Abstract
Infertility represents a growing health problem in industrialized countries. Thus, a greater understanding of the molecular networks involved in this disease could be critical for the development of new therapies. A recent finding revealed that circadian rhythmicity disruption is one of the main causes of poor reproductive outcome. The circadian clock system beats circadian rhythms and modulates several physiological functions such as the sleep-wake cycle, body temperature, heart rate, and hormones secretion, all of which enable the body to function in response to a 24 h cycle. This intricated machinery is driven by specific genes, called “clock genes” that fine-tune body homeostasis. Stress of modern lifestyle can determine changes in hormone secretion, favoring the onset of infertility-related conditions that might reflect disfunctions within the hypothalamic–pituitary–gonadal axis. Consequently, the loss of rhythmicity in the suprachiasmatic nuclei might affect pulsatile sexual hormones release. Herein, we provide an overview of the recent findings, in both animal models and humans, about how fertility is influenced by circadian rhythm. In addition, we explore the complex interaction among hormones, fertility and the circadian clock. A deeper analysis of these interactions might lead to novel insights that could ameliorate the therapeutic management of infertility and related disorders.
Keywords: hormones regulation, clock genes, fertility, reproduction, spermatogenesis
1. Introduction
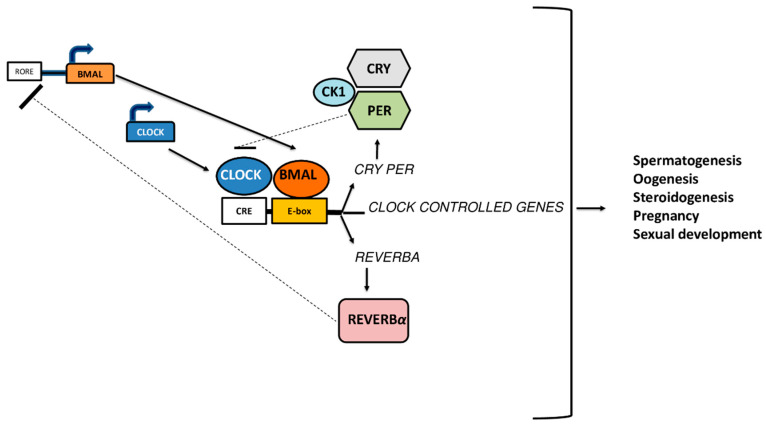
The term ‘’circadian rhythm”, derived from Latin “circa dies”, is used to describe the daily oscillations in gene expression, metabolism, activity patterns and serum hormone levels that occur across 24 h. These physiological processes represent an ubiquitous feature in living organisms, from cyanobacteria to humans, and are finely regulated by the suprachiasmatic nuclei (SCN) of the anterior hypothalamus [1]. In mammals, circadian timing system is generated at cellular levels through a series of interlocked positive and negative transcription/translation feedback loops, leading to the expression of circadian rhythms. The positive loop is composed by Brain and Muscle ARNT-like 1 (BMAL1) gene and Circadian Locomotor Output Cycles Kaput (CLOCK) gene, which encode for BMAL1 and CLOCK proteins, respectively. These proteins form an heterodimer, which finally binds itself to the a cyclic AMP response element (CRE)/E-box element (E-box) of Period (PER), Cryptochrome (CRY) genes, promoting their transcription [2]. Similar to their predecessors BMAL1 and CLOCK, also PER and CRY are able to dimerize, bind to Casein Kinase 1 (CK1), and finally move into the cell nucleus, where they binds to the BMAL1/CLOCK dimer, inhibiting its transcription “closing” the loop (negative loops) [3,4]. In a second transcriptional loop, CLOCK/BMAL1 activate the transcription of genes for the nuclear receptors REV-ERB, also known as NR1D1 (nuclear receptor subfamily 1, group D, member 1). These proteins compete with the retinoic acid-related orphan receptors (ROR) for binding sites ROR-binding elements (RORE) on the BMAL1 gene, providing both positive (ROR) and negative (REV-ERB) transcriptional regulation [4] (Figure 1).
Figure 1.
Autoregulatory feedback loop of clock-specific gene expressions that are involved in fertility processes.
Many data coming from animal studies have demonstrated the role of clock genes and clock-related genes in the regulation of both male and female fertility [5,6]. Indeed, alterations of biological rhythms and disrupted functions of the circadian clocks have been demonstrated to negatively impact reproductive capacity [7]. It is well known that physiological processes governing fertility need to be appropriately tight-timed orchestrated with the external environment to ensure reproductive success. Thus, a fine circadian regulation of reproductive hormones is mandatory for fertility both in males and females. It is interesting to note that the regulation of the estrus cycle, luteinizing hormone (LH) surge, sperm production and maturation, and the timing of insemination and fertilization are regulated by clock genes [6,8]. Furthermore, the timing of peripheral biological rhythm patterns is synchronized with circadian oscillation of melatonin and cortisol [9,10]. Changes in their circulating levels can indirectly impair reproduction, in which proper levels of glucocorticoids are required for normal gonadal function [11].
The aim of this review is to highlight most recent findings on the network connections among circadian rhythms, hormones and fertility.
2. How Fertility Is Influenced by Hormones and Clock Genes?
Originally, the molecular mechanism governing clock gene machinery was identified in the cells of a large number of tissues from several species. Afterwards, some studies demonstrated that clock machinery also had an influence on fertility and reproductive success [12,13]. This interaction is not only one-way, since fertility hormones can also influence clock-gene expression [6], thus indicating a complex network of interactions [14,15].
2.1. Gonadotropins
Fertility is finely regulated by the hypothalamic–pituitary–gonadal axis (HPG axis) and by two hypothalamic neuronal populations, the Kisspeptin neurons and the gonadotropin-releasing hormone (GnRH) neurons [16,17]. The Kisspeptin neurons, located in the anterior ventral periventricular area, are involved in the LH surge while the hypothalamic Kisspeptin neuron population, located in the arcuate nucleus, brings metabolic status information to the HPG axis and released GnRH [18,19]. The GnRH is released in a pulsatile manner by the hypothalamus and acts on the anterior pituitary lobe (Adeno-Hypophysis), regulating the production of gonadotropins and finally releasing follicle-stimulating hormone (FSH) and LH into the bloodstream. In males, LH is mandatory for testosterone production while FSH is involved in sperm production [20,21]. In females, FSH and LH are both involved in the production of steroid hormones (estrogens) by ovarian follicles [22]. Furthermore, the peak of the LH induces the continuous gene and protein expression of BMAL1 in the mouse ovary [23]. The impairment of FSH signaling results in poor spermatogenesis and subfertility in male [20] (Table 1). Moreover, since Kisspeptin signaling is mandatory for the production and activity of Leydig cells, germ cells progression and sperm functions, an alteration of this signaling causes hypogonadotropic hypogonadism [24]
Table 1.
Rhythmicity and physiological effects of hormones in male and female fertility.
| Hormones | Rhythmicity | Effects on Male | Effects on Female | |
|---|---|---|---|---|
| FSH | 24 h circadian rhythm during follicular phase [25] | 24 h circadian rhythm during luteal phase [25] | Sertoli cell tropism and sperm production [20] | Stimulation of estrogens production by ovarian granulosa cells [22] |
| LH | 24 h circadian rhythm during follicular phase [25] | No circadian rhythm in luteal phase [25] | Stimulation of testosterone production by Leydig cells [21] | Stimulation of estrogens production by ovarian granulosa cells [22] |
| Regulation of theca cells androgen production [26] | ||||
| Estrogens | 24 h circadian rhythm during follicular phase [25] | No circadian rhythm in luteal phase [25] | Regulation of ductal and epididymal function [27] | Development and maintenance of secondary sexual characteristics [28] |
| Androgens | 24 h circadian rhythm with a peak in the early morning [26] | Development and maintenance of secondary sexual characteristics [29] | Control of growing follicles [30] | |
| Glucocorticoids | 24 h circadian rhythm with a peak in the morning [31] | Promotion of sperm maturation and steroidogenesis [32] | Regulation of fetal growth and development [33] | |
| Melatonin | 24 h circadian rhythm with a peak in the night [34] | Preservation of spermatogenesis [35,36] | Control of neurological and endocrine systems development [37] | |
| Reduction of free radicals protecting sperm from oxidative damage [35,36] | Protection of the embryo/fetus from metabolic stress [37] | |||
2.2. Estrogens and Androgens
The existence of a link between circadian rhythms and estrogen has been clearly demonstrated [38,39] and a recent study showed that most of female fertility hormones display circadian rhythmicity [25]. During the follicular phase, FSH, LH, estrogen, progesterone, and sex hormone binding-globulin (SHBG) show a peculiar rhythmicity [25] (Table 1). In contrast, during the luteal phase, only FSH and SHBG display such rhythmicity. In females, estrogens stimulate LH surge [22] and determine the appearance of secondary sexual characteristics [28]. It is known that the expression of both Per1 [40] and Per2 [41] is modulated by estrogens. Importantly, a comprehensive study demonstrates that Clock gene is able to influence the activity of the estrogen receptor alpha (ERα), by regulating its transcriptional activity [42]. Furthermore, the link between the circadian system and estrogen synthesis is further strengthened by the discovery that ER receptors are expressed in the SCN [43]. Not only estrogens, but also androgens exert some effects on fertility. Androgens levels show a diurnal oscillation with morning peak levels [26]. In males, androgens are necessary for the development and maintenance of secondary sexual characteristics, libido, growth, prevention of osteoporosis, and spermatogenesis [29]. In females, LH regulates ovarian androgens production [26]. Female hyperandrogenemia (a common feature of the polycystic ovary syndrome, PCOS) is accompanied by an excessive production of androgens that affect Clock gene expression in ovarian rat follicles [44,45]. Metabolic syndrome and obesity in PCOS patients are commonly associated with a decline in reproductive function, disrupted reproductive cycles and attenuated gonadotropins secretion [46,47]. Furthermore, androgens display direct and tissue-specific effects on Per2 gene expression that may account for the effects on the developmental program of the timing system [48].
2.3. Glucocorticoids
Glucocorticoids (GCs) are produced by adrenal glands and are synchronizers of endogenous clocks [9,10]. Their regulation displays a diurnal release pattern, with peak levels at the beginning of the activity phase [31] (Table 1). It was demonstrated that an increase of GCs levels occurring during a stress-mediated response [49] or due to GCs exogenous administration could severely impair different organs including immune [50,51] and reproductive systems [52]. These effects occur through an action on different hypothalamic–pituitary–adrenal (HPA) axis compartments: on the hypothalamus, inhibiting GnRH release; and on the pituitary, influencing gonadotropins synthesis and release [33]. GCs influence ovarian function also indirectly, by altering the levels of circulating gonadotropins and affecting levels of metabolic hormones and growth factors. Some recent discoveries point out that GCs can indirectly cause disruption of ovarian cyclicity, both through the inhibition of Kisspeptin neurons [53] and the stimulation of gonadotropin-inhibitory hormone (GnIH) [54]. On the pituitary gland, GCs inhibit the synthesis and release of LH and FSH, while on testis/ovary, they directly inhibit steroidogenesis and/or gametogenesis and induce apoptosis [55,56].
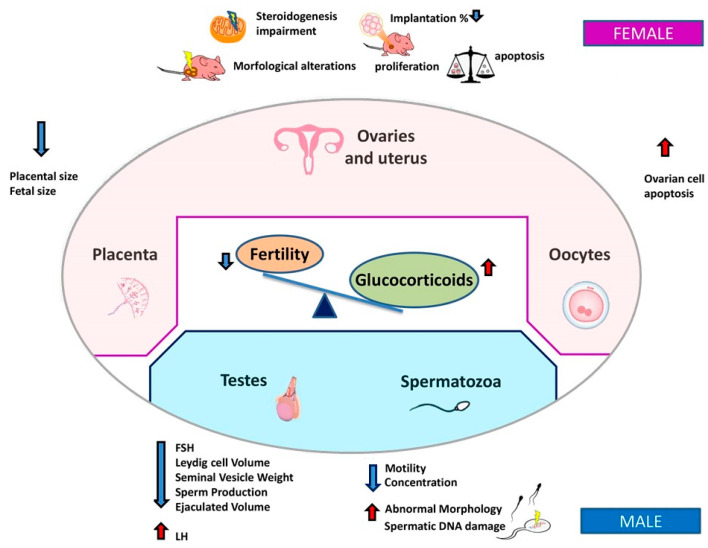
GCs exert their effect through the binding on glucocorticoid receptor (GR), which regulates the expression of glucocorticoids-responsive genes. The exogenous administration of GCs in female mice causes multiple damages on reproductive tissues [57]. In the uterus, it was described that an increase of GCs causes a reduction of embryos implantation rate [58]. Ovaries exposed to GCs treatment undergo several damages such as steroidogenesis alteration, histological impairment [59], and reduction of the total number of germ cells and ovarian volume [60]. In oocytes, GCs impair their reproductive competence, inducing oocytes apoptosis [61]. GCs are also able to compromise placenta integrity, decreasing its weight [62] and size together with a size reduction of newborn mice [63]. (Figure 2).
Figure 2.
Infertility is associated with unbalanced GCs levels. The reciprocal relationship between the disruption of circadian rhythms of GCs and fertility may be either the cause or the effect of female and male infertility.
In male mice, adrenalectomy proved that the homeostatic level of hormones, including GCs, is essential to ensure a correct spermatogenesis, sperm maturation and steroidogenesis [32]. In male rats prenatally exposed to GCs, steroidogenesis is disrupted with a reduction in FSH and testosterone levels and a consequently altered sperm quality (decreased sperm concentration, motility and abnormal morphology). The exposure to betamethasone (BX) causes abnormalities in testes and epididymis, such as a reduction of anogenital distance and the alteration of other histo-morphometric parameters [64]. Moreover, it was demonstrated that not only the F1 generation, with fathers exposed prenatally to the BX, but also the F2 generation displays fertility damage [65]. In order to determine circadian rhythmicity in mouse testes, Chen and co-workers analyzed the expression levels of several circadian clock genes, including Bmal1, Per1, Per2, and Cry1. The expression of these genes was found to be arrhythmic in testes. They also demonstrated that Bmal1 transcript levels and steroidogenic-related genes increased in Leydig cells after treatment with dexamethasone, indicating that circadian clockwork in Leydig cells may play a functional role in the control of testosterone secretion [66].
Regarding human male fertility, many evidences suggest that stress conditions (both physical and psychological) are followed by physiological responses that can impair reproductive functions [67]. In order to test the use of synthetic GCs in the management of male infertility, Prednisone has recently been tested in the treatment of accessory glands inflammation patients with oligospermia. GCs administration causes a considerable improvement in the sperm parameters of these patients, reducing inflammation [68]. However, other studies reported opposite results [69], rendering this question still open. Some trials have also shown that synthetic GCs are able to trigger a disruption in reproductive hormone levels [70], even if there is a lack of studies on this topic. Finally, a discrete number of observational studies suggested that testosterone levels of untreated controls were lowered by GCs [71]. However, randomized controlled trials are still missing.
2.4. Melatonin
Melatonin is a neurohormone secreted by the pineal gland whose secretion is regulated both by dark–light and seasonal cycles that exert pleiotropic actions like growth, development, senescence, and function of cells and organs in both male and female reproductive systems [34] (Table 1).
Human melatonin receptors have been localized in different cells like granulosa cells from preovulatory follicles [72] and in spermatozoa [73]. Melatonin levels have been identified to be lower in semen samples with low and poor fertilization rates [72] and in women with idiopathic infertility [74]. In stress-induced women, in which melatonin suppression is present, the circadian alteration affects fertility and fetus development, increasing the risk of miscarriage, premature birth and low birth weight [75]. It is also known that light emitted from electronic screen devices suppresses melatonin secretion that is linked with a decline of sperm motility and concentration in men [76]. Melatonin is important in the development of the fetal circadian clock, the neurological and endocrine systems, and to protect the embryo/fetus from metabolic stresses that can cause damage to the growing pregnancy. Mothers treated with melatonin can reset and reverse the fetal clock via the adrenal gland [37]. In addition, melatonin has been shown to preserve mouse spermatogenesis and reduce levels of free radicals, protecting sperm from oxidative damage [35,36]. Moreover, melatonin levels may influence the in vitro fertilization outcomes since its levels positively correlate with antral follicle count [77] and could therefore predict embryo quality [78]. However, some data suggest that an excess of melatonin leads to infertility [79]. Indeed, a reduction of melatonin levels can lead to precocious puberty, by affecting its onset [80]. Elevated melatonin levels have been associated with hypogonadism and amenorrhea in women [81,82] and with oligospermia or azoospermia in men [83].
3. Genetic Models of Clock Genes and Fertility
3.1. Female Fertility
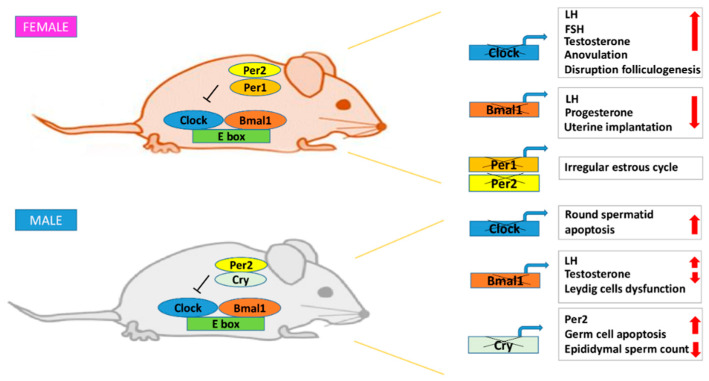
All the information on the role of circadian rhythm in fertility come from knockout mouse studies (Table 2). Per1 and Per2 knockout mice are characterized by a marked decrease in reproductive rate as a consequence of irregularity and acyclicity of the estrous cycle [84,85] (Figure 3). The fertility of young Per1 and Per2 knockout mice does not differ from wild-type mice during mild-age, highlighting that Per1-Per2 absence impairs infertility only in aged mice [84]. It was also demonstrated that Per1-Per2 double knockout mice had a premature depletion of the ovarian follicular reserve, with a consequent decline in reproductive capacity [86]. To deeply understand the impact of clock genes on fertility, a transgenic mouse model of Clock gene was generated (ClockΔ19/Δ19). These mice are characterized by a defective form of CLOCK protein, able to produce the BMAL1-CLOCK dimer, but unable to regulates Per and Cry transcription [87]. In addition to the loss of circadian rhythmicity, they also show higher rate of pregnancy failure [88].
Table 2.
Effect of disrupted genes in female mice mutant models.
| Disrupted Genes | Effects | Ref |
|---|---|---|
| Per1, Per2 | Significant decrease of ovarian follicles in aged mice | [86] |
| Accelerated reproductive aging | [86] | |
| ClockΔ19/Δ19 | Higher rate of pregnancy failure in aged mice | [89] |
| Bmal1 | Delayed puberty | [90] |
| Irregular estrous cycles | [90] | |
| Smaller ovaries and uterus | [90] | |
| Disrupted StAR gene expression | [91] | |
| Lower progesterone levels | [91] |
Figure 3.
Schematic representation of infertility complications due to clock gene disruption on female and male knockout mice.
Bmal1 knockout mice, although able to ovulate, exhibited delayed puberty, irregular estrous cycles and smaller ovaries and uterus [90,92,93] (Figure 3). Bmal1 absence negatively affects progesterone levels [91], causing embryo implantation failure [90]. This detrimental effect was explained with a disrupted expression of the StAR gene, critical for progesterone production regulation. In newborn StAR knockout mice, the adrenal glands lacked their normal cellular architecture and are characterized by high lipid depots. In contrast, testes contained only scattered lipid depots, while the ovaries appeared completely normal. According to this model, mice initially retain some capacity for StAR-independent steroidogenesis; thereafter, progressive lipid accumulation in steroidogenic cells, after hormone stimulation, kills the cells and completely abrogates steroidogenic capacity [91]. This model proves the interlocking and synergistic network of the circadian clock and reproductive systems. Bmal1 knockout and Clock∆19/Δ19 transgenic mice display undetectable LH levels [94] but still undergo ovulation and could become pregnant. This suggests that the circadian system was essential in gating the LH surge but was not required for successful ovulation [6]. Under certain circumstances, transgenic mice lacking LH pulsatility still had ovulation without the classical LH surge [95].
Chu and colleagues have recently shown that leptin receptor (LepR) is required for female fertility. Interestingly, Bmal1 knockout mice showed a reduced expression of LepR and its ligand, reducing also estrogens concentration in granulosa cells. These results suggest that estrogens synthesis is regulated by Bmal1 through the Leptin–LepR pathway [96].
Cholesterol represents an essential substrate for steroid hormone synthesis and genetic mutations altering synthesis and function of proteins involved in cholesterol uptake and mobilization from stored intracellular pools, significantly impact fertility [97].
To highlight the link among cholesterol, fertility and clock genes, an aromatase knockout mouse model ArKO was created [98]. This mutant mouse lacking functional aromatase cytochrome P450 gene (Cyp19) was unable to synthesize endogenous estrogens and, thus, ensure a correct ovulation. These mice had increased levels of testosterone, FSH and LH and are infertile due to the disruption of folliculogenesis and the absence of corpora lutea [99].
In order to better highlight the link among cholesterol, fertility and circadian regulation, a mutant mouse for Clock gene was generated [100]. These mice display reduced and arrhythmic expression levels of the enzyme 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr), that regulates cholesterol synthesis in the liver, facilitating accumulation of cholesterol in this organ [100].
It is therefore clear that most of the factors involved in fertility are influenced, or influence themselves, the biological clock thus constituting a complex “network” of mutual interactions.
3.2. Male Fertility
Circadian genes expression in mouse testes, including the expression of Per1, Per2, Bmal1, Clock, and Cry1, is arrhythmic, suggesting that clock genes have non-circadian functions in spermatogenesis [101]. Instead, clock genes are rhythmically expressed in extra testicular ducts and accessory cells of mouse testis [102].
ClockΔ19/Δ19 transgenic mice show slightly decreased male fertility and reduced newborn mice size [88], while canonical Clock knockout spermatozoa display lower in vitro fertilization, impact on blastula formation, and also lower acrosin (a sperm proteinase essential for fertilization) activity, through up-regulation of SERPINA3K [103,104] (Table 3).
Table 3.
Effect of disrupted genes in male mutant mice models.
| Disrupted Genes | Effects | Ref |
|---|---|---|
| Clock | Significant fertility reduction | [103] |
| Lower in vitro fertility rate | [103] | |
| Lower blastula formation rate | [103] | |
| Lower acrosin activity | [103] | |
| ClockΔ19/Δ19 | Mild sperm fertility in aged mice | [88] |
| Cry | Increase apoptosis of germ cells | [106] |
| Lower epididimal sperm count | [106] | |
| Bmal1 | Total infertility | [92] |
| Disrupted StAR gene expression | [92] | |
| Leydig cell impairment | [105] |
As for female gametes, also in spermatozoa, Bmal1 knockout determines a decrease of the expression of the StAR gene and related proteins [92]. Bmal1 knockout mice are infertile and display low testosterone levels and high serum levels of LH, suggesting a Leydig cell dysfunction. It is well established that in these cells BMAL1 is expressed rhythmically, allowing to postulate a role in testosterone production [92] (Figure 3). A recent study also highlighted that the circadian rhythm of the Leydig cells endocrine functions decreases during aging [105].
The testicular function was studied in Cry1 knockout mice [106]. Cry1 was expressed in Sertoli cells, spermatogonia, spermatocytes and in the interstitium. These mice showed no phenotypic abnormalities, with similar serum and intratesticular testosterone levels to wild-type mice and normal Sertoli and Leydig cells [107]. However, there was an increased number of degenerated and apoptotic germ cells in the testis corresponding to lower epididymal sperm counts and testicular cell apoptosis. In addition, there was an upregulation of Per2 levels in the testis respect to wild-type mice, whereas no differences were observed in the expression of other clock genes [106] (Figure 3).
3.3. Circadian Clock and Sexual Development
Sexual development processes are not exclusively under endocrine control but also own a circadian regulation level [38,44]. Reproductive organs’ circadian regulation influences many sexual developmental processes, like gonadotropin secretion timing, which has been demonstrated to answer to a circadian regulation itself, both regarding GnRH release [108] and gonadotropins secretion [109]. In addition, also the ovulation process was recently identified as having a circadian component, essential for its correct regulation [32].
The age of menarche represents the hallmark of puberty in females. It varies widely between individuals, is considered an heritable trait, and an alteration of its timing has been associated with risks for obesity, type 2 diabetes, cardiovascular disease, and breast cancer. Puberty in human and animals depends on a complex hormonal regulation [110,111]. Moreover, it was demonstrated that girls are more susceptible than boys to develop precocious puberty [112,113]. A strong association between puberty timing and risks to develop breast and endometrial cancers in women and prostate cancer in men has been reported [114]. The timing of menarche can also be influenced by light perception: In fact, in blind women, menarche was reported to be earlier with no differences in menopause when compared to sighted women [115].
Also the microbiome has a role in sex-specific diurnal rhythms. In fact, the absence of microbiome leads to an altered sexual development and growth hormone secretion. The composition and metabolic activity of commensal bacteria show diurnal variations that depend on the circadian clock [116] and vice versa the microbiota can interfere the clock gene expression in the host [117].
In germ-free mouse model, the resulting feminization of male and masculinization of female is likely caused by altered sexual development and growth hormone secretion, associated with differential activation of xenobiotic receptors [116].
Despite this interesting evidence of the circadian component in the modulation of sexual development, the role of clock genes in these processes is still understudied.
4. Effect of Clock Gene Mutation in Humans
Studies with mouse models clearly showed how clock genes can influence fertility, and vice versa. In humans there are mainly indirect evidences about this reciprocal influence in both sexes.
The women shift work influence on circadian regulation is considered one of the main factors causing prolonged waiting time to achieve pregnancy [118], suggesting that the sleep and inherent circadian rhythm disturbances of shift work could lead to menstrual irregularities due to altered levels of FSH, LH and prolactin [12,14,119]. Expression of CLOCK genes (CLOCK, BMAL1, PER, CRY) was analyzed in the full-term human placenta, indicating that placenta works as a peripheral circadian oscillator [37]. Moreover, some studies analyzed if progesterone could have any influence on clock machinery functioning. In details, PER1 levels increase and remain high during the decidualization of the human endometrium. The progesterone receptor activated PER1 transcription by directly binding to its promoter ensuring a correct decidualization [120] (Table 4).
Table 4.
Effect of mutated genes on human female fertility.
| Mutated Genes | Effects | Ref |
|---|---|---|
| PER1 | Attenuated human endometrial decidual transformation | [120] |
| BMAL1 | Damaged decidualization | [121] |
| Aberrant trophoblastic invasion | [121] | |
| BMAL1 polymorphism rs2278TT749 | Associated both with a great number of miscarriages but also with an increased number of pregnancies | [122] |
There are many evidences of clock genes rhythm in human ovary. CLOCK was detected in the granulosa cells of the dominant antral follicle of in vitro fertilization patient, however, its expression was undetectable in preantral and primary ovarian follicles [123]. Even if CLOCK and PER1 were expressed, their mRNA expression levels undergo a decrease with the increase of patient age [124]. Furthermore, it was shown that the expression of PER2 and STAR can be induced in vitro by testosterone, outlining a potential link between CLOCK genes and steroidogenesis, a relation that was highlighted in experimental mouse models [98].
Another recent study investigated the possible link between the expression of circadian rhythm in decidual endometrial cell tissues and the recurrent miscarriage (RM), defined as two or more consecutive losses of a clinical intrauterine pregnancy. With the use of human endometrial stromal cells (HESCs), isolated from decidua of first-trimester pregnancies, it was demonstrated that the expression of BMAL1 was reduced in the endometrial tissues of RM patients [121]. The same study verified that BMAL1 absence in the endometrial cells results in damaged decidualization and in an aberrant trophoblastic invasion, predisposing to RM. Furthermore, a single-nucleotide BMAL1 polymorphism (rs2278TT749 TT) has been found, and it has been associated both with a great number of miscarriages but also with an increased number of pregnancies [122]. CLOCK gene was detected in human healthy fetuses and reached a peak of expression during the 6th week of gestation. Its expression was instead abnormal in spontaneous miscarriage fetuses, compared to healthy fetuses [125].
The same indication comes also from a study regarding the human Han-Chinese population, which has been demonstrated to display some CLOCK gene single nucleotide-polymorphisms that is associated with idiopathic infertility [126]. Going deeply, the rs1801260 polymorphism associates with normal seminal parameters, while rs3817444 associates with both normal and abnormal seminal parameters [126]. Another study within this population highlighted the connection between CLOCK gene polymorphism and semen quality in idiopathic infertility [107]. Heterozygotes and homozygotes males (TC and CC) for a C allele variant at rs3749474 show a significant reduction in seminal volume, compared to TT genotype, and in addition, CC homozygotes have also both significantly lower concentration and sperm motility. Another polymorphism, the rs1801260, in heterozygotes TC genotype, shows a significantly lower motility compared to the TT genotype [127]. In a recent study, a homozygous mutation was identified as likely disease causing in the NPAS2 gene in a family of three brothers from Turkey, affected by Non-Obstructive Azoospermia [128] (Table 5).
Table 5.
Effect of mutated genes on human male fertility.
| Mutated Genes | Effects | Ref |
|---|---|---|
| CLOCK polymorphism rs1801260 | Normal seminal parameters | [126] |
| CLOCK polymorphism rs3817444 | Normal and abnormal seminal parameters | [126] |
| CLOCK polymorphism rs1801260 TC genotype | Lower motility compared to the TT genotype | [127] |
| CLOCK polymorphism rs3749474 CC genotype | Seminal volume reduction, lower concentration and sperm motility compared to TT genotype | [127] |
All these works highlight a potential connection between the Clock gene and human male fertility, but more experimental works and efforts are necessary to discover more about this topic.
5. Conclusions
Infertility represents one of the most important health problems following cancer and cardiovascular diseases. Few studies have explored the relation between circadian rhythms and reproduction and most of them have been made in rodents. However, the discovery of functional clock machinery in many reproductive tissues and the development of clock gene knockout mouse models have revealed the pivotal role of such genes in orchestrating reproductive processes in mammals. Clock genes affect infertility, producing low levels of sex hormones, causing embryo implantation failure and reducing newborn size both in mouse models and in shift-working women.
In this review, we assessed the connection between hormones and fertility from the viewpoint of the circadian system. Although we may not cover the whole field of disease phenotypes, we believe this review highlights the intimacy with which these three physiological aspects interact to maintain homeostasis.
Understanding how the circadian rhythms work, and how genes make them run, is becoming more and more important for human infertility. A tight net of interactors, among which clock genes and hormones are the major players, define a complex network. However, more studies are required to better fix the topic, especially regarding humans. In the years to come, the knowledge of the infertility problem could be increasingly linked to the understanding of this vast network of interactions. Chrono-therapeutical strategies that reset or modify the biological clock may contribute to restore the internal synchrony and thus counteract pathological symptoms of infertility.
Author Contributions
D.P., M.A.V. and A.M.I.: conception and design; F.S. and E.F.: data collection and interpretation; E.F., F.S., F.C., M.A.V. wrote the manuscript. D.P., F.P., D.G., A.M.I. provided suggestions and revised the manuscript for final submission. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by Ministry of University and Research (2017HRTZYA and 2017S55RXB).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Dickmeis T., Weger B.D., Weger M. The circadian clock and glucocorticoids--interactions across many time scales. Mol. Cell. Endocrinol. 2013;380:2–15. doi: 10.1016/j.mce.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Kume K., Zylka M.J., Sriram S., Shearman L.P., Weaver D.R., Jin X., Maywood E.S., Hastings M.H., Reppert S.M. Mcry1 and mcry2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/S0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 3.Nader N., Chrousos G.P., Kino T. Interactions of the circadian clock system and the hpa axis. Trends Endocrinol. Metab. 2010;21:277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Partch C.L., Green C.B., Takahashi J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24:90–99. doi: 10.1016/j.tcb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennaway D.J., Boden M.J., Varcoe T.J. Circadian rhythms and fertility. Mol. Cell Endocrinol. 2012;349:56–61. doi: 10.1016/j.mce.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Sen A., Hoffmann H.M. Role of core circadian clock genes in hormone release and target tissue sensitivity in the reproductive axis. Mol. Cell Endocrinol. 2020;501:110655. doi: 10.1016/j.mce.2019.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan X., Taylor M.J., Cohen E., Hanna N., Mota S. Circadian clock, time-restricted feeding and reproduction. Int J. Mol. Sci. 2020;21 doi: 10.3390/ijms21030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalantaridou S.N., Makrigiannakis A., Zoumakis E., Chrousos G.P. Stress and the female reproductive system. J. Reprod Immunol. 2004;62:61–68. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Morris C.J., Aeschbach D., Scheer F.A. Circadian system, sleep and endocrinology. Mol. Cell Endocrinol. 2012;349:91–104. doi: 10.1016/j.mce.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minnetti M., Hasenmajer V., Pofi R., Venneri M.A., Alexandraki K.I., Isidori A.M. Fixing the broken clock in adrenal disorders: Focus on glucocorticoids and chronotherapy. J. Endocrinol. 2020 doi: 10.1530/JOE-20-0066. [DOI] [PubMed] [Google Scholar]
- 11.Joseph D.N., Whirledge S. Stress and the hpa axis: Balancing homeostasis and fertility. Int J. Mol. Sci. 2017;18:224. doi: 10.3390/ijms18102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills J., Kuohung W. Impact of circadian rhythms on female reproduction and infertility treatment success. Curr. Opin. Endocrinol. Diabetes Obes. 2019;26:317–321. doi: 10.1097/MED.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 13.Boden M.J., Kennaway D.J. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- 14.Angelousi A., Kassi E., Nasiri-Ansari N., Weickert M.O., Randeva H., Kaltsas G. Clock genes alterations and endocrine disorders. Eur J. Clin. Invest. 2018;48:e12927. doi: 10.1111/eci.12927. [DOI] [PubMed] [Google Scholar]
- 15.Miller B.H., Olson S.L., Turek F.W., Levine J.E., Horton T.H., Takahashi J.S. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harter C.J.L., Kavanagh G.S., Smith J.T. The role of kisspeptin neurons in reproduction and metabolism. J. Endocrinol. 2018;238:R173–R183. doi: 10.1530/JOE-18-0108. [DOI] [PubMed] [Google Scholar]
- 17.Yeo S.H., Colledge W.H. The role of kiss1 neurons as integrators of endocrine, metabolic, and environmental factors in the hypothalamic-pituitary-gonadal axis. Front. Endocrinol. 2018;9:188. doi: 10.3389/fendo.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwig M.S., Fraley G.S., Smith J.T., Acohido B.V., Popa S.M., Cunningham M.J., Gottsch M.L., Clifton D.K., Steiner R.A. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of kiss-1 mrna in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 19.Messager S., Chatzidaki E.E., Ma D., Hendrick A.G., Zahn D., Dixon J., Thresher R.R., Malinge I., Lomet D., Carlton M.B., et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via g protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sairam M.R., Krishnamurthy H. The role of follicle-stimulating hormone in spermatogenesis: Lessons from knockout animal models. Arch. Med. Res. 2001;32:601–608. doi: 10.1016/S0188-4409(01)00328-9. [DOI] [PubMed] [Google Scholar]
- 21.Coquelin A., Desjardins C. Luteinizing hormone and testosterone secretion in young and old male mice. Am. J. Physiol. 1982;243:E257–E263. doi: 10.1152/ajpendo.1982.243.3.E257. [DOI] [PubMed] [Google Scholar]
- 22.Goh H.H., Ratnam S.S. The lh surge in humans: Its mechanism and sex difference. Gynecol. Endocrinol. 1988;2:165–182. doi: 10.3109/09513598809023624. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M., Watanabe K., Matsumura R., Anayama N., Miyamoto A., Miyazaki H., Miyazaki K., Shimizu T., Akashi M. Involvement of the luteinizing hormone surge in the regulation of ovary and oviduct clock gene expression in mice. Genes Cells. 2018 doi: 10.1111/gtc.12605. [DOI] [PubMed] [Google Scholar]
- 24.Chianese R., Cobellis G., Chioccarelli T., Ciaramella V., Migliaccio M., Fasano S., Pierantoni R., Meccariello R. Kisspeptins, estrogens and male fertility. Curr Med. Chem. 2016;23:4070–4091. doi: 10.2174/0929867323666160902155434. [DOI] [PubMed] [Google Scholar]
- 25.Rahman S.A., Grant L.K., Gooley J.J., Rajaratnam S.M.W., Czeisler C.A., Lockley S.W. Endogenous circadian regulation of female reproductive hormones. J. Clin. Endocrinol. Metab. 2019;104:6049–6059. doi: 10.1210/jc.2019-00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davison S.L., Bell R. Androgen physiology. Semin Reprod Med. 2006;24:71–77. doi: 10.1055/s-2006-939565. [DOI] [PubMed] [Google Scholar]
- 27.Cooke P.S., Nanjappa M.K., Ko C., Prins G.S., Hess R.A. Estrogens in male physiology. Physiol. Rev. 2017;97:995–1043. doi: 10.1152/physrev.00018.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naftolin F., Garcia-Segura L.M., Horvath T.L., Zsarnovszky A., Demir N., Fadiel A., Leranth C., Vondracek-Klepper S., Lewis C., Chang A., et al. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–116. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- 29.Shea J.L., Wong P.Y., Chen Y. Free testosterone: Clinical utility and important analytical aspects of measurement. Adv. Clin. Chem. 2014;63:59–84. doi: 10.1016/b978-0-12-800094-6.00002-9. [DOI] [PubMed] [Google Scholar]
- 30.Shiina H., Matsumoto T., Sato T., Igarashi K., Miyamoto J., Takemasa S., Sakari M., Takada I., Nakamura T., Metzger D., et al. Premature ovarian failure in androgen receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickmeis T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009;200:3–22. doi: 10.1677/JOE-08-0415. [DOI] [PubMed] [Google Scholar]
- 32.Silva E.J., Vendramini V., Restelli A., Bertolla R.P., Kempinas W.G., Avellar M.C. Impact of adrenalectomy and dexamethasone treatment on testicular morphology and sperm parameters in rats: Insights into the adrenal control of male reproduction. Andrology. 2014;2:835–846. doi: 10.1111/j.2047-2927.2014.00228.x. [DOI] [PubMed] [Google Scholar]
- 33.Whirledge S., Cidlowski J.A. A role for glucocorticoids in stress-impaired reproduction: Beyond the hypothalamus and pituitary. Endocrinology. 2013;154:4450–4468. doi: 10.1210/en.2013-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kratz E.M., Piwowar A. Melatonin, advanced oxidation protein products and total antioxidant capacity as seminal parameters of prooxidant-antioxidant balance and their connection with expression of metalloproteinases in context of male fertility. J. Physiol. Pharmacol. 2017;68:659–668. [PubMed] [Google Scholar]
- 35.Zhang P., Zheng Y., Lv Y., Li F., Su L., Qin Y., Zeng W. Melatonin protects the mouse testis against heat-induced damage. Mol. Hum. Reprod. 2020;26:65–79. doi: 10.1093/molehr/gaaa002. [DOI] [PubMed] [Google Scholar]
- 36.Bejarano I., Monllor F., Marchena A.M., Ortiz A., Lozano G., Jimenez M.I., Gaspar P., Garcia J.F., Pariente J.A., Rodriguez A.B., et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J. Pineal Res. 2014;57:333–339. doi: 10.1111/jpi.12172. [DOI] [PubMed] [Google Scholar]
- 37.Perez S., Murias L., Fernandez-Plaza C., Diaz I., Gonzalez C., Otero J., Diaz E. Evidence for clock genes circadian rhythms in human full-term placenta. Syst Biol. Reprod Med. 2015;61:360–366. doi: 10.3109/19396368.2015.1069420. [DOI] [PubMed] [Google Scholar]
- 38.Simonneaux V., Bahougne T., Angelopoulou E. Daily rhythms count for female fertility. Best Pract Res. Clin. Endocrinol. Metab. 2017;31:505–519. doi: 10.1016/j.beem.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Weiser M.J., Wu T.J., Handa R.J. Estrogen receptor-beta agonist diarylpropionitrile: Biological activities of r- and s-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150:1817–1825. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He P.J., Hirata M., Yamauchi N., Hattori M.A. Up-regulation of per1 expression by estradiol and progesterone in the rat uterus. J. Endocrinol. 2007;194:511–519. doi: 10.1677/JOE-07-0172. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T.J., Sellix M.T., Menaker M., Block G.D. Estrogen directly modulates circadian rhythms of per2 expression in the uterus. Am. J. Physiol. Endocrinol. Metab. 2008;295:E1025–E1031. doi: 10.1152/ajpendo.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li S., Wang M., Ao X., Chang A.K., Yang C., Zhao F., Bi H., Liu Y., Xiao L., Wu H. Clock is a substrate of sumo and sumoylation of clock upregulates the transcriptional activity of estrogen receptor-alpha. Oncogene. 2013;32:4883–4891. doi: 10.1038/onc.2012.518. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez J.D., Chen D., Storer E., Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biol. Reprod. 2003;69:81–91. doi: 10.1095/biolreprod.102.011833. [DOI] [PubMed] [Google Scholar]
- 44.Sellix M.T., Murphy Z.C., Menaker M. Excess androgen during puberty disrupts circadian organization in female rats. Endocrinology. 2013;154:1636–1647. doi: 10.1210/en.2012-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franks S. Polycystic ovary syndrome in adolescents. Int J. Obes (Lond.) 2008;32:1035–1041. doi: 10.1038/ijo.2008.61. [DOI] [PubMed] [Google Scholar]
- 46.Franks S. Genetic and environmental origins of obesity relevant to reproduction. Reprod Biomed. Online. 2006;12:526–531. doi: 10.1016/S1472-6483(10)61177-7. [DOI] [PubMed] [Google Scholar]
- 47.Balasubramanian P., Jagannathan L., Mahaley R.E., Subramanian M., Gilbreath E.T., Mohankumar P.S., Mohankumar S.M. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J. Neuroendocrinol. 2012;24:748–755. doi: 10.1111/j.1365-2826.2011.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mereness A.L., Murphy Z.C., Sellix M.T. Developmental programming by androgen affects the circadian timing system in female mice. Biol. Reprod. 2015;92:88. doi: 10.1095/biolreprod.114.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sapolsky R.M., Romero L.M., Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 50.Hasenmajer V., Sbardella E., Sciarra F., Minnetti M., Isidori A.M., Venneri M.A. The immune system in cushing’s syndrome. Trends Endocrinol. Metab. 2020 doi: 10.1016/j.tem.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Isidori A.M., Venneri M.A., Graziadio C., Simeoli C., Fiore D., Hasenmajer V., Sbardella E., Gianfrilli D., Pozza C., Pasqualetti P., et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (dream): A single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:173–185. doi: 10.1016/S2213-8587(17)30398-4. [DOI] [PubMed] [Google Scholar]
- 52.Vitellius G., Trabado S., Bouligand J., Delemer B., Lombes M. Pathophysiology of glucocorticoid signaling. Ann. Endocrinol. 2018;79:98–106. doi: 10.1016/j.ando.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Luo E., Stephens S.B., Chaing S., Munaganuru N., Kauffman A.S., Breen K.M. Corticosterone blocks ovarian cyclicity and the lh surge via decreased kisspeptin neuron activation in female mice. Endocrinology. 2016;157:1187–1199. doi: 10.1210/en.2015-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirby E.D., Geraghty A.C., Ubuka T., Bentley G.E., Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. USA. 2009;106:11324–11329. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bambino T.H., Hsueh A.J. Direct inhibitory effect of glucocorticoids upon testicular luteinizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology. 1981;108:2142–2148. doi: 10.1210/endo-108-6-2142. [DOI] [PubMed] [Google Scholar]
- 56.Whirledge S., Cidlowski J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35:109–125. [PMC free article] [PubMed] [Google Scholar]
- 57.Li Q.N., Li L., Hou G., Wang Z.B., Hou Y., Liu Z.H., Schatten H., Sun Q.Y. Glucocorticoid exposure affects female fertility by exerting its effect on the uterus but not on the oocyte: Lessons from a hypercortisolism mouse model. Hum. Reprod. 2018;33:2285–2294. doi: 10.1093/humrep/dey322. [DOI] [PubMed] [Google Scholar]
- 58.Rhen T., Grissom S., Afshari C., Cidlowski J.A. Dexamethasone blocks the rapid biological effects of 17beta-estradiol in the rat uterus without antagonizing its global genomic actions. Faseb J. 2003;17:1849–1870. doi: 10.1096/fj.02-1099com. [DOI] [PubMed] [Google Scholar]
- 59.Dare J.B., Arogundade B., Awoniyi O.O., Adegoke A.A., Adekomi D.A. Dexamethasone as endocrine disruptor; type i and type ii (anti) oestrogenic actions on the ovary and uterus of adult wistar rats (rattus novergicus) JBRA Assist. Reprod. 2018;22:307–313. doi: 10.5935/1518-0557.20180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ristic N., Nestorovic N., Manojlovic-Stojanoski M., Trifunovic S., Ajdzanovic V., Filipovic B., Pendovski L., Milosevic V. Adverse effect of dexamethasone on development of the fetal rat ovary. Fundam Clin. Pharmacol. 2019;33:199–207. doi: 10.1111/fcp.12415. [DOI] [PubMed] [Google Scholar]
- 61.Yang G., Chen L., Grant G.R., Paschos G., Song W.L., Musiek E.S., Lee V., McLoughlin S.C., Grosser T., Cotsarelis G., et al. Timing of expression of the core clock gene bmal1 influences its effects on aging and survival. Sci. Transl Med. 2016;8:324ra316. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fowden A.L., Forhead A.J. Glucocorticoids as regulatory signals during intrauterine development. Exp. Physiol. 2015;100:1477–1487. doi: 10.1113/EP085212. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J., Zhang J., Liu R., Gan J., Liu J., Liu W. Endocrine-disrupting effects of pesticides through interference with human glucocorticoid receptor. Environ. Sci. Technol. 2016;50:435–443. doi: 10.1021/acs.est.5b03731. [DOI] [PubMed] [Google Scholar]
- 64.de Barros J.W.F., Borges C.D.S., Missassi G., Pacheco T.L., De Grava Kempinas W. Impact of intrauterine exposure to betamethasone on the testes and epididymides of prepubertal rats. Chem Biol. Interact. 2018;291:202–211. doi: 10.1016/j.cbi.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 65.Borges C.D.S., Pacheco T.L., da Silva K.P., Fernandes F.H., Gregory M., Pupo A.S., Salvadori D.M.F., Cyr D.G., Kempinas W.G. Betamethasone causes intergenerational reproductive impairment in male rats. Reprod Toxicol. 2017;71:108–117. doi: 10.1016/j.reprotox.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Chen H., Gao L., Xiong Y., Yang D., Li C., Wang A., Jin Y. Circadian clock and steroidogenic-related gene expression profiles in mouse leydig cells following dexamethasone stimulation. Biochem. Biophys. Res. Commun. 2017;483:294–300. doi: 10.1016/j.bbrc.2016.12.149. [DOI] [PubMed] [Google Scholar]
- 67.Ilacqua A., Izzo G., Emerenziani G.P., Baldari C., Aversa A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod Biol. Endocrinol. 2018;16:115. doi: 10.1186/s12958-018-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milardi D., Luca G., Grande G., Ghezzi M., Caretta N., Brusco G., De Filpo G., Marana R., Pontecorvi A., Calafiore R., et al. Prednisone treatment in infertile patients with oligozoospermia and accessory gland inflammatory alterations. Andrology. 2017;5:268–273. doi: 10.1111/andr.12300. [DOI] [PubMed] [Google Scholar]
- 69.Bals-Pratsch M., Doren M., Karbowski B., Schneider H.P., Nieschlag E. Cyclic corticosteroid immunosuppression is unsuccessful in the treatment of sperm antibody-related male infertility: A controlled study. Hum. Reprod. 1992;7:99–104. doi: 10.1093/oxfordjournals.humrep.a137568. [DOI] [PubMed] [Google Scholar]
- 70.Rivier C., Vale W. Stimulatory effect of interleukin-1 on adrenocorticotropin secretion in the rat: Is it modulated by prostaglandins? Endocrinology. 1991;129:384–388. doi: 10.1210/endo-129-1-384. [DOI] [PubMed] [Google Scholar]
- 71.Morrison D., Capewell S., Reynolds S.P., Thomas J., Ali N.J., Read G.F., Henley R., Riad-Fahmy D. Testosterone levels during systemic and inhaled corticosteroid therapy. Respir Med. 1994;88:659–663. doi: 10.1016/S0954-6111(05)80062-9. [DOI] [PubMed] [Google Scholar]
- 72.Huang B., Qian C., Ding C., Meng Q., Zou Q., Li H. Fetal liver mesenchymal stem cells restore ovarian function in premature ovarian insufficiency by targeting mt1. Stem Cell Res. Ther. 2019;10:362. doi: 10.1186/s13287-019-1490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elokil A.A., Bhuiyan A.A., Liu H.Z., Hussein M.N., Ahmed H.I., Azmal S.A., Yang L., Li S. The capability of l-carnitine-mediated antioxidant on cock during aging: Evidence for the improved semen quality and enhanced testicular expressions of gnrh1, gnrhr, and melatonin receptors mt 1/2. Poult Sci. 2019;98:4172–4181. doi: 10.3382/ps/pez201. [DOI] [PubMed] [Google Scholar]
- 74.Espino J., Macedo M., Lozano G., Ortiz A., Rodriguez C., Rodriguez A.B., Bejarano I. Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants. 2019;8:338. doi: 10.3390/antiox8090338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nehme P.A., Amaral F., Lowden A., Skene D.J., Cipolla-Neto J., Moreno C.R.C. Reduced melatonin synthesis in pregnant night workers: Metabolic implications for offspring. Med. Hypotheses. 2019;132:109353. doi: 10.1016/j.mehy.2019.109353. [DOI] [PubMed] [Google Scholar]
- 76.Green A., Barak S., Shine L., Kahane A., Dagan Y. Exposure by males to light emitted from media devices at night is linked with decline of sperm quality and correlated with sleep quality measures. Chronobiol. Int. 2020;37:414–424. doi: 10.1080/07420528.2020.1727918. [DOI] [PubMed] [Google Scholar]
- 77.Zheng M., Tong J., Li W.P., Chen Z.J., Zhang C. Melatonin concentration in follicular fluid is correlated with antral follicle count (afc) and in vitro fertilization (ivf) outcomes in women undergoing assisted reproductive technology (art) procedures. Gynecol. Endocrinol. 2018;34:446–450. doi: 10.1080/09513590.2017.1409713. [DOI] [PubMed] [Google Scholar]
- 78.Latif Khan H., Bhatti S., Latif Khan Y., Abbas S., Munir Z., Rahman Khan Sherwani I.A., Suhail S., Hassan Z., Aydin H.H. Cell-free nucleic acids and melatonin levels in human follicular fluid predict embryo quality in patients undergoing in-vitro fertilization treatment. J. Gynecol. Obstet Hum. Reprod. 2020;49:101624. doi: 10.1016/j.jogoh.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Macchi M.M., Bruce J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocrinol. 2004;25:177–195. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Waldhauser F., Boepple P.A., Schemper M., Mansfield M.J., Crowley W.F., Jr. Serum melatonin in central precocious puberty is lower than in age-matched prepubertal children. J. Clin. Endocrinol. Metab. 1991;73:793–796. doi: 10.1210/jcem-73-4-793. [DOI] [PubMed] [Google Scholar]
- 81.Bergiannaki J.D., Soldatos C.R., Paparrigopoulos T.J., Syrengelas M., Stefanis C.N. Low and high melatonin excretors among healthy individuals. J. Pineal Res. 1995;18:159–164. doi: 10.1111/j.1600-079X.1995.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 82.Laughlin G.A., Loucks A.B., Yen S.S. Marked augmentation of nocturnal melatonin secretion in amenorrheic athletes, but not in cycling athletes: Unaltered by opioidergic or dopaminergic blockade. J. Clin. Endocrinol. Metab. 1991;73:1321–1326. doi: 10.1210/jcem-73-6-1321. [DOI] [PubMed] [Google Scholar]
- 83.Karasek M., Pawlikowski M., Nowakowska-Jankiewicz B., Kolodziej-Maciejewska H., Zieleniewski J., Cieslak D., Leidenberger F. Circadian variations in plasma melatonin, fsh, lh, and prolactin and testosterone levels in infertile men. J. Pineal Res. 1990;9:149–157. doi: 10.1111/j.1600-079X.1990.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 84.Pilorz V., Steinlechner S. Low reproductive success in per1 and per2 mutant mouse females due to accelerated ageing? Reproduction. 2008;135:559–568. doi: 10.1530/REP-07-0434. [DOI] [PubMed] [Google Scholar]
- 85.Zheng B., Albrecht U., Kaasik K., Sage M., Lu W., Vaishnav S., Li Q., Sun Z.S., Eichele G., Bradley A., et al. Nonredundant roles of the mper1 and mper2 genes in the mammalian circadian clock. Cell. 2001;105:683–694. doi: 10.1016/S0092-8674(01)00380-4. [DOI] [PubMed] [Google Scholar]
- 86.Zheng Y., Liu C., Li Y., Jiang H., Yang P., Tang J., Xu Y., Wang H., He Y. Loss-of-function mutations with circadian rhythm regulator per1/per2 lead to premature ovarian insufficiencydagger. Biol. Reprod. 2019;100:1066–1072. doi: 10.1093/biolre/ioy245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vitaterna M.H., King D.P., Chang A.M., Kornhauser J.M., Lowrey P.L., McDonald J.D., Dove W.F., Pinto L.H., Turek F.W., Takahashi J.S. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264:719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dolatshad H., Campbell E.A., O’Hara L., Maywood E.S., Hastings M.H., Johnson M.H. Developmental and reproductive performance in circadian mutant mice. Hum. Reprod. 2006;21:68–79. doi: 10.1093/humrep/dei313. [DOI] [PubMed] [Google Scholar]
- 89.King D.P., Zhao Y., Sangoram A.M., Wilsbacher L.D., Tanaka M., Antoch M.P., Steeves T.D., Vitaterna M.H., Kornhauser J.M., Lowrey P.L., et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/S0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boden M.J., Varcoe T.J., Voultsios A., Kennaway D.J. Reproductive biology of female bmal1 null mice. Reproduction. 2010;139:1077–1090. doi: 10.1530/REP-09-0523. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y., Johnson B.P., Shen A.L., Wallisser J.A., Krentz K.J., Moran S.M., Sullivan R., Glover E., Parlow A.F., Drinkwater N.R., et al. Loss of bmal1 in ovarian steroidogenic cells results in implantation failure in female mice. Proc. Natl Acad Sci. USA. 2014;111:14295–14300. doi: 10.1073/pnas.1209249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alvarez J.D., Hansen A., Ord T., Bebas P., Chappell P.E., Giebultowicz J.M., Williams C., Moss S., Sehgal A. The circadian clock protein bmal1 is necessary for fertility and proper testosterone production in mice. J. Biol. Rhythms. 2008;23:26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennaway D.J., Boden M.J., Voultsios A. Reproductive performance in female clock delta19 mutant mice. Reprod Fertil Dev. 2004;16:801–810. doi: 10.1071/RD04023. [DOI] [PubMed] [Google Scholar]
- 94.Miller B.H., Olson S.L., Levine J.E., Turek F.W., Horton T.H., Takahashi J.S. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and clock mutant mice. Biol. Reprod. 2006;75:778–784. doi: 10.1095/biolreprod.106.052845. [DOI] [PubMed] [Google Scholar]
- 95.Kumar P., Sait S.F. Luteinizing hormone and its dilemma in ovulation induction. J. Hum. Reprod Sci. 2011;4:2–7. doi: 10.4103/0974-1208.82351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chu G., Ma G., Sun J., Zhu Y., Xiang A., Yang G., Sun S. Leptin receptor mediates bmal1 regulation of estrogen synthesis in granulosa cells. Animals. 2019;9:899. doi: 10.3390/ani9110899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DeAngelis A.M., Roy-O’Reilly M., Rodriguez A. Genetic alterations affecting cholesterol metabolism and human fertility. Biol. Reprod. 2014;91:117. doi: 10.1095/biolreprod.114.119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Britt K.L., Drummond A.E., Dyson M., Wreford N.G., Jones M.E., Simpson E.R., Findlay J.K. The ovarian phenotype of the aromatase knockout (arko) mouse. J. Steroid Biochem Mol. Biol. 2001;79:181–185. doi: 10.1016/S0960-0760(01)00158-3. [DOI] [PubMed] [Google Scholar]
- 99.Jones M.E., Thorburn A.W., Britt K.L., Hewitt K.N., Wreford N.G., Proietto J., Oz O.K., Leury B.J., Robertson K.M., Yao S., et al. Aromatase-deficient (arko) mice have a phenotype of increased adiposity. Proc. Natl Acad Sci. USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kudo T., Kawashima M., Tamagawa T., Shibata S. Clock mutation facilitates accumulation of cholesterol in the liver of mice fed a cholesterol and/or cholic acid diet. Am. J. Physiol. Endocrinol. Metab. 2008;294:E120–E130. doi: 10.1152/ajpendo.00061.2007. [DOI] [PubMed] [Google Scholar]
- 101.Morse D., Cermakian N., Brancorsini S., Parvinen M., Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol. Endocrinol. 2003;17:141–151. doi: 10.1210/me.2002-0184. [DOI] [PubMed] [Google Scholar]
- 102.Bebas P., Goodall C.P., Majewska M., Neumann A., Giebultowicz J.M., Chappell P.E. Circadian clock and output genes are rhythmically expressed in extratesticular ducts and accessory organs of mice. Faseb j. 2009;23:523–533. doi: 10.1096/fj.08-113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liang X., Cheng S., Jiang X., He X., Wang Y., Jiang Z., Hou W., Li S., Liu Y., Wang Z. The noncircadian function of the circadian clock gene in the regulation of male fertility. J. Biol. Rhythms. 2013;28:208–217. doi: 10.1177/0748730413486873. [DOI] [PubMed] [Google Scholar]
- 104.Cheng S., Liang X., Wang Y., Jiang Z., Liu Y., Hou W., Li S., Zhang J., Wang Z. The circadian clock gene regulates acrosin activity of sperm through serine protease inhibitor a3k. Exp. Biol. Med. 2016;241:205–215. doi: 10.1177/1535370215597199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Baburski A.Z., Sokanovic S.J., Bjelic M.M., Radovic S.M., Andric S.A., Kostic T.S. Circadian rhythm of the leydig cells endocrine function is attenuated during aging. Exp. Gerontol. 2016;73:5–13. doi: 10.1016/j.exger.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Li C., Xiao S., Hao J., Liao X., Li G. Cry1 deficiency leads to testicular dysfunction and altered expression of genes involved in cell communication, chromatin reorganization, spermatogenesis, and immune response in mouse testis. Mol. Reprod Dev. 2018;85:325–335. doi: 10.1002/mrd.22968. [DOI] [PubMed] [Google Scholar]
- 107.Peterlin A., Kunej T., Peterlin B. The role of circadian rhythm in male reproduction. Curr. Opin. Endocrinol. Diabetes Obes. 2019;26:313–316. doi: 10.1097/MED.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 108.Resuehr H.E., Resuehr D., Olcese J. Induction of mper1 expression by gnrh in pituitary gonadotrope cells involves egr-1. Mol. Cell Endocrinol. 2009;311:120–125. doi: 10.1016/j.mce.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 109.Chu A., Zhu L., Blum I.D., Mai O., Leliavski A., Fahrenkrug J., Oster H., Boehm U., Storch K.F. Global but not gonadotrope-specific disruption of bmal1 abolishes the luteinizing hormone surge without affecting ovulation. Endocrinology. 2013;154:2924–2935. doi: 10.1210/en.2013-1080. [DOI] [PubMed] [Google Scholar]
- 110.Elks C.E., Perry J.R., Sulem P., Chasman D.I., Franceschini N., He C., Lunetta K.L., Visser J.A., Byrne E.M., Cousminer D.L., et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 2010;42:1077–1085. doi: 10.1038/ng.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perry J.R., Day F., Elks C.E., Sulem P., Thompson D.J., Ferreira T., He C., Chasman D.I., Esko T., Thorleifsson G., et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514:92–97. doi: 10.1038/nature13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Vries L., Kauschansky A., Shohat M., Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J. Clin. Endocrinol. Metab. 2004;89:1794–1800. doi: 10.1210/jc.2003-030361. [DOI] [PubMed] [Google Scholar]
- 113.Wehkalampi K., Widen E., Laine T., Palotie A., Dunkel L. Patterns of inheritance of constitutional delay of growth and puberty in families of adolescent girls and boys referred to specialist pediatric care. J. Clin. Endocrinol. Metab. 2008;93:723–728. doi: 10.1210/jc.2007-1786. [DOI] [PubMed] [Google Scholar]
- 114.Day F.R., Thompson D.J., Helgason H., Chasman D.I., Finucane H., Sulem P., Ruth K.S., Whalen S., Sarkar A.K., Albrecht E., et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 2017;49:834–841. doi: 10.1038/ng.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Flynn-Evans E.E., Stevens R.G., Tabandeh H., Schernhammer E.S., Lockley S.W. Effect of light perception on menarche in blind women. Ophthalmic Epidemiol. 2009;16:243–248. doi: 10.1080/09286580902863056. [DOI] [PubMed] [Google Scholar]
- 116.Weger B.D., Gobet C., Yeung J., Martin E., Jimenez S., Betrisey B., Foata F., Berger B., Balvay A., Foussier A., et al. The mouse microbiome is required for sex-specific diurnal rhythms of gene expression and metabolism. Cell Metab. 2019;29:362–382.e368. doi: 10.1016/j.cmet.2018.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Montagner A., Korecka A., Polizzi A., Lippi Y., Blum Y., Canlet C., Tremblay-Franco M., Gautier-Stein A., Burcelin R., Yen Y.C., et al. Hepatic circadian clock oscillators and nuclear receptors integrate microbiome-derived signals. Sci. Rep. 2016;6:20127. doi: 10.1038/srep20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bisanti L., Olsen J., Basso O., Thonneau P., Karmaus W. Shift work and subfecundity: A european multicenter study. European study group on infertility and subfecundity. J. Occup Environ. Med. 1996;38:352–358. doi: 10.1097/00043764-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 119.Labyak S., Lava S., Turek F., Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care Women Int. 2002;23:703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y., Meng N., Bao H., Jiang Y., Yang N., Wu K., Wu J., Wang H., Kong S., Zhang Y. Circadian gene per1 senses progesterone signal during human endometrial decidualization. J. Endocrinol. 2019;243:229–243. doi: 10.1530/JOE-19-0284. [DOI] [PubMed] [Google Scholar]
- 121.Lv S., Wang N., Ma J., Li W.P., Chen Z.J., Zhang C. Impaired decidualization caused by downregulation of circadian clock gene bmal1 contributes to human recurrent miscarriagedagger. Biol. Reprod. 2019;101:138–147. doi: 10.1093/biolre/ioz063. [DOI] [PubMed] [Google Scholar]
- 122.Kovanen L., Saarikoski S.T., Aromaa A., Lonnqvist J., Partonen T. Arntl (bmal1) and npas2 gene variants contribute to fertility and seasonality. PLoS ONE. 2010;5:e10007. doi: 10.1371/journal.pone.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen M., Xu Y., Miao B., Zhao H., Luo L., Shi H., Zhou C. Expression pattern of circadian genes and steroidogenesis-related genes after testosterone stimulation in the human ovary. J. Ovarian Res. 2016;9:56. doi: 10.1186/s13048-016-0264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brzezinski A., Saada A., Miller H., Brzezinski-Sinai N.A., Ben-Meir A. Is the aging human ovary still ticking?: Expression of clock-genes in luteinized granulosa cells of young and older women. J. Ovarian Res. 2018;11:95. doi: 10.1186/s13048-018-0471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li R., Cheng S., Wang Z. Circadian clock gene plays a key role on ovarian cycle and spontaneous abortion. Cell Physiol. Biochem. 2015;37:911–920. doi: 10.1159/000430218. [DOI] [PubMed] [Google Scholar]
- 126.Shen O., Ding X., Nie J., Xia Y., Wang X., Tong J., Zhang J. Variants of the clock gene affect the risk of idiopathic male infertility in the han-chinese population. Chronobiol. Int. 2015;32:959–965. doi: 10.3109/07420528.2015.1056305. [DOI] [PubMed] [Google Scholar]
- 127.Zhang J., Ding X., Li Y., Xia Y., Nie J., Yi C., Wang X., Tong J. Association of clock gene variants with semen quality in idiopathic infertile han-chinese males. Reprod Biomed. Online. 2012;25:536–542. doi: 10.1016/j.rbmo.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 128.Ramasamy R., Bakircioglu M.E., Cengiz C., Karaca E., Scovell J., Jhangiani S.N., Akdemir Z.C., Bainbridge M., Yu Y., Huff C., et al. Whole-exome sequencing identifies novel homozygous mutation in npas2 in family with nonobstructive azoospermia. Fertil Steril. 2015;104:286–291. doi: 10.1016/j.fertnstert.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]