Abstract
The interactions between Mycobacterium tuberculosis and host phagocytes such as macrophages and dendritic cells are central to both immunity and pathogenesis. Many receptors have been implicated in recognition and binding of M. tuberculosis such as the mannose receptor, dendritic-cell-specific intercellular adhesion molecule-3 grabbing nonintegrin, dectin-1 and complement receptor 3 as well as Toll-like receptors, scavenger receptors and CD14. While in vitro studies have demonstrated clear roles for particular recep- tor(s), in vivo work in receptor-deficient animals often revealed only a minor, or no role, in infection with M. tuberculosis. The initial encounter of phagocytic cells with myco- bacteria appears to be complex and depends on various parameters. It seems likely that infection with M. tuberculosis does not occur via a single receptor-mediated pathway. Rather, multiple receptors play different roles in M. tuberculosis infection, and the overall effect depends on the expression and availability of a particular receptor on a particular cell type and its triggered downstream responses. Moreover, the role of membrane cholesterol for M. tuberculosis interactions with phagocytes adds to the complexity of mycobacterial recognition and response. This review summarizes current knowledge on non-opsonic receptors involved in binding of mycobacteria and discusses the contribution of individual receptors to the recognition process.
Key Words: Innate immune receptors, Non-opsonic receptors, Mycobacteria
Introduction
Tuberculosis is the world's leading bacterial cause of death, and about one third of the world's population is now infected with Mycobacterium tuberculosis. As a facultative intracellular pathogen, M. tuberculosis enters the host typically via aerosols, and alveolar resident macrophages are considered the first cells to engulf M. tuberculosis and become infected. After this first encounter, dendritic cells and monocyte-derived macrophages also participate in the phagocytic process [1]. Mycobacterial binding to macrophages occurs in cholesterol-rich domains of the host cell plasma membrane [2], and phagocytosis involves different kinds of receptors which either bind to non-opsonized M. tuberculosis or recognize opsonins on the mycobacterial surface. These receptors include C-type lectins such as the mannose receptor (MR), dendritic-cell-specific intercellular adhesion molecule-3 grabbing nonintegrin (DC-SIGN) and dectin-1, complement receptors, surfactant protein receptors, scavenger receptors, and glycosylphosphatidylinisotol (GPI)-anchored receptors such as CD14 [3, 4]. Other surface molecules such as Toll-like receptors (TLRs), are also important for mycobacterial interactions with phagocytic cells and their subsequent activation [5]. Invasion of macrophages by the tubercle bacillus, which is a facultative intracellular pathogen, is critical in the establishment of infection; consequently, M. tuberculosis has evolved several survival strategies and even growth inside its niche in the host cell, the mycobacterial phagosome [6]. M. tuberculosis interferes with the Rab-controlled membrane trafficking and thereby prevents phagosome maturation, which involves the fusion of phagosomes with lysosomes. This process, phagosomal arrest, is critical for M. tuberculosis persistence as it occurs at a stage where no harm can be done to the bacterium while delivery of nutrients continues [reviewed in ref. 6]. It has recently been demonstrated that tryptophan-aspartate-containing coat protein (TACO), also known as coronin 1 or P57, is indispensible for survival of mycobacteria in phagosomes [7]. This protein is normally released from phagosomes prior phagosome fusion with, or maturation into, lysosomes, but mycobacteria can actively retain TACO on phagosomes, thereby preventing their delivery to lysosomes [8]. TACO was shown to be required for calcineurin activation upon mycobacterial infection, thereby blocking their lysosomal delivery: when macrophages from TACO-deficient mice were studied, calcineurin was not activated, resulting in lysosomal transfer and death of internalized mycobacteria [7]. Although the prevailing paradigm states that M. tuberculosis prevents lysosomal fusion, and persists and replicates within the phagosomes of macrophages, a recent study examining an extended time course for up to 7 days of infection arrived at a different conclusion: van der Wel et al. [9] report the escape of mycobacteria into the cytoplasm, which they consider a pathogenic feature of virulent mycobacteria. However, this is contradicted by similar ultrastructural studies on different mycobacterial species, which were found to remain within phagosomes even at late times of infection [10].
The interaction between M. tuberculosis and its host cells is very complex and, although it has been very extensively studied, is far from being understood. The role of the innate immune receptors involved in M. tuberculosis recognition and binding, in particular, has been mostly based on in vitro examination in transfected cells; studies using inhibitors or animals deficient in specific receptors have indicated that these receptors can compensate for each other or are dispensable [11, 12, 13]. This review will focus on the role of membrane-bound (as opposed to soluble) innate immune receptors of phagocytes (fig. 1) that have been shown to recognize mycobacteria (i.e. ligands expressed on the surface of mycobacteria), in a non-opsonic fashion. In contrast to non-opsonic binding, opsonic receptors recognize microbes that are coated with various proteins as target molecules for recognition such as antibodies, complement proteins, lectins and surfactants. It is generally accepted that non-opsonic binding is important during primary infection with inhaled bacteria as serum and complement components are limited in the alveolar space [14]. However, the role of surfactant-opsonized mycobacteria in phagocyte binding is not insignificant [15]. Opsonized or serum-mediated uptake of M. tuberculosis may be more important at later stages of the infection [16, 17]. Also, cytoplasmic innate receptors such as nucleotide-binding oligomerization domain (NOD)-like receptors are important for innate immune recognition of M. tuberculosis [reviewed in ref. 18]. A recent study showed that macrophages and dendritic cells derived from NOD2-deficient mice are impaired in the production of pro-inflammatory cytokines upon mycobacterial infection compared to wild-type cells. However, no differences were observed in vivo with regard to susceptibility to M. tuberculosis infection [18].
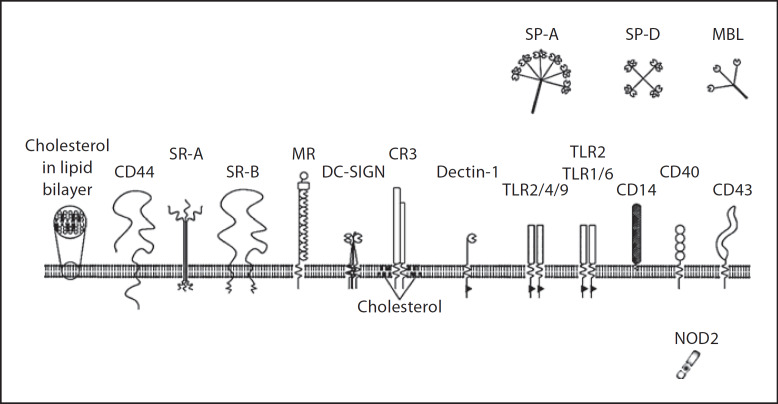
Fig. 1.
Receptors proposed to play a role in recognition and/or uptake of M. tuberculosis (illustration by I. Jastram).
In the present article, we will particularly concentrate on phagocytic membrane receptors involved in the very first line of defence against inhaled mycobacteria. These receptors are germ-line encoded and are also referred to as ‘pattern recognition receptors’ (PRRs), which recognize highly conserved molecular patterns (pathogen-associated molecular patterns) found only in microorganisms. The role of these receptors in M. tuberculosis infection, particularly their interaction with mycobacteria, downstream effector mechanisms, and their relevance in vivo will be discussed.
C-Type Lectins
Carbohydrate-binding C-type lectins play an important role in mycobacterial binding and in driving inflammatory responses due to the presence of carbohydrate-rich mycobacterial surface molecules. C-type lectins comprise a large family of proteins, divided into 17 groups, which contain one or more structurally related C-type lectin-like domains [reviewed in ref. 19]. One distinguishes between soluble lectins and cell-associated (or transmembrane) C-type lectins. Of the soluble C-type lectins, pulmonary surfactant proteins A and D, the collectins SP-A and SP-D, are important for mycobacterial infection: while SP-A increases M. tuberculosis-macrophage interaction by both directly upregulating phagocytosis and serving as a bacterial opsonin, SP-D agglutinates M. tuberculosis and decreases phagocytosis [reviewed in ref. 15]. Also, the soluble mannose-binding lectin which is primarily found in blood, was found to enhance infection of M. tuberculosis by facilitating the entry of mycobacteria into phagocytes, pathogen spread, and the establishment of infection [15]. SP-A, SP-D and mannose-binding lectin generally serve as opsonins and are therefore not the focus of this review.
Of the transmembrane C-type lectins, the MR, DC-SIGN and dectin-1 are most important for infection with M. tuberculosis and will be discussed below. Although complement receptor-3 (CR3), another important macrophage receptor for mycobacterial infections, theoretically belongs to the integrin superfamily, it contains a lectin site that interacts with mycobacterial ligands and will be discussed in this section.
The Mannose Receptor
The MR (CD207) is a type I transmembrane glycoprotein with a large extracellular region containing 8 linear carbohydrate recognition domains (CRDs), and a short cytoplasmic tail containing a non-cannonical tyrosine-based motif involved in phagocytosis, endocytosis and endosomal sorting [20]. The CRDs recognize a broad spectrum of ligands, including mannose-, N-acetylglucosamine- and fucose-terminated glycoconjugates [21, 22]. The MR is expressed predominantly on alveolar macrophages as well as monocyte-derived macrophages and dendritic cells, and represents a prototypic PRR recognizing the most abundant mycobacterial lipoglycan, mannose-capped lipoarabinomannan (ManLAM) [23]. Indeed, phagocytosis of M. tuberculosis by human macrophages occurs primarily via the MR [24]. However, this association depends on the length and abundance of surface-exposed ManLAMs, and the binding and phagocytosis of mycobacteria by the MR is limited to certain virulent strains of the M. tuberculosis complex [24]. It has been shown that higher-order phosphatidyl-myo-inositol mannosides (PIMs), particularly tri-acylated forms, as well as arabinomannans, mannans and mannoproteins can bind to this receptor [25].
It is still not clear whether the MR possesses a signalling function as it lacks defined signalling motifs in its cytoplasmic tail [20]. The binding of M. tuberculosis to the MR via ManLAM does not result in killing of the bacteria, but rather triggers anti-inflammatory pathways by interfering with the lipopolysaccharide-induced positive signals delivered by the TLRs such as IL-12 production [26]. Moreover, engagement of the MR by ManLAM during phagocytosis was shown to be crucial in limiting phagosome-lysosome fusion, allowing the bacteria to reach their unique niche within the host cell, the mycobacterial phagosome [27]. Anti-inflammatory and suppressive mechanisms are also triggered upon mycobacterial engagement of DC-SIGN via ManLAM. The relative importance of MR versus DC-SIGN ligation is briefly discussed below. The biological role of the MR in mycobacterial infections still has to be investigated in M. tuberculosis-infected MR-deficient mice.
Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin
DC-SIGN (CD209) is a type II mannose-binding C-type lectin receptor with a single extracellular CRD that clusters to form tetramers which are required for high-affinity binding of ligands [28]. Its intracellular region contains three motifs: a tyrosine-based internalization motif as well as a tri-acidic amino acid cluster and a di-leucine motif which are thought to be involved in phagocytosis and intracellular trafficking of ligand particles which can ultimately lead to phagosome-lysosome fusion [15].
DC-SIGN is expressed primarily on dendritic cells but has also been found on discrete subsets of macrophages [29] and has been shown to be induced on alveolar macrophages upon mycobacterial infection [30]. Initially described as a receptor for HIV gp120, DC-SIGN has been shown to recognize various microbial pathogens including viruses, bacteria, fungi and parasites [31]. DC-SIGN is not to be considered a classical PRR, but rather serves as an adhesion molecule which can be co-opted by microorganisms to their own advantage, leading to inhibition of the immunostimulatory function of dendritic cells and, hence, to the promotion of pathogen survival [32].
Recent studies revealed the importance of DC-SIGN as the major phagocytic receptor for M. tuberculosis on human dendritic cells by binding to ManLAM [33, 34]. Although some typical mycobacterial macrophage receptors are also expressed on dendritic cells such as the MR or CR3, they seem to be neglected by the tubercle bacillus [34].
It has been demonstrated that DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on LAM [35]. DC-SIGN binds particularly strongly to M. tuberculosis and M. bovis BCG, while mycobacterial strains lacking ManLAM such as the avirulent M. smegmatis are not bound by this receptor [33]. However, DC-SIGN can also bind mycobacterial lipomannan (LM), arabinomannan, the 19-kDa antigen, and lower- and higher-order PIMs [25, 33].
Binding of mycobacteria by DC-SIGN results in an anti-inflammatory immune response by blocking maturation of infected dendritic cells through secreted ManLAM and inducing IL-10 production [33], enabling the intracellular survival of these organisms but not their replication [36]. It is believed that DC-SIGN cooperates with TLR4, and that binding of ManLAM by DC-SIGN triggers signals that interfere with the mycobacterial-induced dendritic cell maturation signals presumably generated by TLR4 [33]. Suppression of the protective pro-inflammatory immune response by M. tuberculosis binding to DC-SIGN was recently confirmed in a study addressing genetic polymorphisms in the human CD209 promoter region: a single-nucleotide polymorphism (SNP) at position −336A/G led to downregulation of CD209 mRNA expression which was associated with significant protection against tuberculosis in the studied individuals [37]. However, this contradicts an earlier study that reported that the two promoter variants −871G and −336A decrease the risk of developing tuberculosis [38]. This discrepancy might be due to the different populations studied.
Although macrophages rather than dendritic cells are the primary target for mycobacterial infection, the specific function of dendritic cells in the cellular immune response seems to be modulated by M. tuberculosis [26]. Moreover, the intracellular behaviour of M. tuberculosis within dendritic cells differs from that in macrophages, with failure to replicate, which is linked to its different portal of entry [36]. It is possible that mycobacterial phagosomes mature to late endosomal/lysosomal stages in dendritic cells as opposed to macrophages where mycobacteria arrest phagosome maturation at an early endosomal stage, which promotes mycobacterial growth [32]. Indeed, it has been demonstrated that DC-SIGN directs phagocytic particles to the phagolysosome in transfected COS-1 fibroblast cells [27]. The observation that macrophages serve as the major intracellular niche to M. tuberculosis as opposed to dendritic cells could be explained by the different trafficking fate of M. tuberculosis after phagocytosis in the two cell types, which is linked to the receptor used: macrophages express high levels of MR while dendritic cells express high DC-SIGN activity, the latter being more potent in processing endocytosed products in lysosomes [27]. Thus, it is more favourable for the survival of the tubercle bacilli to enter the host via macrophages.
M. tuberculosis infection studies of mice lacking the murine DC-SIGN homologue SIGNR1 revealed no difference between wild-type and knockout animals in terms of survival rates and mycobacterial loads in the lungs or distant organs [39]. However, cellular expression and function of DC-SIGN and SIGNR1 are significantly different as SIGNR1 is not present on alveolar macrophages in uninfected mice nor is it induced on lung macrophages during infection. Although SIGNR1 and DC-SIGN display similar binding specificity, the role of SIGNR1 seems to be limited in murine M. tuberculosis infection [39].
Dectin-1
Dectin-1 is a type II transmembrane receptor which contains a single extracellular CRD on a stalk, and a cytoplasmic tyrosine-based activation-like motif which is involved in cellular activation [reviewed in ref. 40]. It is expressed on myeloid cells such as macrophages, dendritic cells and neutrophils, and a subset of T cells. Dectin-1 acts as a PRR and recognizes fungal-cell-wall-derived β-1,3-glucans in a calcium-independent manner and mediates numerous cellular responses, including microbial uptake and killing as well as the production of several cytokines and chemokines, and can directly couple innate and adaptive immune responses [41].
Extensively investigated as a receptor for fungal pathogens, the role of dectin-1 and its associated signalling pathways in any bacterial infection has not been demonstrated until recently when several studies started to point towards a participation of dectin-1, probably in cooperation with TLR2, in the pro-inflammatory response to infection with mycobacteria [42, 43, 44]. Murine bone-marrow-derived macrophages infected with avirulent or attenuated mycobacteria such as M. avium, M. smegmatis, M phlei or M. bovis BCG showed a dectin-1-dependent production of TNF-α. IL-6, and G-CSF as opposed to virulent M. tuberculosis H37Rv [42]. The authors speculate that the limited stimulation of dectin-1 by virulent mycobacteria might be partly responsible for the minimal macrophage pro-inflammatory response induced by these mycobacteria in contrast to avirulent mycobacteria [45]. However, this is controversial as some investigators find no deficiency in inflammatory responses induced by virulent mycobacteria although these responses vary from strain to strain [46, 47].
A study on splenic dendritic cells showed that dectin-1 triggers the production of IL12p40 and IL12p70 independently of TLR2 upon infection with M. tuberculosis through an unknown mechanism involving Syk signalling [43]. Using the non-tuberculous mycobacterium M. abscessus, it could be demonstrated that dectin-1 in direct cooperation with TLR2, was required for phagocytosis of this organism in murine macrophages with subsequent activation of Syk and production of TNF-α. IL-6 and IL12p40 [44]. Although the interaction of dectin-1 with TLR2 was not observed in all of these studies, the collaboration of both receptors and the subsequent pro-inflammatory cytokine production by macrophages does occur via the Syk kinase pathway [48].
While the participation of dectin-1 in mycobacterial infection has begun to be elucidated, it is still not clear what ligand(s) on mycobacteria interact with dectin-1. The presence of β-glucans has not been described in mycobacteria but it is known that M. bovis BCG and M. tuberculosis express α-glucan within the outer capsule [49]. Also, dectin-1 might not be restricted to the recognition of β-glucans as it can also bind an endogenous but undefined ligand on T cells [50]. It has been shown that live mycobacteria bind directly to dectin-1 in a laminarin-inhibitable manner [42, 43, 44], indicating the presence of a ligand for dectin-1 on the bacterial envelope, which might mimic carbohydrate structures present in fungal β-glucans. Inhibition of mycobacterial binding and/or cytokine production by laminarin has also been shown in studies on CR3 (see paragraph below).
Taken together, the function of dectin-1 in host defence upon infection with mycobacteria is still unclear and probably dependent on the cell type being infected and the mycobacterial strain used. In vivo studies on dectin-1-deficient mice are urgently needed to define the biological role and regulatory mechanisms of this receptor for mycobacterial infection.
Complement Receptor 3
M. tuberculosis has been shown to activate the alternative complement pathway and become opsonized by C3b and iC3b which then enables interaction with complement receptors CR1 and CR3/CR4 [51]. Moreover, a C2a-dependent entry pathway specific for mycobacteria has been described [52]. It should be mentioned here that CR3 is the major complement receptor involved in phagocytosis of mycobacteria as this receptor mediates approximately 80% of complement-opsonized M. tuberculosis phagocytosis [51]. Interestingly, CR3 is able to mediate both complement-opsonized as well as non-opsonized phagocytosis of micro-organisms by different mechanisms, both of which have been described for mycobacteria [reviewed in ref. 53].
CR3, also named integrin αMβ2 or CD11b/CD18 or Mac-1, is a heterodimeric surface receptor that belongs to the integrin superfamily. It is expressed on neutrophils, monocytes, natural killer cells and alveolar macrophages. However, resident alveolar macrophages have been shown to express CR3 poorly while differentiation of alveolar macrophages in vitro resulted in increased expression of CR3, which was accompanied by an increased capacity to bind mycobacteria [17]. CR3 displays a broad ligand spectrum due to multiple binding sites; those ligands include complement fragment iC3b, intracellular adhesion molecule-1 (ICAM-1), and bacterial products [reviewed in ref. 53].
It has been shown that mycobacterial antigen 85C is recognized by the I domain of CR3, which also binds to complement molecule iC3b, while the lectin-like C-domain of CR3 binds mycobacterial oligosaccharides such as LAM, which probably is the predominant binding site for mycobacteria [53]. This lectin-like domain has been characterized as a binding site for β-glucans as well as mannose, glucose and N-acetyl-D-glucosamine [54]. Indeed, competition studies showed that the binding of M. tuberculosis was inhibited by the β-glucan laminarin although this might simply represent the broad ligand spectrum of CR3 [53]. Studies on dendritic cells isolated from CD11b–/– mice showed that laminarin-blocked M. tuberculosis triggered IL12p40 production independently of this subunit of CR3 [43].
Binding of mycobacterial PIMs to CR3 has been described as well [55], suggesting that the receptor's lectin site might recognize the mannosyl moiety of PIM. The binding of mycobacteria to the lectin-like domain of CR3 could be blocked by β-glucans, which resulted in a strong inhibition of phagocytosis [53]. Differences in oligosaccharide composition influence the capability of various mycobacterial strains to bind to the lectin-like domain of CR3: studies on CR3-transfected cells revealed that pathogenic mycobacteria, but not non-pathogenic species, were internalized by this receptor [53]. However, phagocytosis of mycobacteria by CR3 does not induce killing of the bacteria [53].
Studies on macrophages isolated from CR3-deficient mice revealed that this receptor plays a role in the uptake of viable M. tuberculosis. However, CR3 does not play any role in the induction of microbicidal mechanisms and subsequent survival of the bacteria [56]. The observation that CR3-deficient and wild-type mice are equally resistant to M. tuberculosis infection suggests that in the absence of CR3, phagocyte infection by mycobacteria can be efficiently supported by alternative receptors [12]. Therefore, the biological role of CR3 in mycobacterial infection in vivo remains uncertain.
Toll-Like Receptors
The TLRs belong to an evolutionary highly conserved family of membrane receptors that have homology to the Drosophila Toll system. The mammalian TLR family now comprises 11 members which recognize a variety of microbial products including bacterial LPS, lipoteichoic acid, peptidoglycans, unmethylated CpG motifs of bacterial DNA or double-stranded RNA of viruses. They are expressed on various immune and non-immune cells including macrophages and dendritic cells [reviewed in ref. 57]. It has recently been demonstrated that the expression of various TLRs is upregulated in whole-blood samples of patients with active pulmonary tuberculosis [58]. The TLRs are type I transmembrane proteins that contain repeated leucine-rich motifs in their extracellular domain which are involved in microbial recognition while their cytoplasmic domains are homologous to the signalling domain of the IL1 receptor. The interaction of M. tuberculosis with TLRs leads to phagocyte activation and may modulate pathways of phagocytosis, but does not directly initiate ingestion of the microbes.
Indeed, activated TLRs trigger intracellular signalling cascades [reviewed in ref. 59], most of which relying heavily on the adapter protein MyD88 and leading to pro-inflammatory and antimicrobial innate immune responses such as NF-κB activation, MAP kinase activation and production of TNF-α, IL-1, IL-12, chemokines and nitric oxide. It is generally believed that a dominance of TLR signalling favours inflammatory and protective pathways as opposed to LAM signalling via C-type lectins (such as MR and DC-SIGN), which favours anti-inflammatory and suppressive mechanisms (discussed in [32]). Moreover, a recent study showed that the LM acylation pattern determines the pro-inflammatory versus anti-inflammatory effects of LM through different PRRs, i.e. TLR2-dependent versus TLR2-independent regulation [60]. However, some of the TLR-induced signals are anti-inflammatory and may mediate feedback inhibition to limit macrophage activation and prevent excessive inflammation: a recent study demonstrated that mycobacterial early secreted antigenic target protein-6 dampened TLR signalling by preventing assembly of the cytosolic MyD88-dependent signalling scaffold [61]. Further, TIR8, a member of the IL-1 receptor family, has recently been shown to play a key role in dampening inflammation and tissue damage in M. tuberculosis infection: TIR8-deficient mice succumbed rapidly to infection with M. tuberculosis [62].
As TLRs themselves do not function as phagocytic receptors, they are often complemented or even rely on other PRRs such as C-type lectins and scavenger receptors for efficient activation and ligand binding [reviewed in ref. 63]. Cross-talk between various receptors including TLR2, TLR4 and the MR for efficient IFN-γ production upon mycobacterial binding has recently been described in a tuberculous pleurisy model [64].
Mycobacteria can activate TLR2, TLR4, TLR9 and TLR1/TLR6 that heterodimerize with TLR2and have all been implicated in recognition of mycobacterial antigens [reviewed in ref. 65]. Mycobacterial induction of TNF-α and IL-12 production was found to be primarily dependent on TLR2 signaling and to a lesser extent TLR4 signalling [66], TLR2 and TLR9 being known to regulate dendritic-cell-derived IL-12 production in murine M. tuberculosis infection [67]. It has been shown that TLR2 recognizes mycobacterial lipoproteins such as the 19-kDa antigen (LpqH), LprA (Rv1270) and LprG (Rv1411) as well as LM and PIMs, while TLR2-dependent cell activation by LAM via phosphoinositide-capped lipoarabinomannan and arabinose-capped lipoarabinomannan has only been described for fast-growing mycobacteria [reviewed in ref. 59]. Cells are activated through TLR4 by binding to a heat-labile factor associated with M. tuberculosis as well as LM [5] and PIM [68], while TLR9 can sense the presence of M. tuberculosis DNA [67]. ManLAM which is mainly recognized by the MR and DC-SIGN, resulting in anti-inflammatory effects, is not recognized by any TLR, suggesting an interesting correlation between LAM-capping structure and its immunomodulatory effects [59].
Although studies on mice infected with M. tuberculosis aerosol demonstrated a role for TLR2 and TLR4 in long-term control of the infection [68, 69]. TLR2 and TLR4 seemed to be redundant in controlling M. tuberculosis infection in another mouse model: only at high infectious doses was reduced survival reported in TLR2-deficient mice [70]. Similar results were obtained in a study using M. avium-infected TLR2- or TLR4-deficient mice [71]. Infected mice deficient in both TLR2 and TLR9 revealed a major cooperation between these two receptors for host resistance to mycobacteria [67]. However, a recent study on mice infected with M. tuberculosis aerosol deficient in all TLRs to which M. tuberculosis products have been assigned as ligands, i.e. TLR2, TLR4 and TLR9, demonstrated that expression of pro-inflammatory cytokines was similar to that in wild-type animals. Also, both wild-type and TLR2/4/9 triple-deficient mice were able to control M. tuberculosis replication, suggesting that TLR2, TLR4 and TL9 are not critical for triggering macrophage effector mechanisms central to anti-mycobacterial defence [72]. TLR2 forms heterodimers with either TLR1 or TLR6, and studies on TLR6-deficient mice showed that these animals are also resistant to high-dose M. tuberculosis aerosol infection [73]. The role of TLR1 in in vivo host response has not been determined yet. Interestingly, studies using mice deficient in the adapter molecule MyD88, which is common to signalling by most TLRs, but also IL1 receptor, succumbed to unrestrained mycobacterial growth while it was not required for the induction of adaptive T cell responses [72].
Taken together, the role of TLRs in immune responses to M. tuberculosis in vivo remains controversial and strongly depends on the experimental setting such as differences in the mycobacterial strains used, differences in the nature and the dose of the microbe, and differences in genetic backgrounds of the receptor-deficient mice. However, TLRs seem to be relevant in human immunity to tuberculosis as a SNP in the human TLR2 gene was associated with higher susceptibility to tuberculous meningitis [74].
Scavenger Receptors
Scavenger receptors belong to a broad family of cell surface transmembrane glycoproteins with multidomain structures classified into 6 subgroups based on their tertiary structure. Initially described to be important for atherogenesis they also play important roles in innate immunity and macrophage regulation. SRs display broad ligand-binding abilities, including recognition of modified lipoproteins, polyanionic molecules as well as Gram-positive and Gram-negative bacteria [reviewed in ref. 75].
Although the role of SRs in non-opsonic recognition and binding of mycobacteria has not been extensively studied, there are a few reports that point towards an implication of SRs in these mechanisms. Initially, class A SRs have been suggested to be important receptors for M. tuberculosis on human monocyte-derived macrophages: when binding to CRs and the MR was blocked, all binding that still persisted could be abrogated when class A SRs were competitively blocked with fucoidin and dextran sulfate [13]. Adipocytes which express numerous SRs can also display macrophage-like characteristics and phagocytose particles. Binding of M. tuberculosis to adipocytes of murine or human origin could be blocked by SR ligands such as polyinosinic acid, fucoidan and oxidized low-density lipoprotein species [76]. Recently, a genome wide RNAi screen for Drosophila macrophage-like cells identified Peste (Pes), a member of the CD36 family of SRs, to be essential for entry of M. fortuitum [77]. Moreover, human class B scavenger receptors were shown to mediate uptake of M. fortuitum, while murine CD36 did not [77].
Although no signal-transducing activity has been attributed to any type of SR, it has recently been shown that CD36 can augment TLR2 signalling in response to a subset of TLR2 ligands [78].
CD14
CD14 is a cell surface lipid-anchored glycan-linked protein without transmembrane and cytoplasmic domains which is predominantly expressed on myelomonocytic cells. It mainly binds LPS of Gram-negative bacteria but can also interact with lipoteichoic acids and peptidoglycan of Gram-positive bacteria [79]. Further, CD14 can bind mycobacterial LAM, which leads to the secretion of IL-8 by macrophages [80]. It has also been shown that M. tuberculosis chaperonin 60.1 partially activates cells via a CD14-dependent mechanism [81]. Earlier studies demonstrated that CD14 can mediate uptake of M. tuberculosis by human microglial cells [82]. Generally, binding of microbial products by CD14 ultimatively leads to phagocytosis, cellular activation and cytokine secretion, but requires cooperation with other receptors and transducer elements such as TLRs [83]. It has been shown that the concentration of soluble CD14 (which is produced by enzymatically cleaved membrane CD14) is significantly increased in patients with pulmonary tuberculosis [84]. Moreover, an SNP in the promoter region of the human CD14 gene, which is associated with elevated levels of soluble CD14 was associated with a higher risk of developing pulmonary tuberculosis [85].
Studies on CD14-deficient murine bone-marrow-derived macrophages demonstrated a significant decrease in TNF-α production upon infection with M. avium compared to control wild-type mice. In vivo infection of these CD14-deficient mice with M. tuberculosis or M. avium revealed no difference in mounting a protective and granulomatous response to mycobacterial infection compared to wild-type control mice [22, 70]. However, a recent study described a role for CD14 in chronic M. tuberculosis infection. Whilst no difference in bacterial load in the lung was found between wild-type and CD14-deficient mice up to 32 weeks after high-dose infection, the wild-type mice started to succumb to infection from 20 weeks onwards while the CD14-knockout mice did not [86]. Therefore, CD14 deficiency seems to protect the animals from chronic M. tuberculosis infection via a reduction in the inflammatory response.
Other Receptors
Other receptors such as CD40, CD43 and CD44, which have been shown to play a role in mycobacterial recognition will be briefly summarized here.
CD40, a member of the TNF receptor family and a co-stimulatory molecule on antigen-presenting cells, has been shown to be involved in M. tuberculosis recognition via hsp70 expressed on mycobacteria [87] leading to stimulation of CC-chemokine production. Another study suggested that CD40 is not directly involved but drives inflammation and enhances immune responses to M. tuberculosis infection in vivo when dendritic cells are stimulated via CD40 although the mycobacterial burden in the lungs is not reduced compared to control animals [88].
CD43, a transmembrane sialoglycoprotein expressed on most hemopoietic cells, was initially studied in vitro in CD43-deficient macrophages, revealing a role of this surface mucin in binding and/or uptake of mycobacteria and in mycobacterium-induced TNF-α production [89]. This CD43-mediated induction of pro-inflammatory cytokines is thought to regulate apoptosis in order to control intracellular growth of mycobacteria [90]; aerosol-infected CD43-knockout mice showed an increased mycobacterial load during both the acute and chronic stage of infection and a more rapid development of granulomas compared to wild-type animals.
CD44, an adhesion molecule which is upregulated during inflammatory response, has also been shown to be a receptor for M. tuberculosis on macrophages that mediates phagocytosis [91]. However, CD44-deficient mice infected with M. tuberculosis showed neither a difference in pulmonary bacterial load compared to wild-type animals nor an altered expression of Th1 immunity to tuberculosis [92]. Nevertheless, CD44 plays an important role in controlling and regulating lung inflammation as absence of this molecule resulted in a substantially increased accumulation of neutrophils in the lung [92].
Cholesterol
Although cholesterol is not a receptor, its role in mycobacterial uptake has emerged in recent years and it is suggested that cholesterol might be directly involved in receptor-mediated phagocytosis. Therefore, studies on interactions between mycobacteria and cholesterol are mentioned below.
Mycobacteria have been demonstrated to display a high binding capacity for cholesterol compared to other microorganisms [2], possibly by direct binding to cholesterol of components of the extremely glycolipid-rich mycobacterial cell wall. Indeed, cholesterol accumulates at the site of M. tuberculosis entry into macrophages, and depleting cells of cholesterol prevents M. tuberculosis internalization [2], indicating that cholesterol accumulation around phagocytic receptors rather than the nature of the receptors itself may dictate M. tuberculosis uptake. Moreover, TACO, a component of the phagosome coat that prevents degradation of mycobacteria in lysosomes, was shown to be associated with cholesterol-rich membrane regions [2]. Therefore, entering the cholesterol-rich domains of macrophages where TACO is predominantly found might ensure mycobacterial intracellular survival in TACO-coated phagosomes [2]. Moreover, cholesterol is also required for mycobacteria to prevent fusion of phagosomes with lysosomes: when pre-existing phagosomes infected with M. avium were depleted of cholesterol, they were reported to mature and fuse with lysosomes [93].
One of the receptors that might confer cholesterol-dependent non-opsonic mycobacterial uptake has been identified in neutrophils as being CR3. It was shown that upon infection with M. kansasii, CR3 and associated GPI-anchored proteins relocate in cholesterol-rich domains where internalization of the bacterium occurs [94].
It has been speculated that hypocholesterolaemia is a major risk factor for developing pulmonary tuberculosis [95], and patients receiving a cholesterol-rich diet showed an accelerated bacteriologic sterilization of sputum cultures. However, it has not yet been determined whether in vivo a similar relocation of cholesterol occurs at sites of M. tuberculosis entry as described in [2].
Another interesting association between cholesterol and infection with M. tuberculosis has been reported only recently: mice deficient in apolipoprotein E (ApoE) succumbed to a low-dose M. tuberculosis aerosol infection only 4 weeks after infection when fed a high-cholesterol diet compared to ApoE-deficient mice on a low-cholesterol diet or wild-type mice on either diet [96]. ApoE-deficient mice display a hypercholesterolaemic phenotype as they are unable to take up blood cholesterol. These highly elevated serum cholesterol levels when fed a high-cholesterol diet were found to be responsible for the extreme M. tuberculosis susceptibility of these mice. The underlying mechanism is not clear but M. tuberculosis can take up and use cholesterol as a source of energy and might therefore have a growth advantage due to the increased nutrient availability. This hypothesis was confirmed by a recent study showing that a mycobacterial gene cluster encoding genes involved in cholesterol catabolism is specifically expressed during survival of M. tuberculosis in macrophages [97].
Conclusions
Taken together, the interaction of mycobacteria with both opsonic and non-opsonic receptors on phagocytic cells is complex and incompletely understood. The role of a particular receptor is often studied in vitro in an artificial system and isolated from the physiological context while data obtained from in vivo studies are often very controversial and the results seem to vary largely in different experimental settings. It is more than likely that in vivo, M. tuberculosis is not internalized by macrophages using a single receptor-mediated pathway. Mycobacteria display numerous and diverse ligands on their surface which might either engage multiple receptors of multiple types simultaneously, or might bind to those receptors that are available on a particular cell or cell type. Moreover, mycobacterial surface molecules might also function to impair the formation of adequate receptor clustering, i.e. cooperation of various receptors to cross-talk and coordinate recognition and entrance of mycobacteria into phagocytes. The role of cholesterol in mycobacterial uptake and survival has been discovered recently and further adds to the complexity of macrophage-mycobacterial interactions (fig. 1).
Various studies propose that progression of tuberculosis following infection is determined by the type of encounter between mycobacteria and host cell receptors such as C-type lectins versus TLR, and consequently the type of host cell such as macrophage versus dendritic cells. However, the question still remains whether M. tuberculosis or the receptor engaged by M. tuberculosis (or both) determine the fate of the phagosome and, consequently, the course of disease. As mentioned above, a dominance of TLR signalling would favour inflammatory and protective pathways while a dominance of signaling via C-type lectins would favour anti-inflammatory and suppressive/non-protective mechanisms. In this context, the LAM capping structure displays an interesting correlation to their immunomodulatory effect: Phosphoinositide-capped lipoarabinomannans are usually considered pro-inflammatory molecules stimulating the production of TNF and IL-12 while ManLAM are rather anti-inflammatory molecules stimulating IL-10 production and inhibiting IL-12 and TNF production [59].
It is possible that the different PRRs on alveolar macrophages outcompete LAM-mediated inhibitory signaling, thus favoring protective effector functions [32]. The overall effect probably depends on various parameters, especially the expression and availability of particular receptors and their triggered downstream responses.
As mentioned before, inconsistencies in the various studies on mycobacterium-receptor function might be due to several technical aspects. The substantial complexity in host defenses explains why specific components have been found to be either essential or redundant. This is dependent on both the nature of the microbe and perhaps also the site of infection (i.e. cell type infected). For example, the phenotype of the macrophages (resident vs. elicited vs. immune activated) as well as the way how mycobacteria are prepared in a particular experimental setting (sonication vs. non-sonication) greatly influences phagocyte-mycobacterium interaction [16, 98]. It has been shown previously that it is important to use experimental infection procedures that most closely mimic natural exposure to obtain results that are physiologically most relevant: while dynamic (shear) binding conditions select more for higher-affinity interactions and imitate the physiological situation better, static binding of mycobacteria to cells would generate more non-specific background binding as demonstrated in a recent in vitro study [99]. In terms of the in vivo system, M. tuberculosis has been shown to be more virulent when administered aerogenically rather than intravenously [100].
As adhesion and internalization are the first steps in mycobacterial pathogenesis, unraveling the role of the receptor(s) involved in these processes is of utmost importance. Inhibition of these events could constitute an efficient form of prophylaxis aimed at blocking invasion of M. tuberculosis.
Acknowledgements
This work was supported by funding from the Deutsche Forschungsgemeinschaft to G.S. (grant SCHA 1470/1-1) and the Wellcome Trust and MRC (South Africa) to G.D.B. R.J.W. receives support from the Wellcome Trust, MRC (UK), European Union and the Bill and Melinda Gates Foundation. G.D.B. is a Wellcome Trust International Senior Research Fellow.
References
- 1.van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15:294–309. doi: 10.1128/CMR.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatfield J, Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages. Science. 2000;288:1647–1650. doi: 10.1126/science.288.5471.1647. [DOI] [PubMed] [Google Scholar]
- 3.Ehlers MR, Daffe M. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends Microbiol. 1998;6:328–335. doi: 10.1016/s0966-842x(98)01301-8. [DOI] [PubMed] [Google Scholar]
- 4.Ernst JD. Macrophage receptors for Mycobacterium tuberculosis. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 6.Vergne I, Chua J, Singh SB, Deretic V. Cell biology of Mycobacterium tuberculosis phagosome. Annu Rev Cell Dev Biol. 2004;20:367–394. doi: 10.1146/annurev.cellbio.20.010403.114015. [DOI] [PubMed] [Google Scholar]
- 7.Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, Huygen K, Miyazaki T, Albrecht I, Massner J, Pieters J. Survival of mycobacteria in macrophages is mediated by coronin 1-dependent activation of calcineurin. Cell. 2007;130:37–50. doi: 10.1016/j.cell.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari G, Langen H, Naito M, Pieters J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 1999;97:435–447. doi: 10.1016/s0092-8674(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 9.van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–1298. doi: 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 10.Jordao L, Bleck CK, Mayorga L, Griffiths G, Anes E. On the killing of mycobacteria by macrophages. Cell Microbiol. 2008;10:529–548. doi: 10.1111/j.1462-5822.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers S, Reiling N, Gangloff S, Woltmann A, Goyert S. Mycobacterium avium infection in CD14-deficient mice fails to substantiate a significant role for CD14 in antimycobacterial protection or granulomatous inflammation. Immunology. 2001;103:113–121. doi: 10.1046/j.1365-2567.2001.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu C, Mayadas-Norton T, Tanaka K, Chan J, Salgame P. Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol. 2000;165:2596–2602. doi: 10.4049/jimmunol.165.5.2596. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerli S, Edwards S, Ernst JD. Selective receptor blockade during phagocytosis does not alter the survival and growth of Mycobacterium tuberculosis in human macrophages. Am J Respir Cell Mol Biol. 1996;15:760–770. doi: 10.1165/ajrcmb.15.6.8969271. [DOI] [PubMed] [Google Scholar]
- 14.Schluger NW. Recent advances in our understanding of human host responses to tuberculosis. Respir Res. 2001;2:157–163. doi: 10.1186/rr53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torrelles JB, Azad AK, Henning LN, Carlson TK, Schlesinger LS. Role of C-type lectins in mycobacterial infections. Curr Drug Targets. 2008;9:102–112. doi: 10.2174/138945008783502467. [DOI] [PubMed] [Google Scholar]
- 16.Stokes RW, Haidl ID, Jefferies WA, Speert DP. Mycobacteria-macrophage interactions. Macrophage phenotype determines the nonopsonic binding of Mycobacterium tuberculosis to murine macrophages. J Immunol. 1993;151:7067–7076. [PubMed] [Google Scholar]
- 17.Stokes RW, Thorson LM, Speert DP. Nonopsonic and opsonic association of Mycobacterium tuberculosis with resident alveolar macrophages is inefficient. J Immunol. 1998;160:5514–5521. [PubMed] [Google Scholar]
- 18.Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21:279–286. doi: 10.1097/QCO.0b013e3282f88b5d. [DOI] [PubMed] [Google Scholar]
- 19.Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–6217. doi: 10.1111/j.1742-4658.2005.05031.x. [DOI] [PubMed] [Google Scholar]
- 20.Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Taylor ME. Structure and function of the macrophage mannose receptor. Results Probl Cell Differ. 2001;33:105–121. doi: 10.1007/978-3-540-46410-5_6. [DOI] [PubMed] [Google Scholar]
- 22.Reiling N, Klug K, Krallmann-Wenzel U, Laves R, Goyert S, Taylor ME, Lindhorst TK, Ehlers S. Complex encounters at the macrophage-mycobacterium interface: studies on the role of the mannose receptor and CD14 in experimental infection models with Mycobacterium avium. Immunobiology. 2001;204:558–571. doi: 10.1078/0171-2985-00093. [DOI] [PubMed] [Google Scholar]
- 23.Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- 24.Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- 25.Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- 26.Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 27.Kang PB, Azad AK, Torrelles JB, Kaufman TM, Beharka A, Tibesar E, DesJardin LE, Schlesinger LS. The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med. 2005;202:987–999. doi: 10.1084/jem.20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernhard OK, Lai J, Wilkinson J, Sheil MM, Cunningham AL. Proteomic analysis of DC-SIGN on dendritic cells detects tetramers required for ligand binding but no association with CD4. J Biol Chem. 2004;279:51828–51835. doi: 10.1074/jbc.M402741200. [DOI] [PubMed] [Google Scholar]
- 29.Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–457. [PubMed] [Google Scholar]
- 30.Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, Herrmann JL, Charles P, Schwartz O, Scheinmann P, Lagrange PH, de Blic J, Tazi A, et al. DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med. 2005;2:e381. doi: 10.1371/journal.pmed.0020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Kooyk Y, Geijtenbeek TB. DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol. 2003;3:697–709. doi: 10.1038/nri1182. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann SH, Schaible UE. A dangerous liaison between two major killers: Mycobacterium tuberculosis and HIV target dendritic cells through DC-SIGN. J Exp Med. 2003;197:1–5. doi: 10.1084/jem.20021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geijtenbeek TB, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M, Amara A, Legres L, Dreher D, Nicod LP, Gluckman JC, et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med. 2003;197:121–127. doi: 10.1084/jem.20021468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maeda N, Nigou J, Herrmann JL, Jackson M, Amara A, Lagrange PH, Puzo G, Gicquel B, Neyrolles O. The cell surface receptor DC-SIGN discriminates between Mycobacterium species through selective recognition of the mannose caps on lipoarabinomannan. J Biol Chem. 2003;278:5513–5516. doi: 10.1074/jbc.C200586200. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann JL, Lagrange PH. Dendritic cells and Mycobacterium tuberculosis: which is the Trojan horse? Pathol Biol (Paris) 2005;53:35–40. doi: 10.1016/j.patbio.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Vannberg FO, Chapman SJ, Khor CC, Tosh K, Floyd S, Jackson-Sillah D, Crampin A, Sichali L, Bah B, Gustafson P, et al. CD209 genetic polymorphism and tuberculosis disease. PLoS ONE. 2008;3:e1388. doi: 10.1371/journal.pone.0001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barreiro LB, Neyrolles O, Babb CL, Tailleux L, Quach H, McElreavey K, Helden PD, Hoal EG, Gicquel B, Quintana-Murci L. Promoter variation in the DC-SIGN-encoding gene CD209 is associated with tuberculosis. PLoS Med. 2006;3:e20. doi: 10.1371/journal.pmed.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wieland CW, Koppel EA, den Dunnen J, Florquin S, McKenzie AN, van Kooyk Y, van der Poll T, Geijtenbeek TB. Mice lacking SIGNR1 have stronger T helper 1 responses to Mycobacterium tuberculosis. Microbes Infect. 2007;9:134–141. doi: 10.1016/j.micinf.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Brown GD. Dectin-1:a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav M, Schorey JS. The β-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL, Brown GD, Sher A. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol. 2007;179:3463–3471. doi: 10.4049/jimmunol.179.6.3463. [DOI] [PubMed] [Google Scholar]
- 44.Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, Takahara K, Lee SJ, Jo EK. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008;10:1608–1621. doi: 10.1111/j.1462-5822.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- 45.Appelberg R, Sarmento A, Castro AG. Tumour necrosis factor-α (TNF-α) in the host resistance to mycobacteria of distinct virulence. Clin Exp Immunol. 1995;101:308–313. doi: 10.1111/j.1365-2249.1995.tb08356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson ST, Williams AJ, Brown JR, Newton SM, Simsova M, Nicol MP, Sebo P, Levin M, Wilkinson RJ, Wilkinson KA. Transmission of Mycobacterium tuberculosis undetected by tuberculin skin testing. Am J Respir Crit Care Med. 2006;173:1038–1042. doi: 10.1164/rccm.200509-1526OC. [DOI] [PubMed] [Google Scholar]
- 47.Newton SM, Smith RJ, Wilkinson KA, Nicol MP, Garton NJ, Staples KJ, Stewart GR, Wain JR, Martineau AR, Fandrich S, et al. A deletion defining a common Asian lineage of Mycobacterium tuberculosis associates with immune subversion. Proc Natl Acad Sci USA. 2006;103:15594–15598. doi: 10.1073/pnas.0604283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, et al. Syk kinase is required for collaborative cytokine production induced through dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dinadayala P, Lemassu A, Granovski P, Cerantola S, Winter N, Daffe M. Revisiting the structure of the anti-neoplastic glucans of Mycobacterium bovis Bacille Calmette-Guérin. Structural analysis of the extracellular and boiling water extract-derived glucans of the vaccine substrains. J Biol Chem. 2004;279:12369–12378. doi: 10.1074/jbc.M308908200. [DOI] [PubMed] [Google Scholar]
- 50.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, 3rd, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 51.Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990;144:2771–2780. [PubMed] [Google Scholar]
- 52.Schorey JS, Carroll MC, Brown EJ. A macrophage invasion mechanism of pathogenic mycobacteria. Science. 1997;277:1091–1093. doi: 10.1126/science.277.5329.1091. [DOI] [PubMed] [Google Scholar]
- 53.Velasco-Velazquez MA, Barrera D, Gonzalez-Arenas A, Rosales C, Agramonte-Hevia J. Macrophage-Mycobacterium tuberculosis interactions: role of complement receptor 3. Microb Pathog. 2003;35:125–131. doi: 10.1016/s0882-4010(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 54.Thornton BP, Vetvicka V, Pitman M, Goldman RC, Ross GD. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 55.Villeneuve C, Gilleron M, Maridonneau-Parini I, Daffe M, Astarie-Dequeker C, Etienne G. Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J Lipid Res. 2005;46:475–483. doi: 10.1194/jlr.M400308-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Rooyakkers AW, Stokes RW. Absence of complement receptor 3 results in reduced binding and ingestion of Mycobacterium tuberculosis but has no significant effect on the induction of reactive oxygen and nitrogen intermediates or on the survival of the bacteria in resident and interferon-γ activated macrophages. Microb Pathog. 2005;39:57–67. doi: 10.1016/j.micpath.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987; quiz 988. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 58.Chang JS, Huggett JF, Dheda K, Kim LU, Zumla A, Rook GA. Myobacterium tuberculosis induces selective up-regulation of TLRs in the mononuclear leukocytes of patients with active pulmonary tuberculosis. J Immunol. 2006;176:3010–3018. doi: 10.4049/jimmunol.176.5.3010. [DOI] [PubMed] [Google Scholar]
- 59.Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D, Yeremeev V, Bihl F, Erard F, Botha T, Drennan M, et al. Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect. 2004;6:946–959. doi: 10.1016/j.micinf.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 60.Doz E, Rose S, Nigou J, Gilleron M, Puzo G, Erard F, Ryffel B, Quesniaux VF. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J Biol Chem. 2007;282:26014–26025. doi: 10.1074/jbc.M702690200. [DOI] [PubMed] [Google Scholar]
- 61.Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol. 2007;8:610–618. doi: 10.1038/ni1468. [DOI] [PubMed] [Google Scholar]
- 62.Garlanda C, Di Liberto D, Vecchi A, La Manna MP, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J Immunol. 2007;179:3119–3125. doi: 10.4049/jimmunol.179.5.3119. [DOI] [PubMed] [Google Scholar]
- 63.Mukhopadhyay S, Herre J, Brown GD, Gordon S. The potential for Toll-like receptors to collaborate with other innate immune receptors. Immunology. 2004;112:521–530. doi: 10.1111/j.1365-2567.2004.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schierloh P, Yokobori N, Aleman M, Landoni V, Geffner L, Musella RM, Castagnino J, Baldini M, Abbate E, de la Barrera SS, Sasiain MC. Mycobacterium tuberculosis-induced γ-interferon production by natural killer cells requires cross-talk with antigen-presenting cells involving Toll-like receptors 2 and 4 and the mannose receptor in tuberculous pleurisy. Infect Immun. 2007;75:5325–5337. doi: 10.1128/IAI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jo EK, Yang CS, Choi CH, Harding CV. Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol. 2007;9:1087–1098. doi: 10.1111/j.1462-5822.2007.00914.x. [DOI] [PubMed] [Google Scholar]
- 66.Means TK, Jones BW, Schromm AB, Shurtleff BA, Smith JA, Keane J, Golenbock DT, Vogel SN, Fenton MJ. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–4082. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 67.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med. 2005;202:1715–1724. doi: 10.1084/jem.20051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abel B, Thieblemont N, Quesniaux VJ, Brown N, Mpagi J, Miyake K, Bihl F, Ryffel B. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169:3155–3162. doi: 10.4049/jimmunol.169.6.3155. [DOI] [PubMed] [Google Scholar]
- 69.Drennan MB, Nicolle D, Quesniaux VJ, Jacobs M, Allie N, Mpagi J, Fremond C, Wagner H, Kirschning C, Ryffel B. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am J Pathol. 2004;164:49–57. doi: 10.1016/S0002-9440(10)63095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, Ehlers S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol. 2002;169:3480–3484. doi: 10.4049/jimmunol.169.7.3480. [DOI] [PubMed] [Google Scholar]
- 71.Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, Sher A. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003;171:4758–4764. doi: 10.4049/jimmunol.171.9.4758. [DOI] [PubMed] [Google Scholar]
- 72.Holscher C, Reiling N, Schaible UE, Holscher A, Bathmann C, Korbel D, Lenz I, Sonntag T, Kroger S, Akira S, et al. Containment of aerogenic Mycobacterium tuberculosis infection in mice does not require MyD88 adaptor function for TLR2, −4 and −9. Eur J Immunol. 2008;38:680–694. doi: 10.1002/eji.200736458. [DOI] [PubMed] [Google Scholar]
- 73.Sugawara I, Yamada H, Li C, Mizuno S, Takeuchi O, Akira S. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol Immunol. 2003;47:327–336. doi: 10.1111/j.1348-0421.2003.tb03404.x. [DOI] [PubMed] [Google Scholar]
- 74.Thuong NT, Hawn TR, Thwaites GE, Chau TT, Lan NT, Quy HT, Hieu NT, Aderem A, Hien TT, Farrar JJ, Dunstan SJ. A polymorphism in human TLR2 is associated with increased susceptibility to tuberculous meningitis. Genes Immun. 2007;8:422–428. doi: 10.1038/sj.gene.6364405. [DOI] [PubMed] [Google Scholar]
- 75.Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001;3:149–159. doi: 10.1016/s1286-4579(00)01362-9. [DOI] [PubMed] [Google Scholar]
- 76.Neyrolles O, Hernandez-Pando R, Pietri-Rouxel F, Fornes P, Tailleux L, Barrios Payan JA, Pivert E, Bordat Y, Aguilar D, Prevost MC, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS ONE. 2006;1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- 78.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 79.Dziarski R, Ulmer AJ, Gupta D. Interactions of CD14 with components of Gram-positive bacteria. Chem Immunol. 2000;74:83–107. doi: 10.1159/000058761. [DOI] [PubMed] [Google Scholar]
- 80.Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, Glauser MP, Tobias PS, Ulevitch RJ. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 81.Lewthwaite JC, Coates AR, Tormay P, Singh M, Mascagni P, Poole S, Roberts M, Sharp L, Henderson B. Mycobacterium tuberculosis chaperonin 60.1 is a more potent cytokine stimulator than chaperonin 60.2 (Hsp 65) and contains a CD14-binding domain. Infect Immun. 2001;69:7349–7355. doi: 10.1128/IAI.69.12.7349-7355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterson PK, Gekker G, Hu S, Sheng WS, Anderson WR, Ulevitch RJ, Tobias PS, Gustafson KV, Molitor TW, Chao CC. CD14 receptor-mediated uptake of nonopsonized Mycobacterium tuberculosis by human microglia. Infect Immun. 1995;63:1598–1602. doi: 10.1128/iai.63.4.1598-1602.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- 84.Hoheisel G, Zheng L, Teschler H, Striz I, Costabel U. Increased soluble CD14 levels in BAL fluid in pulmonary tuberculosis. Chest. 1995;108:1614–1616. doi: 10.1378/chest.108.6.1614. [DOI] [PubMed] [Google Scholar]
- 85.Rosas-Taraco AG, Revol A, Salinas-Carmona MC, Rendon A, Caballero-Olin G, Arce-Mendoza AY. CD14 C(-159)T polymorphism is a risk factor for development of pulmonary tuberculosis. J Infect Dis. 2007;196:1698–1706. doi: 10.1086/522147. [DOI] [PubMed] [Google Scholar]
- 86.Wieland CW, van der Windt GJ, Wiersinga WJ, Florquin S, van der., Poll T CD14 contributes to pulmonary inflammation and mortality during murine tuberculosis. Immunology. 2008;125:272–279. doi: 10.1111/j.1365-2567.2008.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lazarevic V, Myers AJ, Scanga CA, Flynn JL. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity. 2003;19:823–835. doi: 10.1016/s1074-7613(03)00324-8. [DOI] [PubMed] [Google Scholar]
- 88.Demangel C, Palendira U, Feng CG, Heath AW, Bean AG, Britton WJ. Stimulation of dendritic cells via CD40 enhances immune responses to Mycobacterium tuberculosis infection. Infect Immun. 2001;69:2456–2461. doi: 10.1128/IAI.69.4.2456-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fratazzi C, Manjunath N, Arbeit RD, Carini C, Gerken TA, Ardman B, Remold-O'Donnell E, Remold HG. A macrophage invasion mechanism for mycobacteria implicating the extracellular domain of CD43. J Exp Med. 2000;192:183–192. doi: 10.1084/jem.192.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Randhawa AK, Ziltener HJ, Stokes RW. CD43 controls the intracellular growth of Mycobacterium tuberculosis through the induction of TNF-α-mediated apoptosis. Cell Microbiol. 2008;10:2105–2017. doi: 10.1111/j.1462-5822.2008.01194.x. [DOI] [PubMed] [Google Scholar]
- 91.Leemans JC, Florquin S, Heikens M, Pals ST, van der Neut R, Van Der., Poll T CD44 is a macrophage binding site for Mycobacterium tuberculosis that mediates macrophage recruitment and protective immunity against tuberculosis. J Clin Invest. 2003;111:681–689. doi: 10.1172/JCI16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kipnis A, Basaraba RJ, Turner J, Orme IM. Increased neutrophil influx but no impairment of protective immunity to tuberculosis in mice lacking the CD44 molecule. J Leukoc Biol. 2003;74:992–997. doi: 10.1189/jlb.0603301. [DOI] [PubMed] [Google Scholar]
- 93.de Chastellier C, Thilo L. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell Microbiol. 2006;8:242–256. doi: 10.1111/j.1462-5822.2005.00617.x. [DOI] [PubMed] [Google Scholar]
- 94.Peyron P, Bordier C, N'Diaye EN, Maridonneau-Parini I. Nonopsonic phagocytosis of Mycobacterium kansasii by human neutrophils depends on cholesterol and is mediated by CR3 associated with glycosylphosphatidylinositol-anchored proteins. J Immunol. 2000;165:5186–5191. doi: 10.4049/jimmunol.165.9.5186. [DOI] [PubMed] [Google Scholar]
- 95.Perez-Guzman C, Vargas MH. Hypocholesterolemia: a major risk factor for developing pulmonary tuberculosis? Med Hypotheses. 2006;66:1227–1230. doi: 10.1016/j.mehy.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 96.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci USA. 2007;104:1947–1952. doi: 10.1073/pnas.0605728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stokes RW, Norris-Jones R, Brooks DE, Beveridge TJ, Doxsee D, Thorson LM. The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages. Infect Immun. 2004;72:5676–5686. doi: 10.1128/IAI.72.10.5676-5686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hall-Stoodley L, Watts G, Crowther JE, Balagopal A, Torrelles JB, Robison-Cox J, Bargatze RF, Harmsen AG, Crouch EC, Schlesinger LS. Mycobacterium tuberculosis binding to human surfactant proteins A and D, fibronectin, and small airway epithelial cells under shear conditions. Infect Immun. 2006;74:3587–3596. doi: 10.1128/IAI.01644-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.North RJ. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than via the intravenous route. J Infect Dis. 1995;172:1550–1553. doi: 10.1093/infdis/172.6.1550. [DOI] [PubMed] [Google Scholar]