Abstract
We tested the relationship between the capsular and the O-antigen structures and the ability of bacteria to trigger respiratory burst in human polymorphonuclear leukocytes (PMNL). Capsulated and non-capsulated variants as well as capsule-switched derivatives of Klebsiella serotypes bearing or lacking manno(rhamno)biose repeats in their capsular polysaccharides and expressing either mannose-rich or mannose-poor O antigens were tested for their ability to induce respiratory burst and survive in human PMNL. Luminol-enhanced chemiluminescence (CL) was measured to quantify respiratory burst. Intracellular survival was quantified by determining the viable counts of intracellular bacteria. K serotypes and the capsule-switched derivative lacking manno(rhamno)biose induced significantly lower CL than those expressing manno(rhamno)biose. Manno(rhamno)biose-lacking serotypes survived in the cells significantly better than serotypes expressing these repeats. C1q depletion did not affect CL induced by the manno(rhamno)biose-containing serotype, whereas factor B depletion revealed a significantly reduced CL. Likewise, EGTA in the presence of Mg2+ significantly decreased CL, but the values were higher than those induced by the bacterium opsonized with factor B-depleted serum. In the presence of EGTA, Mg2+-treated factor B-depleted serum revealed a significant reduction in the CL response compared with the responses induced by opsonization with factor B-depleted serum alone. These results indicate, in addition to the alternative pathway, a manno(rhamno)biose pattern recognition of Klebsiella by PMNL probably by the complement lectin pathway.
Key Words: Capsular polysaccharides, Complement, Innate immunity, <italic>Klebsiella</italic>, Lipopolysaccharides, Mannose-binding lectin, Polymorphonuclear leukocytes, Respiratory burst
Introduction
Klebsiella pneumoniae is an opportunistic pathogen that is often implicated in pyogenic community-acquired pneumonia in alcoholics and has a high fatality rate if untreated [1, 2, 3, 4, 5, 6, 7]. However, the majority of Klebsiella infections affect hospitalized immunocompromised patients with severe underlying diseases [8, 9]. Its prevalence ranges from 3 to 17% of all such infections, placing them among the eight most frequent pathogens in hospitals [8, 10, 11, 12, 13, 14, 15, 16, 17]. It has been suggested that component(s) of the innate immune system was/were compromised to render the hospitalized patient susceptible to K. pneumoniae infections.
The innate immune system consists of cellular and humoral components. The former include epithelial cells of mucosal surfaces, macrophages and polymorphonuclear leukocytes (PMNL). The latter include serum components, such as natural antibodies, the mannose binding lectin (MBL) and complement proteins, which mediate the bactericidal effect of serum and also opsonophagocytosis of the bacteria, and secretory tissue proteins (opsonins), among which are the surfactant proteins A (SP-A) and D (SP-D) in the lung [18].
Based on the molecular variability in the surface glycoconjugates, the capsule and the lipopolysaccharides (LPS), Klebsiella has been serotyped in 77 capsular and nine O serotypes, respectively [19, 20], that differ significantly in pathogenicity and epidemiological relevance. For example, the K2 serotype has been shown to be significantly more virulent in mice [21, 22] and more common in infectious processes than other serotypes [23, 24]. The O1 serotype is the most common O serotype in strains isolated from human infections [19, 25, 26]. It has been speculated that these differences in pathogenicity and epidemiological relevance of the various serotypes might be in part due to recognition of the capsular or O antigens by components of the innate immune system [27]. Capsular serotypes bearing the mannobiose Manα2/3Man- or rhamnobiose Rhaα2/3Rha repeating units (Di-Man/Rha), termed glycoepitopes, are recognized by the mannose receptor (MR) of the macrophages [22, 27, 28] and SP-A [29]. Such recognition results in a more effective internalization and killing of the bacteria by macrophages. SP-D interacts with non-capsulated strains bearing mannose-rich glycoepitopes of Klebsiella O polysaccharides, impedes the adhesion of the bacteria to epithelial cells and enhances the phagocytosis and subsequent killing of the bacteria by macrophages [30, 31, 32]. Furthermore, Klebsiella serotypes that are captured by macrophages via the MR or following opsonization by SP-A and SP-D trigger a response in the cells, including high production of inflammatory cytokines [29, 32, 33]. Although phagocytosis and killing by alveolar macrophages provide the first line of defense against Klebsiella infections, subsequent infiltration of PMNL and serum at the site of infection also play a pivotal role in eradicating the pathogens.
While the interaction of Klebsiella with MR and the lung collectins SP-A and SP-D occurs in the absence of serum opsonins, the recognition of Klebsiella by PMNL is serum opsonin dependent [34]. Few studies showed marked differences in the potential of the various serotypes to stimulate PMNL in a serum-dependent manner. The capsular serotypes K1, K2, K4 and K5 stimulate the PMNL significantly less than the K3, K6 and K7 serotypes [35, 36]. The molecular basis of this distinct stimulation of the PMNL by the various Klebsiella serotypes is not clear at this stage.
In the present study, we used 18 capsulated and non-capsulated Klebsiella strains and genetically generated capsule-switched derivatives to study their interactions with PMNL. Some of the strains express capsular polysaccharides that contain the di-Man/Rha glycoepitope or the polymannose glycoepitope of O antigen while in contrast to others do not. It will be shown that capsular serotypes that express the di-Man/Rha glycoepitopes stimulate PMNL significantly better than serotypes that lack these glycoepitopes, and that these serotypes are less able to evade intracellular killing by PMNL. It appears that these interactions involve activation of the complement system via the alternative and probably via the MBL pathways.
Materials and Methods
Bacterial Strains and Typing Methods
Capsulated variants of the reference capsular serotypes K21a, K50 and K36 expressing capsular polysaccharides containing di-Man/Rha glycoepitope in their repeating units and of the reference serotypes K2 and K55, whose capsular polysaccharides lack these glycoepitopes, were obtained from the strain collection of the Institute for Infection Medicine, University Hospital Schleswig-Holstein, Campus Kiel, Germany. An additional, wild-type strain of the di-Man/Rha-containing serotype K21a (LO2760) and a strain of the K8 serotype (VA8597) lacking such sequences were isolated from clinical specimens of patients. The strains expressed either polymannose-containing O3 antigen (K50/O3 and K55/O3, reference serotypes; K21/O3, K8/O3 wild-type strains) or O1 antigens (K2/O1 and K21/O1) that lack polymannose. Non-capsulated phase variants K2/n, K21a/n, K50/n, K55/n and K8/n were selected from spontaneous non-mucoid segments of mucoid colonies without mutagenic treatment, as described elsewhere [37]. Such spontaneous phase variation involves the insertion of sequences into one of the glucosyl transferases [Ofek et al., unpubl. observ.]. Additionally, 81 wild-type K. pneumoniae strains that were isolated from clinical specimens of hospitalized patients with pneumonia, sepsis, soft tissue infections or urinary tract infections were included in the analysis.
Capsule-switched derivatives K2(K21a) and K2(K36) that express di-Man/Rha glycoepitopes of the K21a or of the K36 donor strains but retain the genetic background of the K2 recipient were described previously [22, 38, 39]. The capsule-switched derivative K21a(K2) that expresses the capsule of the virulent K2 serotype lacking the di-Man/Rha glycoepitopes was described elsewhere [22].
The K serotypes of the bacteria were determined by the capsule-swelling method using capsule-specific antisera, as described elsewhere [24]. Capsular polysaccharides were quantified by determining the uronic acid content in the Zwittergent extracts of the strains by the uronic acid assay of Blumenkranz and Asboe-Hansen [40], described elsewhere [41].
O serotyping was performed by ELISA using O-type-specific rabbit antibodies [25]. Table 1 summarizes the bacterial strains and their characteristics.
Table 1.
Strains applied in the study and characteristics
| Strain | K serotype | O serotype | Di-Man/Rha glycoepitope in capsule | Polymannose in O antigen |
|---|---|---|---|---|
| NCTC 9143 | K2 [parent of K2(K21a) and K2(K36)] | O1 | – | – |
| KPTA 52 | K2/n (non-capsulated) | O1 | – | – |
| NCTC 9170 | K50 | O3 | + | + |
| K50/3 | K50/n (non-capsulated) | O3 | – | + |
| NCTC 9175 | K55 | O3 | – | + |
| K55/1 | K55/n (non-capsulated) | O3 | – | + |
| KPTA 19 | K21a [parent of K21a(K2)] | O1 | + | – |
| KPTA 20 | K21a/n (non-capsulated) | O1 | – | – |
| VA8597 | K8 | O3 | – | – |
| VA 8597/1 | K8/n | O3 | – | – |
| LO2760 | K21a | O3 | + | – |
| LO2760/a | K21aLO/n (non-capsulated) | O3 | – | – |
| KPTA 37 | K2(K36) | O1 | + | – |
| KPTA 18 | K2(K21a) | O1 | + | – |
| KPTA 25 | K21a(K2) | O1 | + | – |
| NCTC 9156 | K36 | O1 | + | – |
| K36/3 | K36/n (non-capsulated) | O1 | + | – |
| KPTA26 | K2 [parent of K2(K21a) and K2(K36)] | O1 | – | – |
Purification and Characterization of LPS
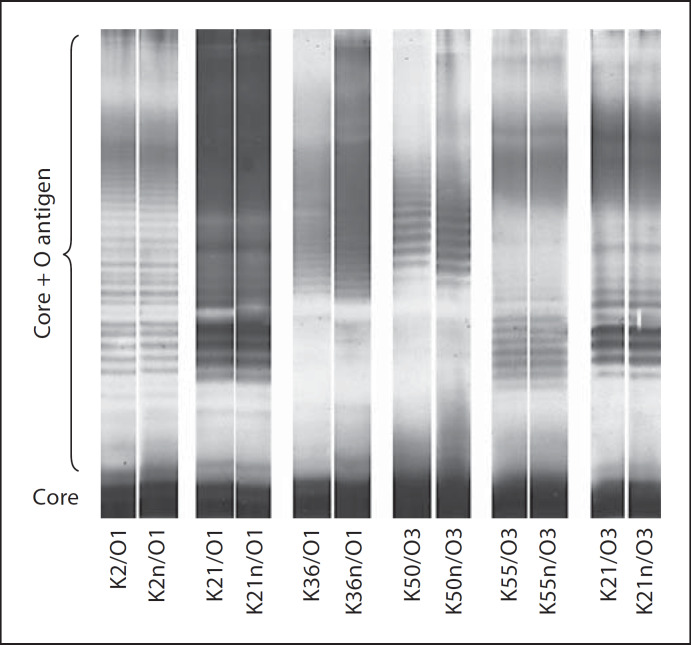
LPS of Gram-negative bacteria consist of lipid A and core antigens, which are integrated in the bacterial outer membrane, and of extracellular sugar repeating units, which form the O antigen. The expression of the O antigens is affected by the genetic background of the bacterium and by different growth conditions. Depending on the length of the sugar repeating units, the LPS molecules move differently fast in gel electrophoresis; each band consist of lipid A, core and O antigens with numerically equal sugar repeating units. In order to prove that the capsule phase variation does not affect LPS expression, the LPS from each parental strain and the corresponding non-capsulated derivative of all Klebsiella strains used were purified using whole-cell lysate and visualized as described previously [31]. The purified preparations were resolved by SDS-PAGE without urea on 5%/10% slab gels and visualized by silver staining. The LPS patterns of capsulated and non-capsulated mutants were shown to be identical, indicating that the capsule phase variation does not affect LPS expression.
Preparation of PMNL
Ficoll® gradient and dextran sedimentation were employed to separate PMNL from heparinized blood of healthy donors (age range 24–56 years), as described elsewhere [24]. Erythrocytes were removed by hypotonic lysis. PMNL were washed twice in Hank's-balanced salt solution (HBSS) and adjusted to 1 × 107 cells/ml.
Complement-Dependent CL Response
Bacteria-induced complement-dependent respiratory burst of the PMNL was recorded by measuring the luminol-enhanced CL response using the Autolumat LB 953-2 (Berthold, Wildbad, Germany), as described previously [24]. CL was induced by adding 10 µl of a bacterial suspension (1 × 109/ml) or zymosan (50 mg/ml) opsonized with normal human serum (NHS) containing natural anti-Klebsiella antibodies [Sahly, unpubl. data] or heat-inactivated human serum (56°C, 30 min; final concentration 10%). The bacterium:leukocyte ratio was approximately 200:1. The CL response was measured every 20 s over a period of 60 min. After curve area integration, the results were expressed as a percentage of CL obtained with zymosan. Each strain was tested at least 3 times with PMNL from 33 healthy donors. The effect of heat-inactivated serum on the CL response was exemplarily tested with K2/O1, K50/O3 and the K21a/O1.
Inhibition of the Classical, MBL and the Alternative Complement Pathways
The activation of complement requires either antibody binding (classical pathway), direct C3 binding to bacterial surfaces (alternative pathway) or mannose-binding-lectin binding to the surface of bacterial polysaccharides (MBL pathway). The first and second cannot occur in the absence of C1q and factor B, respectively. In the presence of Mg2+, EGTA inhibits both the classical and the MBL pathway, because it binds magnesium and calcium, from which the latter is necessary for both the classical and the MBL pathway.
In order to determine whether the alternative pathway is involved in the PMNL stimulation by Klebsiella, factor-B-depleted serum (Calbiochem, Germany) was used to opsonize the bacteria. For a specific inhibition of the classical pathway, the K21a strain was opsonized with C1q-depleted serum (Calbiochem). In order to inhibit the classical and the MBL pathway, 10 mM EGTA was added to the NHS to chelate Ca2+. Because EGTA binds also Mg2+, which is needed for the alternative pathway, 2.5 mM Mg2+ was added to the test. To further verify the role of the MBL pathway, Klebsiella was treated with factor-B-depleted, calcium-chelated serum (EGTA supplemented with Mg2+).
Killing by PMNL
Killing of bacterial strains by PMNL was determined as described previously [24]. 100 µl of bacterial suspension (4 × 107/ml), 400 (µl of HBSS, 400 (µl of PMNL (1 × 107 cells/ml) and 100 (µl ml of serum (final concentration 10% v/v) were prepared in siliconized glass tubes. Vials were incubated in a 37° C shaking water bath. 100 (µl of the various preparations were taken after 0, 30 and 60 min. Ice-cold distilled water (9.9 ml) was added to lyse PMNL, and intracellular survival of the bacteria was quantified by plating serial dilutions on brain-heart infusion agar to enumerate the colony-forming units. Each test was run in duplicate, and each strain was tested at least three times. HBSS was substituted for PMNL in controls.
Statistical Analysis
Significant differences between the CL responses induced by the different bacterial strains and mutants, and the intracellular survival of these strains were evaluated by the Kruskal-Wallis test (nonparametric ANOVA) followed by Dunn's post hoc test.
Results
Presence of Di-Man/Rha Glycoepitopes in the Capsule Enhances Respiratory Burst in PMNL
The amounts of capsular material produced by the capsulated parental strains ranged between 32 and 77 µg of glucuronic acid/109 bacteria. The non-capsulated strains exhibited up to 1.6 µg of glucuronic acid/109 bacteria and had negative quelling reactions with anticapsular antibodies. The capsule-switched derivatives K2(K21a) and K21(K2) produced uronic acids similar to those of the capsulated parental strains of 67 and 73 µg glucuronic acid/109 bacteria. In contrast, the capsule-switched derivative K2(K36) produced higher capsulated parental strains of 92 µg glucuronic acid/109 bacteria.
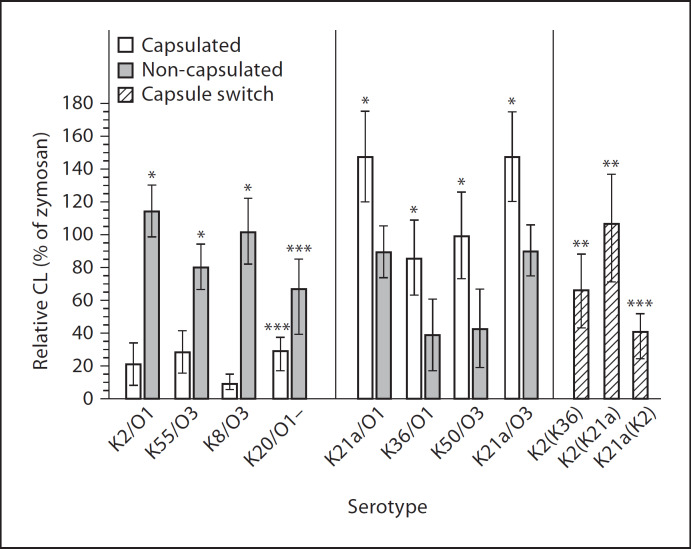
The magnitudes of the CL response (% of zymosan stimulation) induced by the capsulated variants of the serotypes K21a, K36 and K50 and the wild-type strain LO2760 expressing the K21a capsule, all of which contain the di-Man/Rha glycoepitope in their capsular polysaccharides, were 147 ± 27.6, 85 ± 28, 100 ± 26.7 and 103 ± 9%, respectively. These CL responses were significantly higher than those induced by the corresponding non-capsulated variants K21a/n, K36/n and K50/n and the wild-type K21aLO/n (90.7 ± 15.6, 41 ± 26, 43.5 ± 24.3 and 74 ± 5%, respectively; p < 0.001 for each pair of strains; fig. 1).
Fig. 1.
CL response (% of zymosan-induced stimulation) of PMNL from healthy donors after stimulation with serum-pre-opsonized capsulated and non-capsulated strains of the indicated Klebsiella capsular and O serotypes. * p < 0.001, vs. CL responses induced by the corresponding capsulated or non-capsulated strain; ** p < 0.001, vs. CL responses induced by the capsule-switched derivatives K2(K21a) and K2(K36) that produce the heterologous di-Man (K21a)- or di-Rha (K36)-containing capsule vs. the capsulated K2 parental strain; *** p < 0.001, vs. the heterologous K2 capsule.
The capsulated reference serotypes K2 and K55, and the K8 wild-type strain (VA8597), all of which lack the di-Man/Rha glycoepitope, induced CL responses of 22 ± 12, 29.5 ± 12 and 7.8 ± 0.7%, which were significantly lower than the responses induced by the corresponding non-capsulated variants K2/n, K55/n and K8/n (115 ± 15, 80 ± 14 and 100.5 ± 10%, respectively; p < 0.001 for each pair of strains; fig. 1).
The K2(K21a) and K2(K36) capsule-switched derivatives that expressed the heterologous di-Man/Rha-containing K21a or K36 capsule induced significantly higher CL responses than the capsulated parental K2 strain (p < 0.001; fig. 1). Conversely, the capsule-switched derivative K21a(K2) that expresses the K2 capsule lacking the di-Man/Rha glycoepitope induced a significantly lower CL response (39.8 ± 11% of zymosan) than the K21a parental strain (p < 0.001; fig. 1). The CL response induced by the K2(K36) capsule-switched derivative that produces higher amounts of capsular polysaccharides did not significantly differ from that induced by the K36 capsulated strain (fig. 1).
Analysis of 81 additional extended-spectrum β-lactamase-producing strains from clinical specimens revealed that 63 strains expressed the di-Man/Rha glycoepitope in their capsules and stimulated the PMNL at significantly higher levels than the 18 strains that lack these epitopes (96.7 ± 38 and 49.9 ± 43%; respectively, p < 0.001).
The CL Response of PMNL Is Not Influenced by the Structure of the O Antigens
Capsulated strains and their non-capsulated variants exhibited identical smooth LPS structures, indicating that the capsule phase variation does not hamper or modify LPS expression (fig. 2). Previous studies have shown that non-capsulated variants exhibiting alternating mannose in their O antigen (e.g. O3 and O5) bind SP-D with higher affinity than O serotypes lacking such sequences (e.g. O1 and O4) [31]. The magnitude of CL responses by non-capsulated strains could not be related to their O-antigen structure (fig. 1). For instance, one mannose-poor O1 (non-capsulated K2/O1) and one mannose-rich O3 serotype (non-capsulated K8, VA8597) induced high CL responses of 115 ± 15.8 and 100.5 ± 10%, respectively. Two other O1 and two O3 serotypes induced either moderate (K21a/O1 and K55/O3; 90.7 ± 15.6 and 80 ± 14%, respectively) or low CL responses (K36/O1 and K50/O3; 41 ± 26 and 43.5 ± 24.3%, respectively).
Fig. 2.
SDS-PAGE of LPS shows identical patterns of the core region and the core region plus O polysaccharide repeating units from capsulated and non-capsulated Klebsiella serotypes. Black corpera at the bottom of the LPS ladders indicate pure core regions. Each additional thin band indicates core plus sugar repeating unit of increasing number of sugar repeating units. Identical LPS ladders indicate that the capsule phase variation does not affect LPS expression.
Intracellular Killing of the Bacteria by PMNL
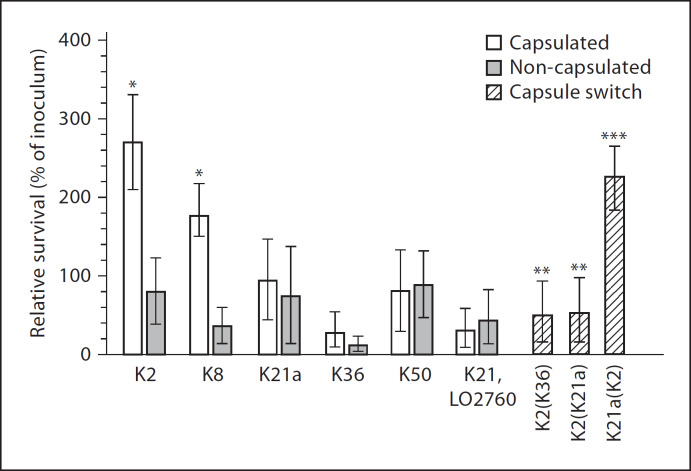
Strains expressing capsule lacking manno(rhamno)-biose glycoepitopes (K2 and K8) and that induced low CL responses survived significantly better in PMNL than serotypes expressing the di-Man/Rha glycoepitopes (K21a, K36 and K50) and stimulating high CL responses.
The magnitude of the intracellular survival of the capsulated variants lacking the di-Man/Rha glycoepitopes was significantly higher (270 ± 73%; 164 ± 13% of inoculum) than that of their corresponding non-capsulated variants (98.5 ± 27.4%; 33.2 ± 22.5% of inoculum) or of the capsulated and non-capsulated variants of the capsular serotypes bearing the di-Man/Rha glycoepitopes (K21a, 103 ± 9.2%; K21a/n, 77 ± 7.2%; K36, 26.4 ± 22.4%; K36/n, 15.6 ± 15.3%; p < 0.001; fig. 3). The exchange of the K2 capsule by that of the di-Man- or di-Rha-containing K21a or K36 capsules resulted in a significant decrease (43 ± 19%; 46.57 ± 21.7% of inoculum) in intracellular survival compared to the capsulated parental K2 strains (p < 0.001). Conversely, the intracellular survival of the capsule-switched derivative K21a(K2) was significantly higher (239 ± 23% of inoculum) than that of the parental K21a strain (p < 0.001). The intracellular survival of the K2(K36) capsule-switched derivative that produces higher amounts of capsular polysaccharides did not significantly differ from that induced by the K36 capsulated strain (fig. 3).
Fig. 3.
Intracellular survival of capsulated and non-capsulated variants of the manno(rhamno)biose-containing serotypes K21a, K36, K50 and the capsule-switched derivatives K2(K21a) and K2(K36) in PMNL as compared to the intracellular survival of the capsulated and non-capsulated variants of the K2 and K8 serotypes, whose capsular polysaccharides lack manno(rhamno)biose glycoepitopes. * p < 0.001, vs. all other tested capsulated and non-capsulated serotypes; ** p < 0.001, vs. the capsulated K2 parental strain; *** p < 0.001, vs. the K21a parent.
Klebsiella-Induced Respiratory Burst in PMNL Occurs via the Alternative and the MBL Complement Pathways
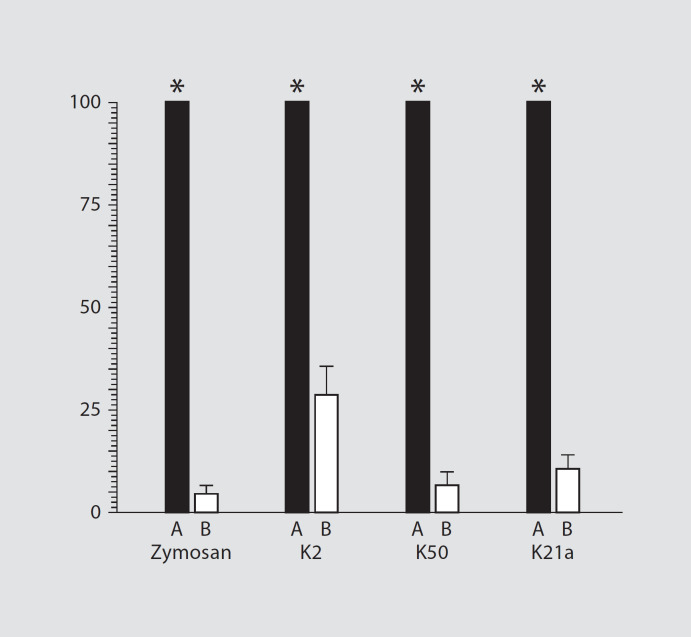
The CL response of PMNL exposed to zymosan or to Klebsiella serotypes K2/O1, K50/O3 or K21a/O1 was markedly abrogated in the presence of heat-inactivated serum as compared to fresh human serum, suggesting involvement of complement (p < 0.0001; fig. 4).
Fig. 4.
CL responses of PMNL after stimulation with zymosan, or the Klebsiella serotypes K2/O1, K50/O3 or K21a/O1. Pre-opso-nization of the zymosan or bacteria with heat-inactivated serum (□) significantly decreased CL responses to values that did not exceed 28% of the values induced by zymosan and bacteria after pre-treatment with NHS (■; p < 0.0001).
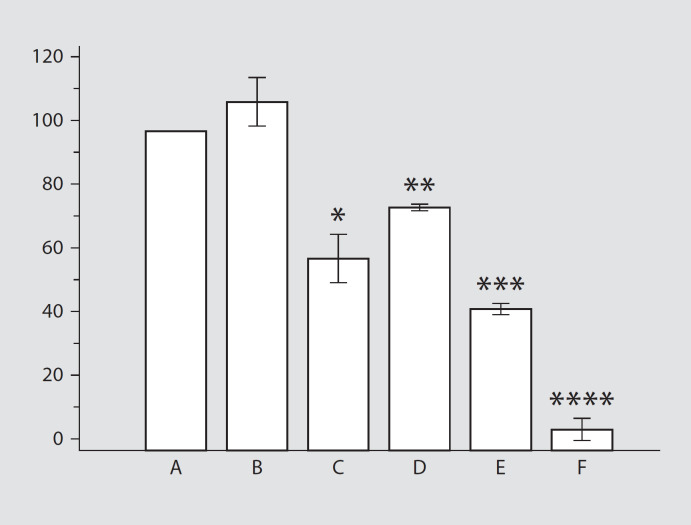
C1q-depleted serum did not affect the CL response (p > 0.05; fig. 5), indicating that complement activation via the classical pathway is not involved in PMNL stimulation by Klebsiella. Depletion of factor B significantly reduced the CL responses to values that were only 60% of the responses induced by NHS-opsonized bacteria (p < 0.001). Addition of EGTA in the presence of Mg2+ to the serum caused a significant reduction in the mean CL response to a mean of 75% of the response obtained with NHS-pretreated bacteria (p < 0.01; fig. 5). Interestingly, the treatment of Klebsiella with factor-B-depleted serum supplemented with EGTA and Mg2+ revealed a significant reduction in CL responses compared to the responses induced by Klebsiella treated with B-depleted serum without EGTA (fig. 5).
Fig. 5.
CL responses after stimulation with the capsulated Klebsiella strains K21a after opsonization with NHS (A), C1q-depleted serum (B), EGTA-treated serum (C), factor-B-depleted serum (D), EGTA-treated factor-B-depleted serum (E) and heat-inactivated serum (F). * p < 0.001, C vs. A and B; ** p < 0.001, vs. A and B; *** p < 0.001, vs. D; **** p < 0.0001, vs. other groups.
Discussion
The bacterial polysaccharide capsule is generally considered as a major virulence factor in the pathogenic potential of the bacterium. For K. pneumoniae this notion was partially revised after it has been shown that the MR of the macrophages and lung SP-A recognize capsular Manα2/3Man or Rhaα2/3Rha (di-Man/Rha) glycoepitopes of a number of capsular serotypes resulting in enhanced binding and ingestion of the bacteria [18, 27]. In contrast, serotypes that lack these di-Man/Rha glycoepitopes are resistant to phagocytosis, non-virulent to mice and are isolated at low frequency from infected patients [21, 25, 38].
In a serum-rich environment, Gram-negative bacteria were affected by two other mechanisms, both of which require the activation of the complement cascade. One mechanism leads to direct killing by the late complement components which permeabilize the outer membrane. The other mechanism, the focus of the present study, involves opsonization of the bacteria leading to enhanced binding, ingestion and killing by PMNL. The opsonization requires the deposition of the C3b fragment on the bacterial surface, with C3b fragment mediating binding and ingestion via the CR3 complement receptor on the phagocytic cells. Invariably, the C3b-mediated interaction of Klebsiella with PMNL triggers an oxidative burst that can be detected as a CL response. In several studies, it was shown that PMNL differently recognize various K. pneumoniae serotypes in the presence of serum. K1, K2, K4 and K5 induced lower respiratory bursts in PMNL than K3 and K7 [35, 36], but the molecular basis of this distinct recognition is not clear at this stage.
In the present study, we correlated the capsular structure of K. pneumoniae to the ability of the organism to trigger a serum-dependent respiratory burst in PMNL. It was demonstrated that the K serotypes K21a, K36 and K50 that express capsular polysaccharides containing di-Man/Rha glycoepitopes induced significantly higher respiratory burst values than the K2, K8 and K55 serotypes that lack these glycoepitopes. Significantly, the respiratory burst induced by the non-capsulated phase variants of the K21a, K36 and K50 capsular types was significantly less than that induced by their parent strains.
These results could be taken to indicate that, in analogy to the recognition of Klebsiella by MR and SP-A, the serum-dependent stimulation of PMNL involves a di-Man/Rha-specific Klebsiella recognition mechanism. This notion is consistent with previous studies showing that the K3 and K7 serotypes, which express Manα2/3Man-containig repeating units, induced significantly higher respiratory burst in PMNL than K1, K2, K4 and K5 serotypes, which lack such glycoepitopes [35, 36].
Because heat inactivation of serum complement factors significantly reduced zymosan- and bacteria-induced respiratory burst in PMNL, it became obvious that the molecular basis of complement activation involves recognition of one or more complement constituents, which are responsible for the deposition of the C3b fragment on the bacterial surface. Apparently, the di-Man/Rha structures of the capsule are responsible for activating the complement by lectin pathways because (i) only capsular serotypes, either of reference strains, wild-type strains or of capsule-switched derivatives bearing such di-Man/Rha structures, induced high CL in PMNL, (ii) depletion of C1q, which is a specific marker for the classical pathway, did not influence the CL response, albeit the presence of anti-Klebsiella antibodies in this serum is unclear, (iii) pretreating the bacteria with factor-B-depleted serum reduced the CL responses significantly, (iv) Klebsiella treated with calcium-chelated serum (EGTA supplemented with Mg2+) to block the calcium-dependent lectin pathway but not the alternative pathway induced significantly diminished CL responses, and (v) the treatment of Klebsiella with factor-B-depleted, calcium-chelated serum (EGTA supplemented with Mg2+) significantly reduced the CL responses compared to the responses induced by Klebsiella treated with B-depleted serum without EGTA.
The relationship between the complement-dependent activation of PMNL and the molecular structure of LPS is not clear at this stage. It was shown that MBL probably binds to Klebsiella O3 antigen, which possesses the mannose homopolysaccharide, and that this binding may play an important role in the ability of the bacteria to activate complement [42]. Because the prominent capsule might mask LPS, it is conceivable that the capsular expression alters the interaction of LPS with complement. In our study, however, the mannose content in the O antigen of LPS apparently did not influence the complement-dependent activation of PMNL because the non-capsulated derivatives of the mannose-rich (O3) and mannose-poor (O1) serotypes stimulated the PMNL similarly (fig. 1). The non-capsulated variants of the di-Man/Rha-containing K serotypes stimulated the PMNL to a much lesser extent than their capsulated parents. Conversely, PMNL stimulation induced by the non-capsulated variants of the K serotypes lacking such glycoepitopes was higher than that induced by the capsulated parental strains, indicating that PMNL activation occurs in a di-Man/Rha-specific manner.
Our studies further emphasize that a better survival of certain Klebsiella serotypes depends on their capsular structures which are not recognized by components of the innate immune system in various body fluids, including serum-rich environments, which invariably are also rich in PMNL during inflammation. Because complement activation may lead to recognition of the bacteria by PMNL via the C3b fragment as well as to direct killing by the late membrane attack complex of the complement system, our study is the first to shed light on the molecular components underlying virulence of this opportunistic pathogen. The virulent serotypes (e.g. K2/O1) escape killing or eradication by the innate immune system by presenting on their surfaces structures that are not recognized by many innate immune components including the lung surfactant proteins, MR of macrophages [28, 29, 30, 31, 32] and, as shown in the present study, by the MBL and alternative pathways of the complement system. In addition to the alternative pathway, the MBL pathway seems to be a major pathway for eradicating di-Man/Rha-containing Klebsiella serotypes from blood and perhaps also during acute inflammation.
References
- 1.Torres A, Serra-Batlles J, Ferrer A, Jimenez P, Celis R, Cobo E, Rodriguez-Roisin R. Severe community-acquired pneumonia; epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144:312–318. doi: 10.1164/ajrccm/144.2.312. [DOI] [PubMed] [Google Scholar]
- 2.Jong GM, Hsiue TR, Chen CR, Chang HY, Chen CW. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest. 1995;107:214–217. doi: 10.1378/chest.107.1.214. [DOI] [PubMed] [Google Scholar]
- 3.Ishida T, Hashimoto T, Arita M, Ito I, Osawa M. Etiology of community-acquired pneumonia in hospitalized patients: a 3-year prospective study in Japan. Chest. 1998;114:1588–1593. doi: 10.1378/chest.114.6.1588. [DOI] [PubMed] [Google Scholar]
- 4.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, McCormack JG, Yu VL. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis. 2002;8:160–166. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;13:986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu CL, Lin FJ, Lee SY, Lee CH, Peng MJ, Chen PJ, Kuo HT. Early evolution of arterial oxygenation in severe community-acquired pneumonia: a prospective observational study. J Crit Care. 2007;22:129–136. doi: 10.1016/j.jcrc.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Song JH, Oh WS, Kang CI, Chung DR, Peck KR, Ko KS, Yeom JS, Kim CK, Kim SW, Chang HH, Kim YS, Jung SI, Tong Z, Wang Q, Huang SG, Liu JW, Lalitha MK, Tan BH, Van PH, Carlos CC, So T;, Asian Network for Surveillance of Resistant Pathogens Study Group Epidemiology and clinical outcomes of community-acquired pneumonia in adult patients in Asian countries: a prospective study by the Asian network for surveillance of resistant pathogens. Int J Antimicrob Agents. 2008;31:107–114. doi: 10.1016/j.ijantimicag.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podschun R, Ullmann U. Klebsiella spp as nosocomial pathogen: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahly H, Podschun R, Ullmann U. Klebsiella infections in the immunocompromised host. Adv Exp Med Biol. 2000;479:237–249. doi: 10.1007/0-306-46831-X_21. [DOI] [PubMed] [Google Scholar]
- 10.Spencer RC. Predominant pathogens found in the European Prevalence of Infection in Intensive Care Study. Eur J Clin Microbiol Infect Dis. 1996;15:281–285. doi: 10.1007/BF01695658. [DOI] [PubMed] [Google Scholar]
- 11.Yinnon AM, Schlesinger Y, Gabbay D, Rudensky B. Analysis of 5 years of bacteraemias: importance of stratification of microbial susceptibilities by source of patients. J Infect. 1997;35:17–23. doi: 10.1016/s0163-4453(97)90857-4. [DOI] [PubMed] [Google Scholar]
- 12.Gikas A, Samonis G, Christidou A, Papadakis J, Kofteridis D, Tselentis Y, Tsaparas N. Gram-negative bacteremia in non-neutropenic patients: a 3-year review. Infection. 1998;26:155–159. doi: 10.1007/BF02771841. [DOI] [PubMed] [Google Scholar]
- 13.Harbarth S, Ruef C, Francioli P, Widmer A, Pittet D. Nosocomial infections in Swiss university hospitals: a multi-centre survey and review of the published experience. Swiss-Noso Network. Schweiz Med Wochenschr. 1999;129:1521–1528. [PubMed] [Google Scholar]
- 14.Vincent JL. Nosocomial infections in adult intensive-care units. Lancet. 2003;361:2068–2077. doi: 10.1016/S0140-6736(03)13644-6. [DOI] [PubMed] [Google Scholar]
- 15.Geffers C, Zuschneid I, Sohr D, Rüden H, Gastmeier P. Microbiological isolates associated with nosocomial infections in intensive care units: data of 274 intensive care units participating in the German Nosocomial Infections Surveillance System (KISS) (in German) Anästhesiol Intensivmed Notfallmed Schmerzther. 2004;39:15–19. doi: 10.1055/s-2004-815713. [DOI] [PubMed] [Google Scholar]
- 16.Gaynes R, Edwards JR, National Nosocomial., Infections Surveillance System Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 17.Kohlenberg A, Schwab F, Geffers C, Behnke M, Rüden H, Gastmeier P. Time-trends for Gram-negative and multidrug-resistant Gram-positive bacteria associated with nosocomial infections in German intensive care units between 2000 and 2005. Clin Microbiol Infect. 2007;14:82–96. doi: 10.1111/j.1469-0691.2007.01879.x. [DOI] [PubMed] [Google Scholar]
- 18.Ofek I, Crouch E, Keisari Y. The role of C-type lectins in the innate immunity against pulmonary pathogens. Adv Exp Med Biol. 2000;479:27–36. doi: 10.1007/0-306-46831-X_3. [DOI] [PubMed] [Google Scholar]
- 19.Hansen DS, Mestre F, Alberti S, Hernandez-Alles S, Alvarez D, Domenech-Sanchez A, Gil J, Merino S, Tomas JM, Benedi VJ. Klebsiella pneumoniae lipopolysaccharide O typing: revision of prototype strains and O-group distribution among clinical isolates from different sources and countries. J Clin Microbiol. 1999;37:56–62. doi: 10.1128/jcm.37.1.56-62.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ørskov I, Ørskov F. Serotyping of Klebsiella. In: Bergan T, Methods in Microbiology, editor. vol 14. London: Academic Press; 1984. pp. pp 143–164. [Google Scholar]
- 21.Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983;40:56–61. doi: 10.1128/iai.40.1.56-61.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ofek I, Kabha K, Athamna A, Frankel G, Wozniak D, Hasty D, Ohman D. Genetic exchange of determinants for capsular polysaccharide biosynthesis between Klebsiella pneumoniae strains expressing serotypes K2 and K21a. Infect Immun. 1993;61:4208–4216. doi: 10.1128/iai.61.10.4208-4216.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podschun R, Heineken P, Ullmann U, Sonntag HG. Comparative investigations of Klebsiella species of clinical origin: plasmid patterns, biochemical reactions, antibiotic resistances and serotypes. Zentralbl Bakteriol Mikrobiol Hyg [A] 1986;262:335–345. doi: 10.1016/s0176-6724(86)80006-2. [DOI] [PubMed] [Google Scholar]
- 24.Sahly H, Aucken H, Benedi VJ, Forestier C, Fussing V, Hansen DS, Ofek I, Podschun R, Sirot D, Tomás JM, Ullmann U. Impairment of respiratory burst in polymorphonuclear leukocytes by extended-spectrum beta-lactamase-producing strains of Klebsiella pneumoniae. Eur J Med Microbiol Infect Dis. 2004;23:20–26. doi: 10.1007/s10096-003-1047-7. [DOI] [PubMed] [Google Scholar]
- 25.Sahly H, Aucken H, Benedi VJ, Forestier C, Fussing V, Hansen DS, Ofek I, Podschun R, Sirot D, Tomás JM, Ullmann U. Increased serum resistance properties in Klebsiella pneumoniae strains producing extended spectrum β-lactamases. Antimicrob Agents Chemother. 2004;48:3477–3482. doi: 10.1128/AAC.48.9.3477-3482.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trautmann M, Ruhnke M, Rukavina T, Held TK, Cross AS, Marre R, Whitfield C. O-antigen seroepidemiology of Klebsiella clinical isolates and implications for immunoprophylaxis of Klebsiella infections. Clin Diagn Lab Immunol. 1997;4:550–555. doi: 10.1128/cdli.4.5.550-555.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahly H, Keisari Y, Crouch E, Sharon N, Ofek I. Recognition of bacterial surface polysaccharides by lectins of the innate immune system and its contribution to defense against infection: the case of pulmonary pathogens. Infect Immun. 2008;76:1322–1332. doi: 10.1128/IAI.00910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Athamna A, Ofek I, Keisari Y, Markowitz S, Dutton GGS, Sharon N. Lectinophagocytosis of encapsulated Klebsiella pneumoniae mediated by surface lectin of guinea pig alveolar macrophages and human monocyte-derived macrophages. Infect Immun. 1991;59:1673–1682. doi: 10.1128/iai.59.5.1673-1682.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabha K, Schmegner J, Keisari Y, Parolis H, Schlepper-Schaefer J, Ofek I. SP-A enhances phagocytosis of Klebsiella by interaction with capsular polysaccharides and alveolar macrophages. Am J Physiol. 1997;272:L344–L352. doi: 10.1152/ajplung.1997.272.2.L344. [DOI] [PubMed] [Google Scholar]
- 30.Ofek I, Mesika A, Kalina M, Keisari Y, Podschun R, Sahly H, Chang D, McGregor D, Crouch E. Surfactant protein D (SP-D) enhances the phagocytosis and killing of unencapsulated phase variants of Klebsiella pneumoniae. Infect Immun. 2001;69:24–33. doi: 10.1128/IAI.69.1.24-33.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahly H, Ofek I, Podschun R, Brade H, He Y, Crouch E. Surfactant protein D binds selectively to Klebsiella pneumoniae lipopolysaccharides containing mannose-rich-antigens. J Immunol. 2002;169:3267–3274. doi: 10.4049/jimmunol.169.6.3267. [DOI] [PubMed] [Google Scholar]
- 32.Kostina E, Ofek I, Crouch E, Friedman R, Sirota L, Klinger G, Sahly H, Keisari Y. Noncapsulated Klebsiella pneumoniae bearing mannose-containing O antigens is rapidly eradicated from mouse lung and triggers cytokine production by macrophages following opsonization with surfactant protein D. Infect Immun. 2005;73:8282–8290. doi: 10.1128/IAI.73.12.8282-8290.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keisari Y, Mesika A, Wang H, Nissimov L, Crouch E, Ofek I. Surfactant protein D-coated Klebsiella pneumoniae stimulates cytokine production in mononuclear phagocytes. J Leukoc Biol. 2001;70:135–141. [PubMed] [Google Scholar]
- 34.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 35.Podschun R, Penner I, Ullmann U. Interaction of Klebsiella capsule type 7 with human polymorphonuclear leucocytes. Microb Pathog. 1992;13:371–379. doi: 10.1016/0882-4010(92)90080-8. [DOI] [PubMed] [Google Scholar]
- 36.Simoons-Smit AM, Verweij-van Vught AM, Kanis IY, MacLaren DM. Chemiluminescence of human leucocytes stimulated by clinical isolates of Klebsiella. J Med Microbiol. 1985;19:333–338. doi: 10.1099/00222615-19-3-333. [DOI] [PubMed] [Google Scholar]
- 37.Matatov R, Goldhar J, Skutelsky E, Sechter I, Perry R, Podschun R, Sahly H, Thankavel K, Abraham S, Ofek I. Inability of encapsulated Klebsiella pneumoniae to assemble functional type 1 fimbriae on their surface. FEMS Microbiol Lett. 1999;179:123–130. doi: 10.1111/j.1574-6968.1999.tb08717.x. [DOI] [PubMed] [Google Scholar]
- 38.Kabha K, Nissimov L, Athamna A, Keisari Y, Parolis H, Parolis LA, Grue RM, Schlepper-Schafer J, Ezekowitz AR, Ohman DE, Ofek I. Relationships among capsular structure, phagocytosis, and mouse virulence in Klebsiella pneumoniae. Infect Immun. 1995;63:847–852. doi: 10.1128/iai.63.3.847-852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahly H, Podschun R, Oelschlaeger TA, Greiwe M, Parolis H, Hastey D, Kekow J, Ullmann U, Ofek I, Sela S. Capsule impedes adhesion and invasion of epithelial cells by Klebsiella pneumoniae. Infect Immun. 2000;68:6744–6749. doi: 10.1128/iai.68.12.6744-6749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 41.Domenico P, Schwartz S, Cunha BA. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang GZ, Sugiyama T, Kato Y, Koide N, Yokochi T. Binding of mannose-binding protein to Klebsiella O3 lipopolysaccharide possessing the mannose homopolysaccharide as the O-specific polysaccharide and its relation to complement activation. Infect Immun. 1995;63:2537–2540. doi: 10.1128/iai.63.7.2537-2540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]