Abstract
New technologies have been recently introduced to improve the monitoring of patients with chronic syndromes such as heart failure. Devices can now be employed to gather large amounts of data and data processing through artificial intelligence techniques may improve heart failure management and reduce costs. The analysis of large datasets using an artificial intelligence technique is leading to a paradigm shift in the era of precision medicine. However, the assessment of clinical safety and the evaluation of the potential benefits is still a matter of debate. In this article, the authors aim to focus on the development of these new tools and to draw the attention to their transition in daily clinical practice.
Keywords: Artificial intelligence, personalised medicine, big data, data analysis, heart failure, patient monitoring, telemedicine, devices
Heart failure (HF) is a complex clinical syndrome associated with a heavy burden of symptoms and a wide range of therapeutic options.[1–5] Despite improvements in HF management over past decades, we still need approaches that might mitigate the effects of this disease, preventing its insidious onset and worsening of symptoms, such as acute decompensations. A trend has recently emerged to extend the diagnostic and therapeutic approaches to include more complex ways of data collection and processing, thereby holding the potential to achieve a greater understanding of HF pathophysiology and to enhance patient care.
Telemedicine – or telehealth – applied to chronic diseases such as HF is rapidly evolving and aims to improve and individualise patient care, as well as reducing financial costs.[6] Telehealth is a broad term that encompasses the different applications of telematics to medicine, allowing diagnosis and/or remote treatment through a set of communication tools.[7] Additionally, a branch of computer science that could enhance future HF management is artificial intelligence (AI), an area of study engaging computers in human processes, such as learning, reasoning or knowledge storage.[8] AI techniques are of great interest in the healthcare and medical field because they can use sophisticated algorithms to analyse large volumes of physiological data obtained from thousands of patients, thus gathering information to assist clinical practice and decision making.[9] In cardiology, AI is currently being investigated in several domains ranging from clinical decision support systems to imaging interpretation.
Some machine learning (ML) techniques allow computers to be trained with information acquired from large datasets that have been previously correctly classified and labelled by medical professionals. Such frameworks teach computers to acquire and develop autonomous rules aiding in classification and interpretation of new inputs, as long as these are similar enough to those used in training datasets. This results in the development of automated decision support systems that facilitate diagnosis and/or prognosis estimation. However, an appropriate classification of telemedical systems based on ML techniques is lacking and profiles of patients who could benefit most from ML telemedicine solutions are unknown and need to be adequately investigated.[10]
A promising application of these technologies is to exploit the relative computing speed now available in computers, which is made possible by parallel processing. This feature can quickly aggregate data from patient electronic health records (EHRs) acquired from multiple sources in order to create a streamlined summary of patient medical problems requiring attention by physician. Therefore, AI systems may perform a thorough search of individual or multiple patient EHRs. This technology could also be used to cross-reference data related to a patient’s family history to find similar patients and to evaluate the diagnoses and treatment responses.[11] Furthermore, the software will be able to integrate relevant genomic, proteomic and metabolomic data and consequently formulate diagnostic work-up and therapeutic regimens, as well as providing recommendations for patient screening.
Such intelligent software solutions could also be used to direct safe patient work-ups and to guide selection of diagnostic tests that maximise effectiveness and safety, addressing questions pertaining to a precise medical problem while minimising health costs.[11] When these approaches are further optimised in the future, they could signify a quantum leap in clinical practice since diagnostic over-testing is a serious problem in modern medicine, often driven by defensive medicine, suboptimal knowledge and the need to increase profit and meet patients’ expectations. This consideration is mainly based on the erronous belief that more testing could be beneficial for the patient.[12]
For example, a study by Sheffield et al. estimated that preoperative cardiac stress testing was unnecessary in more than 56,000 patients referred to elective non-cardiac surgery in a Medicare inpatient claims database.[13] In addition, an increased trend for resting echocardiography testing has been reported in a large Canadian cohort of HF patients, but appropriateness was not assessed.[14] Integrated solutions based on ML and AI have the potential to counter and attenuate such trends, thereby providing significant burden relief on often already overstretched healthcare systems worldwide.
Clinical Applications
There is an increasing interest in the potential for telehealth to support the home-based management of patients with long-term conditions as a means of providing a more patient-centred, cost-efficient and joint service. AI can be used in the field of cardiology in several ways, including determining the most congruous type of imaging study for a specific set of symptoms. If applied properly, AI could reduce inappropriate imaging studies and help physicians adhere to practice guidelines and ever-changing appropriate use criteria. For example, the Imaging in Formation of Optimal Cardiovascular Utilization Strategies (FOCUS) quality improvement initiative of the American College of Cardiology was recently introduced to reduce inadequate use of diagnostic imaging through the use of AI tracking appropriate use criteria.[11]
Another clinical application of the AI that has recently become available is the website-based verification of symptoms platforms available on numerous major medical websites, such as:
https://www.nhsinform.scot/symptoms-and-self-help/a-to-z
https://www.mayoclinic.org/symptom-checker/select-symptom/itt-20009075
https://www.mdlive.com
https://symptoms.webmd.com/default.htm
Patients can access the app online and using the diagnostic tools, they receive a basic assessment of their symptoms. However, these self-diagnostic apparatus intended to help the doctor in decision-making and are not meant to rely only on the patient self-assessment, disjointed from any medical supervision. By means of teleconsultation, the patient may provide the doctor with relevant details of their medical problems by following the steps required by the diagnostic algorithm (Figure 1).
Figure 1: Artificial Intelligence and the Roadmap Towards Precision Medicine.
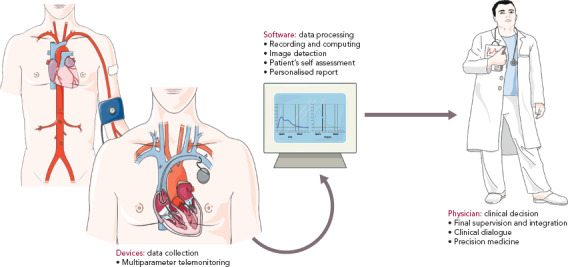
An ideal process is shown starting with the collection of data through wearable or implanted devices that allow telemonitoring of detailed cardiac and vascular function parameters. Data are recorded and computed by tools using sophisticated algorithms in order to provide the physician with personalised reports that further guide the final clinical decision. Source: Dilsizian and Siegel 2014.[11] Reproduced with permission from Servier Medical Art (https://smart.servier.com/).
Prescription Medical Electronics Mobile (PEM Mobile) platforms are mobile apps used for prescription of drugs, available in some public and private hospitals, in order to reduce the number of manual prescriptions associated with digital signature. This mobile app allows the doctor to process prescription drugs via a mobile phone or tablet: the algorithm is able to fully integrate clinical information of pharmacy users, and trace the input from prescribers and pharmacists.[15] Given that the physician is provided with an overview of patients’ medications, this tool enhances the drug-related safety, avoiding overdosing or interactions. This is a relevant issue for HF patients – particularly the elderly – because of the prevalence of several comorbidities requiring multiple treatments. Conveying all prescription data into a single platform helps also to build public registries, which are increasingly useful to understand HF mechanisms.[16] However, PEM Mobile requires more extensive investigations in order to fully provide a quantitative assessment of its clinical benefit. A recent study by Vedanthan et al. investigated possible ways to better control blood pressure (BP) among patients with MI through the use of a smartphone app as a complement to traditional cardiac rehabilitation protocols when compared to patients receiving traditional cardiac rehabilitation alone.[17] The automated positive feedback on in-range arterial BP measurements augments patient adherence to antihypertensive medication, thus fostering an improvement in arterial BP control.
A randomised study in Kenya evaluated whether equipping community healthcare workers with behavioural communication strategies and smartphone technology could facilitate linkage of people with elevated BP to hypertension care programs, therefore lowering BP. The study enrolled 1,460 patients in three arms (usual care, paper-based care [behavioural communication using paper-based tools] and smartphone technology), and systolic arterial BP was evaluated. The strategy combining tailored behavioural communication and mobile health (mHealth) for community health workers led to improved linkage to care, since patients in the arm equipped with a smartphone received tailored messages, alerts and recommendations based on ML of individual assessments.[17]
The Medication Adherence Improvement Support App For Engagement-Blood Pressure (MedISAFE-BP) study is a prospective, randomised controlled trial, with 413 patients, designed to evaluate the impact of the mHealth app on arterial BP and medication adherence. The results may inform the potential effectiveness of this simple system in enhancing cardiovascular disease risk factor assessment and the contribution on the clinical outcomes.[18]
More recently, ML-guided CT angiography (CTA) has been shown to be superior in assessing the diagnostic accuracy of the classification of the coronary stenoses based on the fractional flow reserve assessment when compared to computational-fluid-dynamics-guided CTA assessment.[19] Similarly, ML-based algorithms were able to discriminate pathological versus physiological patterns of hypertrophic cardiac remodelling by ‘learning’ from expert-annotated speckle-tracking echocardiographic datasets, thereby showing potential in the identification of hypertrophic cardiomyopathy.[20] These examples showed how ML-based diagnostic approaches offer many possibilities that may ultimately translate to better patient outcomes and lower financial expenditures.
Devices and Hardware
The use of AI in medicine today is a matter of great interest, especially with respect to diagnostic or predictive analysis of medical data. Several studies investigating telehealth interventions in HF have been published in recent years (Table 1). To date, the most convincing evidence for a telemonitoring device relates to the implantable CardioMEMS device.
Table 1: Completed Studies Incorporating a Telemedicine Programme as an Intervention.
| Trial | Author and Year | ClinicalTrials.gov | Results |
|---|---|---|---|
| CHAMPION | Abraham et al. 2011[21] | NCT00531661 | Reduced rates of hospitalisation with pulmonary pressure monitoring |
| COMPASS-HF | Bourge et al. 2008[23] | NCT00643279 | No significant decrease of HF morbidity with continuous intracardiac pressure monitoring |
| IN-TIME | Hindricks et al. 2014[24] | NCT00538356 | Telemonitoring significantly improves clinical outcomes for HF patients |
| TIM-HF2 | Koehler et al. 2018[29] | NCT01878630 | Reduction of the percentage of days lost due to unplanned cardiovascular hospital admissions |
| COMMIT-HF | Kurek et al. 2017[28] | NCT02536443 | Reduction of long-term mortality by remote monitoring of HF patients with ICDs/CRT-Ds |
| EFFECT | De Simone et al. 2015[26] | NCT01723865 | Reduction of mortality with ICDs |
HF = heart failure.
The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial tested the hypothesis that pulmonary artery pressure guiding HF management with a wireless implantable haemodynamic monitoring system would be a better way to reduce hospital admissions for HF compared to usual guideline-directed medical management alone.[21] This device is implanted into the pulmonary artery (PA) and transmits PA pressures to a central service centre. The physician in charge receives the results, including the trends over time of these measurements. The study was not powered for mortality but showed a significant reduction in HF hospitalisation because of improved HF management. This effect was maintained over a long-term period.[6]
The continuous technical progress of implanted left ventricular assist devices (LVADs) has led to improved clinical outcomes in advanced HF patients. Telemonitoring can be conducted without any active participation by the patient. Since monitoring of LVAD patients is complex and sensitive, it would be necessary to have continuous access to LVAD controller parameters (alarms, rotation speed, energy consumption, flow, pulsatility index), BP, blood coagulation values and concomitant drug treatment. One of the most feared complications of LVAD treatment, especially in the long-term, is transmission infection. This makes prevention particularly important. An algorithm based on image pixelation transmitted by the smartphone of the driveline exit site to the hospital is currently under development to promptly detect inflammation around the site of transmission.[22]
The Chronicle Offers Management to Patients with Advanced Signs and Symptoms of Heart Failure (COMPASS-HF) trial was a prospective, multicentre, randomised, single-blind, parallel-controlled trial of 274 New York Heart Association (NYHA) functional class III or IV HF patients who received an implantable continuous haemodynamic monitor (ICHM). The ICHM is capable of continuously monitoring and storing heart rate, body temperature, patient activity, right ventricular systolic and diastolic pressure, maximal positive and negative rate of change in right ventricular pressure, right ventricular pre-ejection and systolic time intervals, and estimated pulmonary arterial diastolic pressure. The remote examination of the pressure information has occurred at least once a week, in order to undertake appropriate action before the anticipated HF-related event.[23]
The Implant-Based Multiparameter Telemonitoring of Patients with Heart Failure (IN-TIME) trial reported a positive impact on clinical outcomes by using multiparameter monitoring based on information from an ICD device among patients with chronic HF and NYHA class II–III symptoms. By using automatic and daily monitoring of several parameters, such as the incidence of ventricular tachyarrhythmias, biventricular pacing, heart rate variability, patient activity and intracardiac electrogram, a composite clinical score indicating worsening of HF was improved as compared with patients assigned to standard care.[24] Moreover, this multiparameter telemonitoring approach seemed to yield the most absolute benefit in higher-risk populations with worse prognoses.[25]
The Clinical Efficacy of Remote Monitoring in the Management of Heart Failure (EFFECT) study was designed to test the hypothesis that remote monitoring (RM) can reduce death from any cause and cardiovascular hospitalisations in HF patients who receive ICD/CRT-D in current clinical practice. It was a prospective, non-randomised, multicentre trial of RM in a population of consecutive patients who underwent ICD/CRT-D implantation in 25 Italian centres, following current guidelines for the management of chronic HF.[26] The study demonstrated that RM was associated with reduced death and cardiovascular hospitalisation among patients with an implanted ICD/CRT. Furthermore, a subsequent economic analysis of EFFECT study showed that RM was associated with reduced direct healthcare costs compared with standard monitoring.[27]
The Contemporary Modalities In Treatment of Heart Failure (COMMIT-HF) registry evaluated the impact of RM on a long-term prognosis in HF patients. Findings from this investigation, which involved different cardiac device brands for the telemonitoring, strongly suggest that the use of RM improves long-term prognosis.[28] Of note, a significantly lower 1-year mortality was observed in the RM group compared to the usual care group (2.1% versus 11.5%; p<0.0001, respectively), and this result was maintained through a 3-year follow-up (4.9% versus 22.3%; p<0.0001, respectively).
The Telemedical Interventional Management in Heart Failure II (TIM-HF2) trial was a prospective, randomised, controlled, parallel-group, multicentre trial that recruited 1,571 HF patients. The remote patient management intervention consisted of a daily transmission of body weight, systolic and diastolic BP, heart rate, analysis of the heart rhythm, peripheral capillary oxygen saturation and a self-rated health status to the telemedical centre.[29] This trial showed that a structured remote patient management intervention, when used in a well-defined HF population, could reduce the percentage of days lost because of unplanned cardiovascular hospital admissions and all-cause mortality compared to usual monitoring.
Together with CHAMPION, these studies represent valid examples of wide and helpful data collection. However, processing of the information gathered throughout telemedicine in these particular cases could involve more sophisticated algorithms and deep learning to enable even deeper therapeutic pathways. This may stimulate researchers to extend the previous results with new applications.
As a proof-of-concept, AI applications to ECG reading have been recently reported.[30,31] Specifically, a convolutional neural network has allowed detection of patterns of left ventricular dysfunction for asymptomatic patients.[30] However, a limitation is that deep-learning analyses are not able to provide physiological insights, such as meaningful ECG biomarkers.
In addition to the HF studies described here, a new algorithm to monitor HF patients was tested in the Multisensor Chronic Assessment in Ambulatory Heart Failure study (MultiSENSE) study. The HeartLogic index combines data from multiple sensors (first and third accelerometer-based heart sounds, intrathoracic impedance, respiratory rate, the ratio between respiratory rate and tidal volume, nocturnal heart rate and patient activity) integrated with the ICD and has proved to be a sensitive, timely and efficient predictor of HF decompensation.[32] This device calculates daily a composite index by integrating inputs received from the sensors. The activation of the associated alert can enable early detection of clinical deterioration and suggest an action to be taken in patients who are not yet critical, potentially preventing adverse events by stratifying their risk of occurence.[27] Similarly, the use of the HeartLogic sensor alone or in combination with N-terminal of the prohormone brain natriuretic peptide levels in the MultiSENSE study was able to identify time intervals during the natural history of patients with HF in which those individuals were at a significantly higher risk of worsening HF, thus facilitating preventive interventions.[33]
Costs and Sustainability
Telemedicine is believed to have the potential to reduce costs related to healthcare. A recent study evaluated the impact of at-home telemonitoring on healthcare expenditures, number of admissions and length of hospilatisation in patients living with chronic HF, showing a statistically robust positive impact on healthcare costs, and decreasing the number of hospilatisations and their duration. These results were coupled with a reduction in mortality.[34]
A study enrolling patients with comorbidities, such as HF, chronic lung disease and/or diabetes recently showed how home telehealth, when integrated with the health facility’s EHR system, significantly reduced the bed-days-of-care and urgent clinic/emergency room visits, with improvement in glycaemic control, cognitive status and patient satisfaction.[35] Similar observations were consistently confirmed in studies examining integrated telehealth care services among geriatric home care patients and elderly people.[36,37]
Data Management
Health data are defined by the general EU data protection regulation as they contain information on the subject’s past, present and future physical and/or mental health status. For this reason, they represent sensitive and private data. Health data also apply to physiological information collected by cardiac implanted electronic devices, such as implantable defibrillators, pacemakers, CRT devices and implantable loop recorders. Therefore, in the interest of all parties involved in RM, it is important to analyse the legal implications regarding the data produced by RM devices.
Future Applications
V-LAP (Vectorious Medical Technologies) is a miniature, wireless and battery-free microcomputer for cardiac monitoring in HF, able to directly monitor the left atrial pressure. Ex-vivo and animal findings have been published. and the first human study of the device is underway (NCT03775161).[38] The device is designed to be inserted in the interatrial septum and aims to permit data collection on a daily basis. The sensor is not powered by batteries and can work for the life of the patient. It is charged remotely and transmits the patient’s cardiac activity information wirelessly to doctors and to the hospital, where it is analysed by cardiologists.
Dehumanisation
The risk of potential dehumanisation of medicine because of the increased distance between the physician and the patient has to be evaluated with caution. Telehealth should be used as a tool to improve patient care and serve as an adjunct to – rather than a replacement for – face-to-face contact.
Telehealth, in particular, may exert a difference for patients who have physical disabilities or those with financial difficulties in travelling long distances. Telemedicine platforms might offer healthcare providers the opportunity to contact patients across long distances, reaching those in the most isolated locations. The topic of the evolving relationship between the physician and patient has been also recently addressed.[41] Telemedicine, and AI in general, that allow a complete assessment of cardiac and vascular function are becoming a fundamental tool to optimise treatment of chronic diseases, following the Hippocratic principle that “healing is a matter of time, but it is sometimes also a matter of opportunity”.
Conclusion
Telemonitoring includes several tools allowing the electronic transfer of patient data or self-reports to a doctor. So far, telemonitoring has been frequently used in patients with HF, supporting evidence that telemonitoring holds the potential to improve care, quality of life and prognosis. The combination of AI, big data and massive parallel computing offer the ability to create a revolutionary way of practicing evidence-based, cost-effective and personalised medicine. However, barriers to the adoption of AI technologies must be surpassed from regulatory, legal, cultural and political perspectives.
AI represents a different way to yield evidence for clinical practice, and deep learning challenges the usual ways by which the medical community has achieved scientific consensus to date. Because of the epidemic proportion of HF as a clinical syndrome, the ability to process big data from public health registries represents an ongoing opportunity for uncovering the evolving scope of the disease; however, the processing of ‘big medical data’ still must be addressed to build an open resource.[42]
The digitalisation process in cardiology is already with us. Now, clinicians, managers, policy-makers and scientists should start to consider the unique opportunity offered to them to work closely with the patients in order to plan, design, develop, implement and empower a new strategic relationship. Sharing the understanding of common goals that need to be achieved during the journey will allow physicians to provide a better, faster and more personalised treatment, while patients have the role of the ideal partner in forecasting their healthcare plan and wellness-enhancing processes.
References
- 1.Conrad N, Judge A, Tran J et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391:572–80. doi: 10.1016/S0140-6736(17)32520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 4.Seferovic PM, Ponikowski P, Anker SD et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21:1169–86. doi: 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 5.Crespo-Leiro MG, Metra M, Lund LH et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1505–35. doi: 10.1002/ejhf.1236. [DOI] [PubMed] [Google Scholar]
- 6.Eurlings CGMJ, Boyne JJ, de Boer RA et al. Telemedicine in heart failure – more than nice to have? Neth Heart J. 2019;27:5–15. doi: 10.1007/s12471-018-1202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375:154–61. doi: 10.1056/NEJMra1601705. [DOI] [PubMed] [Google Scholar]
- 8.Krittanawong C, Zhang H, Wang Z et al. Artificial intelligence in precision cardiovascular medicine. J Am Coll Cardiol. 2017;69:2657–2664. doi: 10.1016/j.jacc.2017.03.571. [DOI] [PubMed] [Google Scholar]
- 9.Jiang F, Jiang Y, Zhi H et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2:230–43. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leslie SJ, Denvir MA. Clinical decision support software for chronic heart failure. Crit Pathw Cardiol. 2007;6:121–6. doi: 10.1097/HPC.0b013e31812da7cc. [DOI] [PubMed] [Google Scholar]
- 11.Dilsizian SE, Siegel EL. Artificial intelligence in medicine and cardiac imaging: harnessing big data and advanced computing to provide personalized medical diagnosis and treatment. Curr Cardiol Rep. 2014;16:441. doi: 10.1007/s11886-013-0441-8. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg J, Green JB. Over-testing: why more is not better. Am J Med. 2014;127:362–3. doi: 10.1016/j.amjmed.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Sheffield KM, McAdams PS, Benarroch-Gampel J et al. Overuse of preoperative cardiac stress testing in medicare patients undergoing elective noncardiac surgery. Ann Surg. 2013;257:73–80. doi: 10.1097/SLA.0b013e31826bc2f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braga JR, Leong-Poi H, Rac VE et al. Trends in the use of cardiac imaging for patients with heart failure in Canada. JAMA Netw Open. 2019;2:e198766. doi: 10.1001/jamanetworkopen.2019.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patrao L, Deveza R, Martins H. PEM – a new patient centred electronic prescription platform. Procedia Technol. 2013;9:1313–9. doi: 10.1016/j.protcy.2013.12.147. [DOI] [Google Scholar]
- 16.Aimo A, Seghieri C, Nuti S et al. Building medical knowledge from real world registries: the case of heart failure. Int J Cardiol Heart Vasc. 2018;19:98–9. doi: 10.1016/j.ijcha.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vedanthan R, Kamano JH, DeLong AK et al. Community health workers improve linkage to hypertension care in Western Kenya. J Am Coll Cardiol. 2019;74:1897–906. doi: 10.1016/j.jacc.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morawski K, Ghazinouri R, Krumme A et al. Rationale and design of the Medication adherence Improvement Support App For Engagement – Blood Pressure (MedISAFE-BP) trial. Am Heart J. 2017;186:40–7. doi: 10.1016/j.ahj.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Coenen A, Kim YH, Kruk M et al. Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: result from the MACHINE Consortium. Circ Cardiovasc Imaging. 2018;11:e007217. doi: 10.1161/CIRCIMAGING.117.007217. [DOI] [PubMed] [Google Scholar]
- 20.Narula S, Shameer K, Salem Omar AM et al. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. 2016;68:2287–95. doi: 10.1016/j.jacc.2016.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Adamson PB, Bourge RC et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 22.Reiss N, Schmidt T, Boeckelmann M et al. Telemonitoring of left-ventricular assist device patients-current status and future challenges. J Thorac Dis. 2018;10:S1794–s1801. doi: 10.21037/jtd.2018.01.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourge RC, Abraham WT, Adamson PB et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–9. doi: 10.1016/j.jacc.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 24.Hindricks G, Taborsky M, Glikson M et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384:583–90. doi: 10.1016/S0140-6736(14)61176-4. [DOI] [PubMed] [Google Scholar]
- 25.Geller JC, Lewalter T, Bruun NE et al. Implant-based multi-parameter telemonitoring of patients with heart failure and a defibrillator with vs. without cardiac resynchronization therapy option: a subanalysis of the IN-TIME trial. Clin Res Cardiol. 2019;108:1117–27. doi: 10.1007/s00392-019-01447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Simone A, Leoni L, Luzi M et al. Remote monitoring improves outcome after ICD implantation: the clinical efficacy in the management of heart failure (EFFECT) study. Europace. 2015;17:1267–75. doi: 10.1093/europace/euu318. [DOI] [PubMed] [Google Scholar]
- 27.Capucci A, Santini L, Favale S et al. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: a retrospective case series report. ESC Heart Fail. 2019;6:308–18. doi: 10.1002/ehf2.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurek A, Tajstra M, Gadula-Gacek E et al. Impact of remote monitoring on long-term prognosis in heart failure patients in a real-world cohort: Results from all-comers COMMIT-HF Trial. J Cardiovasc Electrophysiol. 2017;28:425–31. doi: 10.1111/jce.13174. [DOI] [PubMed] [Google Scholar]
- 29.Koehler F, Koehler K, Deckwart O et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. 2018;392:1047–57. doi: 10.1016/S0140-6736(18)31880-4. [DOI] [PubMed] [Google Scholar]
- 30.Attia ZI, Kapa S, Lopez-Jimenez F et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25:70–4. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 31.Hannun AY, Rajpurkar P, Haghpanahi M et al. Publisher correction: Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med. 2019;25:530. doi: 10.1038/s41591-019-0359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boehmer JP, Hariharan R, Devecchi FG et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. 2017;5:216–25. doi: 10.1016/j.jchf.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Gardner RS, Singh JP, Stancak B et al. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: Results from the MultiSENSE study. Circ Heart Fail. 2018;11:e004669. doi: 10.1161/CIRCHEARTFAILURE.117.004669. [DOI] [PubMed] [Google Scholar]
- 34.Celler B, Varnfield M, Nepal S et al. Impact of at-home telemonitoring on health services expenditure and hospital admissions in patients with chronic conditions: before and after control intervention analysis. JMIR Med Inform. 2017;5:e29. doi: 10.2196/medinform.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noel HC, Vogel DC, Erdos JJ et al. Home telehealth reduces healthcare costs. Telemed J E Health. 2004;10:170–83. doi: 10.1089/tmj.2004.10.170. [DOI] [PubMed] [Google Scholar]
- 36.Gellis ZD, Kenaley BL, Ten Have T. Integrated telehealth care for chronic illness and depression in geriatric home care patients: the Integrated Telehealth Education and Activation of Mood (I-TEAM) study. J Am Geriatr Soc. 2014;62:889–95. doi: 10.1111/jgs.12776. [DOI] [PubMed] [Google Scholar]
- 37.Finkelstein SM, Speedie SM, Zhou X et al. Perception, satisfaction and utilization of the VALUE home telehealth service. J Telemed Telecare. 2011;17:288–92. doi: 10.1258/jtt.2011.100712. [DOI] [PubMed] [Google Scholar]
- 38.Perl L, Soifer E, Bartunek J et al. A novel wireless left atrial pressure monitoring system for patients with heart failure, first ex-vivo and animal experience. J Cardiovasc Transl Res. 2019;12:290–8. doi: 10.1007/s12265-018-9856-3. [DOI] [PubMed] [Google Scholar]
- 39.Feldman T, Mauri L, Kahwash R et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137:364–75. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 40.Kaye DM, Petrie MC, McKenzie S et al. Impact of an interatrial shunt device on survival and heart failure hospitalization in patients with preserved ejection fraction. ESC Heart Fail. 2019;6:62–9. doi: 10.1002/ehf2.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noseworthy J. The future of care – preserving the patient–physician relationship. N Engl J Med. 2019;381:2265–9. doi: 10.1056/NEJMsr1912662. [DOI] [PubMed] [Google Scholar]
- 42.Topol EJ. The big medical data miss: challenges in establishing an open medical resource. Nat Rev Genet. 2015;16:253–4. doi: 10.1038/nrg3943. [DOI] [PubMed] [Google Scholar]


