Abstract
There is a distinct male predominance in head and neck cancers. The present study aimed to investigate the clinical and pathological features of male and female patients with oral squamous cell carcinoma (OSCC), and to simultaneously conduct a survival analysis. Patients (n = 2573) were identified between January 2008 and December 2018, and subsequently analyzed for characteristics such as age at squamous cell carcinoma diagnosis, lifestyle factors (smoking habit, betel nut chewing and alcohol consumption), pathological American Joint Committee on Cancer (AJCC) anatomic site, AJCC TNM stage, pathological recurrence factor and interval from first diagnosis to recurrence. A case-matched comparison between female (n = 122) and male (n = 2451) patients was conducted. Significant gender differences were noted in age at diagnosis, anatomic site of the tumor, smoking habit, betel nut chewing and alcohol consumption (p < 0.001). There were no significant gender differences in the other clinical and pathological characteristics and survival conditions. In conclusion, female patients with OSCC were older than male patients with OSCC, and mostly had tumors of the oral tongue. Once patients develop OSCC, there was no difference in survival between men and women in a Taiwanese population.
Keywords: head and neck cancer, lifestyle, squamous cell carcinoma of head and neck, survival analysis
1. Introduction
Gender-based differences in head and neck cancers (HNC) have been described in the literature, with a distinct male predominance. A study involving 703 patients with oral squamous cell carcinoma (OSCC) in southern Taiwan from 1985 and 1996 found a 51:1 male-to-female ratio [1], whereas other studies have reported OSCC in 90–93% male and 7–10% female patients [2,3,4]. This disparity in genders may be explained by the lower prevalence of some carcinogenic lifestyle factors, including smoking habits, betel nut chewing and alcohol consumption in women [5]. Concern about the disfiguring effects of these lifestyles factors (including red or brown staining of lips and teeth and foul-smelling breath) has been frequently reported by women, which may account for the sex-related differences in the prevalence of HNC [5].
OSCC accounts for 95% of all oral cancers and is associated with smoking habits and alcohol consumption in Western countries. Betel nut chewing and smoking are predominant risk factors causing OSCC in Taiwan and South Asia [1]. In Taiwan, approximately 2.5 million people are betel nut chewers. The incidence of OSCC was reportedly higher in betel nut chewers than in non-betel nut chewers [6]. A higher mortality rate among patients with OSCC was found to be associated with the increasing prevalence of betel nut chewing [6].
Feghali et al. reported that among patients with OSCC, heavy smokers have more aggressive pathological features and diseases than non-smokers [7]. It would be of interest to investigate whether there are differences in the clinical and pathological characteristics and survival conditions between male and female patients with OSCC, based on differences in carcinogenic lifestyle factors. The present study aimed to investigate the clinical and pathological characteristics of male and female patients, and to conduct survival analysis simultaneously.
2. Materials and Methods
2.1. Patients
This retrospective cohort study was approved by the Institutional Review Board (IRB) and Ethical Committee of Changhua Christian Hospital (IRB number 190408). All clinical data were obtained through review of medical charts and through the cancer registry center. A total of 3248 patients diagnosed with oral cavity and lip squamous cell carcinoma (SCC), who received treatment and follow-up at the Changhua Christian Hospital between 1 January 2008 and 31 December 2018, were identified. Patients were followed up until 30 June 2019. Patients were excluded for the following reasons: not receiving treatment per the American Joint Committee on Cancer (AJCC) cancer treatment guidelines (n = 41), lip cancer not involving mucosa (n = 121), lost to follow-up or incomplete data (n = 102), initially diagnosed with recurrent or distant metastasis (n = 70), or not undergoing surgery at Changhua Christian Hospital (n = 341). Finally, 2571 patients were identified and subsequently analyzed. A case-matched comparison of clinical and pathological characteristics between female (n = 122) and male (n = 2451) patients was conducted.
2.2. Clinical Parameters
The analyzed characteristics of both the groups included age at SCC diagnosis, lifestyle factors (smoking habits, betel nut chewing and alcohol consumption), pathological AJCC anatomic site, AJCC TNM stage, pathological recurrence factor and interval from first SCC diagnosis to recurrence. The anatomic sites were subclassified into alveolar ridge, anterior two-third tongue, buccal mucosa, hard palate, floor of the mouth, retromolar trigone and lip mainly involving the mucosa. Pathological recurrence factors included extranodal spread, close margin (<1 mm or involved margin) and type of tumor cell differentiation. Death information was retrieved from the cancer registry center of Changhua Christian Hospital, and the data were renewed annually by the Health Bureau of Changhua city.
We designed an observational, retrospective study based on gender, to compare the clinical and pathological characteristics and conducted a survival analysis between the groups.
2.3. Statistical Analysis
Continuous variables are presented as mean ± standard deviation (SD), and categorical variables are presented as percentages. Student’s t-test was used to compare continuous variables between cases and controls and the chi-squared test was used to compare differences in categorical variables between the two groups. Estimates of the overall survival (OS) rates were calculated using Kaplan–Meier analyses. Comparisons of group survival functions were conducted using log-rank tests based on OS rates. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed with the statistical package SPSS for Windows (Version 16, IBM, Chicago, IL, USA).
3. Results
Our retrospective study enrolled 2573 patients; 122 women as cases and 2451 men as controls. The clinical and pathological characteristics of the patients are summarized in Table 1 and Table 2.
Table 1.
Age at diagnosis, survival time (follow-up duration was from index data to 30 June 2019), and interval from diagnosis to recurrence in all patients with oral squamous cell carcinoma (OSCC); p-value was tested by Student’s t-test.
| Clinical Characteristics | Gender | Total | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | |||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Age at diagnosis (years) | 122 | 61.7 | 15.1 | 2451 | 56.9 | 10.9 | 2573 | 57.2 | 11.1 | 0.001 |
| Survival time * (months) | 122 | 45.4 | 33.8 | 2451 | 44.7 | 31.0 | 2573 | 44.7 | 31.1 | 0.824 |
| Time from diagnosis to recurrence (months) | 30 | 11.6 | 10.9 | 484 | 13.2 | 12.6 | 514 | 13.1 | 12.5 | 0.496 |
* Survival time was calculated from OSCC diagnosis to death or June 30, 2019.
Table 2.
Stratified data including age at diagnosis and clinical and pathological characteristics of all patients; AJCC: American Joint Committee on Cancer; p-value was tested by chi-square test.
| Clinical and Pathological Characteristics | Gender | Total (n = 2573) |
p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Female (n = 122) |
Male (n = 2451) |
|||||||
| n | % | n | % | n | % | |||
| Age (years) | ≤40 | 12 | 9.8 | 153 | 6.2 | 165 | 6.4 | <0.001 |
| 41–50 | 14 | 11.5 | 547 | 22.3 | 561 | 21.8 | ||
| 51–60 | 33 | 27.0 | 882 | 36.0 | 915 | 35.6 | ||
| 61–70 | 18 | 14.8 | 584 | 23.8 | 602 | 23.4 | ||
| ≥71 | 45 | 36.9 | 285 | 11.6 | 330 | 12.8 | ||
| AJCC anatomic site | Alveolar ridge | 18 | 14.8 | 401 | 16.4 | 419 | 16.3 | <0.001 |
| Anterior tongue | 64 | 52.5 | 669 | 27.3 | 733 | 28.5 | ||
| Buccal mucosa | 23 | 18.9 | 881 | 35.9 | 904 | 35.1 | ||
| Hard palate | 1 | 0.8 | 73 | 3.0 | 74 | 2.9 | ||
| Floor of the mouth | 1 | 0.8 | 76 | 3.1 | 77 | 3.0 | ||
| Retromolar trigone | 4 | 3.3 | 133 | 5.4 | 137 | 5.3 | ||
| Mucosa of the Lips | 11 | 9.0 | 218 | 8.9 | 229 | 8.9 | ||
| Smoking | No | 96 | 78.7 | 454 | 22.5 | 550 | 25.7 | <0.001 |
| Yes | 26 | 21.3 | 1562 | 77.5 | 1588 | 74.3 | ||
| Unknown | 0 | 435 | ||||||
| Betel nut chewing | No | 111 | 91.0 | 808 | 40.1 | 919 | 43.0 | <0.001 |
| Yes | 11 | 9.0 | 1208 | 59.9 | 1219 | 57.0 | ||
| Unknown | 0 | 435 | ||||||
| Alcohol | No | 116 | 95.1 | 844 | 47.5 | 960 | 50.6 | <0.001 |
| Yes | 6 | 4.9 | 931 | 52.5 | 937 | 49.4 | ||
| Unknown | 0 | 676 | ||||||
| AJCC Stage | I | 62 | 50.8 | 942 | 39.3 | 1004 | 39.8 | 0.089 |
| II | 19 | 15.6 | 444 | 18.5 | 463 | 18.4 | ||
| III | 9 | 7.4 | 207 | 8.6 | 216 | 8.6 | ||
| IV | 32 | 26.2 | 805 | 33.6 | 837 | 33.2 | ||
| AJCC Stage | Early (I and II) | 81 | 66.4 | 1433 | 58.5 | 1514 | 58.8 | 0.082 |
| Advance (III and IV) | 41 | 33.6 | 1018 | 41.5 | 1059 | 41.2 | ||
| T stage | 1 | 66 | 54.1 | 1064 | 43.6 | 1130 | 43.9 | 0.129 |
| 2 | 26 | 21.3 | 578 | 23.6 | 604 | 23.5 | ||
| 3 | 5 | 4.1 | 148 | 6.0 | 153 | 5.9 | ||
| 4 | 25 | 20.5 | 653 | 26.7 | 678 | 26.4 | ||
| N stage | 0 | 62 | 70.5 | 1368 | 73.0 | 1430 | 72.9 | 0.247 |
| 1 | 13 | 14.8 | 170 | 9.1 | 183 | 9.3 | ||
| 2 | 11 | 12.5 | 308 | 16.4 | 319 | 16.3 | ||
| 3 | 2 | 2.3 | 28 | 1.5 | 30 | 1.5 | ||
| Extranodal spread | No | 76 | 84.4 | 1539 | 85.4 | 1615 | 85.4 | 0.801 |
| Yes | 14 | 15.6 | 263 | 14.6 | 277 | 14.6 | ||
| Close margin | No | 115 | 94.3 | 2368 | 96.6 | 2483 | 96.5 | 0.197 |
| Yes | 7 | 5.7 | 83 | 3.4 | 90 | 3.5 | ||
| Recurrence | No | 92 | 75.4 | 1967 | 80.3 | 2059 | 80.0 | 0.192 |
| Yes | 30 | 24.6 | 484 | 19.7 | 514 | 20.0 | ||
| Grade | Well | 12 | 10.1 | 423 | 17.5 | 435 | 17.2 | 0.076 |
| Moderately | 98 | 82.4 | 1866 | 77.2 | 1964 | 77.5 | ||
| Poor | 9 | 7.6 | 127 | 5.3 | 136 | 5.4 | ||
Table 1 summarizes the age at diagnosis, survival time (with a follow-up duration from index data to 30 June 2019), and interval from diagnosis to recurrence in all patients. There was a significant difference between men and women in the mean age at diagnosis of cases (p < 0.001). Among cases, the mean age at diagnosis was 61.7 years, whereas it was 56.9 years in controls. There was no significant gender difference in survival time and interval from diagnosis to recurrence.
Table 2 summarizes the data stratified by age at diagnosis and the clinical and pathological characteristics of all patients. In terms of age at diagnosis, the majority of cases were diagnosed in Group 5 (>71 years), followed by Group 3 (51–60 years), whereas the majority of controls were diagnosed in Group 3 (51–60 years). There was a significant difference between cases and controls.
There was a significant difference between men and women (controls and cases) in terms of the anatomic site of the tumor. More than 50% of the cases had tumors of the anterior two-thirds of the tongue, whereas the majority of controls were most likely to have tumors of the buccal mucosa, followed by anterior two-thirds of the tongue.
Smoking habits, betel nut chewing and alcohol consumption were recorded in a significantly lower proportion in women (cases) than in men (controls). There was no significant gender difference in T status, N status or AJCC stage. With regard to recurrence status and recurrence factor, there was no significant gender difference in recurrence, extranodal spread, close margin or tumor differentiation grade.
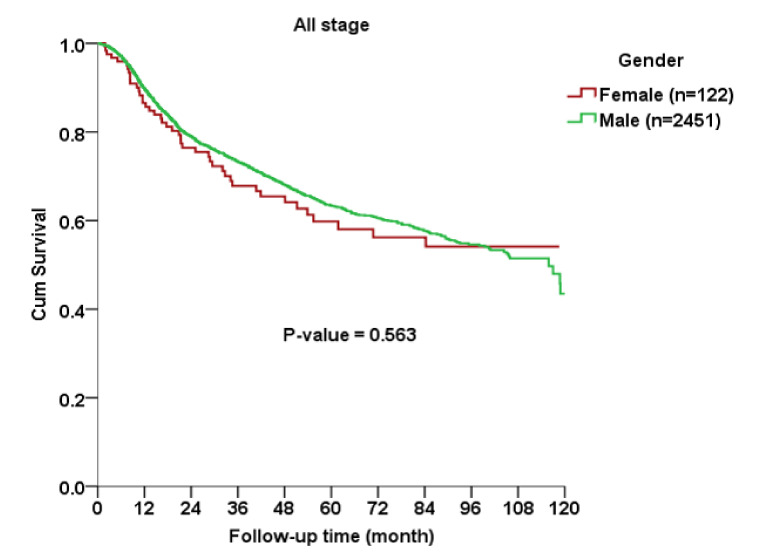
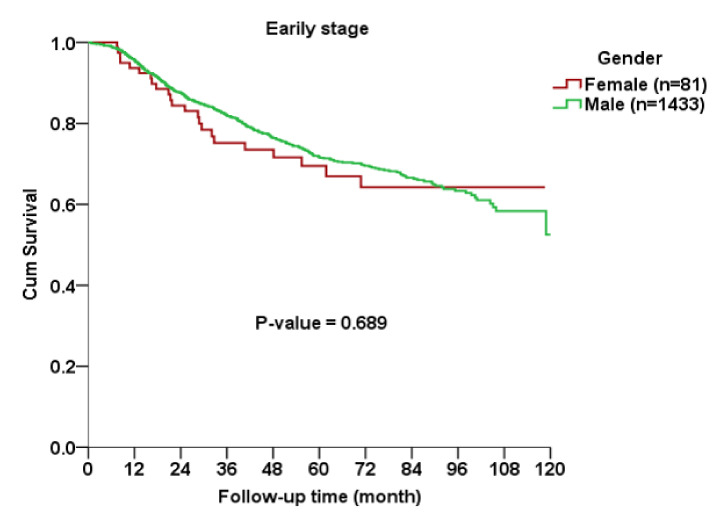
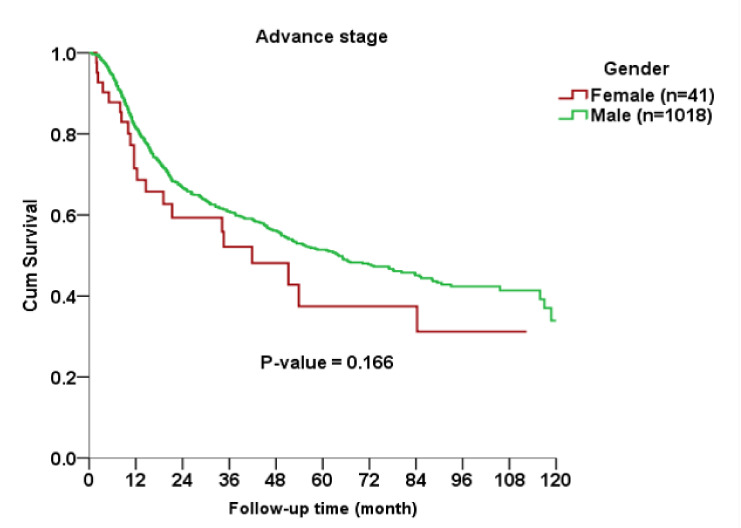
The Kaplan–Meier curve indicated no significant gender difference in OS among all AJCC stages (p = 0.563), early stage (p = 0.689) or advanced stage (p = 0.166) subgroups between cases and controls (Figure 1, Figure 2 and Figure 3).
Figure 1.
Kaplan–Meier curve for overall survival rates between female and male patients with oral squamous cell carcinoma.
Figure 2.
Kaplan–Meier curve for overall survival rates between female and male patients with early-stage oral squamous cell carcinoma.
Figure 3.
Kaplan–Meier curve for overall survival rates between female and male patients with advanced-stage oral squamous cell carcinoma.
4. Discussion
In the present study, the most notable differences between females and males patients with OSCC were found in terms of age at diagnosis and anatomic site of tumors. Older women (>70 years) were more affected than younger women, suggesting an effect of cumulative opportunity to develop OSCC. Male patients with OSCC were mainly in the age group of 51–60 years. Most female patients had tumors of the oral tongue, whereas most male patients had tumors of the buccal mucosa. In addition, male patients had distinct carcinogenic habits, including smoking, betel nut chewing and alcohol consumption. There were no differences in OS between male and female patients, suggesting that once patients have OSCC, they have similar survival conditions, regardless of gender.
Several studies have established that alcohol consumption, cigarette smoking and betel nut chewing contribute to an increased incidence of HNC [8,9,10,11]. These lifestyle factors were prominently found in the male group, which could explain the notable earlier age at diagnosis of OSCC in male patients, compared with that observed in female patients. Kruse et al. also reported that the median age at OSCC diagnosis was higher in female patients than in male patients (65.36 vs. 61.04 years), with a predominance of female patients older than 70 years, similar to that observed in the present study [12].
In contrast, the most common anatomic site of tumors among female patients in Kruse’s study was the gum, while, in our study, it was the tongue. This result of the present study is similar to those of other studies reported around the world [13,14]. Ng et al. demonstrated a general trend of increasing incidence of oral tongue cancer which appears to hold true across most registries [14]. These findings suggest the possible emergence of new etiological or genetic factors driving carcinogenesis in the tongue, which features more strongly in certain geographic areas and in different sexes, accounting for the heterogeneous results observed in the present study [15]. Annertz et al. indicated a continued increasing trend in the incidence of OSCC of the tongue in the 21st century for both the sexes and all age groups, except for young male patients; however, the cause of this trend is controversial [13]. In the present study, the risk factors contributing to tongue OSCC in female patients have not been addressed. The non-smoking, non-drinking and non-betel nuts chewing patients with OSCC may be associated with other etiologies and risk factors. Poor oral hygiene, inadequate dental status and chronic irritation are independent risk factors for OSCC, irrespective of tobacco and alcohol consumption [16]. Human papillomavirus (HPV) has been implicated as a major risk factor in oropharyngeal cancer; however, the prevalence of high-risk HPV in cancer of the oral tongue appears to be low [17,18,19]. None of the known risk factors appear to explain the increasing incidence of OSCC of the oral tongue. Further research is warranted to investigate this issue in the future.
Betel nut chewing is very common lifestyle factor in Taiwan, in particular among blue-collar workers, men, low socioeconomic status people or indigenous people [6]. Betel nut chewers have been shown to have a lower median age at onset (approximately 6–12 years earlier) than non-chewers [20], and this finding could explain why more men were diagnosed with OSCC at a younger age than women in the present study. Furthermore, Su et al. reported that the high incidence of the buccal mucosa, as the site of OSCC may have a strong association with betel nut chewing among male patients [20]. In Japan, betel nut chewing is not as common as in Taiwan. Koyama et al. reported that from 2000 to 2014, a total of 6086 cases of oral cavity cancer were identified. Among all the patients, approximately 40% were women, and tongue cancer accounted for 40% of cases, whereas gum cancer accounted for 32%; buccal mucosa cancer accounted for a small proportion of all cases [21]. Thus, it can be concluded that in OSCC cases, the buccal mucosa is the predominant lesion sites among betel nut chewers. There was no difference in OS between female and male patients with OSCC in our study, although male patients had a distinct lifestyle leading to OSCC. Giraldi et al. reported that the status of pre-diagnosis cigarette smoking and alcohol consumption were not prognostic factors for head and neck specific survival in OSCC, but smoking more than 20 cigarettes per day affected OS among patients with OSCC [22]. On the other hand, Kawakita et al. demonstrated that pre-treatment smoking status did have a prognostic value in patients with OSCC, but this effect was evident only among radiotherapy (RT) or concurrent chemoradiotherapy (CCRT) groups; they concluded that smoking may affect RT or CCRT treatment [23]. In the present day, surgery is still the mainstay treatment in patients with OSCC.
There are several limitations to our study. First, the data of our study were collected from a single medical center in Taiwan, and the people in a single district may have some cultural and geographical features. Second, we did not quantify lifestyle factors—smoking, betel nut chewing and alcohol consumption—and collected the data on these lifestyle factors after the patients had received cancer treatment, which may affect the survival condition. Moreover, a considerable amount of data on lifestyle factors were not available. Although the results were not affected due to distinct gender differences in lifestyle factors, further data analysis could be impeded. Finally, the relatively small sample size of women limited the capacity for further stratified analysis.
5. Conclusions
In conclusion, female patients with OSCC were older than male patients and mostly had tumors on the oral tongue. Once the patients had OSCC, there was no difference in OS between male and female patients in this Taiwanese population.
Acknowledgments
The authors would like to thank Enago for the English language review.
Author Contributions
Conceptualization, N.-C.L. and K.-Y.T.; methodology N.-C.L.; data curation N.-C.L.; writing—original draft preparation, N.-C.L.; writing—review and editing, J.-T.H. and K.-Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen Y.K., Huang H.C., Lin L.M., Lin C.C. Primary oral squamous cell carcinoma: An analysis of 703 cases in southern Taiwan. Oral Oncol. 1999;35:173–179. doi: 10.1016/S1368-8375(98)00101-8. [DOI] [PubMed] [Google Scholar]
- 2.Liao C.T., Tung-Chieh Chang J.T., Wang H.M., Chen I.H., Lin C.Y., Chen T.M., Hsieh L.L., Cheng A.J. Telomerase as an Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Head Neck. 2004;26:504–512. doi: 10.1002/hed.20007. [DOI] [PubMed] [Google Scholar]
- 3.Liao C.T., Chang J.T., Wang H.M., Ng S.H., Hsueh C., Lee L.Y., Lin C.H., Chen I.H., Huang S.F., Cheng A.J., et al. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann. Surg. Oncol. 2008;15:915–922. doi: 10.1245/s10434-007-9761-5. [DOI] [PubMed] [Google Scholar]
- 4.Liao C.T., Kang C.J., Chang J.T., Wang H.M., Ng S.H., Hsueh C., Lee L.Y., Lin C.H., Cheng A.J., Chen I.H., et al. Survival of second and multiple primary tumors in patients with oral cavity squamous cell carcinoma in the betel quid chewing area. Oral Oncol. 2007;43:811–819. doi: 10.1016/j.oraloncology.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y.J., Chang J.T., Liao C.T., Wang H.M., Yen T.C., Chiu C.C., Lu Y.C., Li H.F., Cheng A.J. Head and Neck Cancer in the Betel Quid Chewing Area: Recent Advances in Molecular Carcinogenesis. Cancer Sci. 2008;99:1507–1514. doi: 10.1111/j.1349-7006.2008.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kao S.Y., Lim E. An overview of detection and screening of oral cancer in Taiwan. Chin. J. Dent. Res. 2015;18:7–12. [PubMed] [Google Scholar]
- 7.Al Feghali K.A., Ghanem A.I., Burmeister C., Chang S.S., Ghanem T., Keller C., Siddiqui F. Impact of smoking on pathological features in oral cavity squamous cell carcinoma. J. Can. Res. Ther. 2019;15:582–588. doi: 10.4103/jcrt.JCRT_641_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blot W.J., McLaughlin J.K., Winn D.M., Austin D.F., Greenberg R.S., Preston-Martin S., Bernstein L., Schoenberg J.B., Stemhagen A., Fraumeni J.F. Smoking and Drinking in Relation to Oral and Pharyngeal Cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 9.Hashibe M., Brennan P., Benhamou S., Castellsague X., Chen C., Curado M.P., Dal Maso L., Daudt A.W., Fabianova E., Fernandez L., et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 10.Lee K.W., Kuo W.R., Tsai S.M., Wu D.C., Wang W.M., Fang F.M., Chiang F.Y., Ho K.Y., Wang L.F., Tai C.F., et al. Different impact from betel quid, alcohol and cigarette: Risk factors for pharyngeal and laryngeal cancer. Int. J. Cancer. 2005;117:831–836. doi: 10.1002/ijc.21237. [DOI] [PubMed] [Google Scholar]
- 11.Ko Y.C., Huang Y.L., Lee C.H., Chen M.J., Lin L.M., Tsai C.C. Betel quid chewing, cigarette smoking and alcohol consumption related to oral cancer in Taiwan. J. Oral Pathol. Med. 1995;24:450–453. doi: 10.1111/j.1600-0714.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 12.Kruse A.L., Bredell M., Grätz K.W. Oral Cancer in men and women: Are there differences? Oral Maxillofac. Surg. 2011;15:51–55. doi: 10.1007/s10006-010-0253-6. [DOI] [PubMed] [Google Scholar]
- 13.Annertz K., Anderson H., Palmér K., Wennerberg J. The increase in incidence of cancer of the tongue in the Nordic countries continues into the twenty-first century. Acta Otolaryngol. 2012;132:552–557. doi: 10.3109/00016489.2011.649146. [DOI] [PubMed] [Google Scholar]
- 14.Ng J.H., Iyer N.G., Tan M.H., Edgren G. Changing epidemiology of oral squamous cell carcinoma of the tongue: A global study. Head Neck. 2017;39:297–304. doi: 10.1002/hed.24589. [DOI] [PubMed] [Google Scholar]
- 15.Vettore A.L., Ramnarayanan K., Poore G., Lim K., Ong C.K., Huang K.K., Leong H.S., Chong F.T., Lim T.K., Lim W.K., et al. Mutational landscapes of tongue carcinoma reveal recurrent mutations in genes of therapeutic and prognostic relevance. Genome Med. 2015;7:98. doi: 10.1186/s13073-015-0219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenquist K., Wennerberg J., Schildt E.B., Bladströ>m A., Göran Hansson B.G., Andersson G. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 17.Liang X.H., Lewis J., Foote R., Smith D., Kademani D. Prevalence and significance of human papillomavirus in oral tongue cancer: The Mayo Clinic experience. J. Oral Maxillofac. Surg. 2008;66:1875–1880. doi: 10.1016/j.joms.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Dahlgren L., Dahlstrand H.M., Lindquist D., Högmo A., Björnestål L., Lindholm J., Lundberg B., Dalianis T., Munck-Wikland E. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int. J. Cancer. 2004;112:1015–1019. doi: 10.1002/ijc.20490. [DOI] [PubMed] [Google Scholar]
- 19.Kantola S., Parikka M., Jokinen K., Hyrynkangs K., Soini Y., Alho O.P., Salo T. Prognostic factors in tongue cancer-relative importance of demographic, clinical and histopathological factors. Br. J. Cancer. 2000;83:614–619. doi: 10.1054/bjoc.2000.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su C.C., Yang H.F., Huang S.J., Lian l.B. Distinctive Features of Oral Cancer in Changhua County: High Incidence, Buccal Mucosa Preponderance, and a Close Relation to Betel Quid Chewing Habit. J. Formos. Med. Assoc. 2007;106:225–233. doi: 10.1016/S0929-6646(09)60244-8. [DOI] [PubMed] [Google Scholar]
- 21.Koyama S., Tabuchi T., Okawa S., Morishima T., Ishimoto S., Ishibashi M., Miyashiro I. Oral cavity cancer incidence rates in Osaka, Japan between 2000 and 2014. Oral Oncol. 2020;105:104653. doi: 10.1016/j.oraloncology.2020.104653. [DOI] [PubMed] [Google Scholar]
- 22.Giraldi L., Leoncini E., Pastorino R., Wünsch-Filho V., de Carvalho M., Lopez R., Cadoni G., Arzani D., Petrelli L., Matsuo K., et al. Alcohol and cigarette consumption predict mortality in patients with head and neck cancer: A pooled analysis within the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Ann. Oncol. 2017;28:2843–2851. doi: 10.1093/annonc/mdx486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakita D., Hosono S., Ito H., Oze I., Watanabe M., Hanai N., Hasegawa Y., Tajima K., Murakami S., Tanaka H., et al. Impact of smoking status on clinical outcome in oral cavity cancer patients. Oral Oncol. 2012;48:186–191. doi: 10.1016/j.oraloncology.2011.09.012. [DOI] [PubMed] [Google Scholar]