Abstract
There are more than one million patients worldwide suffering paralysis caused by spinal cord injury (SCI). SCI causes severe socioeconomic problems not only to the patients and their caregivers but also to society; therefore, the development of innovative treatments is crucial. Many pharmacological therapies have been attempted in an effort to reduce SCI-related damage; however, no single therapy that could dramatically improve the serious long-term sequelae of SCI has emerged. Stem cell transplantation therapy, which can ameliorate damage or regenerate neurological networks, has been proposed as a promising candidate for SCI treatment, and many basic and clinical experiments using stem cells for SCI treatment have been launched, with promising results. However, the cell transplantation methods, including cell type, dose, transplantation route, and transplantation timing, vary widely between trials, and there is no consensus regarding the most effective treatment strategy. This study reviews the current knowledge on this issue, with a special focus on the clinical trials that have used stem cells for treating SCI, and highlights the problems that remain to be solved before the widespread clinical use of stem cells can be adopted.
Keywords: stem cell, spinal cord injury, neurogenesis, inflammation, regenerative medicine, transplantation
1. Introduction
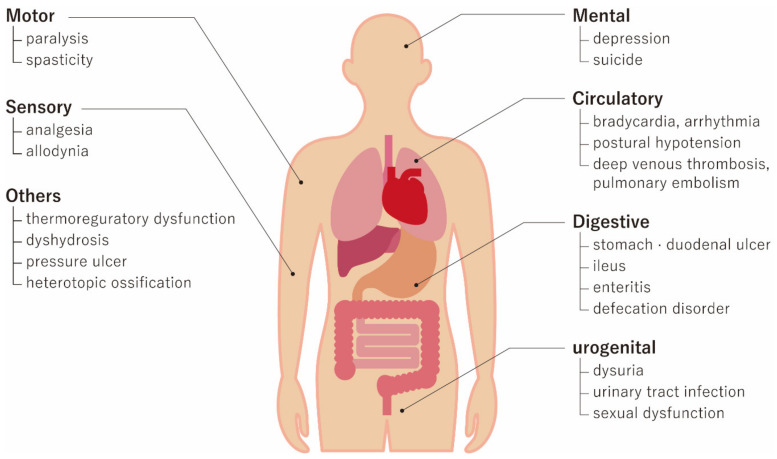
Spinal cord injury (SCI) is mainly caused by severe trauma from traffic accidents, falls, and sports-related injuries, and there are more than one million patients worldwide suffering from SCI-related paralysis. With an increasingly aging society, the number of cases of elderly patients with SCI caused by low-energy falls has increased [1,2]. SCI not only cause motor function deficits, such as paralysis, but also lead to many other severe medical problems, including respiratory, urogenital, and skin problems (Figure 1) [3]. In addition to severe medical problems, SCI patients are generally young and require longer medical and social care, resulting in severe socioeconomic problems not only to the patients and their caregivers but also to society [4]. Therefore, the development of innovative treatments for SCI is eagerly anticipated [5,6,7,8]. However, various medications yielding positive results in animal models of SCI, including methylprednisolone sodium succinate, naloxone, tirilazad mesylate, and nimodipine, have failed to show beneficial effects in human clinical trials [9,10,11]. This failure is often attributed to the fact that SCI involves multiple types of cellular damage that can change over time, making it difficult for a single drug to sufficiently attenuate the damage. Under these circumstances, stem cell therapies have raised significant interest, given that they offer multiple recovery mechanisms for ameliorating SCI-related damage. Numerous experimental studies and clinical trials on the stem cell treatment of SCI have been conducted worldwide [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. However, the clinical trials are conducted in various ways, and there is no consensus regarding the most effective methods, including transplantation timing, cell type, cell dosage, and transplantation route. This review briefly summarizes the pathophysiology of SCI and the therapeutic potential of stem cells and reviews published clinical trials with a specific focus on the different methodologies used in order to highlight unsolved issues in the treatment of SCI using a stem cell approach.
Figure 1.
Systemic medical problems after spinal cord injury (SCI). SCI can cause motor functional deficits of paralysis and increased spasticity. Sensory disturbance includes severe analgesia below the level of injury and allodynia. SCI can also affect sufferers mentally by causing depression and possible suicide. Circulatory, digestive, and urogenital impairments need to be treated, as well as skin problems.
2. Pathophysiology of SCI and Therapeutic Targets
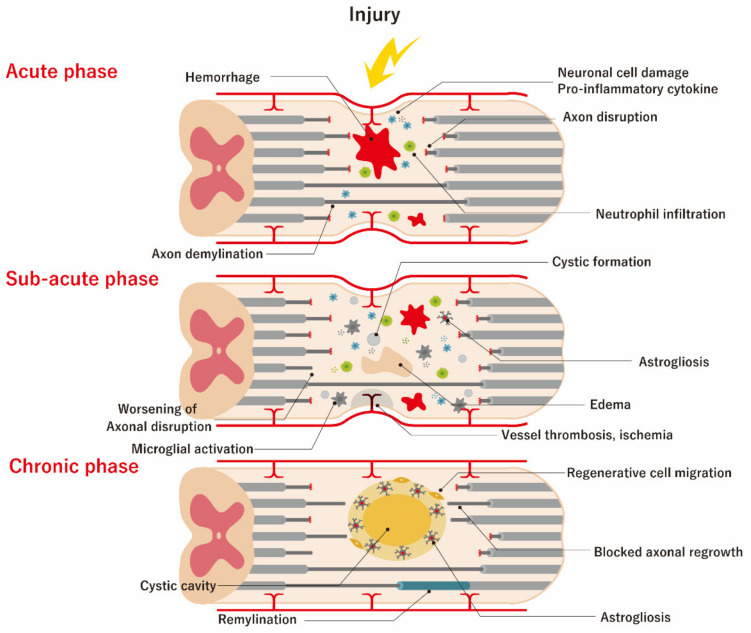
SCI comprises two distinct mechanisms. One is the primary damage caused by the compression and contusion of the spinal cord, resulting in the damage of neuronal and glial cell membranes and the disruption of the microvasculature at the time of injury [49,50]. The other is the secondary damage cascade consisting of tissue swelling from hemorrhage and edema, inflammation, cytotoxic free radical and excitotoxic substance generation, and excessive gliosis, which takes hours to months to develop [51]. Ultra-early surgical interventions, including spinal cord decompression and spine fixation, are routinely performed, but it is often difficult to accomplish complete recovery from SCI, because primary damage has already occurred at the time of injury [6,52]. Therefore, the main current target for SCI treatment is to decrease or halt the secondary damage cascade, which can be divided into acute (within a few days), sub-acute (a few days to 6 months), and chronic (>6 months) phases (Figure 2).
Figure 2.
Pathophysiology of spinal cord injury (SCI). Secondary injury can be divided into three phases, which are acute (within a few days), sub-acute (2 days to 6 months), and chronic (over 6 months). During the acute phase, both vascular and cell membrane damage takes place. Vascular damage can cause hemorrhage and blood spinal cord barrier (BSCB) disruption. The mass effect created by massive hemorrhage can additionally damage the surrounding viable tissues. The BSCB draws the rapid infiltration of inflammatory cells such as neutrophils, resulting in the release of various pro-inflammatory cytokines. Damaged and/or necrotic cells release ATP, potassium ions, and DNA into their microenvironment, which can activate microglia to release additional proinflammatory cytokines and induce the recruitment of more peripheral inflammatory cells. During the sub-acute phase, arterial vessel damage compromises the vascular supply, which can aggravate ischemic damage to the surviving neuronal cells; meanwhile, edema caused by the alteration of vascular membrane permeability leads to further neuronal and vascular damage. Inflammatory cytokines are released from resident and blood-derived cells, and glutamate is released from damaged neuronal cells. The failure of the astrocytic re-uptake of these damage-associated molecular-pattern molecules (DAMPs) can further compromise the neuronal network, resulting in a worsening of demyelination. Inflammatory cytokines can upregulate astrocytes into the active state of astrogliosis, causing them to migrate to the damaged area to isolate it from unaffected areas; this can be considered as a physiological rescue process. In the chronic phase of SCI, the loss of cell volume leads to the vacuo formation of cystic micro-cavitation which is also called as syringomyelia, and this coalesce and forms a barrier for cell migration and regeneration of axon regrowth. In reactive astrogliosis, astrocytes secrete inhibitory chondroitin sulfate proteoglycans, which are initially protective in blocking the DAMPs from spreading, but which eventually interfere with the regeneration and extension of the neuronal network. On the other hand, low-gear reorganization also commences in the chronic phase, including vascular remodeling, alterations in the extracellular matrix composition, regenerative cell migration, and re-organization of neural circuits.
During the acute phase, both vascular and cell membrane damage occurs. Vascular damage can cause hemorrhage and blood spinal cord barrier (BSCB) disruption. Additionally, a mass effect created by hemorrhage can damage surrounding viable tissues. The BSCB promotes the rapid infiltration of inflammatory cells, such as neutrophils, resulting in the release of various proinflammatory cytokines (e.g., tumor necrosis factor-α (TNF-α) or interleukin-1β (IL-1β)) [53]. Damaged and/or necrotic cells release ATP, potassium ions, and DNA into their microenvironment, which can activate microglia to release additional proinflammatory cytokines and induce the recruitment of more peripheral inflammatory cells.
During the sub-acute phase, arterial vessel damage compromises the vascular supply, which can aggravate ischemic damage to the surviving neuronal cells. Additionally, edema caused by the alteration of vascular membrane permeability leads to further neuronal and vascular damage. Inflammatory cytokines (also referred to as the inflammasome) are released from resident and blood-derived cells, and glutamate is released from damaged neuronal cells [54]. The failure of astrocytic re-uptake of these damage-associated molecular-pattern molecules (DAMPs) can further compromise the neuronal network, resulting in a worsening of demyelination [55,56]. Inflammatory cytokines can upregulate astrocytes into the active state of astrogliosis, causing them to migrate to the damaged area to isolate it from unaffected areas, with this considered a physiological rescue process.
In the chronic phase of SCI, the loss of cell volume leads to the vacuo formation of cystic micro-cavitation (referred to as syringomyelia), which coalesces and forms a barrier to cell migration and regeneration of axon regrowth. In astrogliosis, astrocytes secrete inhibitory chondroitin sulfate proteoglycans, which are initially protective in blocking the DAMPs from spreading but eventually interfere with regeneration and extension of the neuronal network. The disruption of BSCB permeability can also be found at this stage and is responsible for the leakage of intravascular components, resulting in chronic inflammation [57]. However, low-gear reorganization also commences in the chronic phase and includes vascular remodeling, alterations in extracellular matrix composition, regenerative cell migration, and re-organization of neural circuits [58,59,60].
3. Mechanisms of Action of Stem Cell Transplantation
Extensive efforts have been applied to elucidate the mode of action of stem cell transplantation in treating SCI, and multiple descriptive reviews have been published [61,62]. Transplanted cells have been shown to exert a variety of neuro- and vascular-protective effects at the different phases of SCI. The cells not only reorganize the neuronal network but also have the capacity for reducing local and systemic inflammation, supporting axonal regeneration and synaptic sprouting, and reducing glial scars. The mechanisms can be sub-categorized into three distinct mechanisms: cell replacement (cell differentiation), functional multipotency (nursing effect), and stem cell regeneration. Cell replacement can be achieved by the differentiation of transplanted cells into neuronal or vascular cells to compensate the lost functions [63,64,65]. Functional multipotency describes the secretion of various trophic factors from transplanted cells that help ameliorate neuronal damage or regenerate new neuronal circuits [66,67,68]. Stem cell regeneration also occurs in the spine, where transplanted cells activate the regeneration of host neuronal stem cells [69].
4. Key Segment of Clinical Trials
4.1. Overview of Clinical Trial Results
For this narrative review, previously reported articles published before April 6, 2020, were obtained through PubMed using the terms “spinal cord injury”, “clinical trial”, and “stem cell therapy”. Understanding that there are many other important unpublished trials that need to be discussed, we attempted to obtain data from other web sources for their addition into this review.
The published clinical trials are listed in Table 1 [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,70] and were divided into acute (cell transplantation within a few days of the insult), sub-acute (cell transplantation within 6 months of the insult), and chronic (cell transplantation >6 months from the insult). Some studies included both sub-acute and chronic patients in a single trial, and in those cases, the patients were divided according to the timing of the treatment (Table 1; column “Patient type”).
Table 1.
List of published clinical trials.
| Reference number | Journal | PMID | Author | Country | Cell Type | Cell Type | Dose | Route | Patient Type | Patient Number | Patient Characteristics (ASIA: A-D, or Others) | Major Functional Outcome | Major Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [12] | Cell Transplant | 29871514 | Xiao | China | Allogenic | UMSC&Scaffold | 4 × 107 | On-spine | Acute | 2 | A | 100% ASIA improvement (C:2) | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Arterial | Sub-acute | 5 | A:4, B:1 | 40% ASIA improvement | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Venous | Sub-acute | 9 | A:7, B:2 | No ASIA improvement, Some SEP/MEP improvement | |
| [14] | Cytotherapy | 19903102 | Pal | India | Autologous | BMSC | 6 × 107/kg | Thecal | Sub-acute | 15 | A11, C4 | No improvement | |
| [15] | Clin Neurol Neurosurg. | 22464434 | Karamouzian | Iran | Autologous | BMSC | 7–12 × 106 | Thecal | Sub-acute | 11 | A | 46% ASIA improvement (C:5), control 15% | |
| [16] | Cytotherapy | 26971680 | Satti | Pakistan | Autologous | BMSC | 1.2 × 106 | Thecal | Sub-acute | 3 | A | Safe | |
| [17] | J Spinal Cord Med | 26208177 | Hur | South Korea | Autologous | AMCS | 9 × 107 | Thecal | Sub-acute | 3 | A2, B1 | ASIA sensory change (improved 2, decline 1) | |
| [18] | Stem Cells | 17464087 | Yoon | South Korea | Autologous | BMMNC | 2 × 108 | Spinal | Sub-acute | 35 | A | 19% ASIA improvement (8% control) | 33% of patients with new pain |
| [19] | Neural Plasticity | 26568892 | Shin | South Korea | Allogenic | NSC | 1 × 108 | Spinal | Sub-acute | 19 | A:17, B:2 | 26% ASIA improvement (A to B:1, A to C:2, B to D:2) | |
| [20] | J Neurotrauma | 28225648 | Anderson | USA | Autologous | Schwann cell | 5, 10, and 15 × 106 | Spinal | Sub-acute | 6 | A | Improvement in FIM and others | |
| [21] | Neurosurgery | 30180779 | Levi | USA | Allogenic | NSC | 4 × 107 | Spinal | Sub-acute | 1 | B | Improvement in motor assessment | |
| [22] | Br J Neurosurg | 21749185 | Bhanot | India | Autologous | BMSC | Spine: 6 to 24 × 107; thecal: 6 to 12 × 107 | Spinal and thecal | Sub-acute | 2 | A | No improvement | |
| [23] | Cell Transplant | 19364066 | Geffner | Ecuador | Autologous | CD34+ | 4 × 108 | Spinal, thecal, and venous | Sub-acute | 3 | A | 67% ASIA improvement (C:2) | |
| [24] | Cytotherapy | 16793729 | Moviglia | Argentina | Autologous | BMSC | 5 to 10 × 109 | Venous | Chronic | 2 | N/A | Motor/SEP recovery | None |
| [25] | Bull Exp Biol Med | 18214319 | Chernykh | Russia | Autologous | BMMNC | 3.6 × 107 | Venous and spinal | Chronic | 18 | N/A | Original Scale improved compare with control | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Arterial | Chronic | 1 | C | 0% ASIA improvement, Some SEP/MEP improvement | |
| [26] | Spinal Cord | 19333245 | Cristante | Brazil | Autologous | CD34+ | 1.5 × 108 | Arterial | Chronic | 29 | Complete | SEP recovery (67%) | |
| [13] | Cell Transplant | 17269439 | Sykova | Czech Rep. | Autologous | BMMNC | 1.0 × 1010 | Venous | Chronic | 5 | A:4, B:1 | 0% ASIA improvement, Some MEP improvement | |
| [27] | Stem Cells Dev | 21303266 | Ra | South Korea | Autologous | AMSC | 4 × 108 | Venous | Chronic | 8 | A & B | 12.5% ASIA improvement (A to C:1) | No SAE |
| [14] | Cytotherapy | 19903102 | Pal | India | Autologous | BMSC | 6 × 107 | Thecal | Chronic | 10 | A9, C1 | 0% ASIA improvement, Some motor/sensory improvement | |
| [28] | Neurorehabil Neural Repair | 20660620 | Kishk | Egypt | Autologous | BMSC | 3–6 × 107 | Thecal | Chronic | 43 | A:40, C:3 | 30% ASIA improvement (control 16%) | Neuropathic pain 24/43 |
| [29] | Cell Transplant | 22507680 | Frolov | Russia | Autologous | CD34+ | 24–51 × 106 | Thecal | Chronic | 20 | N/A | SEP/MEP improvement (15–20%) | |
| [30] | Cell Transplant | 23452836 | El-Kheir | Egypt | Autologous | BMSC | 1.2 × 108 | Thecal | Chronic | 50 | A:15, B:35 | 34% ASIA improvement (control 0%) | |
| [16] | Cytotherapy | 26971680 | Satti | Pakistan | Autologous | BMSC | 1.2 × 106 | Thecal | Chronic | 6 | A | Safe | |
| [17] | J Spinal Cord Med | 26208177 | Hur | South Korea | Autologous | AMCS | 9 × 107 | Thecal | Chronic | 11 | A10, D1 | ASIA sensory change (improved 8) | |
| [31] | Cytotherapy | 28089079 | Vaquero | Spain | Autologous | BMSC | 120 × 106 | Thecal | Chronic | 10 | B:4, C:5, D:1 | Improvement in ASIA score | |
| [32] | Cytotherapy | 29853256 | Vaquero | Spain | Autologous | BMSC | 3 × 108 | Thecal | Chronic | 11 | A3: B:4, C:3, D:1 | 27% ASIA improvement | |
| [33] | J Spinal Cord Med | 16859223 | Lima | Spain | Autologous | Olfactory Mucosa | N/A | Spinal | Chronic | 7 | A | 29% ASIA improvement (A to C:2) | Worsening of sensory:1 |
| [34] | Brain | 18689435 | Mackay-Sim | Spain | Autologous | Olfactory Mucosa | 1.2–2.8 × 107 | Spinal | Chronic | 3 | A | No functional improvement | |
| [35] | Neurosci Lett | 18662744 | Saberi | Iran | Autologous | Schwann cell | 3–4.5 × 106 | Spinal | Chronic | 4 | A:2, C:2 | 25% ASIA improvement (1:C to D) | |
| [36] | Cytotherapy | 18615345 | Deda | Turkey | Autologous | CD34+ | 1 × 107 | Spinal | Chronic | 9 | A | 100% ASIA improvement (B:2, C:7) | |
| [37] | Neurorehabil Neural Repair | 19794133 | Lima | Spain | Autologous | Olfactory Mucosa | N/A | Spinal | Chronic | 20 | A:15, B:5 | 55% ASIA improved (A to B:2, A to C:6, B to C:3) | |
| [38] | Brain Res | 23948102 | Dai | China | Autologous | BMSC | 2 × 107 | Spinal | Chronic | 20 | A | 45% ASIA improvement (A to B:9) | |
| [39] | Stem Cell Res Ther | 25406723 | Mendonca | Brazil | Autologous | BMSC | 4–52 × 106 | Spinal | Chronic | 12 | A | 58% ASIA improvement (B:6, C:1) | |
| [40] | J Transl Med | 25209445 | Cheng | China | Allogenic | UMSC | 4 × 107 | Spinal | Chronic | 10 | A | Improvement in ASIA score (cell: 70%, rehabilitation: 36%, control: 0%) | |
| [41] | Cytotherapy | 29784434 | Vaquero | Spain | Autologous | BMSC | 3 × 108 | Spinal | Chronic | 6 | A:3, B:2, D:1 | Improvement in ASIA score | |
| [42] | Cell Stem Cell | 29859175 | Curtis | USA | Allogenic | NSC | 1.2 × 106 | Spinal | Chronic | 4 | A | EMG improvement | |
| [43] | Neurosurgery | 30180779 | Levi | USA | Allogenic | NSC | 2 × 108 and 4 × 108 | Spinal | Chronic | 24 | A, B | Improvement in motor assessment | |
| [44] | Cell Transplant | 25372344 | Al-Zoubi | USA | Autologous | CD34+ | 7.6 × 107 | Spinal and thecal | Chronic | 19 | A | 47% ASIA improvement (B:7, C:2) | |
| [22] | Br J Neurosurg | 21749185 | Bhanot | India | Autologous | BMSC | Spine: 6 to 24 ×107; thecal: 6 to 12 × 107 | Spinal and thecal | Chronic | 11 | A | 9% ASIA improvement (A to B:1) | |
| [45] | Neurosurgery | 22127044 | Park | South Korea | Autologous | BMSC | Spine: 8 × 106; thecal: 4 × 107 | Spinal and thecal | Chronic | 10 | A:4, B:6 | SEP/MEP improvement (30%) | |
| [46] | Neurosurgery | 26891377 | Oh | South Korea | Autologous | BMSC | Spine: 1.6 × 107; thecal: 3.2 × 107 | Spinal and thecal | Chronic | 20 | B | Original Scale improvement (13%) | |
| [47] | Cytotherapy | 27311799 | Vaquero | Spain | Autologous | BMSC | Spine: 5 to 150 × 106; thecal: 30 × 106 | Spinal and thecal | Chronic | 12 | A | 33% ASIA improvement (B:3, C:1) | |
| [23] | Cell Transplant | 19364066 | Geffner | Ecuador | Autologous | CD34+ | 4 × 108 | Spinal, thecal, and venous | Chronic | 5 | A2, B1, C2 | 75% ASIA improvement | |
| [48] | Exp Clin Transplant | 20353375 | Kumar | India | Autologous | BMMNC | 3–5 × 108 | Thecal | N/A | 264 | A:233, B:7, C:22, D: | 30% ASIA improvement | |
| [70] | Cell Transplant | 22507683 | Sharma | India | Autologous | BMMNC | 1 × 106/kg | Thecal | N/A | 4 | N/A | 25% ASIA improvement |
AMSC, adipose tissue–derived mesenchymal stromal cell; ASIA, the American Spinal Injury Association Impairment Scale; BMSC, bone marrow–derived mesenchymal stromal cell; BMMNC, bone marrow–derived mononuclear cell; CD34+, hematopoietic stem cell showing CD34 positive; EMG, electromyography; MEP, motor evoked potential; N/A, not applicable; NSC, neural stem cells; SAE, severe adverse events; SCI, spinal cord injury; SEP, somatosensory evoked potential; UMSC, umbilical cord–derived mesenchymal stromal cell. Note: Cell dose was corrected to the calculated dose of a patient weighing 60 kg, when the data was provided as cell number per kilogram.
The majority of the clinical trials are in the early stage (phase 1/2), meaning that a small number or none of the patients are set as controls. The trials are mostly performed for severely injured (ASIA A), chronic-stage (>6 months) patients. This decision seems understandable, given that there is no other effective treatment available at this stage for critically handicapped patients. Mesenchymal stromal/stem cells (MSCs) are frequently used, and bone marrow is often selected as the donor source. Autologous cells are more frequently used than allogenic cells, likely due to the safety issues experienced in early trials. Administration routes differ among the trials, but, generally, intrathecal and intraspinal administration are favored over intravenous or intra-arterial routes. Additionally, patient characteristics vary between trials; however, ASIA A, which means complete motor and sensory deficit below the level of injury, is frequently adopted as important inclusion criteria. Although most patients are adults, Sharma et al. [70] reported the results of the intrathecal transplantation of bone-marrow-derived mononuclear cells (BMMNCs) in pediatric patients, where 25% of the patients showed improvements in their ASIA impairment scale classification, whereas the other patients also showed some degree of neurological improvement, including muscle strength, sitting balance, and urine control. Cell dosage also varies widely among trials, ranging in orders of magnitude from 106 to 1010. Some articles report only the safety of the stem cells and their transplantation procedures, whereas others also report functional recovery. Many methods are applied to evaluate functional outcomes, including changes in the ASIA impairment scale classification, Frankel grade, Bartel score, Ashworth scale, functional independence measure assessment (FIM), and electrophysiological improvement (somatosensory evoked potential (SEP); motor-evoked potential (MEP); and electromyography (EMG)). The results of motor function improvement range widely from remarkable recovery to no improvement.
4.1.1. Acute Phase of SCI
Given that most of the animal preclinical experiments are conducted at the acute phase (within 24 h of the injury) [61], the lack of acute-phase clinical trials is somewhat surprising. In the acute phase, it is impossible to obtain enough autologous cells, because they require several weeks for expansion. Xiao et al. [12] reported the use of allogenic umbilical cord-derived MSCs on acute-phase patients, and they transplanted the cells using a collagen scaffold onto the spinal cord ~24 h after the injury. Functional recovery was reported in two complete-injury (ASIA A) patients, who showed recovery to incomplete injury (ASIA C). However, spontaneous recovery is possible in the acute phase of SCI, and the results of stem cell therapy must be further examined and compared with those in control patients.
4.1.2. Sub-Acute Phase of SCI
In this review, we defined the sub-acute phase as the period between 2 days and 6 months after SCI. Autologous MSCs are most frequently administered to sub-acute phase patients; however, allogenic neuronal stem cells obtained from the fetus have also been examined [19]. The results of stem cell therapy differ between trials, with one group of trials reporting no significant recovery [13,14,22], whereas another reported that 46% of ASIA A patients recovered to ASIA C when treated with an intrathecal bone-marrow-derived mesenchymal stromal cell (BMSC) injection relative to only 15% in the control group [15]. Notably, Yoon et al. [18] found that intra-spinal BMMNC injection was effective when the patients were treated within 8 weeks of injury (ASIA improvement: 30%) and ineffective when transplantation was performed >8 weeks after injury (0%). Moreover, they reported that treatment results were higher than those of the matched control (ASIA improvement: 7.6%) [18]. The transplantation route was compared in the same trial, with intra-arterial injection reportedly resulting in better functional recovery than intravenous injection (1.0 × 1010 BMMNCs) [13]. Notably, 33% of patients treated by intraspinal BMMNC injection reported development of new pain [18].
4.1.3. Chronic Phase of SCI
The majority of clinical trials are conducted in the chronic phase, when hope for a spontaneous recovery is minimal. The results vary between trials, with some showing no improvement on the ASIA impairment scale [14,34], whereas others report recovery rates as high as 100% [36]. However, studies that did not report ASIA impairment scale-measured recovery still showed some degree of improvement in other tests, such as SEP or MEP. Additionally, some reports analyzed matched-control patients, with the recovery rate reportedly higher in stem cell treatment groups [25,71]. A randomized study by Cheng et al. [40] randomly divided 34 ASIA A grade SCI patients into three groups (cell transplantation, rehabilitation, and control) and found that only the cell transplantation group showed significant motor, sensory, and urinary recovery as compared with their pretreatment status. El-kheir et al. [30] randomly divided 70 patients into treatment and control groups, and reported that 34% of the patients who received intrathecal BMSC transplantation showed ASIA impairment scale improvement relative to 0% in the control group. Untreated patients have a small chance of spontaneously acquiring a degree of improvement, which requires careful attention when appraising the results of randomized trials, even those studying chronic-phase patients.
4.2. Source Stem Cell Types
Many stem cell types, including MSCs, olfactory ensheathing cells (OECs), Schwann cells, oligodendrocyte progenitor cells (OPCs), neural stem cells (NSCs), embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs), have been intensively examined as promising cell sources and tested in clinical trials. Autologous cells (MSCs, OECs, Schwann cells, and iPSCs) have a lower risk of post-transplant rejection, whereas allogenic cells (MSCs, NSCs, OPCs, ESCs, and iPSCs) have the advantage of easier access due to large-scale manufacturing and standardized stocks. Before the distribution of a given cell type as a commercially available cell source, several factors need to be considered, including safety, efficiency, cost, and the feasibility of large-scale manufacture. There are several basic reports that have compared the use of different cell sources in the treatment of SCI [72,73,74]; however, each stem cell type has its own benefits and drawbacks, and, at present, it is not known which of them will be the most beneficial for SCI treatment.
4.2.1. MSCs and MNCs
The nomenclature conventions and definitions of MSCs (stromal and stem cells) are somewhat convoluted. The International Society for Cell and Gene Therapy (ISCT) Mesenchymal Stromal Cell committee established minimal criteria for a cell to qualify as a mesenchymal stromal cell: (1) it needs to be plastic adherent; (2) express CD73, CD90, and CD105; (3) lack the expression of the hematopoietic and endothelial markers CD11b, CD14, CD19, CD34, CD45, CD79a, and HLA-DR; and (4) be capable of in vitro differentiation into adipocyte, chondrocyte, and osteoblast lineages [75,76]. However, some cell-surface markers later showed an ability to be reversibly upregulated or downregulated according to cell culture conditions [77,78,79]. The use of “stromal” and “stem” to describe MSCs is almost equivalent in the literature, and the ISCT suggests that “mesenchymal stromal cell” should be used to describe bulk unfractionated populations, which include fibroblasts, myofibroblasts, and stem/progenitor cells, whereas “mesenchymal stem cell” should be used for purified stem/progenitor cells [80]. In this review, we use MSCs to describe both stromal and stem cells.
MSCs have demonstrated their ability to ameliorate tissue damage and facilitate functional recovery though immunomodulation, pro-angiogenic signaling, neurotrophic factor secretion, and neural differentiation, and these results have encouraged numerous preclinical experiments using MSCs to treat SCI [27,45,57,81,82,83,84,85,86,87,88,89,90,91]. MSCs can be readily found throughout the body and harvested from bone marrow, abdominal fat, and umbilical cord blood [76]. Bone-marrow-derived MSCs include BMSCs, which require ex vivo expansion, and BMMNCs, which do not. MSCs have several advantages over other stem cells due to the harvesting methods. Additionally, MSCs possess a relatively low risk of tumorigenicity and present no ethical issues [66,68,92,93,94]. Most published clinical trials used BMSCs or BMMNCs, with few using MSCs or MNCs from other sources, such as the umbilical cord or fat (Table 1). Phase I clinical trials using autologous adipose tissue-derived mesenchymal stromal cells (CELLTOP) are ongoing, and preliminary reports reveal a favorable outcome with no safety concerns in the first patient [95].
4.2.2. Hematopoietic Stem Cells
Hematopoietic stem cells expressing CD34 and from both the bone marrow and peripheral blood are also relatively frequently used in clinical trials of SCI treatment [23,26,28,35,43]. Because hematopoietic cells have a long track record as a donor source for bone marrow transplantation against leukemia, a clear methodology and proven long-term safety are the main advantages to their use.
4.2.3. OECs
OECs surround olfactory neurons, with their presumed function as scavengers of pathogens and debris around the border between the central nervous system (CNS) and the nasal mucosa. Additionally, they reportedly express neurotrophic factors that facilitate olfactory regeneration [96]. OECs can be harvested from the nasal mucosa and the olfactory bulb and transplanted into the spinal cord. Clinical trials have demonstrated the safety and feasibility of OEC transplants for SCI treatment, with no increases in severe adverse events reported according to a meta-analysis; however, the efficacy of OECs for this application is considered limited [32,33,36,69,97].
4.2.4. Schwann Cells
Schwann cells act as structural scaffolds for the peripheral nervous system and can promote a microenvironment favorable to neuronal regeneration. Moreover, Schwann cells are neuroprotective and capable of myelinating axons [98,99,100,101]. Several clinical trials, including “The Miami Project to Cure Paralysis (autologous Schwann cell transplantation in subacute spinal cord injury)” have recently been completed [20,34].
4.2.5. NSCs
NSCs are self-renewing, multipotent progenitor cells capable of differentiating into neural cells, oligodendrocytes, and astrocytes [102,103]. Although the cells are mostly be found during fetal development stage of the CNS, they also occur in a limited number of other regions, such as the subventricular zone next to the cerebral ventricle and the central canal of the spinal cord in the adult brain. NSCs provide neuroprotective effects by promoting oligodendrocyte survival and axonal ensheathment [103]. Several clinical trials using NSCs have been reported [19,21,41,42], with one issue involving the early termination of clinical trials sponsored by companies. Stem Cells Inc. launched a clinical trial that showed a degree of motor improvement; however, the trial was terminated prematurely due to business considerations. This serves as an important lesson in functional recovery, cost effectiveness, and profit [42].
4.2.6. ESCs
ESCs possess pluripotency and are considered as one of the most promising cell sources for SCI treatment. ESCs can differentiate into many cell types, including neurons, glial cells, and endothelial cells, under in vitro conditions, and potentially replace the neuronal network damaged in an SCI. However, by definition, ESCs need to be harvested from embryonic cells, which raises significant ethical issues. Geron Corporation launched a phase I clinical trial of a human ESC-based therapy for SCI in 2010, but announced that they will discontinue the clinical trial after transplanting four of the planned 10 patients, ostensibly due to financial considerations, and the results of this trial have not been released. Recently, OPCs derived from ESCs were used in a clinical trial by Asterias Biotherapeutics Inc. (phase I/IIa dose-escalation study, n = 35; NCT02302157). OPC produces neurotrophic factors, stimulates microvasculature re-vascularization, and promotes the remyelination of denuded axons, which are critical for axon regeneration [104,105]. The results had not yet been released at the time of publication.
4.2.7. iPSCs
iPSCs collected from the patient themselves might not require immunosuppressant therapy and successfully avoid the ethical issues associated with ESC harvesting; however, the tumorigenicity of iPSCs is not fully understood [106,107,108]. Various cell types, such as NSCs and MSCs, have been differentiated from iPSCs and transplanted into animal models of SCI [60,109]. Japanese researchers have announced that they are starting a clinical trial using iPSCs soon (http://www.okano-lab.com/okanolab/sekison).
4.3. Cell Dose and Route
Cell dose is among the most important clinical variables; however, it is difficult to determine the optimal dose in humans from the results of animal experiments because of the differences in body weight and spinal cord size. Based on our review of clinical trials, cell doses vary widely among trials, ranging from 106 to 1010 cells.
The application routes can be divided into intra-arterial, intravenous, intrathecal, and intraspinal, with the results of animal experiments comparing the efficacy of each route shown in Table 2 [110,111,112,113,114]. Intravenous transplantation has the advantage of the lowest invasiveness, which enables multiple injections without special equipment. However, despite its efficacy, small amounts of cells are often found in the damaged lesion when this method is used. The intra-arterial approach is superior to intravenous administration in delivering more cells to the lesion; however, ischemic damage caused by cell clusters clogging the artery needs to be avoided. Intrathecal application can also deliver a large number of cells to the spinal cord and is less invasive relative to intraspinal application; however, the rate of cell engraftment is unclear, and complications, such as hydrocephalus and liquorrhea, need to be addressed. The intraspinal approach of direct cell administration achieves the highest level of cell engraftment but requires invasive surgery, and the risk of additional SCI being caused by injection needles should not be underestimated.
Table 2.
Animal experiments comparing the efficacy of different cell administration routes.
| Reference number | Authors (Year) | Rat SCI Model | Timing | Donor Cell | Delivery Routes | Dose | Evaluation | Results |
|---|---|---|---|---|---|---|---|---|
| [110] | Bakshi et al. (2003) | rat cervical SCI | 24 h | rat BMSC | intraventricular intravenous intrathecal |
200 × 104 200 × 104 200 ×104 |
Histology | intraventricular, intrathecal > intravenous |
| [111] | Vaquero et al. (2006) | rat thoracic SCI | 3 mo | rat BMSC | direct intrathecal |
300 × 104 300 × 104 |
motor function |
direct > intrathecal |
| [112] | Paul et al. (2009) | rat cervical SCI | 24 h | human BMSC | direct intravenous intrathecal |
15 × 104 100 × 104 100 × 104 |
Histology | intrathecal > intravenous |
| [113] | Shin et al. (2009) | rat thoracic SCI | 1 wk | human BMSC | intralesional intracisternal intravenous |
30 × 104 100 × 104 100 × 104 |
histology and motor function |
Function: intracisternal > intralesional > intravenous Histology: intralesional > intracisternal > intravenous |
| [114] | Amemori et al. (2015) | rat thoracic SCI | 1 wk | humani PSC-NPC |
direct intrathecal |
50 × 104 50 × 104 |
histology and motor function |
direct > intrathecal |
BMSC, bone marrow–derived mesenchymal stromal cells; iPSC, induced pluripotent stem cells; NPC, neural precursor cells; SCI, spinal cord injury.
4.4. Patient Characteristics and Outcome Measures
The ASIA impairment scale is the most frequently used metric for determining study inclusion, and often, only patients with the ASIA A impairment level (complete motor and sensory loss below the level of injury) are included in the trials. There are several outcome measures adopted by the clinical trials, with the most frequent being the change in the ASIA scale classification. Arguably, the most meaningful outcome measure is yet to be determined. Recent clinical trials launched by companies are seldom completed before early results indicate a failure to meet expectations. The results of these terminated trials are then not reported because they are not deemed beneficial to the funding company [42].
4.5. Results, Pitfalls, and Future Directions
Aside from the large number of experimental studies, clinical trials associated with SCI remains in its infancy. Although the results are somewhat promising, the establishment of the most effective treatment strategies, including cell type, dose, route, and timing, is yet to be realized. Stem cell sheets with/without scaffolds can achieve non-invasive and highly efficient cell delivery and potentially overcome the problem of damage related to direct transplantation.
One pitfall that should be emphasized is that most of these clinical trials are single-centered, investigator-oriented trials. Clinical trials aiming to obtain drug approval are more highly restrictive and include external monitoring to assure good laboratory practices, good clinical practices, and good manufacturing practices established for each country. However, these procedures are often very expensive and differ between countries, which increase the trial threshold for clinical trials. The standardization of the regulations between agencies, such as the United States Food and Drug Administration and the European Medicines Agency, is warranted.
5. Conclusions
The heterogeneous results of clinical trials using stem cells for SCI treatment suggest a need for further assessment and basic experimentation. The biggest movement of clinical trials is that the trials are moving from investigator-oriented academic research to profit-oriented, company-funded research. The results of the studies, as well as their cost effectiveness, will be key to the future development of stem cell research.
Author Contributions
Conceptualization, M.K.; methodology, M.K.; investigation, K.Y. and M.K.; writing—original draft preparation, K.Y.; writing—review and editing, M.K., T.S., and K.H.; supervision, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by AMED under Grant Number JP17bk0104045, and Japan Society for the Promotion of Science Fujita Memorial Fund for Medical Research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Jain N.B., Ayers G.D., Peterson E.N., Harris M.B., Morse L., O’Connor K.C., Garshick E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA. 2015;313:2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson C., Mutch J., Parent S., Mac-Thiong J.M. The changing demographics of traumatic spinal cord injury: An 11-year study of 831 patients. J. Spinal Cord Med. 2015;38:214–223. doi: 10.1179/2045772314Y.0000000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagen E.M. Acute complications of spinal cord injuries. World J. Orthop. 2015;6:17–23. doi: 10.5312/wjo.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeVivo M.J. Causes and costs of spinal cord injury in the United States. Spinal Cord. 1997;35:809–813. doi: 10.1038/sj.sc.3100501. [DOI] [PubMed] [Google Scholar]
- 5.Fehlings M.G., Wilson J.R., O’Higgins M. Introduction: Spinal cord injury at the cutting edge of clinical translation: A focus issue collaboration between NACTN and AOSpine North America. J. Neurosurg. Spine. 2012;17:1–3. doi: 10.3171/2012.6.AOSPINE12632. [DOI] [PubMed] [Google Scholar]
- 6.Furlan J.C., Noonan V., Cadotte D.W., Fehlings M.G. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: An evidence-based examination of pre-clinical and clinical studies. J. Neurotrauma. 2011;28:1371–1399. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Berg M.E., Castellote J.M., Mahillo-Fernandez I., de Pedro-Cuesta J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology. 2010;34:184–192. doi: 10.1159/000279335. [DOI] [PubMed] [Google Scholar]
- 8.Wyndaele M., Wyndaele J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord. 2006;44:523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 9.Bracken M.B., Shepard M.J., Collins W.F., Holford T.R., Baskin D.S., Eisenberg H.M., Flamm E., Leo-Summers L., Maroon J.C., Marshall L.F., et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J. Neurosurg. 1992;76:23–31. doi: 10.3171/jns.1992.76.1.0023. [DOI] [PubMed] [Google Scholar]
- 10.Bracken M.B., Shepard M.J., Holford T.R., Leo-Summers L., Aldrich E.F., Fazl M., Fehlings M.G., Herr D.L., Hitchon P.W., Marshall L.F., et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1-year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. J. Neurosurg. 1988;89:699–706. doi: 10.3171/jns.1998.89.5.0699. [DOI] [PubMed] [Google Scholar]
- 11.Pointillart V., Petitjean M.E., Wiart L., Vital J.M., Lassie P., Thicoipe M., Dabadie P. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38:71–76. doi: 10.1038/sj.sc.3100962. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Z., Tang F., Zhao Y., Han G., Yin N., Li X., Chen B., Han S., Jiang X., Yun C., et al. Significant Improvement of Acute Complete Spinal Cord Injury Patients Diagnosed by a Combined Criteria Implanted with NeuroRegen Scaffolds and Mesenchymal Stem Cells. Cell Transpl. 2018;27:907–915. doi: 10.1177/0963689718766279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sykova E., Homola A., Mazanec R., Lachmann H., Konradova S.L., Kobylka P., Padr R., Neuwirth J., Komrska V., Vavra V., et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transpl. 2006;15:675–687. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 14.Pal R., Venkataramana N.K., Bansal A., Balaraju S., Jan M., Chandra R., Dixit A., Rauthan A., Murgod U., Totey S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy. 2009;11:897–911. doi: 10.3109/14653240903253857. [DOI] [PubMed] [Google Scholar]
- 15.Karamouzian S., Nematollahi-Mahani S.N., Nakhaee N., Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012;114:935–939. doi: 10.1016/j.clineuro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Satti H.S., Waheed A., Ahmed P., Ahmed K., Akram Z., Aziz T., Satti T.M., Shahbaz N., Khan M.A., Malik S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy. 2016;18:518–522. doi: 10.1016/j.jcyt.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Hur J.W., Cho T.H., Park D.H., Lee J.B., Park J.Y., Chung Y.G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2016;39:655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon S.H., Shim Y.S., Park Y.H., Chung J.K., Nam J.H., Kim M.O., Park H.C., Park S.R., Min B.H., Kim E.Y., et al. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: Phase I/II clinical trial. Stem Cells. 2007;25:2066–2073. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- 19.Shin J.C., Kim K.N., Yoo J., Kim I.S., Yun S., Lee H., Jung K., Hwang K., Kim M., Lee I.S., et al. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast. 2015;2015:630932. doi: 10.1155/2015/630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson K.D., Guest J.D., Dietrich W.D., Bartlett Bunge M., Curiel R., Dididze M., Green B.A., Khan A., Pearse D.D., Saraf-Lavi E., et al. Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury. J. Neurotrauma. 2017;34:2950–2963. doi: 10.1089/neu.2016.4895. [DOI] [PubMed] [Google Scholar]
- 21.Levi A.D., Okonkwo D.O., Park P., Jenkins A.L., 3rd, Kurpad S.N., Parr A.M., Ganju A., Aarabi B., Kim D., Casha S., et al. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery. 2018;82:562–575. doi: 10.1093/neuros/nyx250. [DOI] [PubMed] [Google Scholar]
- 22.Bhanot Y., Rao S., Ghosh D., Balaraju S., Radhika C.R., Satish Kumar K.V. Autologous mesenchymal stem cells in chronic spinal cord injury. Br. J. Neurosurg. 2011;25:516–522. doi: 10.3109/02688697.2010.550658. [DOI] [PubMed] [Google Scholar]
- 23.Geffner L.F., Santacruz P., Izurieta M., Flor L., Maldonado B., Auad A.H., Montenegro X., Gonzalez R., Silva F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transpl. 2008;17:1277–1293. doi: 10.3727/096368908787648074. [DOI] [PubMed] [Google Scholar]
- 24.Moviglia G.A., Fernandez Vina R., Brizuela J.A., Saslavsky J., Vrsalovic F., Varela G., Bastos F., Farina P., Etchegaray G., Barbieri M., et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy. 2006;8:202–209. doi: 10.1080/14653240600736048. [DOI] [PubMed] [Google Scholar]
- 25.Chernykh E.R., Stupak V.V., Muradov G.M., Sizikov M.Y., Shevela E.Y., Leplina O.Y., Tikhonova M.A., Kulagin A.D., Lisukov I.A., Ostanin A.A., et al. Application of autologous bone marrow stem cells in the therapy of spinal cord injury patients. Bull. Exp. Biol Med. 2007;143:543–547. doi: 10.1007/s10517-007-0175-y. [DOI] [PubMed] [Google Scholar]
- 26.Cristante A.F., Barros-Filho T.E., Tatsui N., Mendrone A., Caldas J.G., Camargo A., Alexandre A., Teixeira W.G., Oliveira R.P., Marcon R.M. Stem cells in the treatment of chronic spinal cord injury: Evaluation of somatosensitive evoked potentials in 39 patients. Spinal Cord. 2009;47:733–738. doi: 10.1038/sc.2009.24. [DOI] [PubMed] [Google Scholar]
- 27.Ra J.C., Shin I.S., Kim S.H., Kang S.K., Kang B.C., Lee H.Y., Kim Y.J., Jo J.Y., Yoon E.J., Choi H.J., et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011;20:1297–1308. doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 28.Kishk N.A., Gabr H., Hamdy S., Afifi L., Abokresha N., Mahmoud H., Wafaie A., Bilal D. Case control series of intrathecal autologous bone marrow mesenchymal stem cell therapy for chronic spinal cord injury. Neurorehabil. Neural. Repair. 2010;24:702–708. doi: 10.1177/1545968310369801. [DOI] [PubMed] [Google Scholar]
- 29.Frolov A.A., Bryukhovetskiy A.S. Effects of hematopoietic autologous stem cell transplantation to the chronically injured human spinal cord evaluated by motor and somatosensory evoked potentials methods. Cell Transpl. 2012;21:S49–S55. doi: 10.3727/096368912X633761. [DOI] [PubMed] [Google Scholar]
- 30.El-Kheir W.A., Gabr H., Awad M.R., Ghannam O., Barakat Y., Farghali H.A., El Maadawi Z.M., Ewes I., Sabaawy H.E. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transpl. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 31.Vaquero J., Zurita M., Rico M.A., Bonilla C., Aguayo C., Fernandez C., Tapiador N., Sevilla M., Morejon C., Montilla J., et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19:349–359. doi: 10.1016/j.jcyt.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 32.Vaquero J., Zurita M., Rico M.A., Aguayo C., Bonilla C., Marin E., Tapiador N., Sevilla M., Vazquez D., Carballido J., et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: Safety and efficacy of the 100/3 guideline. Cytotherapy. 2018;20:806–819. doi: 10.1016/j.jcyt.2018.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Lima C., Pratas-Vital J., Escada P., Hasse-Ferreira A., Capucho C., Peduzzi J.D. Olfactory mucosa autografts in human spinal cord injury: A pilot clinical study. J. Spinal Cord Med. 2006;29:191–203. doi: 10.1080/10790268.2006.11753874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackay-Sim A., Feron F., Cochrane J., Bassingthwaighte L., Bayliss C., Davies W., Fronek P., Gray C., Kerr G., Licina P., et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: A 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saberi H., Moshayedi P., Aghayan H.R., Arjmand B., Hosseini S.K., Emami-Razavi S.H., Rahimi-Movaghar V., Raza M., Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: An interim report on safety considerations and possible outcomes. Neurosci. Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 36.Deda H., Inci M.C., Kurekci A.E., Kayihan K., Ozgun E., Ustunsoy G.E., Kocabay S. Treatment of chronic spinal cord injured patients with autologous bone marrow-derived hematopoietic stem cell transplantation: 1-year follow-up. Cytotherapy. 2008;10:565–574. doi: 10.1080/14653240802241797. [DOI] [PubMed] [Google Scholar]
- 37.Lima C., Escada P., Pratas-Vital J., Branco C., Arcangeli C.A., Lazzeri G., Maia C.A., Capucho C., Hasse-Ferreira A., Peduzzi J.D. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil. Neural. Repair. 2010;24:10–22. doi: 10.1177/1545968309347685. [DOI] [PubMed] [Google Scholar]
- 38.Dai G., Liu X., Zhang Z., Yang Z., Dai Y., Xu R. Transplantation of autologous bone marrow mesenchymal stem cells in the treatment of complete and chronic cervical spinal cord injury. Brain Res. 2013;1533:73–79. doi: 10.1016/j.brainres.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Mendonca M.V., Larocca T.F., de Freitas Souza B.S., Villarreal C.F., Silva L.F., Matos A.C., Novaes M.A., Bahia C.M., de Oliveira Melo Martinez A.C., Kaneto C.M., et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng H., Liu X., Hua R., Dai G., Wang X., Gao J., An Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014;12:253. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaquero J., Zurita M., Rico M.A., Aguayo C., Fernandez C., Rodriguez-Boto G., Marin E., Tapiador N., Sevilla M., Carballido J., et al. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy. 2018;20:796–805. doi: 10.1016/j.jcyt.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Curtis E., Martin J.R., Gabel B., Sidhu N., Rzesiewicz T.K., Mandeville R., Van Gorp S., Leerink M., Tadokoro T., Marsala S., et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell. 2018;22:941–950 e946. doi: 10.1016/j.stem.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Levi A.D., Anderson K.D., Okonkwo D.O., Park P., Bryce T.N., Kurpad S.N., Aarabi B., Hsieh J., Gant K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma. 2019;36:891–902. doi: 10.1089/neu.2018.5843. [DOI] [PubMed] [Google Scholar]
- 44.Al-Zoubi A., Jafar E., Jamous M., Al-Twal F., Al-Bakheet S., Zalloum M., Khalifeh F., Radi S.A., El-Khateeb M., Al-Zoubi Z. Transplantation of purified autologous leukapheresis-derived CD34+ and CD133+ stem cells for patients with chronic spinal cord injuries: Long-term evaluation of safety and efficacy. Cell Transpl. 2014;23:S25–S34. doi: 10.3727/096368914X684899. [DOI] [PubMed] [Google Scholar]
- 45.Park J.H., Kim D.Y., Sung I.Y., Choi G.H., Jeon M.H., Kim K.K., Jeon S.R. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70:1238–1247. doi: 10.1227/NEU.0b013e31824387f9. [DOI] [PubMed] [Google Scholar]
- 46.Oh S.K., Choi K.H., Yoo J.Y., Kim D.Y., Kim S.J., Jeon S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery. 2016;78:436–447. doi: 10.1227/NEU.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 47.Vaquero J., Zurita M., Rico M.A., Bonilla C., Aguayo C., Montilla J., Bustamante S., Carballido J., Marin E., Martinez F., et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 2016;18:1025–1036. doi: 10.1016/j.jcyt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Kumar A.A., Kumar S.R., Narayanan R., Arul K., Baskaran M. Autologous bone marrow derived mononuclear cell therapy for spinal cord injury: A phase I/II clinical safety and primary efficacy data. Exp. Clin. Transpl. 2009;7:241–248. [PubMed] [Google Scholar]
- 49.Choo A.M., Liu J., Lam C.K., Dvorak M., Tetzlaff W., Oxland T.R. Contusion, dislocation, and distraction: Primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J. Neurosurg. Spine. 2007;6:255–266. doi: 10.3171/spi.2007.6.3.255. [DOI] [PubMed] [Google Scholar]
- 50.LaPlaca M.C., Simon C.M., Prado G.R., Cullen D.K. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13–26. doi: 10.1016/S0079-6123(06)61002-9. [DOI] [PubMed] [Google Scholar]
- 51.Ahuja C.S., Martin A.R., Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016:5. doi: 10.12688/f1000research.7586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson J.R., Forgione N., Fehlings M.G. Emerging therapies for acute traumatic spinal cord injury. CMAJ. 2013;185:485–492. doi: 10.1503/cmaj.121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pineau I., Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- 54.Teng Y.D. Functional Multipotency of Stem Cells and Recovery Neurobiology of Injured Spinal Cords. Cell Transpl. 2019;28:451–459. doi: 10.1177/0963689719850088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu M., Wu W., Li H., Li S., Huang L.T., Yang Y.Q., Sun Q., Wang C.X., Yu Z., Hang C.H. Necroptosis, a novel type of programmed cell death, contributes to early neural cells damage after spinal cord injury in adult mice. J. Spinal Cord Med. 2015;38:745–753. doi: 10.1179/2045772314Y.0000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y., Wang H., Tao Y., Zhang S., Wang J., Feng X. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91–101. doi: 10.1016/j.neuroscience.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 57.Morita T., Sasaki M., Kataoka-Sasaki Y., Nakazaki M., Nagahama H., Oka S., Oshigiri T., Takebayashi T., Yamashita T., Kocsis J.D., et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neuroscience. 2016;335:221–231. doi: 10.1016/j.neuroscience.2016.08.037. [DOI] [PubMed] [Google Scholar]
- 58.Kwon B.K., Tetzlaff W., Grauer J.N., Beiner J., Vaccaro A.R. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 59.Milhorat T.H., Capocelli A.L., Jr., Anzil A.P., Kotzen R.M., Milhorat R.H. Pathological basis of spinal cord cavitation in syringomyelia: Analysis of 105 autopsy cases. J. Neurosurg. 1995;82:802–812. doi: 10.3171/jns.1995.82.5.0802. [DOI] [PubMed] [Google Scholar]
- 60.Nori S., Ahuja C.S., Fehlings M.G. Translational Advances in the Management of Acute Spinal Cord Injury: What is New? What is Hot? Neurosurgery. 2017;64:119–128. doi: 10.1093/neuros/nyx217. [DOI] [PubMed] [Google Scholar]
- 61.Assinck P., Duncan G.J., Hilton B.J., Plemel J.R., Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017;20:637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 62.Badhiwala J.H., Ahuja C.S., Fehlings M.G. Time is spine: A review of translational advances in spinal cord injury. J. Neurosurg. Spine. 2018;30:1–18. doi: 10.3171/2018.9.SPINE18682. [DOI] [PubMed] [Google Scholar]
- 63.Lee J., Kuroda S., Shichinohe H., Ikeda J., Seki T., Hida K., Tada M., Sawada K., Iwasaki Y. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology. 2003;23:169–180. doi: 10.1046/j.1440-1789.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- 64.Novikova L.N., Brohlin M., Kingham P.J., Novikov L.N., Wiberg M. Neuroprotective and growth-promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy. 2011;13:873–887. doi: 10.3109/14653249.2011.574116. [DOI] [PubMed] [Google Scholar]
- 65.Gao S., Guo X., Zhao S., Jin Y., Zhou F., Yuan P., Cao L., Wang J., Qiu Y., Sun C., et al. Differentiation of human adipose-derived stem cells into neuron/motoneuron-like cells for cell replacement therapy of spinal cord injury. Cell Death Dis. 2019;10:597. doi: 10.1038/s41419-019-1772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hofstetter C.P., Schwarz E.J., Hess D., Widenfalk J., El Manira A., Prockop D.J., Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shu Y. Neuronal signaling in central nervous system. Sheng Li Xue Bao. 2011;63:1–8. [PubMed] [Google Scholar]
- 68.Neirinckx V., Cantinieaux D., Coste C., Rogister B., Franzen R., Wislet-Gendebien S. Concise review: Spinal cord injuries: How could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells. 2014;32:829–843. doi: 10.1002/stem.1579. [DOI] [PubMed] [Google Scholar]
- 69.Ceci M., Mariano V., Romano N. Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment. Rev. Neurosci. 2018;30:45–66. doi: 10.1515/revneuro-2018-0020. [DOI] [PubMed] [Google Scholar]
- 70.Sharma A., Gokulchandran N., Chopra G., Kulkarni P., Lohia M., Badhe P., Jacob V.C. Administration of autologous bone marrow-derived mononuclear cells in children with incurable neurological disorders and injury is safe and improves their quality of life. Cell Transpl. 2012;21:S79–S90. doi: 10.3727/096368912X633798. Suppl 1. [DOI] [PubMed] [Google Scholar]
- 71.Feron F., Perry C., Cochrane J., Licina P., Nowitzke A., Urquhart S., Geraghty T., Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- 72.Oh S.K., Jeon S.R. Current Concept of Stem Cell Therapy for Spinal Cord Injury: A Review. Korean J. Neurotrauma. 2016;12:40–46. doi: 10.13004/kjnt.2016.12.2.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruzicka J., Machova-Urdzikova L., Gillick J., Amemori T., Romanyuk N., Karova K., Zaviskova K., Dubisova J., Kubinova S., Murali R., et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transpl. 2017;26:585–603. doi: 10.3727/096368916X693671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gazdic M., Volarevic V., Harrell C.R., Fellabaum C., Jovicic N., Arsenijevic N., Stojkovic M. Stem Cells Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2018:19. doi: 10.3390/ijms19041039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C., Deans R.J., Krause D.S., Keating A., International Society for Cellular T. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 76.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 77.Bellagamba B.C., Grudzinski P.B., Ely P.B., Nader P.J.H., Nardi N.B., da Silva Meirelles L. Induction of Expression of CD271 and CD34 in Mesenchymal Stromal Cells Cultured as Spheroids. Stem Cells Int. 2018;2018:7357213. doi: 10.1155/2018/7357213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stagg J., Pommey S., Eliopoulos N., Galipeau J. Interferon-gamma-stimulated marrow stromal cells: A new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 79.Romieu-Mourez R., Francois M., Boivin M.N., Stagg J., Galipeau J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J. Immunol. 2007;179:1549–1558. doi: 10.4049/jimmunol.179.3.1549. [DOI] [PubMed] [Google Scholar]
- 80.Viswanathan S., Shi Y., Galipeau J., Krampera M., Leblanc K., Martin I., Nolta J., Phinney D.G., Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019–1024. doi: 10.1016/j.jcyt.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 81.Chiba Y., Kuroda S., Maruichi K., Osanai T., Hokari M., Yano S., Shichinohe H., Hida K., Iwasaki Y. Transplanted bone marrow stromal cells promote axonal regeneration and improve motor function in a rat spinal cord injury model. Neurosurgery. 2009;64:991–999. doi: 10.1227/01.NEU.0000341905.57162.1D. [DOI] [PubMed] [Google Scholar]
- 82.Himes B.T., Neuhuber B., Coleman C., Kushner R., Swanger S.A., Kopen G.C., Wagner J., Shumsky J.S., Fischer I. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabil. Neural Repair. 2006;20:278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 83.Kim M., Kim K.H., Song S.U., Yi T.G., Yoon S.H., Park S.R., Choi B.H. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J. Tissue Eng. Regen. Med. 2018;12:e1034–e1045. doi: 10.1002/term.2425. [DOI] [PubMed] [Google Scholar]
- 84.Nakano N., Nakai Y., Seo T.B., Homma T., Yamada Y., Ohta M., Suzuki Y., Nakatani T., Fukushima M., Hayashibe M., et al. Effects of bone marrow stromal cell transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS ONE. 2013;8:e73494. doi: 10.1371/journal.pone.0073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Otero L., Zurita M., Bonilla C., Aguayo C., Vela A., Rico M.A., Vaquero J. Late transplantation of allogeneic bone marrow stromal cells improves neurologic deficits subsequent to intracerebral hemorrhage. Cytotherapy. 2011;13:562–571. doi: 10.3109/14653249.2010.544720. [DOI] [PubMed] [Google Scholar]
- 86.Paradisi M., Alviano F., Pirondi S., Lanzoni G., Fernandez M., Lizzo G., Giardino L., Giuliani A., Costa R., Marchionni C., et al. Human mesenchymal stem cells produce bioactive neurotrophic factors: Source, individual variability and differentiation issues. Int. J. Immunopathol. Pharm. 2014;27:391–402. doi: 10.1177/039463201402700309. [DOI] [PubMed] [Google Scholar]
- 87.Wang L.J., Zhang R.P., Li J.D. Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochir. (Wien.) 2014;156:1409–1418. doi: 10.1007/s00701-014-2089-6. [DOI] [PubMed] [Google Scholar]
- 88.Watanabe S., Uchida K., Nakajima H., Matsuo H., Sugita D., Yoshida A., Honjoh K., Johnson W.E., Baba H. Early transplantation of mesenchymal stem cells after spinal cord injury relieves pain hypersensitivity through suppression of pain-related signaling cascades and reduced inflammatory cell recruitment. Stem Cells. 2015;33:1902–1914. doi: 10.1002/stem.2006. [DOI] [PubMed] [Google Scholar]
- 89.Wu S., Suzuki Y., Ejiri Y., Noda T., Bai H., Kitada M., Kataoka K., Ohta M., Chou H., Ide C. Bone marrow stromal cells enhance differentiation of cocultured neurosphere cells and promote regeneration of injured spinal cord. J. Neurosci. Res. 2003;72:343–351. doi: 10.1002/jnr.10587. [DOI] [PubMed] [Google Scholar]
- 90.Ye Y., Feng T.T., Peng Y.R., Hu S.Q., Xu T. The treatment of spinal cord injury in rats using bone marrow-derived neural-like cells induced by cerebrospinal fluid. Neurosci. Lett. 2018;666:85–91. doi: 10.1016/j.neulet.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 91.Zhao T., Yan W., Xu K., Qi Y., Dai X., Shi Z. Combined treatment with platelet-rich plasma and brain-derived neurotrophic factor-overexpressing bone marrow stromal cells supports axonal remyelination in a rat spinal cord hemi-section model. Cytotherapy. 2013;15:792–804. doi: 10.1016/j.jcyt.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 92.Abrams M.B., Dominguez C., Pernold K., Reger R., Wiesenfeld-Hallin Z., Olson L., Prockop D. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor. Neurol. Neurosci. 2009;27:307–321. doi: 10.3233/RNN-2009-0480. [DOI] [PubMed] [Google Scholar]
- 93.Neuhuber B., Timothy Himes B., Shumsky J.S., Gallo G., Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 94.Suh H.I., Min J., Choi K.H., Kim S.W., Kim K.S., Jeon S.R. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir. (Wien.) 2011;153:1003–1010. doi: 10.1007/s00701-011-0945-1. [DOI] [PubMed] [Google Scholar]
- 95.Bydon M., Dietz A.B., Goncalves S., Moinuddin F.M., Alvi M.A., Goyal A., Yolcu Y., Hunt C.L., Garlanger K.L., Del Fabro A.S., et al. CELLTOP Clinical Trial: First Report From a Phase 1 Trial of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury. Mayo Clin. Proc. 2020;95:406–414. doi: 10.1016/j.mayocp.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 96.Boyd J.G., Doucette R., Kawaja M.D. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. Faseb J. 2005;19:694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- 97.Li L., Adnan H., Xu B., Wang J., Wang C., Li F., Tang K. Effects of transplantation of olfactory ensheathing cells in chronic spinal cord injury: A systematic review and meta-analysis. Eur. Spine J. 2015;24:919–930. doi: 10.1007/s00586-014-3416-6. [DOI] [PubMed] [Google Scholar]
- 98.Bunge M.B., Wood P.M. Realizing the maximum potential of Schwann cells to promote recovery from spinal cord injury. Handb. Clin. Neurol. 2012;109:523–540. doi: 10.1016/B978-0-444-52137-8.00032-2. [DOI] [PubMed] [Google Scholar]
- 99.Duncan I.D., Aguayo A.J., Bunge R.P., Wood P.M. Transplantation of rat Schwann cells grown in tissue culture into the mouse spinal cord. J. Neurol. Sci. 1981;49:241–252. doi: 10.1016/0022-510X(81)90082-4. [DOI] [PubMed] [Google Scholar]
- 100.Saberi H., Firouzi M., Habibi Z., Moshayedi P., Aghayan H.R., Arjmand B., Hosseini K., Razavi H.E., Yekaninejad M.S. Safety of intramedullary Schwann cell transplantation for postrehabilitation spinal cord injuries: 2-year follow-up of 33 cases. J. Neurosurg. Spine. 2011;15:515–525. doi: 10.3171/2011.6.SPINE10917. [DOI] [PubMed] [Google Scholar]
- 101.Zhu T., Tang Q., Gao H., Shen Y., Chen L., Zhu J. Current status of cell-mediated regenerative therapies for human spinal cord injury. Neurosci. Bull. 2014;30:671–682. doi: 10.1007/s12264-013-1438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karimi-Abdolrezaee S., Eftekharpour E., Wang J., Morshead C.M., Fehlings M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parr A.M., Kulbatski I., Zahir T., Wang X., Yue C., Keating A., Tator C.H. Transplanted adult spinal cord-derived neural stem/progenitor cells promote early functional recovery after rat spinal cord injury. Neuroscience. 2008;155:760–770. doi: 10.1016/j.neuroscience.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 104.Sharp J., Frame J., Siegenthaler M., Nistor G., Keirstead H.S. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Keirstead H.S., Nistor G., Bernal G., Totoiu M., Cloutier F., Sharp K., Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J. Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li J.Y., Christophersen N.S., Hall V., Soulet D., Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 107.Nori S., Okada Y., Nishimura S., Sasaki T., Itakura G., Kobayashi Y., Renault-Mihara F., Shimizu A., Koya I., Yoshida R., et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015;4:360–373. doi: 10.1016/j.stemcr.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsuji O., Miura K., Okada Y., Fujiyoshi K., Mukaino M., Nagoshi N., Kitamura K., Kumagai G., Nishino M., Tomisato S., et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc. Natl. Acad. Sci. USA. 2010;107:12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzuki H., Ahuja C.S., Salewski R.P., Li L., Satkunendrarajah K., Nagoshi N., Shibata S., Fehlings M.G. Neural stem cell mediated recovery is enhanced by Chondroitinase ABC pretreatment in chronic cervical spinal cord injury. PLoS ONE. 2017;12:e0182339. doi: 10.1371/journal.pone.0182339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bakshi A., Hunter C., Swanger S., Lepore A., Fischer I. Minimally invasive delivery of stem cells for spinal cord injury: Advantages of the lumbar puncture technique. J. Neurosurg. Spine. 2004;1:330–337. doi: 10.3171/spi.2004.1.3.0330. [DOI] [PubMed] [Google Scholar]
- 111.Vaquero J., Zurita M., Oya S., Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: Systemic or local administration? Neurosci. Lett. 2006;398:129–134. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 112.Paul C., Samdani A.F., Betz R.R., Fischer I., Neuhuber B. Grafting of human bone marrow stromal cells into spinal cord injury: A comparison of delivery methods. Spine (Phila Pa 1976) 2009;34:328–334. doi: 10.1097/BRS.0b013e31819403ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shin D.A., Kim J.M., Kim H.I., Yi S., Ha Y., Yoon D.H., Kim K.N. Comparison of functional and histological outcomes after intralesional, intracisternal, and intravenous transplantation of human bone marrow-derived mesenchymal stromal cells in a rat model of spinal cord injury. Acta Neurochir. (Wien.) 2013;155:1943–1950. doi: 10.1007/s00701-013-1799-5. [DOI] [PubMed] [Google Scholar]
- 114.Amemori T., Ruzicka J., Romanyuk N., Jhanwar-Uniyal M., Sykova E., Jendelova P. Comparison of intraspinal and intrathecal implantation of induced pluripotent stem cell-derived neural precursors for the treatment of spinal cord injury in rats. Stem Cell Res. 2015;6:257. doi: 10.1186/s13287-015-0255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]