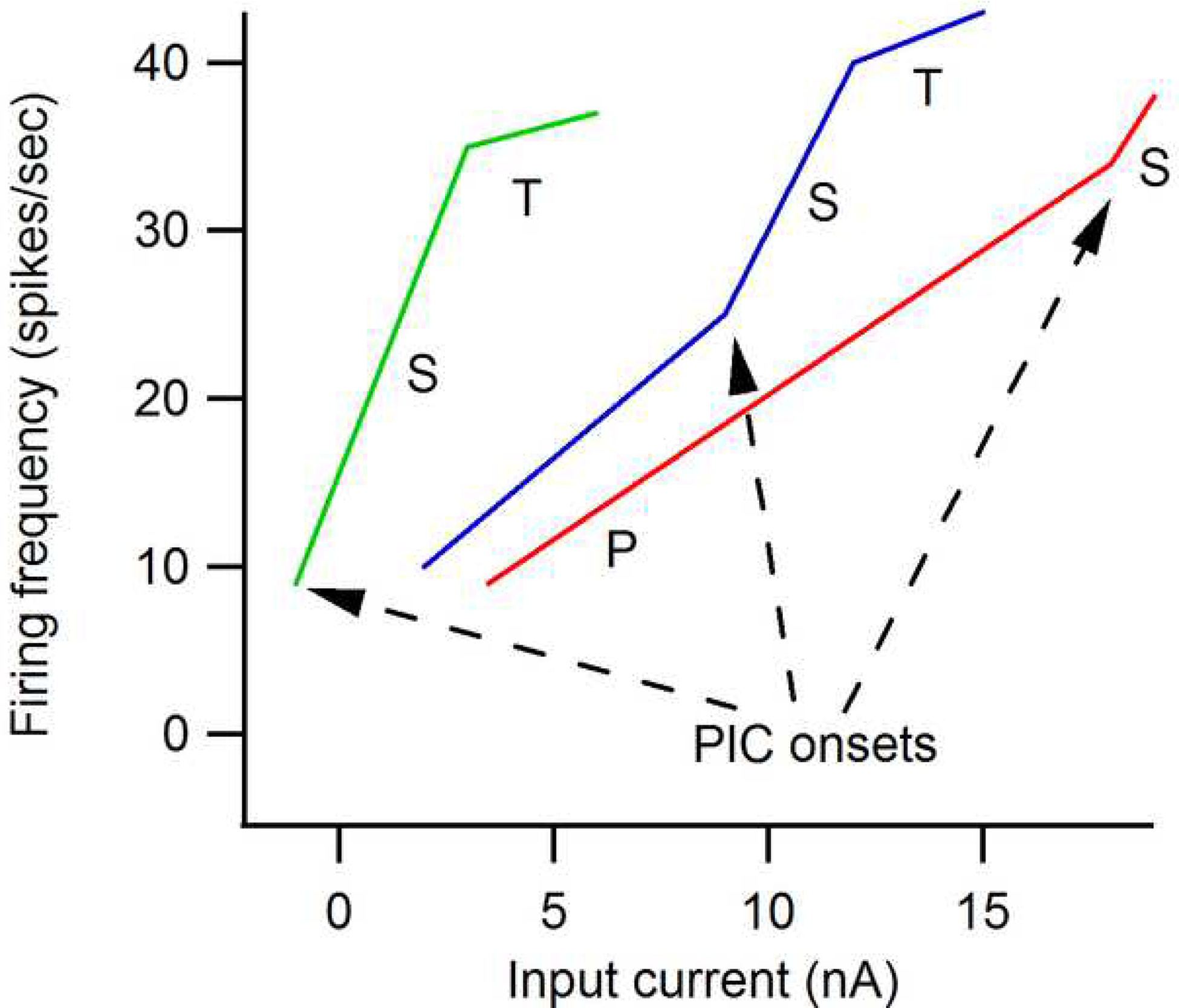
Transformation of the motoneuron frequency-current (F-I) function by neuromodulatory input. As monoaminergic input from the brainstem increases from low (red trace) to medium (blue) to high (green), the threshold of the cell is lowered markedly. In addition the PIC becomes larger and its threshold is lowered. As a result, the primary range disappears and firing is dominated by the PIC. There is an initial acceleration in firing (the secondary range) followed by a more shallow but usually still positive tertiary range (this phenomenon has variously been referred to as rate limiting, saturation and the “preferred” firing range).
Figure 7 summarizes the firing behaviors that occur in a motoneuron with a medium to strong PIC during a slowly increasing and decreasing voluntary drive: 1: initial acceleration (secondary range); 2: preferred firing range (tertiary range); and 3) onset-offset hysteresis (offset at lower current than onset). Note that the decline is linear because the PIC remains on and does not deactivate until the cell returns below its threshold level - this is a consequence of the hysteresis. It is not unusual however to see some deceleration in firing just as de-recruitment approaches. The 4th hallmark, not illustrated here, is self-sustained firing in response to a brief input. Each of these 4 hallmarks of PIC behavior is present in human motor unit firing patterns (Kiehn and Eken, 1997; Gorassini et al., 2002b, a; Walton et al., 2002) and indeed the acceleration in discharge has been consistently seen in a wide range of studies (e.g. Person and Kudina, 1972; De Luca et al., 1982; Romaiguere et al., 1989). Figure 8 shows results from motor units in the biceps brachii muscle of a healthy person, illustrating the hallmarks of Fig. 7. The tendency for self-sustained firing is likely also a major contributor to the enhancement in force demonstrated by Collins and colleagues in response to electrical stimulation of muscle afferents (Collins et al., 2002a; Collins et al., 2002b; Nickolls et al., 2004). This is a strong phenomenon that may provide a marked increase in efficacy and fatigue resistance for functional electrical stimulation of humans with spinal injury or other diseases of the CNS.
Figure 7:
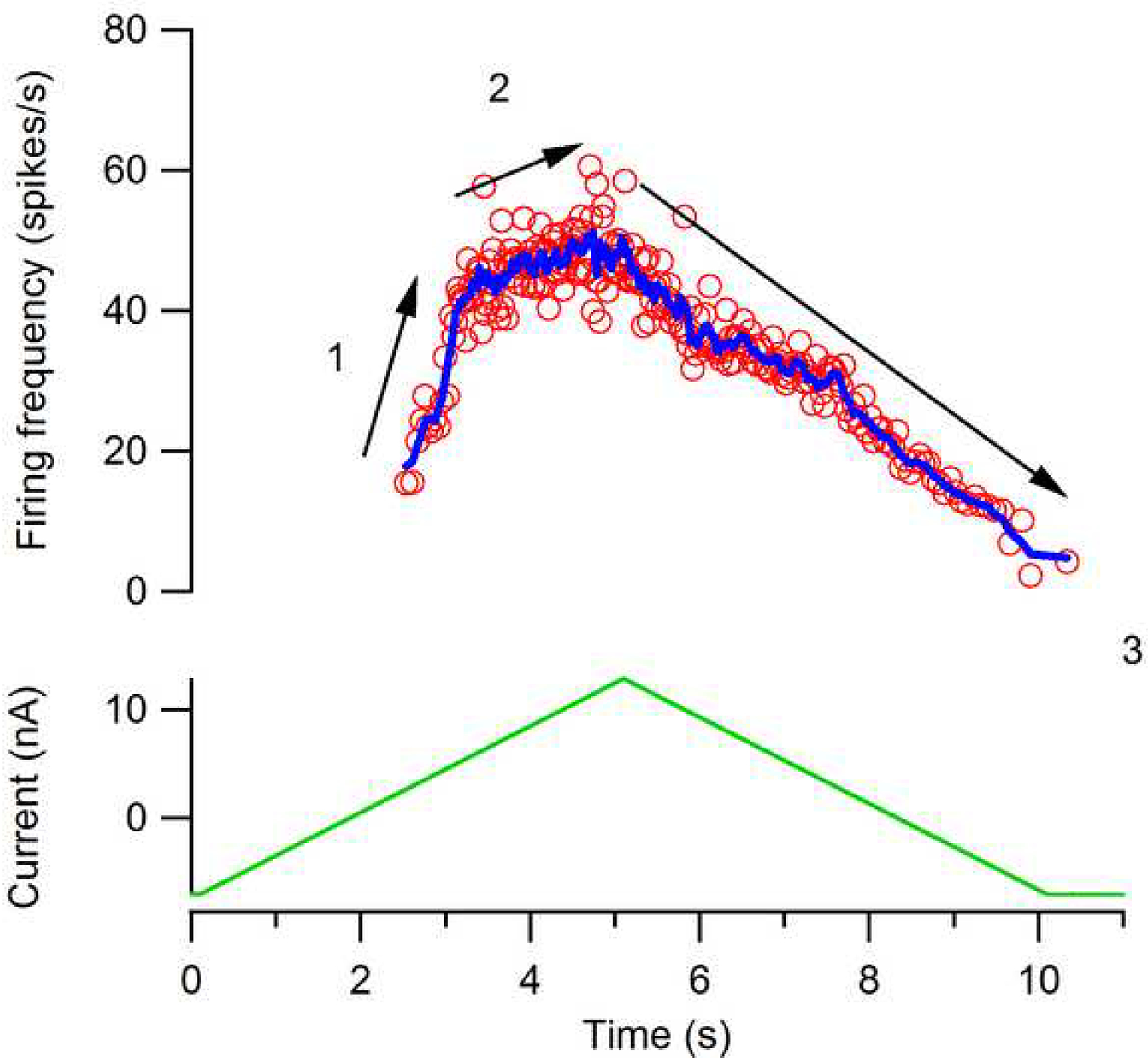
A typical relation between input and firing for a feline motoneuron with a strong PIC. Each of the phases labeled 1 through 3 has been detected in human motor unit firing patterns. 1: Initial acceleration (secondary range). 2: Preferred firing range (i.e. tertiary range or rate limiting). 3: Offset at a lower input than onset (i.e. hysteresis). A de-acceleration in firing rate is sometimes evident right at de-recruitment - see Fig. 8.
Figure 8:
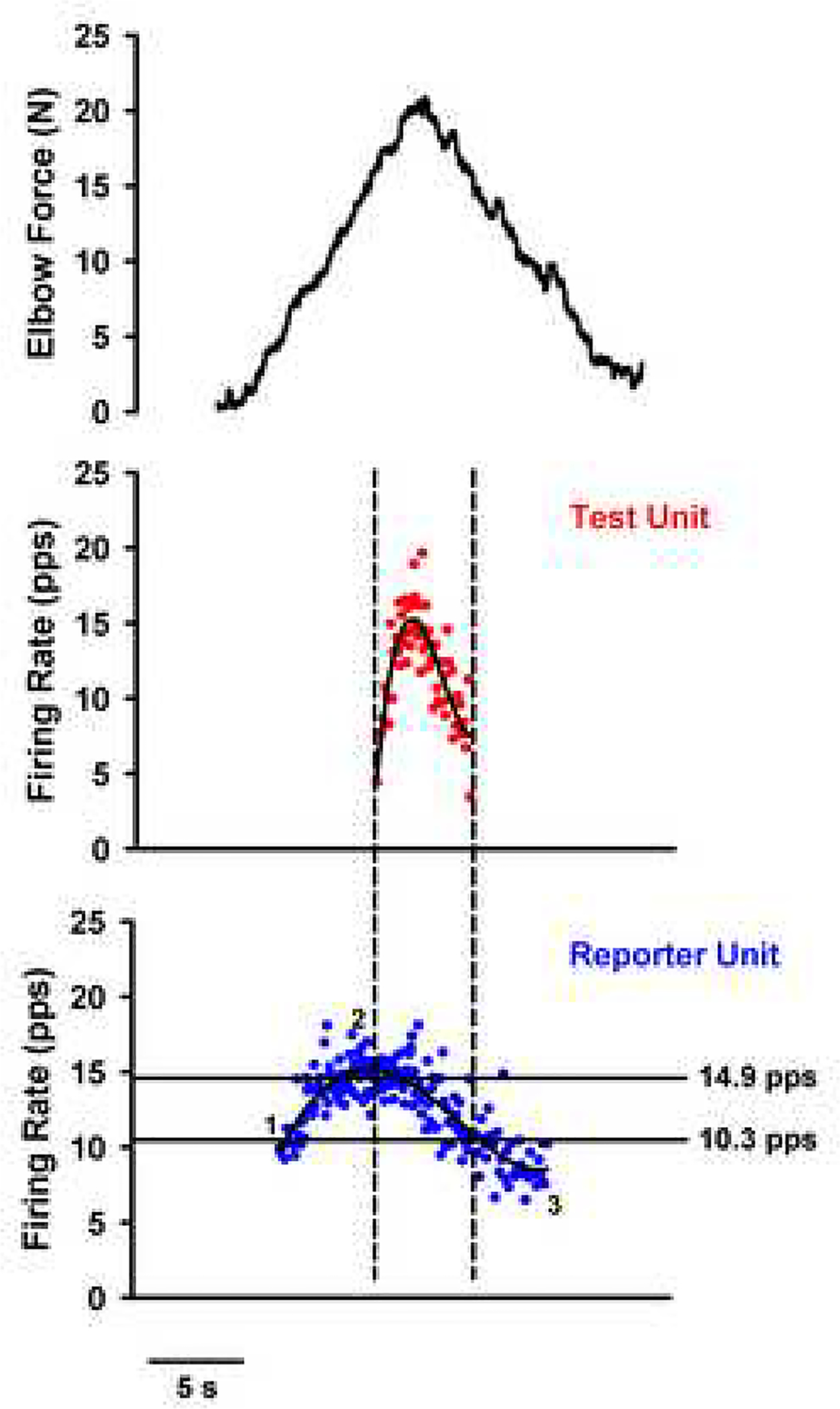
Example of human motor unit firing patterns from a pair of motor units in the biceps brachii of a healthy subject. On the lowest panel, the “reportor” unit exhibits the same firing behaviors as in the motoneuron in Fig. 7: 1: Initial acceleration, 2: Preferred firing range and 3: Hysteresis. Note also there is de-acceleration at de-recruitment. This figure also illustrates the paired motor unit method for estimating PIC amplitude. Vertical dashed lines indicate times of recruitment and de-recruitment of the higher-threshold (test) unit; horizontal lines indicate the corresponding lower-threshold (reporter) unit firing rates (estimate of synaptic input to test unit) at these times. The lower-threshold (reporter) unit provides an estimate of average synaptic drive and thus the differences in its frequency of firing (Δ F) at recruitment vs. . Top panel, black: Volitional elbow flexion force in N (y-axis) in relation to time (x-axis); middle panel, red: firing rates of Test Unit in pulses per second (y-axis) in relation to time (x-axis), average in black; bottom panel, blue: firing rates of Reporter Unit in pulses per second (y-axis) in relation to time (x-axis), average in black.Note Δ F for this subject was 4.6 Hz. Unpublished data from Mottram.
A serious concern with examining motor unit firing patterns is that the pattern of synaptic input is not known - perhaps this pattern itself has a steep slope, a saturation, and a linear decline. For this reason, the paired motor unit technique was introduced by Eken and Kiehn and by Gorassini and colleagues (Kiehn and Eken, 1997; Gorassini et al., 2002a). The power of this approach is evident in Fig. 8, which shows the force of the elbow flexor muscles generated by a healthy subject (top panel) during the rising and falling phase of a voluntary ramp contraction performed with the elbow flexor muscles. The middle panel shows a higher threshold (test) motor unit, and the lower panel shows a lower threshold (reporter) motor unit recruited during the ramp contraction.
In the paired motor unit analysis technique, (Figure 8) the firing frequency of a lower-threshold motor unit of the pair (reporter unit; lower panel) is used to estimate the synaptic drive to the motoneuron pool, including the drive to a second, higher-threshold (test; middle panel) motor unit of the pair. The degree to which a motoneuron PIC helps to sustain the discharge of the motor unit (in this case the test unit) is determined from the reduction in reporter unit firing at the de-recruitment of the test unit compared with recruitment of the test unit (ΔF). This ΔF value corresponds to the reduction in synaptic drive needed to counteract the intrinsic PIC and thus is used as an indirect measure of this current ((Gorassini et al., 2004; Powers et al., 2008); for assumptions used with this technique).
The estimate of PIC amplitudes in the test motor unit depends critically on whether the firing rate of the reporter unit is an accurate measure of synaptic drive to the motoneuron pool and specifically, to the test motor unit under study This approach depends on the premise that the firing rate of a motoneuron is proportional to the underlying synaptic current, given the assumption that the frequency-current (F-I) relation for current reaching the soma is similar for injected current and for currents reaching the soma from synaptic inputs (effective somatic current) (Binder-Macleod and Lee, 1996). If so, then the firing rate of a motor unit is potentially a fairly accurate measure of the drive to the motoneuron pool including the test unit, (assuming the two motor units are receiving common synaptic inputs (Bennett et al., 2001a)). The paired motor unit technique remains the most effective method for detecting and quantifying the contribution of the PIC to motoneuron firing.
These ΔF estimates indicate that about 40% of the firing rate modulation of motor units during slowly varying isometric contractions is due to the PIC (Gorassini et al., 2002a). This is likely an underestimate. The hysteresis estimated by ΔF only reflects a portion of the PIC. This point is difficult to appreciate without a thorough understanding of current-voltage relations in motoneurons, but suffice it to say that the hysteresis measured by the ΔF only reflects that portion of the PIC that exceeds the current required to offset the cells input conductance (i.e. the conductance that sets the resting membrane potential; input resistance is its inverse) (Powers et al., 2008). In fact in cells where the PIC is not large enough to induce hysteresis, it still amplifies synaptic input. As yet there are no methods to measure this amplification in human motor units.
Thus a reasonable conclusion at present is that the PIC generates more than half of the firing modulation in human motor units during these slow, isometric voluntary contractions. This conclusion is entirely consistent with PIC behavior in animal preparations, but probably has not reached the level of certainty that can be ascribed to the AHP and the role of the NaPIC in action potential generation. Moreover, motor unit firing patterns are variable and do not always exhibit the hallmarks of PICs (e.g. Fuglevand et al., 2006). One problem is uncertainty about how strong monoaminergic input is in different motor behaviors. In fact it is possible that a strong PIC is only essential in posture and moderate to strong voluntary movements. In addition, as emphasized above, inhibition can readily control or suppress the PICs in motor pools that would interfere with the task (e.g. antagonists). It should also be emphasized that the PIC is unlikely to be a dominant component in all motor behaviors. Studies of central pattern generators (CPGs) underlying locomotion and scratching in animal preparations show that these CPGs likely activate motoneurons via different mechanisms. In the scratch reflex, much of the PIC appears to be deactivated by a strong inhibition (Perreault, 2002; Berg et al., 2007) and the oscillations are driven by pre-motor spinal circuits, perhaps using local neuromodulators or via activation of the NMDA glutamate receptor, which would allow voltage-dependent amplification on its own without the PIC. In locomotion, PICs appear to be involved (Brownstone et al., 1994) but likely interact with local neuromodulatory inputs (Muennich and Fyffe, 2004; Miles et al., 2007) and NMDA glutamate receptors (Hochman et al., 1994).
Perhaps the most promising approach for assessing effects of 5HT and NE in humans is to use pharmacology, i.e. to use drugs that block or mimic the effects of 5HT and NE. As yet, the only published studies, by Hornby et al (2004) using baclofen and Walton et al (Walton et al., 2002) using caffeine, are consistent with the presence of PICs in motoneurons. For example in the Walton et al study, caffeine increased the percentage of motor units exhibiting self-sustained firing, which is consistent with caffeine’s action of increasing NE in the CNS. In Hornby et al, baclofen eliminated the initial steep portion of firing rate modulation and decreased maximal voluntary contraction, suggesting a decreased PIC as expected from the effects of baclofen on PICs in animal preparations (Svirskis and Hounsgaard, 1998; Li et al., 2004c). Yet baclofen also has a strong presynaptic inhibitory effect on sensory afferents that occurs at very low doses (Li et al., 2004c). Further studies using pharmacological agents that directly impact NE and 5HT are needed. In addition, studies of these drugs in primate models are also greatly needed, as these studies would allow effects of PICs on connections between individual cortical cells and motoneurons to be examined.
Although further work is needed to quantify PIC effects on human motor unit firing patterns, the evidence presented above supports a major role for the PIC in many normal motor behaviors. Fuglevand et al (2006) have criticized our emphasis on the PIC, noting the lack of direct measures of both the PIC and of the level of monoaminergic activity in humans. Yet, as emphasized at the start of this section, assessments of the AHP in human motoneurons are similarly indirect. A strong argument in favor of the PIC is that it accounts for several features of the motor unit firing data (ΔF, the hallmarks noted in Fig. 2 and the presence of self-sustained firing). Consider for example the hallmarks of firing rate acceleration during the ascending phase and then linear declines during the descending phase. While it is true that nonlinear summation, due to shunting from open ion channels, occurs in motoneurons (Cushing et al., 2005) this explanation cannot explain why the decline in rate is linear and does not mirror the behavior on the rising phase. An alternative explanation using organization of ionotropic input (Heckman and Binder, 1993a) also fails to explain the linear descending behavior. In contrast, the PIC provides a natural explanation for both ascending and descending phases. Moreover, the evidence favoring a major role for PICs from animal preparations is very strong, with perhaps the most important being the clear modulation of raphespinal neurons with locomotor speed. No other known mechanism has this explanatory power and thus we conclude that it is reasonably likely that the PIC plays a major role in determining motor unit firing patterns in humans.
Nonetheless it should be emphasized that the process of comparing human motor unit firing patterns to cellular results from animal preparations remains difficult. Research into alternative interpretations remains very important. One might speculate for example that the monosynaptic corticospinal input in humans is stronger than seen even in non-human primates and thus may provide good recruitment and rate modulation with little requirement for brainstem neuromodulation to enhance motoneuron excitability. Systematic and realistic computer simulations of motoneurons coupled with careful comparison to human and animal data are needed to help resolve these issues.
One final point to consider is the effect of monoamines on the resting membrane potential. As noted above, studies in animal preparations clearly show that resting potential depolarization and lowering of action potential threshold (Krawitz et al., 2001) accompanying facilitation of the PIC by both 5HT and NE. As yet no methods are available for estimating these effects in humans, though careful examination of recruitment threshold changes with monoaminergic drugs might be a viable approach.
Implications for pathology
Spinal injury
If the neuromodulatory input from the 5HT and NE systems has such a potent effect on recruitment and rate modulation of motoneurons, then sudden loss of this neuromodulatory system should produce a large drop in their net excitability. Consistent with this expectation, complete transections of the spinal cord immediately and dramatically reduce motoneuron excitability (Hounsgaard et al., 1988; Miller et al., 1996), Excitability becomes so low that there is almost a complete loss of reflex responses in extensor motor pools (Baldissera et al., 1981; Miller et al., 1996). Flexion reflexes can be generated in flexor motor pools, but even these are much weaker than when the cord is intact (Nygren and Olson, 1976; Kehne et al., 1985). Note that these flexion reflexes are weak despite a dramatic increase in the excitability in the interneurons in the dorsal horn that mediate the flexion reflex, which of course are released from monoaminergic inhibition. The dominant role of loss of monoaminergic input for this reduction in excitability is revealed by administration of monoaminergic agonists in acute spinal animals, which restore motoneuron excitability (Hounsgaard et al., 1988; Miller et al., 1996; Harvey et al., 2006a; Li et al., 2007). It is thus likely that a significant portion of the spinal shock following spinal injury in human subjects is due to loss of monoaminergic input. As time passes after the injury, human patients begin to exhibit spasms, suggesting that motoneuron excitability might undergo a chronic adaptation.
A series of elegant studies using the in vitro rat sacral cord preparation by Bennett and colleagues have indeed established that motoneuron PICs undergo a remarkable plastic transformation in the weeks following full spinal transection in the rat (Bennett et al., 2001b; Bennett et al., 2001a; Bennett et al., 2004; Gorassini et al., 2004; Li et al., 2004b; Harvey et al., 2006b; Li et al., 2007). Despite the continued lack of 5HT and NE (which both decrease to a few percent of their normal levels), both the NaPIC and CaPIC more than double in amplitude within 8 weeks after spinal transection, while levels of 5HT and NE remain very low. Moreover, this emergence of a strong PIC matches closely the time course of the emergence of spasms (Bennett et al., 2001b; Bennett et al., 2004; Li et al., 2004b). An essential role for the PIC in spasms is strongly supported by the studies showing that spasms are eliminated by blocking PICs. However, PICs and associated spasms cannot be activated on their own. Because of their voltage dependence, PICs require a brief depolarizing trigger to be initiated. This is provided by sensory inputs to interneuronal systems that activate motoneurons, and thus classical considerations of changes in afferent transmission after injury are still important (Bennett et al., 2004; Li et al., 2004b). The mechanisms of this remarkable chronic adaptation in PICs are under investigation. One striking aspect of the chronic changes in motoneurons is that other properties of the motoneuron do not appear to change much - for example, data from several studies indicate that the resting membrane potential of motoneurons does not change post-injury (e.g. Button et al., 2008). Thus the adaptation does not occur for all effects of 5HT and NE but is limited to the PIC.
ALS
Studies of changes in motoneurons in the neurodegenerative disease amyotrophic lateral sclerosis (ALS) are also underway. Work in motoneuron cultured from embryos shows that PICs may again be involved, in a way that is likely maladaptive. NaPICs are upregulated early in life, by 10 days post birth in a standard transgenic mouse model of ALS (van Zundert et al., 2008) or before (Kuo et al., 2004; Kuo et al., 2005). It is quite possible the resulting excessive excitability contributes to the degenerative process. It is notable that the elegant studies of axonal excitability in human subjects by Bostock, Burke and colleagues also suggest that the NaPIC is upregulated in human ALS patients (Mogyoros et al., 1998). The potential role of the CaPIC is not clear at this point and is presently being investigated (unpublished data, Quinlan, Manuel, ElBasiouny and Heckman). Anatomical changes also occur, with an increase in dendritic branching at the same time PICs are increasing (i.e. by 10 days post birth) (Bories et al., 2007; Amendola and Durand, 2008), These anatomical changes may be compensation for increased PICs but much further study of this issue is required. The potential role of neuromodulation in upregulating PICs in this disease remains to be investigated.
Hemiparetic cerebral stroke
Stretch reflexes in stroke patients are hyperexcitable, perhaps because motoneurons themselves become hyperexcitable. A careful examination using the paired motor unit technique (Gorassini et al. 2002a) during voluntary ramp contractions with elbow flexor muscles of healthy control subjects and spastic-paretic stroke survivors revealed no differences in the amplitude of the PIC across spastic-paretic and non-spastic-paretic muscle (Mottram et al, in review). Instead, it was suggested that the enhanced stretch reflexes observed at rest in spastic-paretic stroke survivors are due to the presence of a low-level depolarizing synaptic drive to the resting spastic-paretic motoneuron pool. The source of this depolarized drive appears to involve ionotropic input but enhanced brainstem neuromodulatory input may also be involved, via the effects of 5HT and NE on the resting membrane potential (see above).
Potential drug therapies
One of the most interesting implications of changes in monoaminergic systems in spinal injury, ALS and stroke is the possibility of employing drugs that affect various receptor subtypes for 5HT and NE for therapeutic purposes. In fact, one of the major anti-spasticity drugs presently in use, tizandine, likely acts in this manner. This agent acts at NE alpha 2 receptors to restore a measure of inhibition to high threshold afferent input. For motoneurons, recent studies indicate that the PIC is controlled by different 5HT receptors (5HT2) than the resting membrane potential (perhaps 5HT6 or 7) (Li et al., 2007). Thus independent control of these two parameters may provide highly effective control of spasticity. Another important point to keep in mind with administration of any monoaminergic drug is that the normal cycle of NE and 5HT is high during the day and low during the night. Regimens that mimic this diurnal cycle might prove especially effective.
Conclusion
The ionotropic input system definitely influences excitability of the motoneuron by influencing its depolarization toward recruitment threshold. For the motor pool as a whole, ionotropic inputs can also increase net excitability (i.e. gain from synaptic input to force or EMG output). This effect occurs either by imposing a background drive such that additional input recruits larger motor units or by using an input system that is preferentially distributed to high force units (e.g. the vestibulospinal input). Yet the neuromodulatory effects of the descending monoaminergic systems have the potential to induce much larger changes in excitability both of individual motoneurons and the motor pool as a whole. This point is especially important in the clinic. Consider for example the standard tendon tap reflex: its amplitude may be dominated by the level of monoaminergic input to the cord, which affects the amplitude of the NaPIC, resulting in amplification of the ionotropic Ia EPSP. This NaPIC amplification may well outweigh the effect of depolarization of the resting membrane potential by increased tonic activity in descending ionotropic inputs. Moreover, an increase in monoaminergic drive will itself depolarize motoneurons via neuromodulatory mechanisms. This example emphasizes an additional important point: a highly transient input like the tendon tap or the H-reflex will only be amplified by the fast NaPIC not the slower activating CaPIC. The latter will however play an important role in tendon vibration reflexes and this input has been used to demonstrate the presence of self-sustained firing in human motor units. The most important point however is a clear: as illustrated in Figure 9, motoneuron excitability is determined both by ionotropic inputs and by neuromodulatory inputs, with the latter likely being the more powerful. Thus it is essential to consider the effect of neuromodulatory input in a wide range of normal and pathological motor behaviors.
Figure 9:
Motoneuron excitability is controlled by two types of synaptic input, ionotropic and neuromodulatory input. As indicated by the size of the arrows, the neuromodulatory system has a more powerful effect.
ACKNOWLEDGEMENT
The authors’ work related to this review was supported by NIH grants (NS034382 and NS51462) to CJH and NIH NRSA Fellowships to JS and KQ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alaburda A, Perrier JF, Hounsgaard J (2002) Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol 508:219–226. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Fyffe RE (2007) The continuing case for the Renshaw cell. J Physiol 584:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE (1998) Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha- motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol 393:69–83. [PubMed] [Google Scholar]
- Amendola J, Durand J (2008) Morphological differences between wild-type and transgenic superoxide dismutase 1 lumbar motoneurons in postnatal mice. J Comp Neurol 511:329–341. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML (2001) A neural circuit for circadian regulation of arousal. Nat Neurosci 4:732–738. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M (1981) Integration in spinal neuronal systems. In: Handbook of Physiology The Nervous System. Motor control (Brooks VB, ed), pp 509–595. Bethesda: American Physiological Society. [Google Scholar]
- Bennett DJ, Li Y, Siu M (2001a) Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol 86:1955–1971. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M (1998) Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80:2023–2037. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Harvey PJ, Gorassini M (2001b) Evidence for plateau potentials in tail motoneurons of awake chronic spinal rats with spasticity. J Neurophysiol 86:1972–1982. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Sanelli L, Cooke CL, Harvey PJ, Gorassini MA (2004) Spastic long-lasting reflexes in the awake rat after sacral spinal cord injury. J Neurophysiol 91:2247–2258. [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J (2007) Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315:390–393. [DOI] [PubMed] [Google Scholar]
- Binder-Macleod SA, Lee SCK (1996) Catchlike property of human muscle during isovelocity movements. J Appl Physiol 80:2051–2059. [DOI] [PubMed] [Google Scholar]
- Binder MD (2002) Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons. Brain Res Brain Res Rev 40:1–8. [DOI] [PubMed] [Google Scholar]
- Binder MD (2003) Intrinsic dendritic currents make a major contribution to the control of motoneurone discharge. J Physiol 552:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Stuart DG (1981) Motor unit-muscle receptor interactions: design features of the neuromuscular control system In: Spinal and supraspinal mechanisms of voluntary motor control and locomotion (Desmedt JE, ed), pp 72–98: Basel Karger. [Google Scholar]
- Bories C, Amendola J, Lamotte d’Incamps B, Durand J (2007) Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci 25:451–459. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Sullivan MC, Coulter JD (1982) Organization of descending serotonergic projections to the spinal cord. In: Descending pathways to the spinal cord Progress in Brain Research (Kuypers HGJM, Martin GF, eds), pp 239–265. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Gossard JP, Hultborn H (1994) Voltage-dependent excitation of motoneurones from spinal locomotor centres in the cat. Exp Brain Res 102:34–44. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ (1992) On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res 90:441–455. [DOI] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK (2008a) Multiple modes of amplification of synaptic inhibition to motoneurons by persistent inward currents. J Neurophysiol 99:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK (2008b) Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons. J Neurophysiol 99:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE (1981) Motor units: anatomy, physiology, and functional organization In: Handbook of Physiology, The Nervous System, Motor Control (Brooks VB, ed), pp 345–422. Bethesda, MD: American Physiological Society. [Google Scholar]
- Button DC, Kalmar JM, Gardiner K, Marqueste T, Zhong H, Roy RR, Edgerton VR, Gardiner PF (2008) Does elimination of afferent input modify the changes in rat motoneurone properties that occur following chronic spinal cord transection? J Physiol 586:529–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB, Eikenberg DC, Hieble JP, Langer SZ, Lefkowitz RJ, Minneman KP, Molinoff PB, Ruffolo RR, Trendelenberg U (1994) International union of pharmacology nomenclature of adrenoceptors. Pharmacol Rev 46:121–136. [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM (2000) Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci 12:1635–1646. [DOI] [PubMed] [Google Scholar]
- Cathers I, O’Dwyer N, Neilson P (2004) Variation of magnitude and timing of wrist flexor stretch reflex across the full range of voluntary activation. Exp Brain Res 157:324–335. [DOI] [PubMed] [Google Scholar]
- Charles KJ, Calver AR, Jourdain S, Pangalos MN (2003) Distribution of a GABAB-like receptor protein in the rat central nervous system. Brain Res 989:135–146. [DOI] [PubMed] [Google Scholar]
- Collins DF, Burke D, Gandevia SC (2002a) Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol 538:289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Gorassini M, Bennett D, Burke D, Gandevia SC (2002b) Recent evidence for plateau potentials in human motoneurones. Adv Exp Med Biol 508:227–235. [DOI] [PubMed] [Google Scholar]
- Cope TC, Clark BD (1991) Motor-unit recruitment in the decerebrate cat: several unit properties are equally good predictors of order. J Neurophysiol 66:1127–1138. [DOI] [PubMed] [Google Scholar]
- Cushing S, Bui T, Rose PK (2005) Effect of nonlinear summation of synaptic currents on the input-output properties of spinal motoneurons. J Neurophysiol 94:3465–3478. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP (1982) Behavior of human motor units in different muscles during linearly varying contractions. J Physiol Lond 329:113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK (2006) Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol 570:355–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Dai Y (2004) Monoamines increase the excitability of spinal neurones in the neonatal rat by hyperpolarizing the threshold for action potential production. J Physiol 557:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Seki K, Votaw S (2002) Roles of primate spinal interneurons in preparation and execution of voluntary hand movement. Brain Res Brain Res Rev 40:53–65. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Winter DA, Patla AE (1993) Models of recruitment and rate coding organization in motor-unit pools. J Neurophysiol 70:2470–2488. [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Dutoit AP, Johns RK, Keen DA (2006) Evaluation of plateau-potential-mediated ‘warm up’ in human motor units. J Physiol 571:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe RE (1991) Spatial distribution of recurrent inhibitory synapses on spinal motoneurons in the cat. J Neurophysiol 65:1134–1149. [DOI] [PubMed] [Google Scholar]
- Fyffe REW (2001) Spinal motoneurons: synaptic inputs and receptor organization In: Motor biology of the spinal cord (Cope TC, ed), pp 21–46. London: CRC press. [Google Scholar]
- Garraway SM, Hochman S (2001a) Modulatory actions of serotonin, norepinephrine, dopamine, and acetylcholine in spinal cord deep dorsal horn neurons. J Neurophysiol 86:2183–2194. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Hochman S (2001b) Serotonin increases the incidence of primary afferent-evoked long-term depression in rat deep dorsal horn neurons. J Neurophysiol 85:1864–1872. [DOI] [PubMed] [Google Scholar]
- Gawrylewski A (2008) Hooked on a hunt. The Scientist 22:38–40. [Google Scholar]
- Gilmore J, Fedirchuk B (2004) The excitability of lumbar motoneurones in the neonatal rat is increased by a hyperpolarization of their voltage threshold for activation by descending serotonergic fibres. J Physiol 558:213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ (2002a) Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87:1850–1858. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ (2002b) Intrinsic activation of human motoneurons: reduction of motor unit recruitment thresholds by repeated contractions. J Neurophysiol 87:1859–1866. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF (2004) Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127:2247–2258. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallen P, Saitoh K, Kozlov A, Robertson B (2008) Neural bases of goal-directed locomotion in vertebrates--an overview. Brain Res Rev 57:2–12. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Pinter MJ (1984) An investigation of threshold properties among cat spinal alpha-motoneurones. J Physiol 357:453–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haftel VK, Prather JF, Heckman CJ, Cope TC (2001) Recruitment of cat motoneurons in the absence of homonymous afferent feedback. J Neurophysiol 86:616–628. [DOI] [PubMed] [Google Scholar]
- Hammar I, Jankowska E (2003) Modulatory effects of alpha1-,alpha2-, and beta -receptor agonists on feline spinal interneurons with monosynaptic input from group I muscle afferents. J Neurosci 23:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li X, Li Y, Bennett DJ (2006a) 5-ht2 receptor activation facilitates a persistent sodium current and repetitive firing in spinal motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 96:1158–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ (2006b) Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. J Neurophysiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ (1994) Computer simulations of the effects of different synaptic input systems on the steady-state input-output structure of the motoneuron pool J Neurophysiol 71:1727–1739. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD (1988) Analysis of effective synaptic currents generated by homonymous Ia afferent fibers in motoneurons of the cat J Neurophysiol 60:1946–1966. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD (1991) Computer simulation of the steady-state input-output function of the cat medial gsatrocnemius motoneuron pool. J Neurophysiol 65:952–967. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD (1993a) Computer simulations of motoneuron firing rate modulation. J Neurophysiol 69:1005–1008. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD (1993b) Computer simulations of the effects of different synaptic inputs systems on motor unit recruitment. J Neurophysiol 70:1827–1840. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Lee RH, Brownstone RM (2003) Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends in Neurosciences 26:688–695. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ (2005) Persistent inward currents in motoneuron dendrites: Implications for motor output. Muscle & Nerve 31:135–156. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD (2008a) Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol-London 586:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J (2008b) Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14:264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Mendell LM (1981) Functional organization of motoneuron pool and its inputs In: Handbook of Physiology, The Nervous System, Motor Control. (Brooks VB, ed), pp 423–507. Bethesda, MD: American Physiological Society. [Google Scholar]
- Hochman S, Jordan LM, Schmidt BJ (1994) TTX-resistant NMDA receptor-mediated voltage oscillations in mammalian lumbar motoneurons. J Neurophysiol 72:2559–2562. [DOI] [PubMed] [Google Scholar]
- Hochman S, Garraway SM, Machacek DW, Shay BL (2001) 5-HT recpeptors and the neuromodulatory control of spinal cord function In: Motor Neurobiology of the Spinal Cord (Cope TC, ed), pp 47–87. London: CRC Press. [Google Scholar]
- Holstege JC, Kuypers HG (1987) Brainstem projections to spinal motoneurons: an update. Neuroscience 23:809–821. [DOI] [PubMed] [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG (2002) Motoneurons: A preferred firing range across vertebrate species? Muscle Nerve 25:632–648. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Heckman CJ, Harvey RL, Rymer WZ (2004) Changes in voluntary torque and electromyographic activity following oral baclofen. Muscle & Nerve 30:784–795. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O (1993) Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol Lond 468:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O (1988) Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol 405:345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71:533–554. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Lindstrom S, Wigstrom H (1979) On the function of recurrent inhibition in the spinal cord. Exp Brain Res 37:399–403. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB (2003) Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol 552:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP (2004) Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143:77–95. [DOI] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Heckman CJ (2008) Summation of excitatory and inhibitory synaptic inputs by motoneurons with highly active dendrites. J Neurophysiol 99:1643–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ (2007) Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10:363–369. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA (1993) 5-HT and motor control: a hypothesis. Trends Neurosci 16:346–352. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA (2002) Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40:45–52. [DOI] [PubMed] [Google Scholar]
- Jankowska E (1992) Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38:335–378. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Slawinska U, Hammar I (2002) On organization of a neuronal network in pathways from group II muscle afferents in feline lumbar spinal segments. J Physiol 542:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH (2000) Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci 12:701–714. [DOI] [PubMed] [Google Scholar]
- Jones SM, Lee RH (2006) Fast amplification of dynamic synaptic inputs in spinal motoneurons in vivo. J Neurophysiol 96:2200–2206. [DOI] [PubMed] [Google Scholar]
- Jordan LM, Liu J, Hedlund PB, Akay T, Pearson KG (2008) Descending command systems for the initiation of locomotion in mammals. Brain Res Rev 57:183–191. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Gallager DW, Davis M (1985) Spinalization unmasks clonidine’s alpha 1-adrenergic mediated excitation of the flexor reflex in rats. J Neurosci 5:1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D (2006) The motoneurone and its muscle fibres. Oxford: Oxford University Press. [Google Scholar]
- Kernell D, Hultborn H (1990) Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res 507:176–179. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T (1997) Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78:3061–3068. [DOI] [PubMed] [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, McCrea DA (2001) State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. J Physiol 532:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ (2005) Increased persistent Na+ current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol-London 563:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ (2006) Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol-London 574:819–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Johnson MD, Heckman HM, Heckman CJ (2003) Active dendritic integration of inhibitory synaptic inputs in vivo. J Neurophysiol 90:3617–3624. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Schonewille M, Siddique T, Schults ANA, Fu RG, Bar PR, Anelli R, Heckman CJ, Kroese ABA (2004) Hyperexcitability of cultured spinal motoneurons from presymptomatic ALS mice. J Neurophysiol 91:571–575. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ (1996) Influence of voltage-sensitive dendritic conductances on bistable firing and effective synaptic current in cat spinal motoneurons in vivo. J Neurophysiol 76:2107–2110. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ (1998a) Bistability in spinal motoneurons in vivo: Systematic variations in persistent inward currents. J Neurophysiol 80:583–593. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ (1998b) Bistability in spinal motoneurons in vivo: Systematic variations in rhythmic firing patterns. J Neurophysiol 80:572–582. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ (1999a) Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha(1) agonist methoxamine. J Neurophysiol 81:2164–2174. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ (1999b) Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol 82:2518–2527. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ (2000) Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci 20:6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ (2003) Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol 89:27–39. [DOI] [PubMed] [Google Scholar]
- Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ (2007) Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol 97:1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ (2004a) Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91:767–783. [DOI] [PubMed] [Google Scholar]
- Li Y, Harvey PJ, Li X, Bennett DJ (2004b) Spastic long-lasting reflexes of the chronic spinal rat studied in vitro. J Neurophysiol 91:2236–2246. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Harvey PJ, Bennett DJ (2004c) Effects of baclofen on spinal reflexes and persistent inward currents in motoneurons of chronic spinal rats with spasticity. J Neurophysiol 92:2694–2703. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL (1993) Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J Physiol 461:213–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Garcia JA (2006) Serotonergic modulation of spinal sensory circuits. Curr Top Med Chem 6:1987–1996. [DOI] [PubMed] [Google Scholar]
- Lu Y, Perl ER (2007) Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J Physiol 582:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D (2005) How much afterhyperpolarization conductance is recruited by an action potential? A dynamic-clamp study in cat lumbar motoneurons. J Neurosci 25:8917–8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D (2006) The afterhyperpolarization conductance exerts the same control over the gain and variability of motoneurone firing in anaesthetized cats. J Physiol 576:873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D (2007) Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69:291–316. [DOI] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM (2007) Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci U S A 104:2448–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Rymer WZ, Heckman CJ (1995) 5-HT 1b/1d agonist CGS-12066B attenuates clasp knife reflex in the cat. J Neurophysiol 74:453–456. [DOI] [PubMed] [Google Scholar]
- Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ (1996) Restoration of extensor excitability in the acute spinal cat by the 5-HT2 agonist DOI. J Neurophysiol 75:620–628. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D, Bostock H (1998) Strength-duration properties of sensory and motor axons in amyotrophic lateral sclerosis. Brain 121 (Pt 5):851–859. [DOI] [PubMed] [Google Scholar]
- Moritz AT, Newkirk G, Powers RK, Binder MD (2007) Facilitation of somatic calcium channels can evoke prolonged tail currents in rat hypoglossal motoneurons. J Neurophysiol 98:1042–1047. [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Christou EA, Meyer FG, Enoka RM (2005) Frequency modulation of motor unit discharge has task-dependent effects on fluctuations in motor output. J Neurophysiol 94:2878–2887. [DOI] [PubMed] [Google Scholar]
- Muennich EA, Fyffe RE (2004) Focal aggregation of voltage-gated, Kv2.1 subunit-containing, potassium channels at synaptic sites in rat spinal motoneurones. J Physiol 554:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R (2002) G protein pathways. Science 296:1636–1639. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Nichols CD (2008) Serotonin receptors. Chem Rev 108:1614–1641. [DOI] [PubMed] [Google Scholar]
- Nickolls P, Collins DF, Gorman RB, Burke D, Gandevia SC (2004) Forces consistent with plateau-like behaviour of spinal neurons evoked in patients with spinal cord injuries. Brain 127:660–670. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP (2002) A small-systems approach to motor pattern generation. Nature 417:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren L-G, Olson L (1976) On spinal noradrenaline receptor supersensitivity: correlation between nerve terminal densities and flexor reflexes various times after intracisternal 6-hydroxydopamine. Brain Res 116:455–470. [DOI] [PubMed] [Google Scholar]
- Perreault MC (2002) Motoneurons have different membrane resistance during fictive scratching and weight support. J Neurosci 22:8259–8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J (2003) 5-HT(2) Receptors Promote Plateau Potentials in Turtle Spinal Motoneurons by Facilitating an L-Type Calcium Current. J Neurophysiol 89:954–959. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Cotel F (2008) Serotonin differentially modulates the intrinsic properties of spinal motoneurons from the adult turtle. J Physiol 586:1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Mejia-Gervacio S, Hounsgaard J (2000) Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol 528 Pt 1:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person RS, Kudina LP (1972) Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32:471–483. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD (2001) Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143:137–263. [DOI] [PubMed] [Google Scholar]
- Powers RK, Nardelli P, Cope TC (2008) Estimation of the contribution of intrinsic currents to motoneuron firing based on paired motoneuron discharge records in the decerebrate cat. J Neurophysiol 100:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL (2000) Synaptic control of motoneuronal excitability. Physiol Rev 80:767–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaiguere P, Vedel JP, Pagni S, Zenatti A (1989) Physiological properties of the motor units of the wrist extensor muscles in man. Exp Brain Res 78:51–61. [DOI] [PubMed] [Google Scholar]
- Rudomin P (2002) Central control of information transmission through the intraspinal arborizations of sensory fibers examined 100 years after Ramon y Cajal. Prog Brain Res 136:409–421. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM (2000) The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53:689–710. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE (1980a) Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol 43:1296–1318. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE (1980b) Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol 43:1700–1724. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J (1998) Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol 79:45–50. [DOI] [PubMed] [Google Scholar]
- Theiss RD, Heckman CJ (2005) Systematic variation in effects of serotonin and norepinephrine on repetitive firing properties of ventral horn neurons. Neuroscience 134:803–815. [DOI] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ (2007) Persistent inward currents in rat ventral horn neurones. J Physiol-London 580:507–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, El Manira A, Wallen P, Svirskis G, Hounsgaard J (2005) Cellular signalling properties in microcircuits. Trends Neurosci 28:534–540. [DOI] [PubMed] [Google Scholar]
- van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown RH Jr., Constantine-Paton M, Bellingham MC (2008) Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci 28:10864–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE (1978) Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol 41:1203–1216. [DOI] [PubMed] [Google Scholar]
- Walton C, Kalmar JM, Cafarelli E (2002) Effect of caffeine on self-sustained firing in human motor units. J Physiol 545:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]


