Abstract
The purpose of this review article is to discuss the differences between ventricular repolarization in males and females in terms of morphology, possible mechanism, and practical significance. The interest in the subject increased when it became known that in comparison to men, women have a higher incidence of torsade de pointes (tdp) and a greater lengthening of QT‐interval after administration of class III antiarrhythmic drugs.
Before puberty, the QT intervals and the patterns of ventricular repolarization in boys and girls are similar. At puberty, in boys the QT interval shortens, and a typical male pattern of ventricular repolarization develops. This pattern is characterized by a higher amplitude of the J‐point, a shorter and steeper ST segment course, a steeper ascent, and a higher amplitude of the T wave. This pattern is prevalent in >90% of young males. With increasing age the prevalence of the male pattern in males declines gradually and drops to 14% in the oldest age group. The rise and fall of the prevalence of the male pattern appears to parallel the rise and decline of testosterone in males. The female pattern of ventricular repolarization is prevalent in about 80% of females in all age groups.
The hormonal effects on ventricular repolarization have been studied in normal and castrated rabbits of both sexes. The available evidence indicates that the females have greater divergence of L calcium current among different layers of the myocardium and a lower density of the repolarizing Kr and Ks currents. The clinical significance of the repolarization differences among genders remains to be determined. Of particular interest is the question whether the males with female pattern are at the same risk of tdp as the females or whether the females with male pattern are at lower risk of tdp than the females with female pattern.
The fact that women have longer QTc than men has been known, at least, since 1920. 1 Other differences between the electrocardiogram (ECG) of men and women were elicited later. In 1953, Lepeschkin and Surawicz 2 described the results of their analysis of the ECG in 50 normal men and 50 normal women. One of the conclusions of this study stated that: “The electrocardiogram of women is characterized not only by a longer duration of Q‐T and its components Q‐oT (where o stands for onset) and Q‐aT (where a stands for apex) but also by a longer and more horizontal RS‐T segment than that of men. The point when the T wave reaches one‐half of its final amplitude is situated nearer to the apex of T in women than in men. These characteristics make it possible to distinguish the electrocardiogram of a woman from that of man.” In all probability, this information failed to attract much attention because we have not found in the literature any mentions of the fact that an experienced electrocardiographer can predict with some measure of success the gender of an adult person by looking at its normal ECG. Subsequent reports of the differences between the ECG of men and women appeared in the Scandinavian literature. 3 , 4 In one of these studies, 3 the method of ECG analysis proposed in 1968 by Punsar et al. included measurements of the amplitude of the ST‐segment junction, the ST‐segment slope, the T wave ascent, and the T wave amplitude. More recently, investigators from United States 5 , 6 , 7 and Argentina 8 compared the gender differences of the variables describing amplitude, velocity, and duration of ventricular repolarization. The combined results of these and the earlier studies have established that a typical male ECG can be differentiated from a typical female ECG by the following characteristics:
-
1
Higher takeoff of the ST segment i.e., greater amplitude of the ST junction
-
2
Shorter period between the J‐point and the onset of T wave
-
3
Steeper slope of the ST segment
-
4
Steeper ascent of the T wave
-
5
Higher T wave amplitude.
The question whether the descent of T wave is also steeper in males than in females remains unsettled. Lepeschkin and Surawicz 2 found no significant difference between the duration of the descending T wave branch (a T‐T interval) in men and women. Lehmann and Yang reported that the descending Txyz limbs were more concordant between genders than maximum Txyz and the slope of T ascent but “still steeper in men.” 9 The illustrated tracings in their paper show that these differences were small, and could be possibly explained by differences in T wave amplitude. In the study of Bidoggia et al. 8 the interval from the apex to the end of T was longer in males than in females in 1 age group, but did not differ from each other in the remaining 4 age groups.
It is important to emphasize that the stated gender‐ and age‐dependent morphological repolarization differences refer only to the average values. In practice, typical male and typical female ECG patterns are encountered in all age groups in both genders. Therefore, even the experienced electrocardiographers cannot achieve a perfect score when guessing the gender or age category by visual inspection of the normal ECG. 10
QUANTITATIVE DIFFERENCES BETWEEN TYPICAL MALE AND TYPICAL FEMALE PATTERNS
In the study of Lepeschkin and Surawicz 2 the durations of the Q‐oT, Q‐aT, and oT‐aT intervals were about 7% longer in women than in men. In 1984 Lundh 3 established that men had significantly higher ST‐junction amplitude (Fig. 1), higher ST‐segment inclination, and higher T wave amplitude (Fig. 2) than women. Storstein et al. 4 found that ST elevation in right precordial leads was lower in normal female than in normal male students. Lehmann and Yang 9 used digitized 12‐lead ECGs from 426 normal male and 127 female adults with heart rates of 60, 70, and 80 beats/min to derive X, Y, and Z lead voltages, and then generated for each of the above heart rate category by gender, resultant spatial vector amplitudes (ST‐Txyz) and instantaneous slopes (dV/dt xyz) at successive 4 ms intervals following the J‐point. They found that at each heart rate, the spatial ST‐T vector voltage time trajectory was steeper in males than in females, beginning virtually from the J‐point. Within each heart rate category there was an early intersex divergence of ST‐Txyz trajectories with men exhibiting 2‐ to 3‐fold greater dV/dt xyz values during the ST segment and achieving greater maximum Txyz and dV/dt xyz values than women (Fig. 3). This figure suggests that if the initial portion of the ST segment could be considered as a straight line the ST angle would be considerably greater in males than in females.
Figure 1.
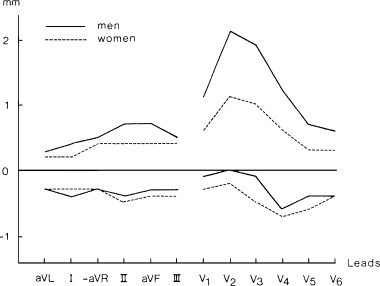
Normal limits of ST‐junction amplitude (mm) in men and women. Upper and lower limits are given as 2.5% and 97.5% percentiles.1mm = 0.1 mV. From Lundh B: On the normal scalar ECG. A new classification system considering age, sex and heart position. Acta Med Scand. Supplement 1984;1–145. With permission.
Figure 2.
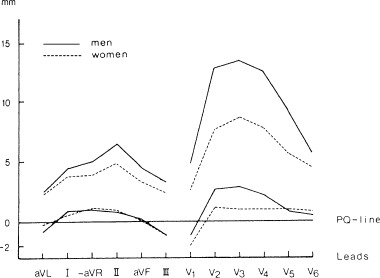
Normal limits of the T wave amplitude (mm) in 163 men and 194 women. Lower and upper limits correspond to 2.5% percentile and the 97.5% percentile, respectively.1 mm = 0.1 mV. From Lundh B: On the normal scalar ECG. A new classification system considering age, sex, and heart position. Acta Med Scand. Supplement 1984;1–145. With permission.
Figure 3.
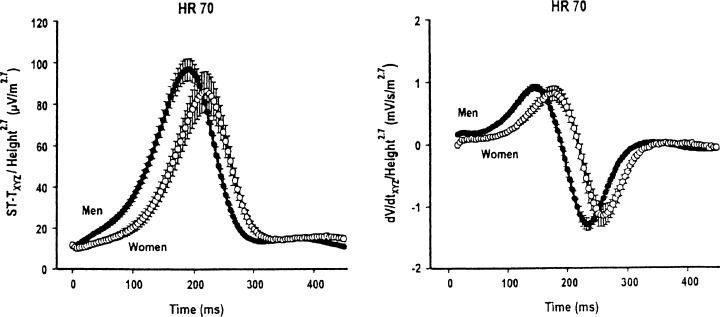
ST‐Txyz and dV/dt xyz waveforms for heart rate 70 beats/min normalized by height (in meters) to the 2.7 power, in men and women. From Lehman MH, Yang H: Sexual dimorphism in the electrocardiographic dynamics of human repolarization in the time domain. Circulation 2001;104:32–38. With permission.
Bidoggia et al. 8 analyzed ECG repolarization variables in 250 normal healthy males and 250 normal healthy females between the ages of 20 and 80 years and distributed into five groups according to age. They found that in the precordial lead exhibiting the highest T wave amplitude, the course of the repolarization was faster and the duration of repolarization shorter in males than in females. The parameters with the highest individual weight were the J‐point amplitude and the ST angle. Figure 4 (left panel) shows that the J‐point amplitude was significantly greater in males than in females with the greater difference in the younger than in the older subjects. Figure 4 (middle panel) shows that in all age groups the ST segment was shorter in males than in females, and Figure 4 (right panel) shows that in all age groups the ST angle was larger in males than in females with a greater difference between younger than between older subjects.
Figure 4.
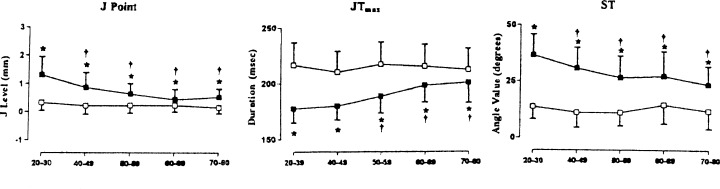
Mean and SD values of the repolarization variables analyzed in men (solid squares) and women (open squares) in different age groups. The sex‐dependent differences decreased as a function of age, mainly because of age‐dependent changes in men. *P < 0.001 vs. women; +P < 0.05 vs. 20–39 years age group. Left panel‐J‐point. Middle panel‐JT duration; Right panel‐ST angle. From Bidoggia H, Maciel JP, Capalozza N, et al.: Sex‐dependent electrocardiographic pattern of cardiac repolariztion. Am Heart J 2000;140:43–46. With permission
To define the characteristic male and female patterns Bidoggia et al. 8 used the following four variables: The J‐point amplitude, the ST‐angle, the duration of JT segment and the maximal T wave amplitude. Combining different values of these variables, these investigators computed eight different scores ranging from –3 (least male) to +4 (most male). They showed that most women had scores −3 to −1 and most males had scores 2–4, and reported that the score decreased smoothly in males between 40 and 60 years of age, but remained nearly constant in women of all ages. Thus, the scores from 0 to +4 were present in 92.5% of young men, in 64.6% of older men, and in <10% of all women. Importantly, these results revealed that the scores in both genders were heterogeneous, and that the heterogeneity was greater in males than in females.
In our recent study of early ventricular repolarization patterns, 11 we examined normal ECGs of 529 males and 544 females, age 5 to 96 years, subdivided into nine age groups in each gender. Unlike Bidoggia et al., 8 we used only two variables, namely the J‐point amplitude and the ST angle to define three separate repolarization patterns. We believe that the other two descriptors use by Bidoggia et al., 8 i.e., the duration of J‐T segment and the T wave amplitude are less discriminating because they are strongly influenced by the differences in heart rate. We inspected only the 4 precordial leads V1, V2, V3, and V4. In children with negative T wave in the leads V1–3, we included lead V5.
We measured the T wave amplitude in the lead with the tallest T wave. The pattern was considered as female when the J‐point amplitude was <0.1 mV in each of the 4 leads (Fig. 5(A)). The pattern was considered as male when the J‐point was ≥0.1 mV in at least one of the four leads and when the ST angle was ≥20° in at least one of these leads (Fig. 5(B)). In most cases, both criteria were met in the same lead. In a variant of a male pattern that was present in 6% of cases, there was no visible transition between the ST segment and the ascent of the T wave (Fig. 5(C)). We considered the pattern as indeterminate when the J‐point was ≥0.1 mV but the ST angle was <20° in each of the 4 leads (Fig. 5(D)). We found that the ECG readers to whom we explained the criteria were able to recognize visually without measurements the typical male and the typical female patterns in most normal ECGs.
Figure 5.
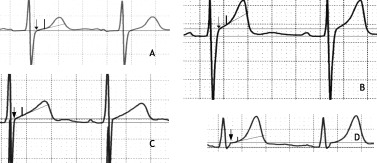
Method of pattern determination in representative electrocardiogram complexes of lead V3.The two horizontal lines represent the Q‐Q line and the line parallel to the Q‐Q line at the level of J‐point, respectively. The arrow marks the J‐point, the short vertical line marks the point 60 ms after the J‐point, the oblique line connects the J‐point with the above point. (A) Female pattern: the J‐point is at the level of the Q‐Q line and the ST angle is 19°. (B) Male pattern: the J‐point is >0.1 mV above the Q‐Q line, and the ST angle is 36°. (C) Variant of the male pattern in which the T wave ascends at the J‐point; the J‐point is >0.1 mV above the Q‐Q line, and the angle between the line parallel to the Q‐Q line at the level of the J‐point and the ascent of the T wave is 29°. (D). Intermediate pattern: the J‐point is >0.1 mV above the Q‐Q line, and the ST angle is 15°. From Surawicz B, Parikh SR: Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiol 2002; 40:1870–1876. With permission.
Figure 6 shows the prevalence of the male, the female, and the indeterminate pattern in nine age groups of males and females. The number of males and females in each group is similar. It can be seen that in females the three patterns are similarly distributed in all age groups, except in children in whom the prevalence of the male pattern is higher and that of the female pattern is lower than in the remaining age groups. The prevalence of the male pattern increased in male adolescents and young adults, reaching 91% in the age group 17 to 24 years of age. With further increase in age, prevalence of the male pattern gradually declined and was accompanied by a corresponding increase in the prevalence of the female pattern. At more advanced ages, the female pattern became predominant.
Figure 6.
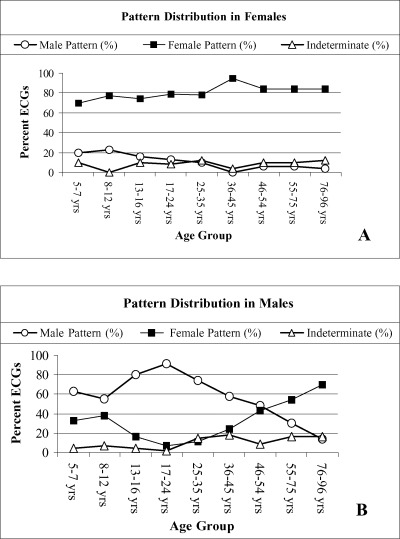
Pattern distribution in different age groups of females and males. ECG = electrocardiograms. From Surawicz B, Parikh SR: Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiol 2002;40:1870–1876. With permission.
In the ECGs with female pattern, the J‐point was <0.1 mV in all cases, and the ST angle was <20° in 88.4% of all cases. The two variables were distributed similarly. Thus, the male pattern was present in 76.3% of subjects with a J‐point > −0.1 mV and in 82.2% of all subjects with an ST angle >−20°. Conversely, the female pattern was present in 85.7% of all subjects with a J‐point <0.1 mV and in 89.6% of all subjects with an ST angle <20°.
Table 1 shows the distribution of heart rate, QTc interval, and T wave amplitude among different age groups in both genders. The heart rate was significantly faster in females than in males in four of nine age groups and was not significantly different in the other five age groups. The QTc interval was significantly longer in women than in men in the age groups from 17 to 75 years and was not significantly different in children up to age 16 and in the age group 76 to 96 years (Fig. 7). The T wave amplitude was significantly greater in males than in females in all age groups except for the age group 5 to 7 years.
Table 1.
Distribution of Heart Rate, QTc Duration and T Wave Amplitude
| Age Group in years | Male Mean HR (SD) | Female Mean HR (SD) | p Value | Male Mean QTc (SD) | Female Mean QTc (SD) | p Value | Male Mean T (SD) | Female Mean T (SD) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| 5–7 | 89.6 | 87.2 | 0.334 | 403.2 | 402.6 | 0.84 | 5.3 | 4.9 | 0.297 |
| (11.7) | (12.9) | (16.4) | (10.0) | (2.1) | (2.3) | ||||
| 8–12 | 77.5 | 82.6 | 0.023 | 408.8 | 409.7 | 0.725 | 5.2 | 4.2 | 0.005 |
| (11.9) | (11.1) | (11.9) | (14.9) | (2.2) | (1.6) | ||||
| 13–16 | 71.8 | 75.8 | 0.134 | 407.7 | 411.9 | 0.118 | 4.5 | 3.7 | 0.037 |
| (13.6) | (14.7) | (13.6) | (14.9) | (2.3) | (1.5) | ||||
| 17–24 | 70.3 | 73.0 | 0.23 | 401.8 | 408.6 | 0.033 | 5.3 | 3.0 | <0.001 |
| (11.2) | (10.4) | (14.6) | (15.5) | (2.1) | (1.2) | ||||
| 25–35 | 70.3 | 74.5 | 0.046 | 405.7 | 411.9 | 0.042 | 3.7 | 3.0 | 0.024 |
| (12.0) | (9.3) | (15.8) | (14.6) | (1.8) | (1.4) | ||||
| 35–45 | 70.1 | 73.5 | 0.11 | 403.6 | 411.4 | 0.003 | 4.2 | 2.5 | <0.001 |
| (10.8) | (9.6) | (11.3) | (13.1) | (1.6) | (1.3) | ||||
| 46–54 | 70.1 | 74.3 | 0.061 | 409.4 | 417.3 | 0.002 | 4.0 | 2.8 | <0.001 |
| (13.9) | (11.8) | (14.4) | (14.5) | (1.9) | (1.3) | ||||
| 55–75 | 64.1 | 70.5 | 0.001 | 408.0 | 412.6 | 0.004 | 4.3 | 2.6 | <0.001 |
| (10.4) | (12.4) | (11.8) | (10.2) | (1.7) | (1.1) | ||||
| 76–98 | 67.5 | 72.2 | 0.032 | 415.1 | 415.5 | 0.98 | 4.2 | 3.4 | 0.007 |
| (14.8) | (11.7) | (15.6) | (14.4) | (2.1) | (1.4) |
HR = Heart rate; QTc = Corrected QT Interval; Mean T = Mean T wave Voltage (x0.1 mV).
Figure 7.
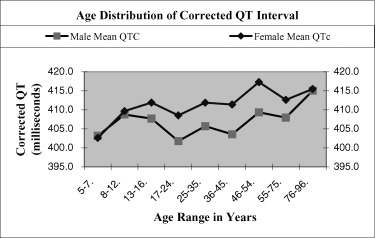
Average QTc intervals in nine age groups of males and females.
EFFECT OF HEART RATE
In our study 11 the heart ranged from 45 to 96 beats per minute (bpm) in adults and up to 110 bpm in children. We found that differences in heart rate from 10 to 65 bpm resulted in no change in pattern in 95% of 494 subjects in whom ECGs with different heart rates were recorded at separate times. In the gender comparison study of Lehmann and Yang 9 slopes of ST segment at three different heart rates (60, 70, and 80 bpm) illustrated in Figure 5 in their article show no detectable differences. However, maximum T wave amplitude and maximum (and minimum) dV/dt xyz were affected by differences in heart rate.
HORMONAL HYPOTHESIS
There is no difference between QT intervals of male and female infants at birth 12 and in children before puberty. 13 The available evidence suggests that although the female hormones are not responsible for the longer QT interval in women, the male hormones are responsible for the shorter QT interval in men at the onset of puberty. 8 In support of the androgen hypothesis, Bidoggia et al. 14 found that repolarization in castrated males was slower than in normal males, whereas the repolarization in virilized women was faster than that in normal women. They also reported that intramuscular testosterone administration restored male ECG pattern of repolarization in three castrated males.
The results of our study 11 are consistent with the hypothesis that the physiologic differences between early ventricular repolarization in males and females are strongly influenced by changes in the availability or activity of the male sex hormones. The age‐dependent changes in prevalence of male pattern in males appeared to parallel the rise of testosterone blood levels in males during puberty, and the decline of testosterone in elderly males. 15 , 16
In females after puberty, we found no significant age‐dependent differences in the distribution of patterns. Earlier studies showed no differences in the corrected QT intervals in women among the three phases of menstrual cycle 17 and no significant effect of hormonal replacement on the duration of the QTc interval in postmenopausal women. 18 However, in one study in postmenopausal women with cardiovascular disease receiving hormone replacement therapy there was a statistically significant inverse relation between the changes in androstenedione blood levels and the change in the mean QT interval. 19
We do not dismiss the possibility that female hormones can modulate ventricular repolarization because it has been shown that the drug‐induced QT prolongation in women was greater during menses and ovulatory cycles than during the luteal phase. 20 The differences, however, are relatively small.
The discussion of the channelopathies responsible for the congenital long QT syndrome, which is more prevalent in females, 21 , 22 is beyond the scope of our review. It needs to be mentioned, however, that the effect of hereditary genetic factors on the QT interval has been discerned also among apparently normal adults. 23 , 24 In a recent study Pietila et al. 23 found that in the healthy middle‐aged Finnish women, two mutations of the HERG K897T gene were associated with significantly longer QTc and T peak‐T end intervals compared with genotypes without such mutations.
MECHANISMS
Estrogen and androgen receptors are present in cardiac myocytes from many species, including normal women and men. The sex hormones belong to a large family of endogenous signaling molecules that can bind to specific receptors. The ligand‐receptor complexes are transported to the cell nucleus where they modulate gene expression in the cells. For example, such genomic pathway is believed to permit androgens to produce hypertrophy by increasing amino‐acid incorporation into proteins. 25 Also some of the estrogen effects are believed to act in a similar manner. 26 In addition to the genomic pathways the sex hormones are known to exert direct nongenomic effects on the function of the membrane channels, including calcium flux. 26 At the present time the understanding of the mechanisms by which the sex hormones modulate repolarization remains incomplete, and appears to differ among species. 26 Some of the established findings are as follows: (1) There is transmural dispersion of LICa in female but not in male rabbit ventricle. 27 This transmural gradient is due to greater whole‐cell LICa conductance in epicardium than in endocardium in female ventricle. Gonadectomy, estradiol or dihydrotestosterone (DHT) have no effect on LICa in male rabbits, but in females castration eliminates the transmural LICa gradient. 27 (2) Among the three major repolarizing K+ currents in the rabbit ventricular muscle, Liu et al. 28 found no difference in I to density between genders, but I Kr and I Ks densities were lower in the female than in the male rabbit ventricular muscle. 28 It has been suggested that the above gender‐related differences in the transmural gradient of LICa combined with smaller I Kr and I Ks densities could explain the prolonged repolarization and greater transmural dispersion of repolarization in female compared to male rabbit ventricle. 26 Conversely, the greater density of I Kr and I ks could explain the protective effect of testosterone against the QT prolongation by quinidine in DHT‐treated animals 29 and against the excess ventricular action potential prolongation and early afterdepolarizations induced by I K‐blocking antiarrhythmic drugs in female rabbits and orchiectomized male rabbits. 30
UNANSWERED QUESTIONS
Much work remains to be done to firmly establish the genesis of the “repolarization gender gap.” 10 Although the androgens appear to play the major role in creating the difference between the male and the female pattern of early ventricular repolarization, questions remain. In particular, it is difficult to explain the heterogeneity of patterns beginning in childhood and persisting to variable degree in all age groups in both genders. Are the typical male patterns in women caused by an excess of, or an exaggerated response to testosterone? Are the female patterns in males caused by deficiency of or low responsiveness to testosterone?Figure 8 shows two ECGs with a typical male pattern, one in a nonagenarian male and the other in a nonagenarian female. Do these subjects retain the effects of testosterone, or are their ECG patterns modulated by some other hormonal or nonhormonal factors?
Figure 8.
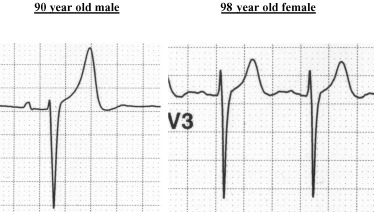
Examples of male pattern in a nonagenarian male and a nonagenarian female.
Of great interest to the clinician is the electrocardiographic pattern of “early repolarization,” 31 , 32 seen predominantly in the age categories of males with high prevalence of male pattern. It is tempting to speculate that this pattern is an exaggerated (“supernormal”) typical male pattern.
At the other end of the spectrum of repolarization patterns are the ubiquitous low amplitude or flat T waves, often dubbed as “nonspecific T changes.” We would like to propose that in most cases, in the absence ventricular hypertrophy, such changes represent exaggerated female patterns rather than markers of myocardial damage. In all likelihood, the low or flat T waves unaccompanied by increased QRS amplitude should be considered as normal variants of a female pattern particularly in women and in the elderly men. Thus a case can be made for the inclusion of the “gender pattern” among the routinely reported computed intervals and axes in all ECGs.
POSSIBLE PRACTICAL SIGNIFICANCE
Women have a steeper course of QT/RR relation than men, i.e., a greater QTc difference at longer than at shorter R‐R intervals. 33 This is similar to the reversed use‐dependence characterizing the Class III antiarrhythmic drugs, and may be a factor predisposing to bradycardia‐dependent arrhythmias.
Women are known to be at higher risk of torsade de pointes in various conditions associated with prolonged QT. 10 , 34 , 35 , 36 Also, drugs that prolong repolarization, e.g., Class III antiarrhythmic drugs cause a greater increase in QT duration in women than in men. 37 It will be of interest to examine whether these gender differences are caused by differences in prevalence of the male and female patterns among genders, that is, whether females with male patterns share the protection afforded to males, and males with female patterns share the risks inherent in females.
REFERENCES
- 1. Bazzett H. An analysis of the time‐relations of electrocardiograms. Heart 1920;7: 353–370 [Google Scholar]
- 2. Lepeschkin E, Surawicz B. The duration of the Q‐U interval, and its components in electrocardiograms of normal persons. Am Heart J 1953;46: 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Lundh B. On the normal scalar ECG. A new classification system considering age, sex, and heart position. Thesis in Acta Med Scand Suppl 1984, pp 1–145. [PubMed]
- 4. Storstein L, Bjornstad H, Meen DH. Electrocardiographic findings according to sex in athletes and controls. Cardiology 1991;79: 227–36. [DOI] [PubMed] [Google Scholar]
- 5. Merri M, Benhorin J, Alberti M, et al Electrocardiographic quantitation of ventricular repolarization. Circulation 1998;80: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 6. Gambill CL, Wilkins ML, Haisty WK Jr, et al T‐wave amplitudes in normal populations. J Electrocardiol 1995;29: 191–197. [DOI] [PubMed] [Google Scholar]
- 7. Yang H, Elko P, Fromm BS, et al Maximal ascending and descending slopes of the T‐waves in men and women J of Electrocardiol 1997;30: 267–276. [DOI] [PubMed] [Google Scholar]
- 8. Bidoggia H, Maciel JP, Capalozzi N, et al Sex‐dependent electrocardiographic pattern of cardiac repolarization. Am Heart J 2000;140: 430–436. [DOI] [PubMed] [Google Scholar]
- 9. Lehmann MH, Yang H. Sexual dimorphism in the electrocardiographic dynamics of human repolarization. Characterization in true time domain. Circulation 2001;104: 32–38. [DOI] [PubMed] [Google Scholar]
- 10. Surawicz B. Puzzling gender repolarization gap. J Cardiovasc Electrophysiol 2001;12: 613–615. [DOI] [PubMed] [Google Scholar]
- 11. Surawicz B, Parikh SR. Prevalence of male and female patterns of early ventricular repolarization in the normal ECG of males and females from childhood to old age. J Am Coll Cardiol 2002;40: 1870–1876. [DOI] [PubMed] [Google Scholar]
- 12. Stramba‐Badiole M, Spagniolo D, Bosi G, et al Are gender differences in QTc present at birth? Am J Cardiol 1995;75: 1277–1278. [PubMed] [Google Scholar]
- 13. Rautaharju PM, Zhou SH, Wong S, et al Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol 1992;8: 690–695. [PubMed] [Google Scholar]
- 14. Bidoggia H, Maciel JP, Capalozza N, et al Sex differences in the electrocardiographic pattern of cardiac repolarization: Possible role of testosterone. Am Heart J 2000;140: 678–683. [DOI] [PubMed] [Google Scholar]
- 15. Odell WD. Endocrinology of sexual maturation In De Groot LJ, Jameson JL. (eds.): Endocrinology, 4th ed Philadelphia , PA , WB Saunders, 2001;103: 2207–2212. [Google Scholar]
- 16. Nankin HR, Calkins JH. Decreased bioavailable testosterone in aging and impotent men. J Clin Endocrinol Metab 1986;63: 1418–1420. [DOI] [PubMed] [Google Scholar]
- 17. Burke JH, Ehlert FA, Kruse JT, et al Gender‐specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol 1997;79: 178–181. [DOI] [PubMed] [Google Scholar]
- 18. Larsen JA, Tung RH, Sadananda R, et al Effects of hormone replacement therapy on QT interval. Am J Cardiol 1998;82: 993–995. [DOI] [PubMed] [Google Scholar]
- 19. Nowinski K, Pripp U, Carlstroem K, et al Repolarization measures and their relation to sex hormones in postmenopausal women with cardiovascular disease receiving hormone replacement therapy. Am J Cardiol 2002;90: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez I, Kilburn MJ, Liu X‐K, et al Drug‐induced QT prolongation in women during the menstrual cycle. JAMA 2001;285: 1322–1326. [DOI] [PubMed] [Google Scholar]
- 21. Lehmann MH, Timothy KW, Frankovich D, et al Age‐gender influence on the rate‐corrected QT interval and the QT‐heart rate relation in families with genotypically characterized long QT syndrome. J Am Coll Cardiol 1997;29: 93–99. [DOI] [PubMed] [Google Scholar]
- 22. Zareba W, Moss AJ, Le Cessie S, et al Risk of cardiac events in family members of patients with long QT syndrome. J Am Coll Cardiol 1995;26: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 23. Pietila E, Fodstadt H, Niskasaari BM, et al Association between HERG K897T polymorphism and QT interval in middle‐aged Finnish women. J Am Coll Cardiol 2002;40: 511–514. [DOI] [PubMed] [Google Scholar]
- 24. Friedlander Y, Lapidos T, Sinnreich R, et al Genetic and environmental sources of QT interval variability in Israeli families: the Kibbutz Settlement Family Study. Clin Genet 1999;56: 200–209. [DOI] [PubMed] [Google Scholar]
- 25. Marsh JD, Lehmann MH, Ritchie RH, et al Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 1998: 98: 256–261. [DOI] [PubMed] [Google Scholar]
- 26. Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc Res 2002;53: 740–751. [DOI] [PubMed] [Google Scholar]
- 27. Pham TV, Robinson RB, Danilo PJ, et al Effects of gonadal steroids on gender‐related differences in transmural dispersion of L‐type calcium current. Cardiovasc Res 2002;53: 752–762. [DOI] [PubMed] [Google Scholar]
- 28. Liu XK, Katchman A, Drici MD, et al Gender differences in the cycle length‐dependent QT and potassium currents in rabbits. J Pharmacol Exp Ther 1998;285: 672–679. [PubMed] [Google Scholar]
- 29. Drici MD, Burklow TR, Haridasse V, et al Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation 1999;94: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 30. Pham TV, Sosunov EA, Gainulin RZ, et al Impact of sex and gonadal steroids on prolongation of ventricular repolarization and arrhythmias induced by IK‐blocking drugs. Circulation 2001;103: 2207–2212. [DOI] [PubMed] [Google Scholar]
- 31. Grant RP, Estes EH, Doyle JT. Special vector electrocardiography. The clinical characteristics of S‐T and T vectors. Circulation 1951;3: 182–197. [DOI] [PubMed] [Google Scholar]
- 32. Mehta M, Jain AC, Mehta A. Early repolarization. Clin Cardiol 1999;22: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kligfield P, Lax KG, Okin PM. QT interval‐heart rate relation during exercise in normal men and women: definition by linear regression. J Am Coll Cardiol 1996;28: 1547–1555. [DOI] [PubMed] [Google Scholar]
- 34. Makkar RR, Fromm BS, Steinman RT, et al Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA 1993;270: 2590–2597. [DOI] [PubMed] [Google Scholar]
- 35. Kawasaki R, Machado C, Reinoehl J, et al Increased propensity of women to develop torsades de pointes during complete heart block. J Cardiovasc Electrophysiol 1995;6: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 36. Reinoehl J, Frankovich D, Machado C, et al Probucol‐associated tachyarrhythmic events and QT prolongation: Importance of gender. Am Heart J 1996;131: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 37. Lehmann MH, Hardy S, Archibald D, et al JTc prolongation with d,l‐sotalol in women versus men. Am J Cardiol 1999;83: 354–359. [DOI] [PubMed] [Google Scholar]


