Abstract
A 55‐year‐old, previously healthy man presented with an episode of wide QRS tachycardia that had left bundle branch morphology and left superior axis. His electrocardiogram in sinus rhythm showed characteristic Brugada pattern with coved type ST‐segment (J‐point) elevation in leads V1–V2, mild QRS widening of 110 ms, and left axis deviation. The mechanism of the tachycardia was shown to be bundle branch reentry. Baseline H‐V interval of 68 ms additionally lengthened to 119 ms after intravenous procainamide administration indicating significant conduction system disease. The tachycardia was no longer inducible after successful ablation of the right bundle branch.
Keywords: Brugada syndrome, conduction system disease, bundle branch reentrant ventricular tachycardia
The Brugada syndrome is a genetically transmitted primary arrhythmogenic disorder with a high risk of sudden death. The syndrome is characterized by a right bundle branch block pattern and ST‐segment (J‐point) elevation in the right precordial leads of the electrocardiogram (ECG). 1 Sudden death in this syndrome is typically caused by fast polymorphic ventricular tachycardia (VT) or ventricular fibrillation (VF). 2 The present report describes a patient with characteristic Brugada ECG pattern who presented with bundle branch reentrant (BBR) VT.
CASE REPORT
A 55‐year‐old, previously healthy man, presented to the hospital with an episode of fast (200 beats/min) wide QRS tachycardia treated by cardioversion (Fig. 1(A)). His past medical history was unremarkable. He had no family history of sudden death or cardiac arrhythmia. Serial ECGs recorded in sinus rhythm showed dynamic J‐point and coved type ST‐segment elevation in leads V1–V2 compatible with the Brugada pattern, mild QRS widening of 110 ms, left axis deviation, and PR‐interval of 200 ms (Fig. 2). Physical and neurological examination, laboratory tests, echocardiogram, and coronary angiogram were normal. Electrophysiological study was remarkable for mild His‐ventricular (H‐V) interval prolongation of 68 ms and reproducibly inducible sustained monomorhpic VT with surface ECG morphology identical to the clinical arrhythmia. The tachycardia mechanism was consistent with BBR VT based on the following criteria: (1) left bundle branch block (LBBB) morphology and left superior axis (Figs. 1(A)–(C)), (2) AV dissociation (Fig. 1(C)), (3) H preceding every V with the H‐V interval (112 ms) greater than that recorded during sinus rhythm (Figs. 1(B) and (C)), (4) H before the right bundle deflection (Fig. 1(B)), (5) spontaneous changes in the HH intervals preceded similar changes in the VV intervals (Fig. 1(C)), and (6) reproducible spontaneous termination of the VT with retrograde conduction block to H (V with no H) (Fig. 1(C)). Polymorphic VT/VF was not inducible at baseline. Administration of intravenous procainamide (10 mg/kg over a 30‐minute period) resulted in marked ST‐segment (J‐point) elevation in leads V1–V2 additionally supporting the relation of the ECG findings to the Brugada phenotype. The H‐V interval lengthened to119 ms indicating significant conduction system disease. Sustained polymorphic VT was induced with triple ventricular extrastimuli. Right bundle branch was subsequently ablated. The BBR VT was no longer inducible. The patient underwent implantable cardioverter‐defibrillator placement. He remained free from arrhythmia over a follow‐up of 9 months.
Figure 1.
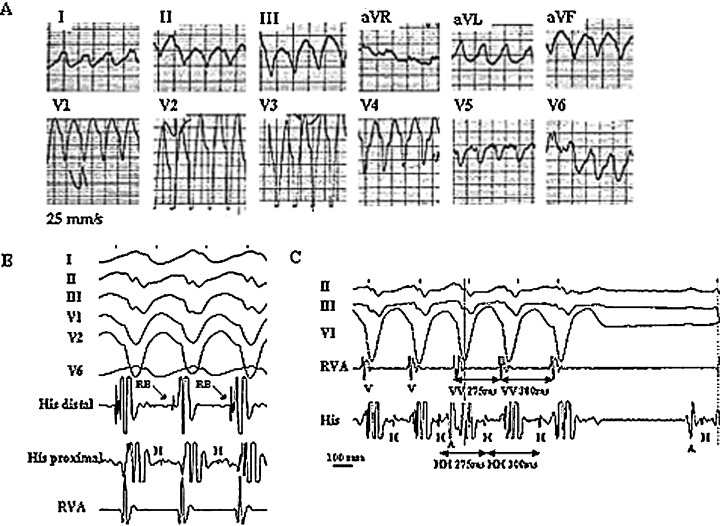
Twelve‐lead ECG during the spontaneous episode of tachycardia (A). Surface ECG leads I, II, III, V1, V2, V6 (B) or II, III, V1 (C) and intracardiac recordings from the His bundle (His) and right ventricular apex (RVA) obtained during induced tachycardia of the same ECG morphology. The recordings are consistent with bundle branch reentrant ventricular tachycardia. See text for discussion. A, H, RB, and V denote atrial, His bundle, right bundle, and ventricular electrograms, respectively.
Figure 2.

Twelve‐lead ECG demonstrating dynamic pattern of right bundle branch block and coved type of ST segment (J‐point) elevation in leads V1‐V2 compatible with Brugada phenotype (A). Disappearance of the Brugada ECG manifestations on the third day of hospitalization (B).
DISCUSSION
It is well recognized that fast polymorphic VT/VF is the underlying cause of sudden death and syncope in patients with the Brugada syndrome. 2 The proposed mechanism of the arrhythmia is functional reentry (phase 2 reentry) caused by marked transmural dispersion of ventricular repolarization due to heterogeneous loss of the action potential dome. 3 Occurrence of monomorphic VT in patients with the Brugada syndrome has been previously reported, 4 , 5 although BBR VT has never been described as a part of arrhythmia spectrum associated with this syndrome. Boersma et al. recently described a patient with the Brugada syndrome who presented with monomorphic VT that had LBBB morphology and superior axis. 5 Similar to our case, their patient showed baseline intraventricular conduction abnormalities manifested by QRS duration of 160 ms, left anterior fascicular block, and prolonged H‐V interval of 70 ms. The VT was inducible with programmed stimulation. However, the authors do not report if BBR mechanism was sought during electrophysiological study.
Whether BBR VT and Brugada ECG pattern observed in our patient are related phenomena is unknown. BBR is a well‐known mechanism of VT in patients with abnormal His‐Purkinje conduction. Significant conduction delay within the His‐Purkinje system is required to sustain BBR. 6 His‐Purkinje abnormalities manifested through intraventricular conduction defects and H‐V interval prolongation are commonly found in patients with the Brugada syndrome. 2 Mutations in the SCN5A gene encoding voltage‐gated Na+ channel α subunit have been associated with both the Brugada syndrome and isolated progressive cardiac conduction defect (Lenegre‐Lev disease) phenotypes. 7 , 8 Moreover, it has recently been shown that a single gene mutation may produce both these phenotypes. 9 It is plausible, therefore, that the electrophysiological substrates for BBR VT and the Brugada syndrome may share a common genotype and that BBR VT may be a potential mechanism of arrhythmia in patients with this syndrome.
REFERENCES
- 1. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical an electrocardiogarphic syndrome: A multicenter report. J Am Coll Cardiol 1992;20: 1391–1396. [DOI] [PubMed] [Google Scholar]
- 2. Alings M, Wilde AA. Brugada syndrome: Clinical data and suggested pathophysiological mechanism. Circulation 1999;99: 666–673. [DOI] [PubMed] [Google Scholar]
- 3. Yan G‐X, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST‐segment elevation. Circulation 1999;100: 1660–1666. [DOI] [PubMed] [Google Scholar]
- 4. Shimada M, Miyazaki T, Miyoshi S, et al Sustained monomorphic ventricular tachycardia in a patient with Brugada syndrome. Jpn Circ J 1996;60: 364–370. [DOI] [PubMed] [Google Scholar]
- 5. Boersma LVA, Jaarsma W, Jessurun ER, et al Brugada Syndrome: A case report of monomorphic ventricular tachycardia. PACE 2001;24: 112–115. [DOI] [PubMed] [Google Scholar]
- 6. Tchou P, Mehdirad AA. Bundle branch reentry ventricular tachycardia. PACE 1995;18: 1427–1437. [DOI] [PubMed] [Google Scholar]
- 7. Chen Q, Kirsch GE, Zhang D, et al Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 1998;392: 293–296 [DOI] [PubMed] [Google Scholar]
- 8. Schott JJ, Alshinawi C, Kyndt F, et al Cardiac conduction defects associate with mutations in SCN5A. Nat Genet 1999;23: 20. [DOI] [PubMed] [Google Scholar]
- 9. Kyndt F, Probst V, Potet F, et al Novel SCN5A mutation leading either to isolated cardiac conduction defect or Brugada syndrome in a large French family. Circulation 2001;104: 3081–3086. [DOI] [PubMed] [Google Scholar]


