Abstract
Obesity contributes significantly to the global health burden. A better understanding of adipogenesis, the process of fat formation, may lead to the discovery of novel treatment strategies. However, it is of concern that the regulation of adipocyte differentiation has predominantly been studied using the murine 3T3-L1 preadipocyte cell line and murine experimental animal models. Translation of these findings to the human setting requires confirmation using experimental models of human origin. The ability of mesenchymal stromal/stem cells (MSCs) to differentiate into adipocytes is an attractive model to study adipogenesis in vitro. Differences in the ability of MSCs isolated from different sources to undergo adipogenic differentiation, may be useful in investigating elements responsible for regulating adipogenic differentiation potential. Genes involved may be divided into three broad categories: early, intermediate and late-stage regulators. Preadipocyte factor-1 (Pref-1) is an early negative regulator of adipogenic differentiation. In this review, we briefly discuss the adipogenic differentiation potential of MSCs derived from two different sources, namely adipose-derived stromal/stem cells (ASCs) and Wharton’s Jelly derived stromal/stem cells (WJSCs). We then discuss the function and suggested mechanisms of action of Pref-1 in regulating adipogenesis, as well as current findings regarding Pref-1’s role in human adipogenesis.
Keywords: adipogenesis, Pref-1, adipose-derived stromal/stem cells, Wharton’s jelly derived stromal/stem cells, transcription factor, differentiation, Notch signaling, MAPK kinase signaling
1. Introduction
Over one third of the world’s population is overweight or obese [1,2]. Based on current trends, estimations predict that 60% of the global population will be obese or overweight by 2030 [1,2]. This is a huge concern, as obesity is a major risk factor for several comorbidities such as diabetes mellitus, hypertension, cardiovascular disease and cancer [3,4,5]. The strong association of obesity with chronic comorbidities contributes significantly to overburdened health care systems globally [6,7]. The obvious need for novel interventions to combat obesity has resulted in an active interest in identifying novel therapeutic targets to manage obesity and treat obesity-associated metabolic disorders.
Identifying novel therapeutic targets requires a good understanding of the regulatory elements involved during adipogenesis (fat cell formation). It is; however, of concern that the regulation of adipocyte differentiation has predominantly been studied using the murine 3T3-L1 preadipocyte cell line and murine experimental animal models. Translation of these findings to the human setting is uncertain and requires confirmation using experimental models of human origin.
Mesenchymal stromal/stem cells (MSCs) have the ability to differentiate into various mesodermal cell lineages, such as osteoblasts, myoblasts and adipocytes [8,9]. The ability of MSCs to differentiate into adipocytes is an attractive model to study adipogenesis in vitro. Furthermore, differences in the ability of MSCs isolated from different sources to undergo adipogenic differentiation, may be useful in investigating the different elements responsible for this process. In this review, we briefly discuss the different types of adipose tissue and the ability of MSCs to differentiate into adipocytes, focusing on adipose-derived stromal/stem cells (ASCs) and Wharton’s jelly derived stromal/stem cells (WJSCs). The function and suggested mechanisms of action of Pref-1 in regulating adipogenesis, as well as current findings regarding Pref-1′s role in human adipogenesis, is then reviewed.
2. Adipose Tissue
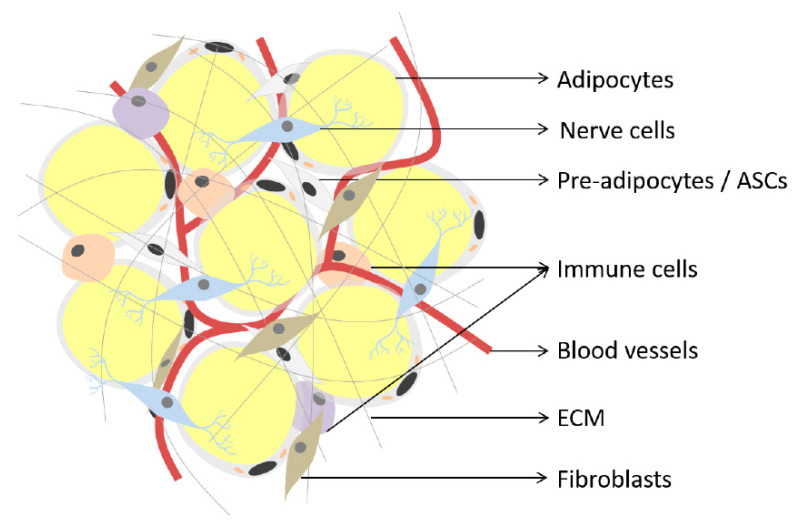
Adipose tissue is an endocrine organ comprised of terminally differentiated adipocytes, preadipocytes, ASCs, fibroblasts, endothelial cells, nerve cells and immune cells, which function together to maintain homeostasis within the tissue (Figure 1) [10,11]. There are two main types of adipose tissue in mammals: white and brown adipose tissue [12,13,14], which differ regarding their origin, function and morphology.
Figure 1.
Schematic representation of adipose tissue. Adipose tissue is comprised of adipocytes, preadipocytes, adipose-derived stromal/stem cells (ASCs), immune cells, nerve cells, fibroblasts, blood vessels and extracellular matrix (ECM), among others. Image created by C.d.S.
White adipose tissue (WAT) is found throughout the body [12,13,15], and is mainly involved in energy storage. WAT is further subdivided into visceral and subcutaneous adipose tissue. Visceral WAT is located mainly around organs, while subcutaneous WAT is located directly under the skin [13]. Brown adipose tissue (BAT) is involved in energy expenditure and thermogenesis in infants, and is located at specific sites including the upper back (interscapular region) and around the kidneys (perirenal) [13,16]. A unique feature of brown adipocytes is the expression of uncoupling protein 1 (UCP1), a mitochondrial protein implicated in energy expenditure [16,17]. During cold exposure, the sympathetic nervous system is stimulated and norepinephrine is released which results in BAT producing heat through non-shivering thermogenesis [12]. To do so, fatty acids are released through lipolysis and used by UCP1 for heat production [13,18].
A second brown adipocyte type, known as beige adipocytes, has also been described [16,19,20,21]. Several factors induce the differentiation of precursors, located within WAT depots, into beige adipocytes. Beige adipocytes are also referred to as brite or brown-in-white adipocytes. Factors that induce beige adipocyte differentiation include chronic cold acclimatization, exercise, and long-term treatment with proliferator-activated receptor gamma (PPARγ) agonists, among others [16,22].
Mature white adipocytes are large, roundish cells which are characterized by a single large lipid droplet which occupies most of the cytosol and contains few mitochondria (Figure 2) [19]. Energy is stored in the form of triglycerides within the unilocular lipid droplet [16]. Brown adipocytes are more ellipsoid in shape, mitochondria rich and contain multiple lipid droplets within the cytosol (Figure 2) [12]. Beige adipocytes are roundish cells that, similar to brown adipocytes, are UCP-positive, mitochondria rich and contain multiple lipid droplets [13,16,23].
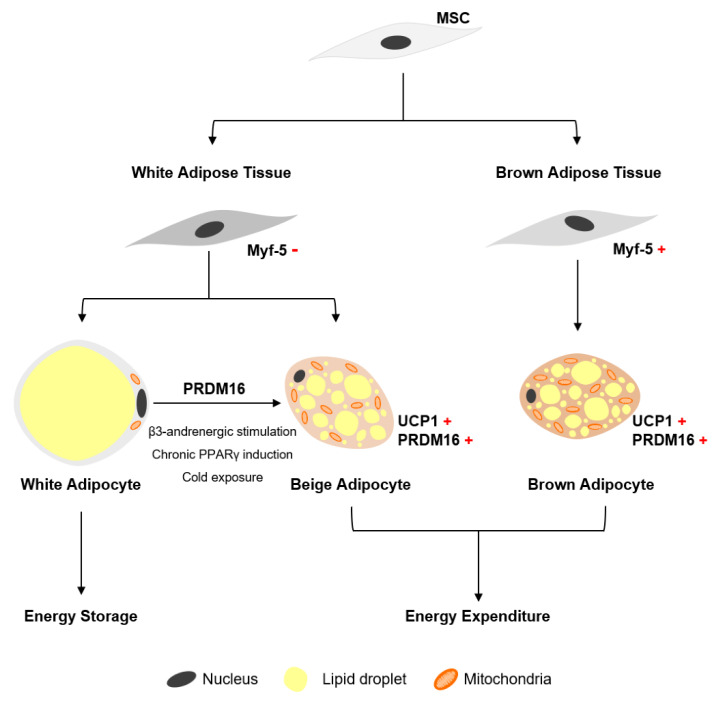
Figure 2.
Origin of adipocytes. White and beige adipocytes arise from different precursor cells in their respective adipose tissue depots. White adipocytes are involved in energy storage and contain one unilocular lipid droplet which occupies most of the cytosol. Beige and brown adipocytes are involved in energy expenditure through UCP1. MSC, mesenchymal stromal/stem cell; Myf-5, myogenic factor 5; PRDM16, PR domain containing 16; UCP1, uncoupling protein 1 Image adapted by C.d.S. from Sarjeant and Stephens et al. (2012) [12].
White adipocytes originate from MSCs within adipose tissue, also known as ASCs, that do not express myogenic factor 5 (Myf-5) (Figure 2) [12,13]. In contrast, brown adipocytes arise from Myf-5-expressing (Myf-5-positve) myotomal precursors (Figure 2) [16,19]. Beige adipocytes arise from a unique set of precursors, still to be defined, that reside within WAT depots [24]. Wang and colleagues (2013) neatly demonstrated, using a pulse-chase fate-mapping technique, that beige adipocytes are not the product of trans-differentiation of pre-existing white adipocytes in WAT depots [25]. The origin of adipocytes has not been fully elucidated, and several other cells, such as vascular endothelial cells and neural crest cells, have been implicated as possible precursors of both brown and white adipocytes [19].
3. Mesenchymal Stromal/Stem Cells
MSCs are a heterogeneous population containing a subset of multipotent adult stem cells that were first discovered by Friedenstein and colleagues in murine bone marrow [26,27,28], referred to by the authors as bone marrow-derived MSCs (BM-MSCs). Since their initial discovery, MSCs have been found to be present in most human adult tissues where they play an important role in cell replacement and regeneration, secretion of bioactive molecules and immune regulation [26,29]. The adipogenic, osteogenic and chondrogenic differentiation capacity of multipotent MSCs is well described [28,30], but studies suggest that MSCs also have the ability to differentiate into other cell types such as myocytes and neural cells, albeit that this is not yet well described/accepted [28,29].
Bone marrow is a common source of MSCs; however, bone marrow aspiration is invasive and contains a small number of MSCs (0.001–0.01% of total nucleated cells) [31]. Furthermore, the number of MSCs present in bone marrow declines with age [26,32]. These factors make bone marrow a less attractive source for isolating MSCs for cell therapy applications. On the other hand, adipose tissue obtained from liposuction and abdominoplasty procedures and the post-natal umbilical cord (UC), are generally considered biological waste, and their use is consequently associated with minimal ethical concerns [33,34,35]. This, has resulted in adipose tissue and UC gaining popularity as sources of MSCs [36,37,38]. The reported MSC yields from bone marrow and UC are highly variable, but seem to be comparable [39]. However, the efficiency of isolating MSCs from adipose tissue is significantly greater [39].
The high variability in yields when MSCs are isolated from UC or placenta potentially results from the anatomical complexity of these tissues. MSCs can be isolated from various anatomical regions of the UC, including the amniotic epithelial membrane, the sub-amnion cord lining, intervascular gelatinous connective tissue, known as Wharton’s jelly (WJ), and the perivascular region surrounding the umbilical blood vessels [40,41,42]. MSCs can also be isolated from the fetal side of placenta, the cord-placenta junction or the maternal side of placenta [40]. The yield and the differences in MSC properties may thus be due to differences in the anatomical regions from which these cells are isolated.
Adipogenic Differentiation Capacity of hASCs and hWJSCs
Adipogenic differentiation is one of the criteria that need to be fulfilled when characterizing MSCs isolated from different tissue sources [43]. Consequently, a large number of studies have compared the adipogenic differentiation potential of MSCs isolated from different tissues. It is clear from these studies that the differentiation characteristics of MSCs differ based on the tissue source or the anatomical site from which the MSCs are isolated [30,44]. The adipogenic differentiation potential of MSCs isolated from different human-derived tissue sources is summarized in Table 1. We have limited ourselves to those studies that either use ASCs or BM-MSCs as reference cultures. As mentioned previously, ASCs are known for their superior adipogenic differentiation potential, while BM-MSCs were the first MSC cell type to be identified/isolated, and are thus often used as a reference standard in MSC studies.
Table 1.
Summary of the adipogenic differentiation potential of MSCs isolated from human tissues. The sources are listed from left to right according to decreasing adipogenic potential observed in the respective studies (direct comparisons).
| Study | Tissue Sources Compared | Reference | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amable et al., 2014 | AT | > | BM | > | WJSC | [46] | ||||||||||||
| Brohem et al., 2013 | AT | > | BM | [49] | ||||||||||||||
| Hu et al., 2013 | AT | = | WJSC | [47] | ||||||||||||||
| Li et al., 2015 | AT | = | BM | [50] | ||||||||||||||
| Liu et al., 2009 | AT | > | BM | [51] | ||||||||||||||
| Manini et al., 2011 | AT | > | BM | > | Dermis | [52] | ||||||||||||
| Maurney et al., 2007 | AT | = | BM | [53] | ||||||||||||||
| Mohamed-Ahmed et al., 2018 | AT | > | BM | > | Dermis | [54] | ||||||||||||
| Noël et al., 2007 | AT | = | BM | [55] | ||||||||||||||
| Ragni et al., 2013 | AT | > | BM | > | UCB | > | WJSC | > | AF | > | PV | [45] | ||||||
| Sakaguchi et al., 2005 | AT | = | SV | > | BM | = | PS | > | Muscle | [56] | ||||||||
| Xu et al., 2017 | AT | > | BM | [30] | ||||||||||||||
| Baksh et al., 2007 | UC | > | BM | [57] | ||||||||||||||
| Barlow et al., 2015 | BM | > | PC | [58] | ||||||||||||||
| Batsali et al., 2017 | BM | > | WJSC | [59] | ||||||||||||||
| Isobe et al., 2015 | BM | = | SF | > | SHED | > | DP | [60] | ||||||||||
| Zhu et al., 2012 | > | BM | [61] | |||||||||||||||
AF, amniotic fluid; AT, adipose tissue; BM, bone marrow; DP, dental pulp; nFS, neonatal foreskin; SHED, exfoliated deciduous teeth; PS, periosteum; PV, perivascular region of UC; SF, synovium fluid; SV, synovium; PC, placenta; UCB, umbilical cord blood; UC, umbilical cord; =, no difference in adipogenic potential was observed.
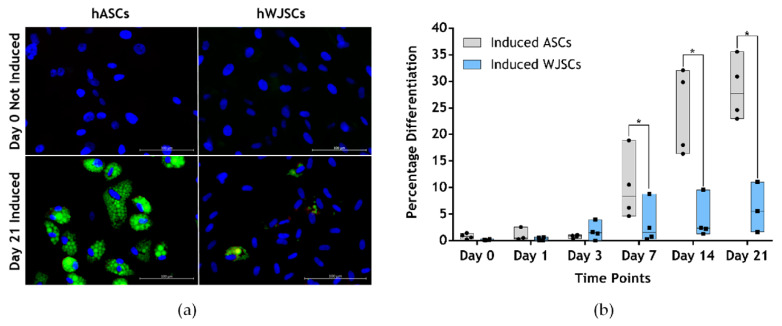
Very few studies have directly compared the adipogenic differentiation potential of human ASCs (hASCs) and human WJSCs (hWJSCs) in the same study (Table 1). Ragni and colleagues (2013) [45] and Amable and colleagues (2014) [46] showed that hASCs displayed enhanced adipogenic differentiation when compared to hWJSCs, while Hu and colleagues (2013) [47] observed no difference between these two cell types. Our group has observed that hWJSCs differentiate poorly into adipocytes. On average, 2.91% ± 2.72% of hWJSCs (n = 3 independent primary cultures) differentiated into adipocytes after a 21-day adipogenic induction period, compared to 28.50% ± 2.91% (n = 3) in the case of hASCs (Figure 3a,b). It is clear from our observation and the studies summarized in Table 1 that although hASCs and hWJSCs display similar morphological and phenotypic characteristics [47,48], they differ with respect to their adipogenic differentiation potential. hASCs and hWJSCs also differ with respect to their proliferation rates (hWJSCs have a higher proliferation rate) and cytokine secretion profiles [47].
Figure 3.
Adipogenic differentiation potential of hASCs and hWJSCs. (a) Microscopy images of Day 0 (prior to induction) and Day 21 induced hASCs and hWJSCs. Cells were stained with a nuclear dye Vybrant® DyeCycle Violet (blue) and a lipophilic dye Nile Red (green). Scale bars: 100 μm. Magnification: 20×. (b) The percentage of hASCs and hWJSCs that differentiated into adipocytes was determined via a flow cytometric Nile Red assay [62]. Each dot or square within the floating bars represents an independent hASC and hWJSC culture. Four cultures of each were included in the study. The horizontal lines within the bars represent the population median. Statistical significance between the two cell types at the various time points is displayed with an asterisk when * p < 0.05 C. hASCS and hWJSCs displayed the following phenotype: CD36+/CD44+/CD45-/CD73+/CD90+/CD105+ (not shown). Unpublished, original data.
The reasons behind the differences observed in differentiation potential between hASCs and hWJSCs are not known, and require further investigation. However, the differences could be exploited as an in vitro model to understand the molecular regulators of adipogenesis. It is likely that several factors determine the ability of MSCs to differentiate into a specific cell type. Pierdomenico and colleagues (2011) suggested that the physiological environment of MSCs affects their differentiation capabilities [63]. These investigators reported that hWJSCs, which were isolated from umbilical cord collected from infants of diabetic mothers, displayed improved adipogenic differentiation capacity compared to hWJSCs isolated from umbilical cord obtained from infants of non-diabetic donors [63]. Xu and colleagues (2017) suggested that MSC fate is controlled by the methylation status of transcription factor genes, and that epigenetic memory plays a role in the differential differentiation capacities of MSCs derived from different sources [30].
4. Adipogenesis
Adipogenesis is a complex, multi-step process in which precursor cells differentiate into either mature brown or white adipocytes [12,15,46]. Studies using the 3T3-L1 cell line have shown that white adipogenesis consists of several phases including (i) cell commitment; (ii) mitotic clonal expansion; and (iii) terminal differentiation [64,65]. The stages in humans are less well defined.
During the cell commitment stage, MSCs commit to undergo differentiation into preadipocytes [64]. Murine preadipocytes then undergo two rounds of mitosis during mitotic clonal expansion [65], which is an important step as the unwinding of DNA allows transcription factors to bind and initiate a well-controlled cascade required for terminal white adipogenic differentiation (summarized in Figure 4) [66,67,68]. Brown/beige adipocyte differentiation and activation is also regulated by sequential activation of a series of transcription factors specific to each of these adipocyte types. However, several different pathways, depending on the stimulus received, may be involved in brown and beige adipogenic differentiation [24,69].
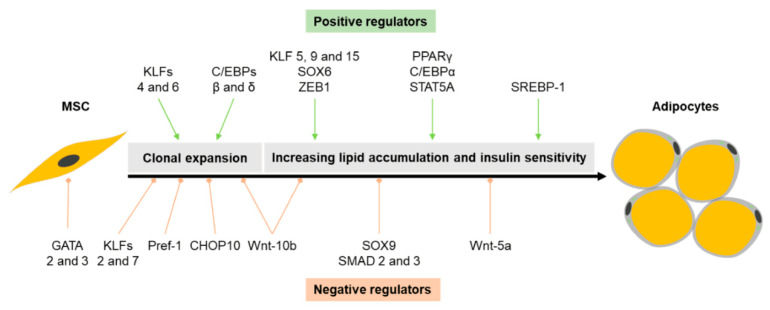
Figure 4.
Positive and negative regulators of white adipogenesis. Adipogenesis is tightly regulated by several transcription factors that are expressed at different stages during the differentiation pathway. Image adapted (with permission) by C.d.S., K.K. and M.A. from Sarjeant and Stephens et al. (2012) [12]. MSC, Mesenchymal stromal/stem cell; AP-1, activating protein-1; KLFs, Krüppel-like factors; C/EBP, CAAT-enhancer binding proteins; PPARγ, Peroxisome proliferator-activated receptor gamma; STAT, Signal transducer and activator of transcription; SREBP-1, sterol regulatory element binding protein 1; Pref-1, preadipocyte factor 1; Wnt, Wingless/Integrated protein; SOX 6 and 9; Sex-Determining Region Y-Box 6 and 9; SMAD 2 and 3, Mothers against decapentaplegic homolog 2 and 3; CHOP10, C/EBP homologous protein 10; ZEB1, Zinc finger E-box-binding homeobox 1.
4.1. Transcriptional Regulation of White Adipogenesis
Murine preadipocytes express high levels of preadipocyte factor-1 (Pref-1), CCAAT/enhancer binding protein (C/EBP) homologous protein (CHOP) and GATA transcription factor (Figure 4). Elevated levels of these molecules as well other negative regulators of adipogenesis such as sex Determining Region Y-Box 9 (SOX 9) and Mothers against decapentaplegic homolog 2 and 3 (SMAD 2 and 3) suppress adipogenic differentiation, hence the requirement for down-regulation of these molecules in order for adipogenic differentiation to proceed [12,70]. Pref-1 is one of the key negative regulators of adipogenesis [71,72,73,74]. C/EBPα and PPARγ are the main transcription factors that regulate adipogenesis [67,68], with PPARγ being the defining master regulator [12,13,67,68,75,76]. Up-regulation of C/EBPα and PPARγ ends the mitotic clonal expansion phase and leads to terminal differentiation [70]. During terminal differentiation, end-stage adipogenesis-associated genes are up-regulated, allowing preadipocytes to acquire all the morphological, genetic and biochemical characteristics of a mature adipocyte. These features include the up-regulation of enzymes responsible for fatty acid biosynthesis and transport, as well as triglyceride synthesis.
In vitro adipogenic differentiation of MSCs is induced by exposing the cells to a cocktail of compounds consisting of dexamethasone (DXM), 3-isobutyl-1-methylxantine (IBMX), insulin and fetal bovine serum (FBS). Insulin acts through the insulin-like growth factor 1 (IGF-1) receptor to initiate mitotic clonal expansion and also plays a role in promoting lipid accumulation [13,64]. FBS down-regulates the early transcription factor CHOP10, releasing C/EBPβ, which in turn activates C/EBPα and PPARγ [77]. IBMX, a phosphodiesterase inhibitor, activates the cAMP-dependent protein kinase pathway and up-regulates Pref-1, C/EBPβ and C/EBPδ [78]. The synthetic glucocorticoid, DXM, up-regulates C/EBPβ and PPARγ, while down-regulating Pref-1 [13,70,72]. Once activated, PPARγ binds to and activates the promoters of adipocyte specific genes such as fatty acid binding protein 4 [FABP4, also known as adipocyte protein 2 (aP2), an adipocyte selective fatty acid binding protein], and phosphoenolpyruvate carboxylase (PEPCK, which is involved in gluconeogenesis) [79]. Other positive regulators of adipogenesis include sterol regulatory element binding protein-1 (SREBP-1), signal transducer and activator of transcription (STAT) transcription factor 3 and STAT 5A, SOX6, ZEB1, among others [12,13,79].
4.2. Transcriptional Regulation of Brown Adipogenesis
There are several genes that distinguish brown adipocytes from white adipocytes (reviewed in [24,69]). In addition to Ucp1, some other brown adipocyte-associated genes include Ppara (fatty acid metabolism), Cidea (lipid droplet remodelling), Dio2 (thyroid hormone metabolism), Elovl3 (fatty acid elongase), Pgc1α (mitochondrial biogenesis) and Cpt1b (fatty acid transport) [24]. Central to the regulation of different pathways, such as brown adipocyte differentiation and adaptive thermogenesis, is the PR domain zinc finger 16 (Prdm16)/Cebpβ complex. PRDM16 is not expressed by white adipocytes and plays an essential role in regulating brown adipogenic differentiation [24,80,81].
Under the control of the sympathetic nervous system (SNS), cold exposure induces Myf5+ mesodermal precursors or beige/brown adipocyte precursors residing in WAT depots to differentiate into beige brown adipocytes [24,80,82,83,84]. Adrenergic signals, such as noradrenaline and catecholamines, are released, which bind to β-adrenergic receptors to activate the downstream differentiation pathways [82,85,86]. Brown and beige adipogenic differentiation requires the up-regulation of early transcription factors, such as early B-cell factor-2 (EBF2) [87], PPARγ coactivator 1A (PGC1α) [88], nuclear factor I-A (NFIA) [89] and others. All of these transcription factors play a role in the up-regulation of adipogenesis-associated genes such as C/EBPβ and PPARγ. The brown adipogenesis master regulator, PRDM16, binds to the active form of C/EBPβ [90] to regulate several processes, such as browning of WAT, fatty acid oxidation and mitochondrial metabolism [69]. Foxhead P1 (Foxp1) acts as a negative regulator of brown/beige adipogenic differentiation and thermogenesis by preventing the PRDM16/CEBPβ complex from up-regulating PPARγ and β3-AR expression [82].
5. Preadipocyte Factor 1 (Pref-1)
5.1. Brief Overview on the Discovery of Pref-1
In 1987, Helman and colleagues [91,92] investigated genes expressed during differentiation of neuroendocrine tissue. The investigators found two genes, pG2 and pG8, that were highly expressed in both normal and neoplastic neuroendocrine tissue, with pG2 expression being limited to the adrenal gland. A year later, Fay and colleagues discovered a novel protein in amniotic fluid, referred to as fetal antigen-1 (FA-1) [93]. In 1993, Laborda and colleagues reported on a novel protein (delta-like; dlk) belonging to the epidermal growth factor-like superfamily, which is expressed in tumors with neuroendocrine features such as neuroblastoma, pheochromocytoma, and certain small cell lung carcinoma cell lines [94]. The expression of the delta-like (dlk) protein in normal tissue was limited to the adrenal gland and placenta [94]. In the same year, Smas and Sul [73] identified Pref-1 as a novel regulator of adipocyte differentiation. The term “Preadipocyte Factor” was assigned based on the observation that the mRNA was highly expressed in the preadipocyte 3T3-L1 cell line, but not in mature adipocytes. Since then, sequence alignments have shown that pG2, FA1, dlk1 and Pref-1 are the product of the same gene, with varied post transcriptional modifications [95,96].
5.2. Pref-1 Structural Characteristics
Pref-1 is a paternally imprinted gene located in an imprinted region of mouse chromosome 12 [97] and on the long arm of chromosome 14 (14q32.2) in humans [71]. The Pref-1 gene encodes a 385 amino acid protein which exhibits homology to the Notch/Delta/Serrata family of EGF-like proteins [73,74,98,99]. Transmembrane Pref-1 contains an extracellular domain, a juxtamembrane region, a transmembrane domain and a cytoplasmic domain (Figure 5) [74,99,100]. Similar to canonical Notch-ligands, the extracellular domain of Pref-1 contains 6 EGF-repeats (Figure 5), but lacks the N-terminal Delta-Serrate-LAG-2 (DSL) domain found in classical canonical Notch ligands [99,100]. The N-terminal EGF repeats however possess Delta and OSM-11 (DOS) domains common to all Notch ligands [101]. The intracellular domain of Pref-1 is shorter than the classical Notch ligands [102,103]. The Pref-1 protein ranges in size between 50 and 60 kDa due to post translational modifications, such as N-linked glycosylation [99].
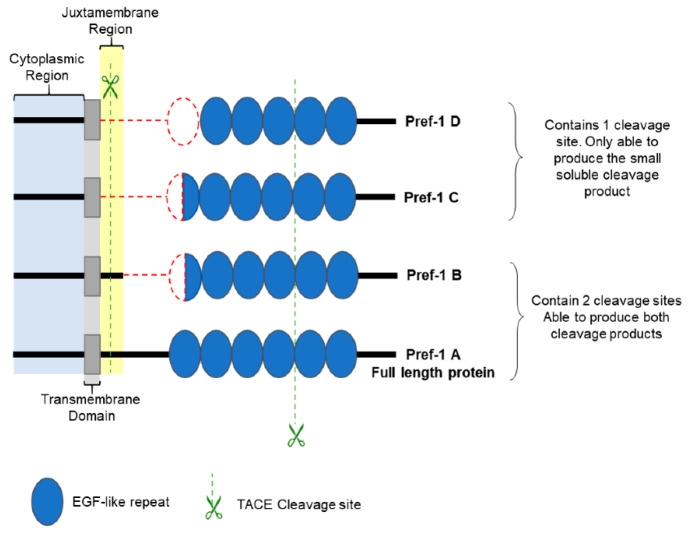
Figure 5.
Structure of the different Pref-1 isoforms. The Pref-1 protein can be found in four isoforms (Pref-1 A, B, C and D) due to alternative splicing. The red dotted line indicates the missing sections due to in-frame deletions. Green arrows indicate the cleavage sites for the generation of the soluble forms of the protein. Pref-1, Preadipocyte factor 1; EGF, Epidermal growth factor. Image created by C.d.S.
Pref-1 has two tumor necrosis factor alpha converting enzyme (TACE) cleavage sites within its extracellular domain, one in the juxtamembrane region and one near the fourth EGF-like repeat (Figure 5). Pref-1 cleavage is regulated by protein kinase C [100]. Cleavage at the juxtamembrane region results in a full-length soluble fraction. Cleavage of the full length soluble fraction at the second cleavage site near the fourth EGF-like repeat produces a large 50 kDa soluble protein fragment as well as a smaller soluble 25 kDa fragment [104,105]. Only the 50 kDa soluble form of the cleaved protein has been implicated as an inhibitor of adipogenesis [74,100,105,106]. The function of the small soluble unit is currently unknown.
Alternative splicing gives rise to four Pref-1 isoforms, Pref-1 A–D (Figure 5) [98]. The Pref-1A isoform is the full-length protein capable of producing both soluble products upon cleavage. Isoforms B to D contain in-frame deletions in the extracellular domain. Nevertheless, despite these deletions, Pref-1 B is capable of producing both soluble forms, as the deletion does not affect either cleavage site (Figure 5). Pref-1 C and D are only capable of producing the small soluble fragment, as the deletion removes the cleavage site within the juxtamembrane region (Figure 5) [74,99].
5.3. The Role of Pref-1 in Adipogenesis
The 50 kDa soluble Pref-1 fraction acts in an autocrine/paracrine fashion to regulate both BAT and WAT differentiation [100]. The effect of Pref-1 on adipocyte differentiation was predominantly studied in a murine 3T3-L1 preadipocyte cell line by either over-expressing Pref-1 [73,97,104,107,108,109], by exposure to soluble Pref-1 [106,108,110] or by knocking down Pref-1 gene expression using Pref-1 anti-sense sequences [72,73,107,108,111]. Over-expression of Pref-1 resulted in impaired adipogenic differentiation, while increased adipogenic differentiation was observed when the Pref-1 gene was knocked down. The effect of Pref-1 on adipogenic differentiation seems to be cell-type specific, since Nueda and colleagues (2007) found that over-expression of dlk1 in C3H10T1/2 cells, a murine mesenchymal cell line, potentiates adipogenic differentiation [109]. Similar experimental strategies were employed in vivo using animal models. The expression of adipogenic differentiation-associated genes increased and adipogenic differentiation was accelerated in Pref-1 null mice [71,97], while transgenic mice that over-expressed Pref-1 showed decreased expression of adipogenic differentiation markers, as well as adipose tissue mass [110,112,113].
The role of Pref-1 in BAT differentiation is less well studied. Armengol and colleagues (2012) reported high levels of Pref-1 expression in fetal BAT, which gradually declined after birth [114]. Although these investigators did not observe changes in fetal BAT development in Pref-1 null mice, they noticed that genes specific to brown fat thermogenesis were over-expressed [114]. Furthermore, high levels of Pref-1 expression were observed in C/EBPα-null mice, an experimental model of impaired fetal BAT differentiation, as well as in vitro in primary mouse preadipocytes [114]. Over-expression of Pref-1 in C/EBPα-null mice and primary mouse preadipocytes was accompanied by decreased expression of thermogenic markers such as UCP1 and PPARγ co-activator-α (PGC-1α) [114]. Armengol and colleagues (2012) concluded that Pref-1 could play an important role in controlling thermogenesis-associated gene expression [114]. Zhang and colleagues (2010) observed that treatment of insulin receptor substrate 1 (IRS-1)-deficient brown preadipocytes with bone morphogenetic protein-7 (BMP7) resulted in a significant decrease in Pref-1 expression, which resulted in initiation of adipogenic differentiation. IRS-1-deficient brown preadipocytes express high basal levels of Pref-1 [115]. In a recent study, Rhee and colleagues (2019) investigated the role of Pref-1 in browning of white adipose tissue [116]. Their findings support previous findings suggesting that Pref-1 plays a role in controlling thermogenesis, and they proposed that TACE-mediated cleavage of Pref-1 regulates adipose tissue browning [116].
Pref-1 Expression during hASC and hWJSC Adipogenic Differentiation
The role of Pref-1 in adipogenic differentiation has mainly been investigated using the murine 3T3-L1 preadipocyte cell line, which expresses high levels of endogenous Pref-1 [72,73]. Endogenous expression of Pref-1 in hASCs and hWJSCs has not been well studied. Zhang and colleagues (2011) observed Pref-1 (Dlk1) expression in 7 of 15 primary hWJSC cultures [117]. There was no correlation between Pref-1 expression and the ability of the cultures to undergo adipogenic differentiation. However, these investigators did find that the hWJSC cultures that displayed poor adipogenic differentiation did not express Pref-1 [117]. Karagianni and colleagues (2013) found that only a small sub-population of the cells present in primary hWJSC cultures expressed Pref-1 [118]. Furthermore, Pref-1 gene expression varied greatly between different primary hWJSC cultures. These investigators did not observe significant changes in Pref-1 expression over a 21-day adipogenic differentiation period, and concluded that the impaired adipogenic differentiation observed in vitro was unrelated to endogenous Pref-1 [118]. Interestingly, high levels of Pref-1 have been observed in umbilical cord blood plasma when compared to maternal plasma [118,119]. Lee and colleagues (2015) were unable to detect Pref-1 in hWJSCs [120], but observed that MSC-like cells isolated from umbilical cord blood expressed high levels of Pref-1 [120,121].
Mitterberger and colleagues (2012) found that the majority of hASCs express Pref-1 and that the expression of Pref-1 decreases during adipogenic differentiation [122]. These investigators also reported that knockdown of the pref-1 gene resulted in enhanced adipogenic differentiation [122]. To our knowledge this is the only study that has reported on expression of endogenous Pref-1 in hASCs. Zwierzina and colleagues (2015) indicated that approximately 30% of stromal vascular fraction (SVF) cells, isolated from subcutaneous adipose tissue, express cell surface Pref-1 [123]. All SVF cells stained positive for intracellular Pref-1. However, only the cells that were csPref1Neg/csCD34Pos (cs, cell surface) displayed strong proliferation and adipogenic differentiation [123].
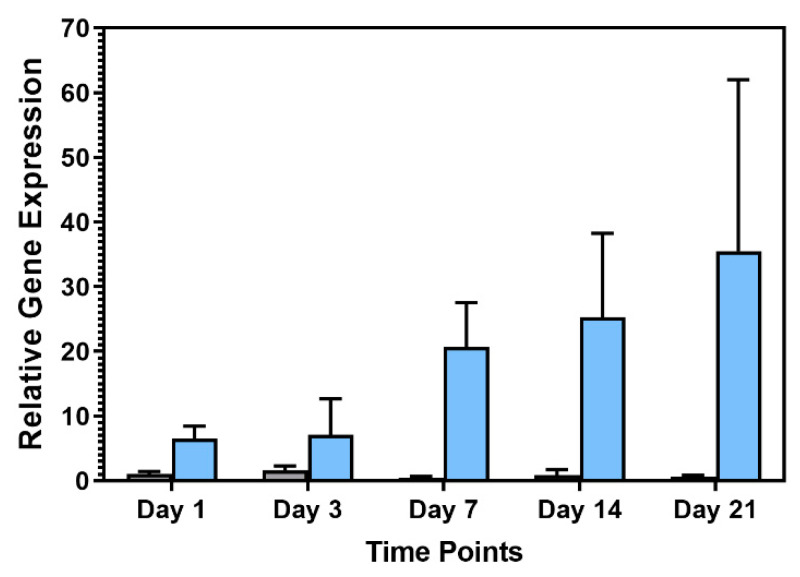
Given the differences observed in the adipogenic differentiation potential of hASCS and hWJSCs, and the importance of Pref-1 during adipogenesis, our laboratory investigated Pref-1 gene expression in primary hASC and hWJSC cultures by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) over a 21-day period. Similar to findings reported by Karagianni and colleagues (2013) [118], we found higher levels of Pref-1 gene expression in hWJSCs relative to hASCs (Figure 6). Karagianni and colleagues (2013) investigated Pref-1 gene expression at various time points during adipogenic differentiation of umbilical cord (UC)-derived MSCs, BM-MSCs and ASCs [118]. These authors observed no differences in Pref-1 gene expression levels during adipogenic differentiation of UC-MSCs and BM-MSCs [118]. Morganstein and colleagues (2010) monitored relative Pref-1 mRNA levels at several time points during adipogenic differentiation of MSCs isolated from fetal blood and fetal bone marrow [124]. Both MSC types (fetal blood and fetal BM) displayed an increase in Pref-1 gene expression during adipogenic differentiation (compared to the baseline Pref-1 gene expression levels observed for the respective undifferentiated cells) [124]. Up-regulation of Pref-1 gene expression was noticeably lower in the fetal blood-derived MSCs when compared to levels observed during adipogenic differentiation of fetal BM-derived MSCs. A gradual increase in Pref-1 expression was observed until day 14 of adipogenic differentiation for both the fetal blood-derived MSCs and the fetal BM-derived MSCs, after which Pref-1 gene expression levels decreased [124]. The authors of this review are not aware of any other studies that have investigated the role of Pref-1 during of adipogenic differentiation of MSCs isolated from adipose tissue, UC, blood and BM.
Figure 6.
Relative levels of Pref-1 mRNA expression in hASCs (grey bars) and hWJSCs (blue bars). Data is shown as Pref-1 expression relative to not induced cells at each time point (n = 6 independent isolations for all time points with exception to day 3 where n = 3). Gene expression levels were normalized to the following reference genes: PPIA, TBP, YWHAZ (ΔCt), after which they were normalized to control (not induced) cells (ΔΔCt). Error bars represent the standard error of the mean (SEM). Unpublished, original data.
6. Pref-1 Mechanism of Action
It is well established that sustained over-expression of Pref-1 prevents adipogenic differentiation. Therefore, Pref-1, needs to be down-regulated for adipogenic differentiation to occur [74,99,100]. However, the exact mechanism by which Pref-1 inhibits adipogenesis has yet to be elucidated. Two pathways have been implicated in Pref-1’s mechanism of action, the mitogen activated protein kinases (MAPK) signaling pathway and the Notch signaling pathway.
6.1. Pref-1 and the MAPK Kinase (MEK)/ERK Signaling Pathway
Mitogen-activated protein kinases (MAPKs) play an important role in cell differentiation, proliferation and death by transmitting extracellular signals received by cell surface receptors to transcription factors within the nucleus in order to regulate transcription [125,126]. MAPKs are serine/threonine kinases which can be grouped into three main sub-families: extracellular signal-regulated kinases (ERKs), Jun amino-terminal kinases (JNKs) and stress-activated kinases (p38/SAPKs). The activity of these kinases is regulated by phosphorylation cascades [125,126,127].
Adipocyte differentiation involves cellular growth, proliferation and differentiation, all of which are regulated by MAPKs. It is thus not surprising that MAPK-dependent pathways, especially pathways involving ERK1/2, are involved in adipogenesis. Binding of insulin or IGF-1 to cell surface receptors such as the insulin growth factor receptor, activates the ERK1/2 pathway and promotes adipogenic differentiation [128,129,130]. Insulin-induced adipogenic differentiation is reported to be concentration dependent [131]. Although the exact role of MAPKs in adipogenesis is not fully understood, it is hypothesized that initiation of adipogenic differentiation (mitotic clonal expansion) requires the rapid initial activation of the ERK1/2 pathway [125]. This hypothesis is supported by several studies that have confirmed that initiation of adipogenic differentiation requires early activation of the ERK1/2 pathway [125,132,133,134]. Tang and colleagues (2003) [65] and Kim and colleagues (2007) [129] showed that activation (phosphorylation) of MAPKs occurs within hours of induction of adipogenic differentiation, after which MAPK activity gradually returned to basal levels. Sustained ERK1/2 activation mediates phosphorylation of the master regulator PPARγ, which consequently results in decreased transcriptional activity and adipogenic differentiation [65,128,135]. Down-regulation of ERK1/2 activation is thus needed for adipogenic differentiation to proceed [65,136]. The presence of ERK inhibitors such as PD98059 or UO126 reverses the negative impact of ERK phosphorylation (activation) on adipogenic differentiation [65,136,137].
Using Pref-1 null mouse embryo fibroblasts (MEFs), Kim and colleagues (2007) showed that Pref-1 directly activates the MAPK/ERK (MEK)1/2 and ERK1/2 pathways [129]. An initial, rapid activation (phosphorylation) of ERK1/2, was observed during differentiation of wildtype MEFs into adipocytes. This was followed by a second increase, although lower in intensity, in ERK phosphorylation. The second wave of ERK activation corresponded to Pref-1 expression levels in the wildtype MEFs, but (the second wave) was absent in Pref-1 null MEFs. Enhanced adipocyte differentiation was observed for Pref-1 null MEFs compared to wildtype MEFs. The introduction (supplementation) of soluble Pref-1 during adipogenic differentiation of Pref-1 null MEFs resulted in the restoration of the second wave of ERK activation, which resulted in decreased C/EBPα and PPARγ2 expression and consequently decreased adipogenic differentiation [129]. Nueda and colleagues (2007) observed a similar pattern of ERK activation (initial high level activation followed by a second wave of lower ERK activation) during adipogenic differentiation in C3H10T1/2 cells transfected to express high levels of Pref-1 [109]. The exact manner in which Pref-1 mediates ERK1/2 phosphorylation has, however, not been fully elucidated.
Wang and colleagues (2010) reported that Sox9 is not only involved in chondrocyte and osteogenic differentiation, but also potentially plays a role in adipogenic differentiation [97]. Sox9 is activated by the MEK/ERK pathway [99] and Wang and colleagues (2010) observed that Pref-1-mediated ERK phosphorylation precedes the induction of Sox9 [97]. Activation of Sox9 prevents up-regulation of C/EBPβ and C/EBPδ, which in turn prevents the activation of C/EBPα and PPARγ [97], and ultimately suppresses adipogenic differentiation [99,129,138].
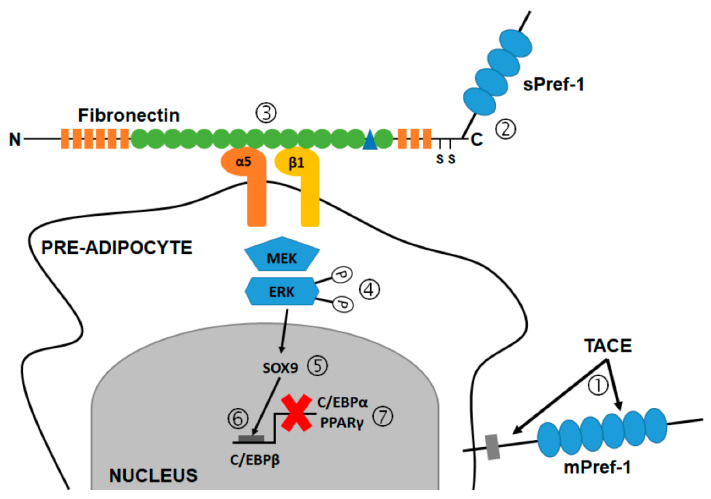
Fibronectin is a ubiquitous and essential component of the ECM, and plays a role in regulating cellular processes such as cell adhesion, migration, proliferation and differentiation [97,139,140]. Fibronectin is a ligand for many integrin receptor proteins, including the classical fibronectin receptor α5β1 [140]. Wang and colleagues (2010) showed that Pref-1 interacts with the C-terminal region of fibronectin and suggested that the interaction of Pref-1 with fibronectin plays an important role in Pref-1′s inhibition of adipogenesis [97]. Increased adipogenic differentiation was observed when interaction of fibronectin with the α5β1 integrin was disrupted either through treatment with RGD peptides, which compete for binding to α5β1 integrin, or through knock-down of the α5 integrin subunit, or fibronectin, using small interfering RNA (siRNA) transfection [97]. Exposing the cells in which either the α5 integrin subunit or fibronectin was knocked-down, to soluble Pref-1, did not result in a significant decrease in adipogenic differentiation. Wang and colleagues (2010) concluded that the 50 kDA soluble Pref-1 fraction binds to the C-terminal of fibronectin, and thereby facilitates the interaction of Pref-1 with preadipocytes resulting in the activation of the MEK/ERK pathway, which in turn activates Sox9, and thereby inhibits adipogenesis [97,100]. The current suggested mechanism of Pref-1 action is summarized in Figure 7.
Figure 7.
Schematic illustration of the suggested mechanism of Pref-1-mediated inhibition of adipogenesis involving the MEK/ERK pathway. (1) TACE-mediated cleavage of membrane bound Pref-1. (2) 50 kDA soluble Pref-1 binds to the C-terminus of fibronectin. (3) Fibronectin binds to the α5β1 integrin receptor and the fibronectin/sPref-1 complex (4) activates the MEK/ERK pathway, which in turn (5) activates SOX9. (6) SOX9 binds to the C/EBPβ promoter, preventing up-regulation of C/EBPβ and (7) consequently up-regulation of C/EBPα and PPARγ. Figure adapted by C.D. from Hudak and Sul (2013) [100]. C/EBPα, CAAT-enhancer binding protein alpha; C/EBPβ, CAAT-enhancer binding protein beta; ERK, Extracellular signal-regulated kinases; MEK, Mitogen-activated protein kinase kinase; mPref-1, membrane-bound preadipocyte factor-1; sPref-1, soluble preadipocyte factor-1; PPARγ, Peroxisome proliferator-activated receptor gamma; TACE, Tumor necrosis factor alpha converting enzyme.
There is however currently no consensus regarding the above-mentioned mechanism, for although binding of fibronectin to the α5β1 integrin appears to be important for adipogenesis to occur, how this is impacted by the binding of Pref-1 to fibronectin remains unclear. Zhang and colleagues (2003) suggested that Pref-1 expression impairs ERK1/2 phosphorylation through the down-regulation of IGF-1 [131]. Ruiz-Hidalgo and colleagues (2002) did not observe an increase in ERK1/2 phosphorylation upon induction of Pref-1 expression [141]. Nueda and colleagues (2007) showed that Pref-1 can have both pro- and anti-adipogenic effects, depending on the cell line used [109]: C3H10T1/2 cells displayed poor adipogenic differentiation compared to 3T3-L1 cells. However, the adipogenic capacity of C3H10T1/2 cells increased when Pref-1 was up-regulated through transfection with Pref-1 cDNA constructs encoding the extracellular portion of the protein, while down-regulation of Pref-1 had no effect on these cells. When the same experiments were repeated using 3T3-L1 cells, Pref-1 over-expression inhibited adipogenesis and down-regulation enhanced adipogenesis [109].
6.2. Pref-1 and the Notch Signaling Pathway
The Notch signaling pathway present in most animals is highly conserved and is involved in regulating cell fate, proliferation, differentiation and death [142]. Notch refers to a cell-surface receptor that interacts with Notch ligands expressed by surrounding cells [142]. There are five canonical Notch ligands: three Delta-like ligands (DLL1, DLL3, DLL4) and two Jagged ligands (Jagged 1 and 2). The extracellular domain of canonical Notch ligands contains a series of EGF-repeats, a Delta-Serrate-LAG-2 (DSL) domain and an N-terminal domain [143]. The active intracellular domain of the Notch receptor is cleaved by a γ-secretase during Notch ligand/receptor interactions, and relocates to the nucleus where it affects the expression of several transcription factors, such as Hes1 and Hey-1 [102,142,144,145].
Initial studies investigated the effect of Pref-1 on Notch signaling in Drosophilia melanogaster [103] and Candida elegans [146]. Bray and colleagues (2008) reported that membrane-bound Pref-1 was able to antagonize Notch signaling, while soluble Pref-1 had no effect [103]. These findings were contradicted by Komatsu and colleagues (2008), who observed activation of Notch signaling by soluble Pref-1 in Candida elegans [146].
Several studies suggest that Notch signaling is involved in the regulation of proliferation and adipogenic differentiation of both white and brown adipocyte progenitor cells [147]. However, the reported findings are often at odds with one another, with some suggesting that Notch signaling does not play an essential role [97,148], potentiates [145,149,150,151,152,153,154] or inhibits adipogenic differentiation [153,155,156,157,158,159,160]. Studies that have reported on the effects of Notch signaling on Pref-1 expression and adipogenic differentiation are summarized in Table 2. It is clear from the differences observed that carefully designed studies, preferably conducted in vivo, are needed to clarify the role of Notch signaling in this context.
Table 2.
Summary of the impact of Notch signaling on Pref-1 expression and adipogenic differentiation.
| Cell Type | Experimental Approach | Impact on | References | ||
|---|---|---|---|---|---|
| Notch signaling | Adipogenic Differentiation | Pref-1 Expression | |||
| 3T3-L1 | Exposure to soluble Jagged1 | Up-regulated | Decreased | Not determined | [152] |
| hes1 over-expression | Up-regulated | Decreased | Not determined | ||
| hes1 knock-down | Down-regulated | Decreased | Up-regulated | ||
| 3T3-L1 | Notch4 over-expression | Up-regulated | Increased | Down-regulated | [149] |
| 3T3-L1 | Adipogenic differentiation. No specific treatment. | Decreases over time | Increased (compared to C3H10T1/2) | Not determined | [109] |
| dlk1 knock-down | Up-regulated | Not assessed | Down-regulated | ||
| C3H10T1/2 | Adipogenic differentiation. No specific treatment. | Remained unchanged | Decreased (compared to 3T3-L1 cells) | Up-regulated | |
| dlk1 over-expression | Down-regulated | Increased | Up-regulated | ||
| dlk1 knock-down | Up-regulated | Increased | Down-regulated | ||
| Notch1 knock-down | Down-regulated | Decreased | Down-regulated | ||
| dlk1 over-expression in Notch1 knock-down cells | Down-regulated | Decreased | Up-regulated | ||
| 3T3-L1 | Transfection with miRNA (miR-139–5p mimic) | Initially increased. Gradually decreased. | Decreased | Up-regulated | [154] |
| Co-transfection with pcDNA3.1_NICD (over-expressing Notch) | Up-regulated | Increased | Not determined | ||
| Mouse BM-derived MSCs | Inhibition of γ-secretase | Decreased | Increased | Decreased | [162] |
| Human BM-derived MSCs | Adipogenic differentiation. No specific treatment. | Decreased | Increased | Decreased | [158] |
| Inhibition of γ-secretase | Decreased | Increased | Not measured | ||
siRNA, small interfering RNA; miRNA, micro-RNA; BM, bone marrow
Ross and colleagues (2004) indicated that Notch signaling can either potentiate or inhibit 3T3-L1 preadipocyte differentiation [152]. Jagged1-mediated activation of the Notch signaling pathway, as well as over-expression of hes1, resulted in decreased C/EBPα and PPARγ expression, and consequently decreased adipogenic differentiation [152]. However, the investigators reported in the same study that knock-down of hes1 resulted in up-regulation of Pref-1, resulting in inhibition of adipogenesis [152]. The investigators attributed their contradictory findings to Hes-1 having a dual role by promoting adipogenic differentiation through Hes-1-mediated down-regulation of inhibitory proteins such as Pref-1, followed by inhibition of adipogenic differentiation by preventing up-regulation of C/EBPα and PPARγ, downstream of Pref-1 (target unknown) [152].
Baladron and colleagues (2004) found that addition of soluble Pref-1 fragments, and over-expression of Pref-1 in 3T3-L1 preadipocytes, resulted in decreased Hes-1 expression and consequently decreased endogenous Notch activity [161]. Furthermore, these investigators supported their findings by showing that knock-down of Pref-1 resulted in increased Hes-1 expression, suggesting activation of Notch signaling [161]. The investigators only investigated the interaction between Hes-1 and Pref-1 and did not report on any end-stage adipogenesis markers.
Lai and colleagues (2013) reported that the over-expression of Notch 4 resulted in increased proliferation of 3T3-L1 preadipocytes. Furthermore, over-expression of Notch 4 enhanced the adipogenic differentiation of 3T3-L1 preadipocytes by down-regulating Pref-1 and up-regulating C/EBPβ and PPARγ [149].
Nueda and colleagues (2007) investigated the effect of Notch signaling in two different cell lines, 3T3-L1 preadipocytes and mesenchymal C3H10T1/2 cells, which differ with respect to their endogenous Notch1 levels [109]. Garces and colleagues (1997) showed that down-regulation of Notch1, using a Notch1 antisense cDNA construct, prevented adipogenic differentiation in 3T3-L1 cells [151]. Use of the same strategy to knock-down Notch1 in C3H10T1/2 cells, resulted in decreased adipogenic differentiation [109]. Expression of Pref-1 was unable to overcome the negative effect of decreased Notch signaling on adipogenic differentiation, as it was unable to restore adipogenic differentiation of the Notch1-knock-down cells. The investigators concluded that a certain level of Notch1 expression is necessary for cells to undergo adipogenic differentiation [109].
Using primary mouse ASCs (mASCs), Huang and colleagues (2010) showed that inhibition of Notch signaling by inhibition of γ-secretase activity resulted in the down-regulation of Pref-1 and up-regulation of PPARγ, consequently enhancing adipogenic differentiation [162]. Mi and colleagues (2015) showed that the microRNA (miRNA), miR-139-5p, inhibits adipogenic differentiation of 3T3-L1 preadipocytes by directly targeting the untranslated regions (UTRs) of Notch1, resulting in down-regulation of Hes-1 and Hey-1, and up-regulation of Pref-1 [154].
Song and colleagues (2015) reported down-regulation of Notch gene expression during adipogenic differentiation of human BM-MSCs (hBM-MSCs) [158]. Inhibition of Notch signaling resulted in enhanced adipogenic differentiation in hBM-MSCs [155,158]. Interestingly, Vujovic and colleagues (2007) only observed an effect when adipogenic differentiation was induced using only DXM. No effect was observed when differentiation was induced with the complete adipogenic induction cocktail [155].
7. Conclusions
Our current knowledge of adipogenesis stems mainly from studies using either the murine 3T3-L1 preadipocytic cell line or murine experimental animal models. It is clear from these studies that Pref-1 plays an important regulatory role during murine adipocyte differentiation. However, the role of Pref-1 during adipogenic differentiation in humans is not well established. Furthermore, it is unclear if the results from murine studies can be directly translated to the human setting, and more studies using cells of human origin are needed. In this regard, the observed differences in the ability of human MSCs of different origins to undergo adipogenesis in vitro, provides an excellent model to study human adipocyte differentiation.
Acknowledgments
We want to acknowledge and thank the clinical staff (surgeons and nursing staff) at a private hospital in Pretoria, South Africa, for their assistance in collecting lipoaspirates and umbilical cord tissue from which we isolated the MSCs.
Abbreviations
| AF | Amniotic fluid |
| AP-1 | Activating protein-1 |
| aP2 | Adipocyte protein 2 |
| ASCs | Adipose-derived stromal/stem cells |
| AT | Adipose tissue |
| BAT | Brown adipose tissue |
| BM | Bone marrow |
| BM-MSCs | Bone marrow-derived MSCs |
| BMP7 | Bone morphogenetic protein-7 |
| C/EBP | CAAT-enhancer binding proteins |
| cAMP | Cyclic Adenosine Monophosphate |
| CHOP | C/EBP homologous protein |
| cs | Cell surface |
| DLK | Delta-like |
| DLL | Delta-like ligands |
| DOS | Delta and OSM-11 |
| DP | Dental pulp |
| DSL | Delta-Serrate-LAG-2 |
| DXM | Dexamethasone |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ERKs | Extracellular signal-regulated kinases |
| FA-1 | fetal antigen-1 |
| FABP4 | Fatty acid-binding protein 4 |
| FBS | Fetal bovine serum |
| Foxp1 | Foxhead P1 |
| hASCs | Human-derived ASCs |
| hBM-MSCs | Human-derived BM-MSCs |
| hWJSCs | Human-derived WJSCs |
| IBMX | 3-Isobutyl-1-methylxantine |
| IGF-1 | Insulin-like growth factor 1 |
| IRS-1 | Insulin receptor substrate 1 |
| JNKs | Jun amino-terminal kinases |
| kDa | kilo Dalton |
| KLFs | Krüppel-like factors |
| LDL | Low-density lipoprotein |
| MAPK | Mitogen-activated protein kinase |
| mASCs | Mouse ASCs |
| MEFs | Mouse embryo fibroblasts |
| MEK | Mitogen-activated protein kinase kinase |
| mPref-1 | Membrane bound Pref-1 |
| mRNA | Messenger ribonucleic acid |
| MSCs | Mesenchymal stromal/stem cells |
| Myf-5 | Myogenic factor 5 |
| nFS | Neonatal foreskin |
| PC | Placenta |
| PECK | Phosphoenolpyruvate carboxylase |
| PGC-1α | PPARγ co-activator-alpha |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| PRDM16 | PR domain containing 16 |
| Pref-1 | Preadipocyte factor-1 |
| PS | Periosteum |
| PV | Perivascular region |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| SAPKs | Stress-activated kinases |
| SF | Synovium fluid |
| SHED | Exfoliated deciduous teeth |
| SMAD | Mothers against decapentaplegic homolog |
| SOX | Sex determining region Y-box |
| SV | Synovium |
| sPref-1 | Soluble Pref-1 |
| SREBP-1 | Sterol regulatory element binding protein 1 |
| STAT | Signal transducer and activator of transcription |
| SVF | Stromal vascular fraction |
| TACE | Tumor necrosis factor alpha converting enzyme |
| UC | Umbilical cord |
| UCB | Umbilical cord blood |
| UCP1 | Uncoupling protein 1 |
| UTR | Untranslated region |
| WAT | White adipose tissue |
| WJSCs | Wharton’s jelly derived stromal/stem cells |
| Wnt | Wingless/integrated protein |
| ZEB1 | Zinc finger E-box binding homeobox 1 |
Author Contributions
Conceptualization, M.S.P. and C.D.; Methodology, C.d.S., K.K. and C.D.; Writing—original draft preparation, C.d.S. and C.D.; Writing—review and editing, K.K., M.A.A., M.S.P.; Visualization (images), C.d.S., M.A.A. and C.D.; Supervision, C.D., M.A.A. and M.S.P.; Funding acquisition, M.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the South African Medical Research Council in terms of the SAMRC’s Flagship Award Project [SAMRC-RFAUFSP-01-2013/STEM CELLS], the SAMRC Extramural Unit for Stem Cell Research and Therapy, and the Institute for Cellular and Molecular Medicine of the University of Pretoria.
Conflicts of Interest
The authors have no conflicts of interest declare.
References
- 1.Chooi Y.C., Ding C., Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi: 10.1016/j.metabol.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Haider N., Larose L. Harnessing adipogenesis to prevent obesity. Adipocyte. 2019;8:98–104. doi: 10.1080/21623945.2019.1583037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Sowers J.R., Ren J. Targeting autophagy in obesity: From pathophysiology to management. Nat. Rev. Endocrinol. 2018;14:356–376. doi: 10.1038/s41574-018-0009-1. [DOI] [PubMed] [Google Scholar]
- 4.Gadde K.M., Martin C.K., Berthoud H.-R., Heymsfield S.B. Obesity: Pathophysiology and management. J. Am. Coll. Cardiol. 2018;71:69–84. doi: 10.1016/j.jacc.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotchen T.A. Obesity-related hypertension: Epidemiology, pathophysiology, and clinical management. Am. J. Hypertens. 2010;23:1170–1178. doi: 10.1038/ajh.2010.172. [DOI] [PubMed] [Google Scholar]
- 6.Baleta A., Mitchell F. Country in focus: Diabetes and obesity in South Africa. Lancet Diabetes Endocrinol. 2014;2:687–688. doi: 10.1016/S2213-8587(14)70091-9. [DOI] [PubMed] [Google Scholar]
- 7.Tremmel M., Gerdtham U.-G., Nilsson P., Saha S. Economic burden of obesity: A systematic literature review. Int. J. Environ. Res. Public Health. 2017;14:435. doi: 10.3390/ijerph14040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rastegar F., Shenaq D., Huang J., Zhang W., Zhang B.-Q., He B.-C., Chen L., Zuo G.-W., Luo Q., Shi Q., et al. Mesenchymal stem cells: Molecular characteristics and clinical applications searched and summarized relevant literature. World J. Stem Cells. 2010;2:67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roobrouck V.D., Clavel C., Jacobs S.A., Ulloa-Montoya F., Crippa S., Sohni A., Roberts S.J., Luyten F.P., Van Gool S.W., Sampaolesi M., et al. Differentiation potential of human postnatal mesenchymal stem cells, mesoangioblasts, and multipotent adult progenitor cells reflected in their transcriptome and partially influenced by the culture conditions. Stem Cells. 2011;29:871–882. doi: 10.1002/stem.633. [DOI] [PubMed] [Google Scholar]
- 10.Kuri-Harcuch W., Velez-delValle C., Vazquez-Sandoval A., Hernández-Mosqueira C., Fernandez-Sanchez V. A cellular perspective of adipogenesis transcriptional regulation. J. Cell. Physiol. 2019;234:1111–1129. doi: 10.1002/jcp.27060. [DOI] [PubMed] [Google Scholar]
- 11.Cristancho A.G., Lazar M.A. Forming functional fat: A growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarjeant K., Stephens J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012;4:a008417. doi: 10.1101/cshperspect.a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park A. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells. 2014;6:33. doi: 10.4252/wjsc.v6.i1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheja L., Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019;15:507–524. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 15.Ali A.T., Hochfeld W.E., Myburgh R., Pepper M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013;92:229–236. doi: 10.1016/j.ejcb.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K., Maretich P., Kajimura S. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab. 2018;29:191–200. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G., Meyer J.G., Cai W., Softic S., Li M.E., Verdin E., Newgard C., Schilling B., Kahn C.R. Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol. Cell. 2019;74:844–857.e7. doi: 10.1016/j.molcel.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fedorenko A., Lishko P.V., Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenwald M., Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte. 2014;3:4–9. doi: 10.4161/adip.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinoda K., Luijten I.H.N., Hasegawa Y., Hong H., Sonne S.B., Kim M., Xue R., Chondronikola M., Cypess A.M., Tseng Y.-H., et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 2015;21:389–394. doi: 10.1038/nm.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz T.J., Huang T.L., Tran T.T., Zhang H., Townsend K.L., Shadrach J.L., Cerletti M., McDougall L.E., Giorgadze N., Tchkonia T., et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cedikova M., Kripnerová M., Dvorakova J., Pitule P., Grundmanova M., Babuska V., Mullerova D., Kuncova J. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016;2016:1–11. doi: 10.1155/2016/6067349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harms M., Seale P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q.A., Tao C., Gupta R.K., Scherer P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquez-Curtis L.A., Janowska-Wieczorek A., McGann L.E., Elliott J.A.W. Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology. 2015;71:181–197. doi: 10.1016/j.cryobiol.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Friedenstein A.J., Piatetzky-Shapiro I.I., Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- 28.Zolocinska A. The expression of marker genes during the differentiation of mesenchymal stromal cells. Adv. Clin. Exp. Med. 2018;27:717–723. doi: 10.17219/acem/68386. [DOI] [PubMed] [Google Scholar]
- 29.Kobolak J., Dinnyes A., Memic A., Khademhosseini A., Mobasheri A. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods. 2016;99:62–68. doi: 10.1016/j.ymeth.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Xu L., Liu Y., Sun Y., Wang B., Xiong Y., Lin W., Wei Q., Wang H., He W., Wang B., et al. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017;8:275. doi: 10.1186/s13287-017-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu W., Zhuang Y., Gan Y., Wang X., Tang T., Dai K. Comparison and characterization of enriched mesenchymal stem cells obtained by the repeated filtration of autologous bone marrow through porous biomaterials. J. Transl. Med. 2019;17:377. doi: 10.1186/s12967-019-02131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Charif N., Mainard D., Bensoussan D., Stoltz J.-F., de Isla N. Donor’s age dependent proliferation decrease of human bone marrow mesenchymal stem cells is linked to diminished clonogenicity. Biomed. Mater. Eng. 2014;24:47–52. doi: 10.3233/BME-140973. [DOI] [PubMed] [Google Scholar]
- 33.Orbay H., Tobita M., Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012;2012:1–9. doi: 10.1155/2012/461718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagamura-Inoue T. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells. 2014;6:195. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baer P.C. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J. Stem Cells. 2014;6:256. doi: 10.4252/wjsc.v6.i3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoogduijn M.J., Dor F.J.M.F. Mesenchymal stem cells: Are we ready for clinical application in transplantation and tissue regeneration? Front. Immunol. 2013;4:144. doi: 10.3389/fimmu.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hassan G., Kasem I., Soukkarieh C., Aljamali M. A simple method to isolate and expand human umbilical cord derived mesenchymal stem cells: Using explant method and umbilical cord blood serum. Int. J. Stem Cells. 2017;10:184–192. doi: 10.15283/ijsc17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies J.E., Walker J.T., Keating A. Concise review: Wharton’s jelly: The rich, but enigmatic, source of mesenchymal stromal cells. Stem Cells Transl. Med. 2017;6:1620–1630. doi: 10.1002/sctm.16-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vangsness C.T., Sternberg H., Harris L. Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical application: A literature review of different harvest sites. Arthrosc. J. Arthrosc. Relat. Surg. 2015;31:1836–1843. doi: 10.1016/j.arthro.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Beeravolu N., McKee C., Alamri A., Mikhael S., Brown C., Perez-Cruet M., Chaudhry G.R. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J. Vis. Exp. 2017;(122):55224. doi: 10.3791/55224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bharti D., Shivakumar S.B., Park J.-K., Ullah I., Subbarao R.B., Park J.-S., Lee S.-L., Park B.-W., Rho G.-J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018;372:51–65. doi: 10.1007/s00441-017-2699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramanian A., Fong C.-Y., Biswas A., Bongso A. Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton’s jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS ONE. 2015;10:e0127992. doi: 10.1371/journal.pone.0127992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 44.Kwon A., Kim Y., Kim M., Kim J., Choi H., Jekarl D.W., Lee S., Kim J.M., Shin J.-C., Park I.Y. Tissue-specific differentiation potency of mesenchymal stromal cells from perinatal tissues. Sci. Rep. 2016;6:23544. doi: 10.1038/srep23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ragni E., Viganò M., Parazzi V., Montemurro T., Montelatici E., Lavazza C., Budelli S., Vecchini A., Rebulla P., Giordano R., et al. Adipogenic potential in human mesenchymal stem cells strictly depends on adult or foetal tissue harvest. Int. J. Biochem. Cell Biol. 2013;45:2456–2466. doi: 10.1016/j.biocel.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 46.Amable P.R., Teixeira M.V.T., Carias R.B.V., Granjeiro J.M., Borojevic R. Gene expression and protein secretion during human mesenchymal cell differentiation into adipogenic cells. BMC Cell Biol. 2014;15:46. doi: 10.1186/s12860-014-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu L., Hu J., Zhao J., Liu J., Ouyang W., Yang C., Gong N., Du L., Khanal A., Chen L. Side-by-side comparison of the biological characteristics of human umbilical cord and adipose tissue-derived mesenchymal stem cells. Biomed Res. Int. 2013;2013:1–12. doi: 10.1155/2013/438243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Kim J.-H., Jo C.H., Kim H.-R., Hwang Y. Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical Cord, and adipose tissue. Stem Cells Int. 2018;2018:1–12. doi: 10.1155/2018/8429042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brohem C.A., de Carvalho C.M., Radoski C.L., Santi F.C., Baptista M.C., Swinka B.B., de A Urban C., de Araujo L.R.R., Graf R.M., Feferman I.H.S., et al. Comparison between fibroblasts and mesenchymal stem cells derived from dermal and adipose tissue. Int. J. Cosmet. Sci. 2013;35:448–457. doi: 10.1111/ics.12064. [DOI] [PubMed] [Google Scholar]
- 50.Li C., Wu X., Tong J., Yang X., Zhao J., Zheng Q., Zhao G., Ma Z. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res. Ther. 2015;6:55. doi: 10.1186/s13287-015-0066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu T.M., Martina M., Hutmacher D.W., Hui J.H.P., Lee E.H., Lim B. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2006;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 52.Manini I., Gulino L., Gava B., Pierantozzi E., Curina C., Rossi D., Brafa A., D’Aniello C., Sorrentino V. Multi-potent progenitors in freshly isolated and cultured human mesenchymal stem cells: A comparison between adipose and dermal tissue. Cell Tissue Res. 2011;344:85–95. doi: 10.1007/s00441-011-1139-0. [DOI] [PubMed] [Google Scholar]
- 53.Mauney J.R., Nguyen T., Gillen K., Kirker-Head C., Gimble J.M., Kaplan D.L. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28:5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mohamed-Ahmed S., Fristad I., Lie S.A., Suliman S., Mustafa K., Vindenes H., Idris S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018;9:168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noël D., Caton D., Roche S., Bony C., Lehmann S., Casteilla L., Jorgensen C., Cousin B. Cell specific differences between human adipose-derived and mesenchymal–stromal cells despite similar differentiation potentials. Exp. Cell Res. 2008;314:1575–1584. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 56.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 57.Baksh D., Yao R., Tuan R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 58.Barlow S., Brooke G., Chatterjee K., Price G., Pelekanos R., Rossetti T., Doody M., Venter D., Pain S., Gilshenan K., et al. Comparison of human placenta- and bone marrow–derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1108. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 59.Batsali A.K., Pontikoglou C., Koutroulakis D., Pavlaki K.I., Damianaki A., Mavroudi I., Alpantaki K., Kouvidi E., Kontakis G., Papadaki H.A. Differential expression of cell cycle and WNT pathway-related genes accounts for differences in the growth and differentiation potential of Wharton’s jelly and bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2017;8:102. doi: 10.1186/s13287-017-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isobe Y., Koyama N., Nakao K., Osawa K., Ikeno M., Yamanaka S., Okubo Y., Fujimura K., Bessho K. Comparison of human mesenchymal stem cells derived from bone marrow, synovial fluid, adult dental pulp, and exfoliated deciduous tooth pulp. Int. J. Oral Maxillofac. Surg. 2016;45:124–131. doi: 10.1016/j.ijom.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 61.Zhu X., Shi W., Tai W., Liu F. The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived mesenchymal stem cells. Cell Tissue Res. 2012;350:277–287. doi: 10.1007/s00441-012-1453-1. [DOI] [PubMed] [Google Scholar]
- 62.Durandt C., van Vollenstee F.A., Dessels C., Kallmeyer K., de Villiers D., Murdoch C., Potgieter M., Pepper M.S. Novel flow cytometric approach for the detection of adipocyte subpopulations during adipogenesis. J. Lipid Res. 2016;57:729–742. doi: 10.1194/jlr.D065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pierdomenico L. Diabetes mellitus during pregnancy interferes with the biological characteristics of Wharton’s jelly mesenchymal stem cells. Open Tissue Eng. Regen. Med. J. 2011;4:103–111. doi: 10.2174/1875043501104010103. [DOI] [Google Scholar]
- 64.Gregoire F.M., Smas C.M., Sul H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 65.Tang Q.-Q., Otto T.C., Lane M.D. Mitotic clonal expansion: A synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ambele M.A., Dessels C., Durandt C., Pepper M.S. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 2016;16:725–734. doi: 10.1016/j.scr.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 67.de Sá P.M., Richard A.J., Hang H., Stephens J.M. Comprehensive Physiology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2017. Transcriptional regulation of adipogenesis; pp. 635–674. [DOI] [PubMed] [Google Scholar]
- 68.Lee J.-E., Schmidt H., Lai B., Ge K. Transcriptional and epigenomic regulation of adipogenesis. Mol. Cell. Biol. 2019;39:e00601-18. doi: 10.1128/MCB.00601-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shapira S.N., Seale P. Transcriptional control of brown and beige fat development and function. Obesity. 2019;27:13–21. doi: 10.1002/oby.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang E., Kim C. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules. 2019;24:1157. doi: 10.3390/molecules24061157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moon Y.S., Smas C.M., Lee K., Villena J.A., Kim K.-H., Yun E.J., Sul H.S. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol. Cell. Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smas C.M., Chen L., Zhao L., Latasa M.-J., Sul H.S. Transcriptional repression of Pref-1 by glucocorticoids promotes 3T3-L1 adipocyte differentiation. J. Biol. Chem. 1999;274:12632–12641. doi: 10.1074/jbc.274.18.12632. [DOI] [PubMed] [Google Scholar]
- 73.Smas C.M., Sul H.S. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-L. [DOI] [PubMed] [Google Scholar]
- 74.Wang Y., Kim K.-A., Kim J.-H., Sul H.S. Pref-1, a preadipocyte secreted factor that inhibits adipogenesis. J. Nutr. 2006;136:2953–2956. doi: 10.1093/jn/136.12.2953. [DOI] [PubMed] [Google Scholar]
- 75.Moseti D., Regassa A., Kim W.-K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016;17:124. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosen E.D., Sarraf P., Troy A.E., Bradwin G., Moore K., Milstone D.S., Spiegelman B.M., Mortensen R.M. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/S1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 77.Huang H., Lane M.D., Tang Q.-Q. Effect of serum on the down-regulation of CHOP-10 during differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2005;338:1185–1188. doi: 10.1016/j.bbrc.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 78.Jing K., Heo J.-Y., Song K.-S., Seo K.-S., Park J.-H., Kim J.-S., Jung Y.-J., Jo D.-Y., Kweon G.-R., Yoon W.-H., et al. Expression regulation and function of Pref-1 during adipogenesis of human mesenchymal stem cells (MSCs) Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2009;1791:816–826. doi: 10.1016/j.bbalip.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Rosen E.D., Walkey C.J., Puigserver P., Spiegelman B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 80.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H.M., Erdjument-Bromage H., et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seale P., Kajimura S., Spiegelman B.M. Transcriptional control of brown adipocyte development and physiological function--of mice and men. Genes Dev. 2009;23:788–797. doi: 10.1101/gad.1779209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu P., Huang S., Ling S., Xu S., Wang F., Zhang W., Zhou R., He L., Xia X., Yao Z., et al. Foxp1 controls brown/beige adipocyte differentiation and thermogenesis through regulating β3-AR desensitization. Nat. Commun. 2019;10:5070. doi: 10.1038/s41467-019-12988-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu J., Boström P., Sparks L.M., Ye L., Choi J.H., Giang A.-H., Khandekar M., Virtanen K.A., Nuutila P., Schaart G., et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sidossis L.S., Porter C., Saraf M.K., Børsheim E., Radhakrishnan R.S., Chao T., Ali A., Chondronikola M., Mlcak R., Finnerty C.C., et al. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bachman E.S. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 86.Ghorbani M., Teimourian S., Farzad R., Namvar Asl N. Apparent histological changes of adipocytes after treatment with CL 316,243, a β-3-adrenergic receptor agonist. Drug Des. Devel. Ther. 2015;9:669. doi: 10.2147/DDDT.S73891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rajakumari S., Wu J., Ishibashi J., Lim H.-W., Giang A.-H., Won K.-J., Reed R.R., Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 89.Hiraike Y., Waki H., Yu J., Nakamura M., Miyake K., Nagano G., Nakaki R., Suzuki K., Kobayashi H., Yamamoto S., et al. NFIA co-localizes with PPARγ and transcriptionally controls the brown fat gene program. Nat. Cell Biol. 2017;19:1081–1092. doi: 10.1038/ncb3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kajimura S., Seale P., Kubota K., Lunsford E., Frangioni J.V., Gygi S.P., Spiegelman B.M. Initiation of myoblast to brown fat switch by a PRDM16–C/EBP-β transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Helman L.J., Thiele C.J., Linehan W.M., Nelkin B.D., Baylin S.B., Israel M.A. Molecular markers of neuroendocrine development and evidence of environmental regulation. Proc. Natl. Acad. Sci. USA. 1987;84:2336–2339. doi: 10.1073/pnas.84.8.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Helman L.J., Sack N., Plon S.E., Israel M.A. The sequence of an adrenal specific human cDNA, pG2. Nucleic Acids Res. 1990;18:685. doi: 10.1093/nar/18.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fay T.N., Jacobs I., Teisner B., Poulsen O., Chapman M.G., Stabile I., Bohn H., Westergaard J.G., Grudzinskas J.G. Two fetal antigens (FA-1 and FA-2) and endometrial proteins (PP12 and PP14) isolated from amniotic fluid; preliminary observations in fetal and maternal tissues. Eur. J. Obstet. Gynecol. Reprod. Biol. 1988;29:73–85. doi: 10.1016/0028-2243(88)90167-0. [DOI] [PubMed] [Google Scholar]
- 94.Laborda J., Sausville E.A., Hoffman T., Notario V. Dlk, a putative mammalian homeotic gene differentially expressed in small cell lung carcinoma and neuroendocrine tumor cell line. J. Biol. Chem. 1993;268:3817–3820. [PubMed] [Google Scholar]
- 95.Jensen C.H., Krogh T.N., Hojrup P., Clausen P.P., Skjodt K., Larsson L.-I., Enghild J.J., Teisner B. Protein Structure of Fetal Antigen 1 (FA1). A novel circulating human epidermal-growth-factor-Like protein expressed in neuroendocrine tumors and its relation to the gene products of Dlk and pG2. Eur. J. Biochem. 1994;225:83–92. doi: 10.1111/j.1432-1033.1994.00083.x. [DOI] [PubMed] [Google Scholar]
- 96.Lee Y.L., Helman L., Hoffman T., Laborda J. Dlk, pG2 and Pref-1 mRNAs encode similar proteins belonging to the EGF-like superfamily. Identification of polymorphic variants of this RNA. Biochim. Biophys. Acta Gene Struct. Expr. 1995;1261:223–232. doi: 10.1016/0167-4781(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y., Zhao L., Smas C., Sul H.S. Pref-1 interacts with fibronectin to inhibit adipocyte differentiation. Mol. Cell. Biol. 2010;30:3480–3492. doi: 10.1128/MCB.00057-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smas C.M., Green D., Sul H.S. Structural characterization and alternate splicing of the gene encoding the preadipocyte EGF-like protein Pref-1. Biochemistry. 1994;33:9257–9265. doi: 10.1021/bi00197a029. [DOI] [PubMed] [Google Scholar]
- 99.Sul H.S. Minireview: Pref-1: Role in adipogenesis and mesenchymal cell fate. Mol. Endocrinol. 2009;23:1717–1725. doi: 10.1210/me.2009-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hudak C.S., Sul H.S. Pref-1, a gatekeeper of adipogenesis. Front. Endocrinol. (Lausanne). 2013;4:79. doi: 10.3389/fendo.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kopan R., Ilagan M.X.G. The canonical notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Falix F.A., Aronson D.C., Lamers W.H., Gaemers I.C. Possible roles of DLK1 in the Notch pathway during development and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2012;1822:988–995. doi: 10.1016/j.bbadis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Bray S.J., Takada S., Harrison E., Shen S.C., Ferguson-Smith A.C. The atypical mammalian ligand Delta-like homologue 1 (Dlk1) can regulate Notch signalling in Drosophila. BMC Dev. Biol. 2008;8:11. doi: 10.1186/1471-213X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smas C.M., Chen L., Sul H.S. Cleavage of membrane-associated Pref-1 generates a soluble inhibitor of adipocyte differentiation. Mol. Cell. Biol. 1997;17:977–988. doi: 10.1128/MCB.17.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y., Sul H.S. Ectodomain shedding of preadipocyte Factor 1 (Pref-1) by tumor necrosis factor alpha converting enzyme (TACE) and inhibition of adipocyte differentiation. Mol. Cell. Biol. 2006;26:5421–5435. doi: 10.1128/MCB.02437-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mei B., Zhao L., Chen L., Sul H.S. Only the large soluble form of preadipocyte factor-1 (Pref-1), but not the small soluble and membrane forms, inhibits adipocyte differentiation: Role of alternative splicing. Biochem. J. 2002;364:137–144. doi: 10.1042/bj3640137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bauer S.R., Ruiz-Hidalgo M.J., Rudikoff E.K., Goldstein J., Laborda J. Modulated expression of the epidermal growth factor-like homeotic protein dlk influences stromal-cell–pre-B-cell interactions, stromal cell adipogenesis, and pre-B-cell interleukin-7 requirements. Mol. Cell. Biol. 1998;18:5247–5255. doi: 10.1128/MCB.18.9.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Garcés C., Ruiz-Hidalgo M.J., Bonvini E., Goldstein J., Laborda J. Adipocyte differentiation is modulated by secreted delta-like (dlk) variants and requires the expression of membrane-associated dlk. Differentiation. 1999;64:103–114. doi: 10.1007/s002580050265. [DOI] [PubMed] [Google Scholar]
- 109.Nueda M.L., Baladrón V., Sánchez-Solana B., Ballesteros M.Á., Laborda J. The EGF-like protein dlk1 inhibits Notch signaling and potentiates adipogenesis of mesenchymal cells. J. Mol. Biol. 2007;367:1281–1293. doi: 10.1016/j.jmb.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 110.Lee K., Villena J.A., Moon Y.S., Kim K.-H., Lee S., Kang C., Sul H.S. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor–1 (Pref-1) J. Clin. Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nueda M.-L., García-Ramírez J.J., Laborda J., Baladrón V. Dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3-L1 Cells. J. Mol. Biol. 2008;379:428–442. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 112.Villena J.A., Choi C.S., Wang Y., Kim S., Hwang Y.-J., Kim Y.-B., Cline G., Shulman G.I., Sul H.S. Resistance to high-fat diet-induced obesity but exacerbated insulin resistance in mice overexpressing preadipocyte factor-1 (Pref-1): A new model of partial lipodystrophy. Diabetes. 2008;57:3258–3266. doi: 10.2337/db07-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Charalambous M., Da Rocha S.T., Radford E.J., Medina-Gomez G., Curran S., Pinnock S.B., Ferrón S.R., Vidal-Puig A., Ferguson-Smith A.C. DLK1/PREF1 regulates nutrient metabolism and protects from steatosis. Proc. Natl. Acad. Sci. USA. 2014;111:16088–16093. doi: 10.1073/pnas.1406119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Armengol J., Villena J.A., Hondares E., Carmona M.C., Sul H.S., Iglesias R., Giralt M., Villarroya F. Pref-1 in brown adipose tissue: Specific involvement in brown adipocyte differentiation and regulatory role of C/EBPδ. Biochem. J. 2012;443:799–810. doi: 10.1042/BJ20111714. [DOI] [PubMed] [Google Scholar]
- 115.Zhang H., Schulz T.J., Espinoza D.O., Huang T.L., Emanuelli B., Kristiansen K., Tseng Y. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol. Cell. Biol. 2010;30:4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rhee M., Kim J., Lee M.-W., Yoon K., Lee S. Preadipocyte factor 1 regulates adipose tissue browning via TNF-α-converting enzyme-mediated cleavage. Metabolism. 2019;101:153977. doi: 10.1016/j.metabol.2019.153977. [DOI] [PubMed] [Google Scholar]
- 117.Zhang X., Hirai M., Cantero S., Ciubotariu R., Dobrila L., Hirsh A., Igura K., Satoh H., Yokomi I., Nishimura T., et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow to adipose tissue. J. Cell. Biochem. 2011;112:1206–1218. doi: 10.1002/jcb.23042. [DOI] [PubMed] [Google Scholar]
- 118.Karagianni M., Brinkmann I., Kinzebach S., Grassl M., Weiss C., Bugert P., Bieback K. A comparative analysis of the adipogenic potential in human mesenchymal stromal cells from cord blood and other sources. Cytotherapy. 2013;15:76–88.e2. doi: 10.1016/j.jcyt.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 119.Briana D.D., Papathanasiou A.-E., Gavrili S., Georgantzi S., Marmarinos A., Christou C., Voulgaris K., Gourgiotis D., Malamitsi-Puchner A. Preadipocyte factor-1 in maternal, umbilical cord serum and breast milk: The impact of fetal growth. Cytokine. 2019;114:143–148. doi: 10.1016/j.cyto.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 120.Lee S., Park B.-J., Kim J.Y., Jekarl D., Choi H.Y., Lee S.Y., Kim M., Kim Y., Park M.-S. The effect of fibroblast growth factor on distinct differentiation potential of cord blood–derived unrestricted somatic stem cells and Wharton’s jelly–derived mesenchymal stem/stromal cells. Cytotherapy. 2015;17:1723–1731. doi: 10.1016/j.jcyt.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 121.Kluth S.M., Buchheiser A., Houben A.P., Geyh S., Krenz T., Radke T.F., Wiek C., Hanenberg H., Reinecke P., Wernet P., et al. DLK-1 as a marker to distinguish unrestricted somatic stem cells and mesenchymal stromal cells in cord blood. Stem Cells Dev. 2010;19:1471–1483. doi: 10.1089/scd.2010.0070. [DOI] [PubMed] [Google Scholar]
- 122.Mitterberger M.C., Lechner S., Mattesich M., Kaiser A., Probst D., Wenger N., Pierer G., Zwerschke W. DLK1(PREF1) is a negative regulator of adipogenesis in CD105+/CD90+/CD34+/CD31−/FABP4− adipose-derived stromal cells from subcutaneous abdominal fat pats of adult women. Stem Cell Res. 2012;9:35–48. doi: 10.1016/j.scr.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 123.Zwierzina M.E., Ejaz A., Bitsche M., Blumer M.J.F., Mitterberger M.C., Mattesich M., Amann A., Kaiser A., Pechriggl E.J., Hörl S., et al. Characterization of DLK1(PREF1)+/CD34+ cells in vascular stroma of human white adipose tissue. Stem Cell Res. 2015;15:403–418. doi: 10.1016/j.scr.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 124.Morganstein D.L., Wu P., Mane M.R., Fisk N.M., White R., Parker M.G. Human fetal mesenchymal stem cells differentiate into brown and white adipocytes: A role for ERRα in human UCP1 expression. Cell Res. 2010;20:434–444. doi: 10.1038/cr.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bost F., Aouadi M., Caron L., Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87:51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 126.Morrison D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012;4:a011254. doi: 10.1101/cshperspect.a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gehart H., Kumpf S., Ittner A., Ricci R. MAPK signalling in cellular metabolism: Stress or wellness? EMBO Rep. 2010;11:834–840. doi: 10.1038/embor.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu E., Kim J.B., Sarraf P., Spiegelman B.M. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 129.Kim K.-A., Kim J.-H., Wang Y., Sul H.S. Pref-1 (Preadipocyte Factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation. Mol. Cell. Biol. 2007;27:2294–2308. doi: 10.1128/MCB.02207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smith P.J., Wise L.S., Berkowitz R., Wan C., Rubin C.S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 1988;263:9402–9408. [PubMed] [Google Scholar]
- 131.Zhang H., Nøhr J., Jensen C.H., Petersen R.K., Bachmann E., Teisner B., Larsen L.K., Mandrup S., Kristiansen K. Insulin-like growth factor-1/Insulin bypasses Pref-1/FA1-mediated inhibition of adipocyte differentiation. J. Biol. Chem. 2003;278:20906–20914. doi: 10.1074/jbc.M300022200. [DOI] [PubMed] [Google Scholar]
- 132.Sale E.M., Atkinson P.G., Sale G.J. Requirement of MAP kinase for differentiation of fibroblasts to adipocytes, for insulin activation of p90 S6 kinase and for insulin or serum stimulation of DNA synthesis. EMBO J. 1995;14:674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boney C.M., Smith R.M., Gruppuso P.A. Modulation of insulin-like growth factor I mitogenic signaling in 3T3- L1 preadipocyte differentiation. Endocrinology. 1998;139:1638–1644. doi: 10.1210/endo.139.4.5920. [DOI] [PubMed] [Google Scholar]
- 134.Bost F., Caron L., Marchetti I., Dani C., Le Marchand-Brustel Y., Binétruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem. J. 2002;361:621–627. doi: 10.1042/bj3610621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Camp H.S., Tafuri S.R. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. J. Biol. Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 136.Jaiswal R.K., Jaiswal N., Bruder S.P., Mbalaviele G., Marshak D.R., Pittenger M.F. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen-activated protein kinase. J. Biol. Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 137.Font de Mora J., Porras A., Ahn N., Santos E. 3T3-L1 adipocytic differentiation. Mol. Cell. Biol. 1997;17:6068–6075. doi: 10.1128/MCB.17.10.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y., Sul H.S. Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9. Cell Metab. 2009;9:287–302. doi: 10.1016/j.cmet.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.To W.S., Midwood K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pankov R. Fibronectin at a glance. J. Cell Sci. 2002;115:3861–3863. doi: 10.1242/jcs.00059. [DOI] [PubMed] [Google Scholar]
- 141.Ruiz-Hidalgo M. Dlk modulates mitogen-activated protein kinase signaling to allow or prevent differentiation. Exp. Cell Res. 2002;274:178–188. doi: 10.1006/excr.2001.5464. [DOI] [PubMed] [Google Scholar]
- 142.Kopan R. Notch signaling. Cold Spring Harb. Perspect. Biol. 2012;4:a011213. doi: 10.1101/cshperspect.a011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.D’Souza B., Meloty-Kapella L., Weinmaster G. Canonical and non-canonical notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nueda M., Gómez M.G.-, Rodríguez-ca M., Baladrón V. DLK proteins modulate NOTCH signaling to influence a brown or white 3T3-L1 adipocyte fate. Sci Rep. 2018;8:16923. doi: 10.1038/s41598-018-35252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Searfoss G.H., Jordan W.H., Calligaro D.O., Galbreath E.J., Schirtzinger L.M., Berridge B.R., Gao H., Higgins M.A., May P.C., Ryan T.P. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional γ-secretase inhibitor. J. Biol. Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 146.Komatsu H., Chao M.Y., Larkins-Ford J., Corkins M.E., Somers G.A., Tucey T., Dionne H.M., White J.Q., Wani K., Boxem M., et al. OSM-11 facilitates LIN-12 Notch signaling during Caenorhabditis elegans vulval development. PLoS Biol. 2008;6:e196. doi: 10.1371/journal.pbio.0060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shan T., Liu J., Wu W., Xu Z., Wang Y. Roles of Notch signaling in adipocyte progenitor cells and mature adipocytes. J. Cell. Physiol. 2017;232:1258–1261. doi: 10.1002/jcp.25697. [DOI] [PubMed] [Google Scholar]
- 148.Nichols A.M., Pan Y., Herreman A., Hadland B.K., De Strooper B., Kopan R., Huppert S.S. Notch pathway is dispensable for adipocyte specification. Genesis. 2004;44:40–44. doi: 10.1002/gene.20061. [DOI] [PubMed] [Google Scholar]
- 149.Lai P., Tsai C., Tseng M. Active form Notch4 promotes the proliferation and differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 2013;430:1132–1139. doi: 10.1016/j.bbrc.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 150.Urs S., Turner B., Tang Y., Rostama B., Small D., Liaw L. Effect of soluble Jagged1-mediated inhibition of Notch signaling on proliferation and differentiation of an adipocyte progenitor cell model. Adipocyte. 2012;1:46–57. doi: 10.4161/adip.19186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Garcés C., Ruiz-Hidalgo M.J., De Mora J.F., Park C., Miele L., Goldstein J., Bonvini E., Porrás A., Laborda J. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J. Biol. Chem. 1997;272:29729–29734. doi: 10.1074/jbc.272.47.29729. [DOI] [PubMed] [Google Scholar]
- 152.Ross D.A., Rao P.K., Kadesch T. Dual Roles for the Notch Target Gene Hes-1 in the Differentiation of 3T3-L1 Preadipocytes. Mol. Cell. Biol. 2004;24:3505–3513. doi: 10.1128/MCB.24.8.3505-3513.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ba K., Yang X., Wu L., Wei X., Fu N., Fu Y., Cai X., Yao Y., Ge Y., Lin Y. Jagged-1-mediated activation of notch signalling induces adipogenesis of adipose-derived stem cells. Cell Prolif. 2012;45:538–544. doi: 10.1111/j.1365-2184.2012.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mi L., Chen Y., Zheng X., Li Y., Zhang Q., Mo D., Yang G. MicroRNA-139-5p Suppresses 3T3-L1 preadipocyte differentiation through Notch and IRS1/PI3K/Akt insulin signaling pathways. J. Cell. Biochem. 2015;116:1195–1204. doi: 10.1002/jcb.25065. [DOI] [PubMed] [Google Scholar]
- 155.Vujovic S., Henderson S.R., Flanagan A.M., Clements M.O. Inhibition of γ-secretases alters both proliferation and differentiation of mesenchymal stem cells. Cell Prolif. 2007;40:185–195. doi: 10.1111/j.1365-2184.2007.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Osathanon T., Subbalekha K., Sastravaha P., Pavasant P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol. Int. 2012;36:1161–1170. doi: 10.1042/CBI20120288. [DOI] [PubMed] [Google Scholar]
- 157.Ugarte F., Ryser M., Thieme S., Fierro F.A., Navratiel K., Bornhäuser M., Brenner S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp. Hematol. 2009;37:867–875.e1. doi: 10.1016/j.exphem.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 158.Song B., Chi Y., Li X., Du W., Han Z.-B., Tian J., Li J., Chen F., Wu H., Han L., et al. Inhibition of Notch signaling promotes the adipogenic differentiation of mesenchymal stem cells through autophagy activation and PTEN-PI3K/AKT/mTOR pathway. Cell. Physiol. Biochem. 2015;36:1991–2002. doi: 10.1159/000430167. [DOI] [PubMed] [Google Scholar]
- 159.Lei T., Bi Y., Gao M.J., Gao S.M., Zhou L.L., Zheng H.L., Chen X.D. HES1 inhibits adipogenesis of porcine mesenchymal stem cells via transcriptional repression of FAD24. Domest. Anim. Endocrinol. 2013;45:28–32. doi: 10.1016/j.domaniend.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 160.Ross D.A., Hannenhalli S., Tobias J.W., Cooch N., Shiekhattar R., Kadesch T. Functional analysis of Hes-1 in preadipocytes. Mol. Endocrinol. 2006;20:698–705. doi: 10.1210/me.2005-0325. [DOI] [PubMed] [Google Scholar]
- 161.Baladrón V., Ruiz-Hidalgo M.J., Nueda M.L., Díaz-Guerra M.J.M., García-Ramírez J.J., Bonvini E., Gubina E., Laborda J. Dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp. Cell Res. 2005;303:343–359. doi: 10.1016/j.yexcr.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 162.Huang Y., Yang X., Wu Y., Jing W., Cai X., Tang W., Liu L., Liu Y., Grottkau B.E., Lin Y. γ-secretase inhibitor induces adipogenesis of adipose-derived stem cells by regulation of Notch and PPAR-γ. Cell Prolif. 2010;43:147–156. doi: 10.1111/j.1365-2184.2009.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]