Abstract
Introduction
Loss‐of‐function (LoF) mutations in the SCN5A gene cause multiple phenotypes including Brugada Syndrome (BrS) and a diffuse cardiac conduction defect. Markers of increased risk for sudden cardiac death (SCD) in LoF SCN5A mutation carriers are ill defined. We hypothesized that late potentials and fragmented QRS would be more prevalent in SCN5A mutation carriers compared to SCN5A‐negative BrS patients and evaluated risk markers for SCD in SCN5A mutation carriers.
Methods
We included all SCN5A loss‐of‐function mutation carriers and SCN5A‐negative BrS patients from our center. A combined arrhythmic endpoint was defined as appropriate ICD shock or SCD.
Results
Late potentials were more prevalent in 79 SCN5A mutation carriers compared to 39 SCN5A‐negative BrS patients (66% versus 44%, p = .021), while there was no difference in the prevalence of fragmented QRS. PR interval prolongation was the only parameter that predicted the presence of a SCN5A mutation in BrS (OR 1.08; p < .001). Four SCN5A mutation carriers, of whom three did not have a diagnostic type 1 ECG either spontaneously or after provocation with a sodium channel blocker, reached the combined arrhythmic endpoint during a follow‐up of 44 ± 52 months resulting in an annual incidence rate of 1.37%.
Conclusion
LP were more frequently observed in SCN5A mutation carriers, while fQRS was not. In SCN5A mutation carriers, the annual incidence rate of SCD was non‐negligible, even in the absence of a spontaneous or induced type 1 ECG. Therefore, proper follow‐up of SCN5A mutation carriers without Brugada syndrome phenotype is warranted.
Keywords: Brugada syndrome, fragmented QRS, late potentials, SCN5A
1. INTRODUCTION
Brugada syndrome (BrS) is a hereditary arrhythmogenic heart disease associated with an increased risk for ventricular arrhythmia and sudden cardiac death (SCD). (Brugada & Brugada, 1992) The diagnosis of BrS can be made if a diagnostic type 1 pattern is observed (Priori et al., 2013). Loss‐of‐function (LoF) mutations in the cardiac sodium channel (NaV1.5) encoded by SCN5A are found in up to 25% of BrS index patients, while mutations in other genes that affect the transient outward current Ito and calcium current have only been described sporadically (Le Scouarnec et al., 2015). The prevailing phenotype in loss‐of‐function SCN5A mutations is a progressive cardiac conduction disorder (Probst et al., 2006). Typically, SCN5A mutations cause both atrial and ventricular conduction slowing, while these findings are not (or to a lesser extent) observed in SCN5A‐negative BrS patients. As shown previously, this is evident from the resting 12 lead ECG by an increased PR interval and QRS duration (Maury et al., 2013; Smits et al., 2002). Data on other aspects of the genotype–phenotype relationship have only been described in small cohorts. In this regard, we wanted to focus on two parameters that correspond to ventricular conduction abnormalities: late potentials (LP) on signal averaged ECG (saECG) and fragmented QRS (fQRS) on the 12 lead ECG. LP are high frequency low amplitude electrocardiographic signals that were historically developed for risk stratification in patients after acute myocardial infarction as they correspond to delayed and fragmented activation of abnormal myocardial tissue (Simson, 1981). LP were associated with a higher event rate in BrS in some small studies (Ajiro, Hagiwara, & Kasanuki, 2005; Huang et al., 2009; Ikeda et al., 2001). The second parameter that has been proposed as an additional risk marker is fQRS (Morita et al., 2008). It corresponds to myocardial scar in ischemic heart disease, but has been correlated with local epicardial conduction delay in an experimental model of BrS (Morita et al., 2008). Evaluation of a genotype–phenotype correlation in these studies was hampered by a low number of genotyped patients and SCN5A mutation carriers. We hypothesized that the presence of LP and fQRS would be another feature that is highly dependent on the presence of a SCN5A mutation in BrS patients. Therefore, we evaluated both LP and fQRS in a relatively large cohort of LoF SCN5A mutation carriers. Furthermore, data on risk stratification for SCD in LoF SCN5A mutation carriers without diagnostic type 1 ECG are scarce. Therefore, we evaluated markers that point to an increased risk for SCD in LoF SCN5A mutation carriers.
2. METHODS
2.1. Study population
We retrospectively analyzed the database of the Center for Hereditary Heart Diseases of the University Hospitals Leuven to identify all BrS and conduction disease patients who underwent genetic testing for at least SCN5A and family members of patients with a known SCN5A mutation who were tested for the mutation present in the family. The cohort consisted of four different patient groups: SCN5A LoF mutation carriers with BrS (SCN5A+BrS+), SCN5A LoF mutation carriers without BrS (SCN5A+BrS−), BrS patients without SCN5A mutation (SCN5A−BrS+) and genotype negative family members of patients with SCN5A LoF mutation (controls). Positive phenotype for BrS was defined according to the 2013 expert consensus document as the presence of a type 1 ST segment elevation in the right precordial leads in the standard or higher positions that was present either spontaneously or after provocation with a sodium channel blocker (Priori et al., 2013). All patients with a SCN5A mutation underwent a provocative test with a sodium channel blocker (most frequently ajmaline, some older cases with flecainide or procainamide) if resting ECG did not show a type 1 ECG. Ajmaline testing was executed by a stepwise infusion of 10 mg every 2 min up to a maximum of 1 mg/kg (Rolf et al., 2003). Only patients that underwent saECG were retained. A combined clinical endpoint of SCD was defined as the combination of appropriate ICD shock, documented ventricular fibrillation or SCD. Patients were classified as symptomatic if they experienced probable arrhythmic syncope or the combined endpoint. The research protocol was approved by the local ethics committee.
2.2. Late potential measurement
All patients underwent saECG (Figure 1) in a supine position, at 40‐Hz high pass filtering using Frank leads and a fast Fourier transform filter on a MAC‐5000 resting ECG analysis system (Marquette, GE Healthcare, Chicago, IL, USA). The test was performed during daytime. Details on the methodology have been published previously (Timmermans et al., 1994). Recommended normal values for the different LP parameters were used: filtered QRS duration (filQRS) ≤124 ms in males and ≤116 ms in females, root mean square voltage of the last 40 ms (RMS40) ≥16 μV in males, and ≥15 μV in females, low amplitude signals <40 μV (LAS40) ≤42 ms for both genders (Marcus, Zareba, & Sherrill, 2007). LP were considered positive if ≥2 parameters were fulfilled (Figure 1).
Figure 1.
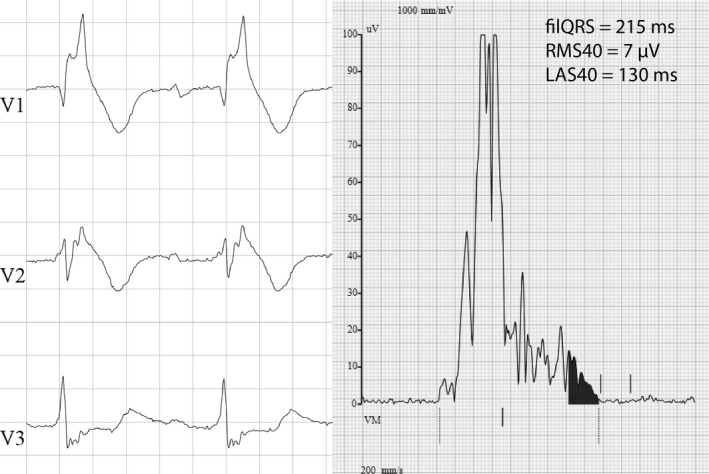
Example of fragmented QRS (left panel) and late potentials (right panel) in a patient with a spontaneous type 1 ECG pattern and subsequent appropriate ICD shock
2.3. ECG measurements
Of 12 lead ECG's were recorded using MAC 5500 (Marquette, GE Healthcare, Chicago, IL, USA). Automated analysis was done using the widely used ‘GE Marquette 12SLTM ECG Analysis Program’. As such, automated measurements of PR interval, QRS duration, QT interval corrected for heart rate with Fridericia's formula (QTc), and RR interval were obtained. Fragmented QRS was measured according to the method specific for BrS patients proposed by Morita (Morita et al., 2008). In short, the number of spikes inside the QRS complex in leads V1–V3 was counted. Fragmented QRS was present if ≥4 spikes in one lead or ≥8 spikes in lead V1, V2, and V3 were observed (Figure 1).
2.4. Mutational analysis
Different mutation detection techniques including direct Sanger sequencing and panel‐based next generation sequencing were used to detect single nucleotide variation and small insertions/deletions. Variant interpretation and classification was done according to a previously published algorithm specific for the SCN5A gene (Kapplinger et al., 2015). Variants that were scored as ‘probably pathogenic’ and ‘variant of unknown significance favor pathogenic’ were considered as (likely) disease‐causing. All variants were also assessed by the non‐SCN5A‐specific ACMG‐AMP criteria, but since these criteria might be too stringent in this context, final classification of the disease‐causing potential of SCN5A variants was not based on these criteria (Richards et al., 2015). The functional effect of the mutations was classified as follows: truncating mutation (introduction of stop codon, frameshift or splice site mutations with assumed 100% reduction in peak sodium current of the mutated channel), missense mutation with ≥90% and ≤90% reduction in peak sodium current of the mutated channel (Meregalli et al., 2009). This classification was based upon reports in literature.
2.5. Statistical analysis
All continuous variables are expressed as mean ± standard deviation and categorical variables as number (percentage). Student t test, Chi‐square test and One‐way ANOVA were used where appropriate. Tukey's multiple comparison test was used for post hoc testing of the ANOVA data. Binary logistic regression (enter method) was used for evaluating predictors of carrying a SCN5A mutation and the combined endpoint. Only variables with a p‐value <.05 in the univariate analysis were entered into the multivariate model. A p‐value <.05 was considered statistically significant. Data were analyzed with SPSS 24.0. Graphics were created with Graphpad Prism 6.0.
3. RESULTS
3.1. Study population
The study population consisted of four different groups amounting to a total of 156 patients (Table 1). We included 79 SCN5A mutation carriers of whom 44 demonstrated a type 1 ECG spontaneously or after provocation with a sodium channel blocker (SCN5A+BrS+) and 35 did not (SCN5A+BrS−). Another 39 were diagnosed with BrS but did not carry a mutation in SCN5A (SCN5A−BrS+). Finally, 38 family members of index patients with a SCN5A mutation did not carry the familial mutation and served as controls. A spontaneous type 1 ECG was equally frequent in BrS patients with and without SCN5A mutation. ICD's were implanted in 43 patients. In Brugada syndrome, conventional indications were used to decide on ICD implantation. In the SCN5A+BrS− group, ICD's were implanted in 10 patients: five for induction of VF during electrophysiological study (EPS), three for syncope with negative EPS, one for syncope with positive EPS and one for out of hospital cardiac arrest. Patients with 12 different mutations were included in this cohort (Table 2). A SCN5A mutation was detected in 42% of the included probands (25 of 60). In total, 45 patients were included with a truncating mutation, three with a missense mutation with ≥90% peak INa reduction, 20 with ≤90% peak INa reduction and 11 with unknown functional effect. The BrS minor genes were tested in a subset of the probands (20 SCN5A−BrS+ and 9 SCN5A+BrS+), but no mutation was identified in any of these BrS minor genes.
Table 1.
Patient demographics and characteristics
| SCN5A+ | SCN5A−BrS+ (N = 39) | Controls (N = 38) | p‐valuea | ||
|---|---|---|---|---|---|
| BrS+ (N = 44) | BrS− (N = 35) | ||||
| Age | 40 ± 16 | 32 ± 18 | 46 ± 13 | 38 ± 14 | .001 b |
| Males | 23 (52%) | 17 (49%) | 26 (67%) | 21 (55%) | .42 |
| Follow‐up (months) | 50 ± 58 | 45 ± 51 | 63 ± 73 | ‐ | .43 |
| Spontaneous type 1 | 16 (36%) | 0 | 16 (41%) | ‐ | <.001 |
| Atrial fibrillation or flutter | 1 (2%) | 6 (15%) | 1 (3%) | 0 | .015 c |
| Symptomatic | 9 (21%) | 7 (20%) | 8 (21%) | 0 | .99 |
| Combined endpoint | 1 (2.3%) | 3 (8.6%) | 1 (2.6%) | 0 | .32 |
| ICD | 19 (43%) | 10 (29%) | 14 (36%) | 0 | .41 |
| Beta‐blocker | 4 (9%) | 4 (11%) | 5 (13%) | 4 (11%) | .96 |
BrS, Brugada syndrome; ICD, implantable cardioverter defibrillator; N, number.
Bold p‐values indicate significant results.
The p‐value is calculated between the four groups for age, gender, and beta‐blocker use and between the three disease groups for the other variables.
p‐value Tukey post hoc test <.05 between SCN5A +BrS− versus SCN5A−BrS+.
p‐value significant between SCN5A +BrS− and both SCN5A +BrS+ and SCN5A−BrS+.
Table 2.
SCN5A mutations in this cohort
| Exon | DNA change | Protein change | Number of patients | Novel | Peak Na+ current reduction | Comments | ExAC | Clinvarc | SCN5A‐specific classification | ACMG‐AMP criteria | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BrS+ | BrS− | ||||||||||
| Intron 5 | c.611+1G>A | 8 | 1 | No | T | 2 families | 0/41380 | 1P | PP | P | |
| 9 | c.1045G>A | p.(Asp349Asn) | 3 | 0 | No | ? | 2 families | 2/120416 | 1VUS | VUS FP | VUS |
| 9 | c.1100G>A | p.(Arg367His) | 1 | 0 | No | 100% | 0/118952 | ‐ | PP | P | |
| 9 | c.1127G>A | p.(Arg376His) | 6 | 6a | No | 70% | 2 families | 0/107056 | 1P | PP | P |
| 10 | c.1312A>T | p.(Met438Leu) | 1 | 0 | Yes | ? | 0/117992 | ‐ | PP | VUS | |
| 15 | c.2268_2271del | p.(Phe756Leufs*8) | 1 | 3 | Yes | T | 0/120482 | ‐ | PP | LP | |
| 16 | c.2466G>A | p.(Trp822*) | 4b | 2 | Yes | T | c.2465G>A or p.Trp822* described | 0/117272 | ‐ | PP | P |
| 16 | c.2658T>A | p.(His886Gln) | 4 | 3b | Yes | ? | 3 families | 0/121102 | ‐ | PP | P |
| Intron 25 | c.4437+5G>A | 0 | 2 | No | T | 0/39242 | 1VUS | PP | VUS | ||
| Intron 27 | c.4813+3_4813+6dup | 13 | 11 | No | T | 8 families | 0/115016 | 1LP | PP | P | |
| 28 | c.4895G>A | p.(Arg1632His) | 2 | 6 | No | 0%d | 2 families | 1/121382 | ‐ | VUS FP | P |
| 28 | c.4978A>G | p.(Ile1660Val) | 1 | 1 | No | 100% | 3/121412 | 1LP | VUS‐FP | LP | |
BrS, Brugada syndrome; ExAC, exome aggregation consortium; FP, favor pathogenic; LP, likely pathogenic; P, pathogenic; PP, probably pathogenic; T, truncating mutation (introduction of stop codon, frameshift or splice site mutation); VUS, variant of unknown significance.
Two patients reached the composite endpoint.
One patient reached the composite endpoint.
In clinvar, annotations based only on literature are not reported here.
Loss of function of this mutation is based on altered channel kinetics.
3.2. ECG parameters to distinguish between the different groups
First, we tested whether ECG parameters differed between the four predefined groups (Table 3). Late potentials were more prevalent in the three disease groups compared to controls (p < .001). The presence of LP was 68% and 63% in BrS patients with SCN5A mutation and BrS‐negative SCN5A mutation carriers respectively, while they were only positive in 44% of BrS patients without SCN5A mutation (p = .063). Since LP were prevalent in the same order of magnitude independent of disease expression in SCN5A mutation carriers, we then grouped them together and compared them with SCN5A‐negative BrS patients. SCN5A mutation carriers had both a longer filQRS and LAS40, while RMS40 was comparable compared to SCN5A−BrS+ patients (Figure 2). This resulted in positive LP in 66% of the mutation carriers compared to 44% of the SCN5A−BrS+ patients (p = .021). There was no difference in fQRS between the four groups (p = .19). Likewise, fQRS was equally present in patients with LoF SCN5A mutation (14%) and SCN5A− Brugada patients (8%; p = .33). Finally, there was no difference in LP parameters and fQRS according to the type of functional defect.
Table 3.
Electrocardiographic parameters
| SCN5A+ | SCN5A−BrS+ (N = 39) | Controls (N = 38) | p‐value | ||
|---|---|---|---|---|---|
| BrS+ (N = 44) | BrS− (N = 35) | ||||
| PR (ms) | 199 ± 28 | 186 ± 28 | 157 ± 22 | 151 ± 24 | <.001 a , b , c , d |
| QRS (ms) | 117 ± 22 | 112 ± 15 | 106 ± 15 | 94 ± 10 | <.001 a , b , d , e |
| RR (ms) | 919 ± 136 | 1010 ± 163 | 853 ± 164 | 917 ± 152 | <.001 a , b |
| QTc (ms) | 416 ± 30 | 406 ± 20 | 405 ± 18 | 414 ± 21 | .071 |
| filQRS (ms) | 136 ± 23 | 131 ± 16 | 123 ± 11 | 114 ± 10 | <.001 a , b , d |
| RMS40 (μV) | 17 ± 12 | 20 ± 11 | 20 ± 9 | 37 ± 20 | <.001 b , d , e |
| LAS40 (ms) | 52 ± 17 | 46 ± 13 | 42 ± 11 | 34 ± 11 | <.001 a , b , d |
| LP ≥ 2/3 | 30 (68%) | 22 (63%) | 17 (44%) | 5 (13%) | <.001 b , d , e |
| fQRS | 5 (11%) | 6 (17%) | 3 (8%) | 1 (3%) | .19 |
BrS, Brugada syndrome; filQRS, filtered QRS duration; fQRS, fragmented QRS; LAS40, duration of low amplitude signals < 40 μV; LP, late potentials; RMS40, root mean square of the last 40 ms of the QRS interval.
Bold p‐values indicate significant results.
p‐value Tukey post hoc test <.05 between SCN5A+BrS+ and SCN5A−BrS+.
p‐value Tukey post hoc test <.05 between SCN5A+BrS+ and controls.
p‐value Tukey post hoc test <.05 between SCN5A+BrS− and SCN5A−BrS+.
p‐value Tukey post hoc test <.05 between SCN5A+BrS− and controls.
p‐value Tukey post hoc test <.05 between SCN5A+BrS+ and SCN5A+BrS+.
p‐value Tukey post hoc test <.05 between SCN5A−BrS+ and controls.
Figure 2.
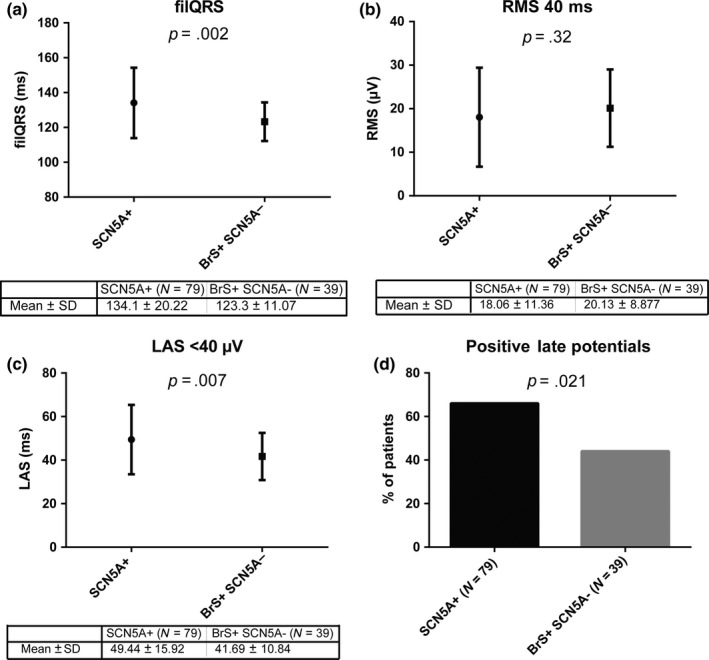
The different components of late potentials are illustrated in panels (a) (filQRS = filtered QRS duration), (b) (RMS 40 ms = root means square voltage of the last 40 ms) and (c) (LAS <40 μV = duration of low amplitude signals below 40 μV) for SCN5A loss‐of‐function mutation carriers and BrS patients without SCN5A mutation (BrS+ SCN5A−). Panel (d) shows that late potentials were more prevalent in SCN5A mutation carriers. N, number; SD, standard deviation
3.3. Predictors of SCN5A mutation in Brugada syndrome
ECG predictors for carrying a SCN5A mutation were evaluated in 83 BrS patients (Table 4). While in the univariate model, five variables were predictive of carrying a SCN5A mutation including filQRS, LAS40, and the presence of LP, only PR interval was a predictor in the multivariate model. Receiver operator characteristic (ROC) curve showed an area under the curve of 89 ± 3%. PR interval ≥ 180 ms had a sensitivity of 68% and specificity of 90% for carrying a SCN5A mutation in BrS patients. First degree AV block was highly specific for carrying a SCN5A mutation (specificity 97%, sensitivity 52%).
Table 4.
Predictors of the presence of a SCN5A mutation in BrS
| Univariate analysis | Multivariate analysisa | |||||
|---|---|---|---|---|---|---|
| OR | CI | p‐value | OR | CI | p‐value | |
| Age diagnosis | 0.97 | 0.94–1.00 | .056 | |||
| Male gender | 0.55 | 0.23–1.33 | .19 | |||
| Syncope | 0.86 | 0.29–2.57 | .79 | |||
| Spontaneous type 1 ECG | 0.82 | 0.34–1.99 | .66 | |||
| PR (ms) | 1.08 | 1.04–1.11 | <.001 | 1.08 | 1.04–1.11 | <.001 |
| QRS (ms) | 1.04 | 1.01–1.07 | .017 | 0.96 | 0.90–1.03 | .24 |
| RR (ms) | 1.003 | 1–1.01 | .052 | |||
| QTc (ms) | 1.02 | 0.999–1.04 | .062 | |||
| filQRS (ms) | 1.06 | 1.02–1.09 | .004 | 1.03 | 0.93–1.13 | .59 |
| RMS40 (μV) | 0.97 | 0.92–1.01 | .17 | |||
| LAS40 (ms) | 1.06 | 1.02–1.10 | .003 | 1.09 | 0.99–1.20 | .10 |
| LP ≥ 2/3 | 2.77 | 1.13–6.79 | .026 | 0.43 | 0.05–3.60 | .46 |
| fQRS | 1.54 | 0.34–6.90 | .57 | |||
BrS, Brugada syndrome; CI, confidence interval; filQRS, filtered QRS duration; fQRS, fragmented QRS; LAS40, duration of low amplitude signals < 40 μV; LP, late potentials; OR, odds ratio; RMS40, root mean square of the last 40 ms of the QRS interval.
Bold p‐values indicate significant results.
There was no interaction between the different components of LP and the presence of LP.
3.4. Predictors of SCD in LoF SCN5A mutation carriers
Only five patients (all males) in the whole cohort reached the endpoint during a mean follow‐up of 50 ± 60 months. Of these, four were carrier of a SCN5A mutation (follow‐up 44 ± 52 months) resulting in an annual incidence rate of 1.37%. Three out of these four patients with SCN5A mutation did not have a diagnostic type 1 ECG either at baseline or after provocation with a sodium channel blocker. Several parameters were a predictor of the endpoint in univariate analysis including both filQRS and LAS40 (Table 5). In multivariate analysis, no predictors were significantly associated with events.
Table 5.
Predictors of the combined endpoint in SCN5A mutation carriers
| Endpoint (N = 4) | No endpoint (N = 75) | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|
| OR | CI | p‐value | OR | CI | p‐value | |||
| Age diagnosis | 31 ± 13 | 37 ± 17 | 0.98 | 0.92–1.04 | .47 | |||
| Male gender | 4 (100%) | 36 (48%) | >100 | 0–∞ | .998 | |||
| Syncope | 0 | 12 (16%) | <0.001 | 0–∞ | .999 | |||
| Spontaneous type 1 ECG | 1 (25%) | 15 (20%) | 1.33 | 0.13–13.74 | .81 | |||
| PR (ms) | 223 ± 40 | 192 ± 28 | 1.04 | 1–1.08 | .049 | 1.02 | 0.97–1.07 | .53 |
| QRS (ms) | 134 ± 34 | 114 ± 18 | 1.04 | 0.998–1.08 | .066 | |||
| RR (ms) | 999 ± 97 | 957 ± 157 | 5.08 | 0.012–2105 | .6 | |||
| QTc (ms) | 443 ± 66 | 410 ± 22 | 1.03 | 1.001–1.06 | .038 | 1.00 | 0.94–1.05 | .85 |
| filQRS (ms) | 163 ± 36 | 133 ± 18 | 1.05 | 1.008–1.084 | .017 | 1.03 | 0.96–1.12 | .38 |
| RMS40 (μV) | 12 ± 4 | 18 ± 12 | 0.89 | 0.73–1.09 | .25 | |||
| LAS40 (ms) | 68 ± 43 | 48 ± 13 | 1.05 | 1–1.101 | .049 | 1.01 | 0.93–1.09 | .83 |
| LP ≥ 2/3 | 3 (75%) | 49 (65%) | 1.59 | 0.16–16 | .69 | |||
| fQRS | 5 (50%) | 9 (12%) | 7.3 | 0.92–59 | .06 | |||
CI, confidence interval; filQRS, filtered QRS duration; fQRS, fragmented QRS; LAS40, duration of low amplitude signals < 40 μV; LP, late potentials; OR, odds ratio; RMS40, root mean square of the last 40 ms of the QRS interval.
Bold p‐values indicate significant results.
3.5. Phenotype of SCN5A +BrS− patients with sudden death
Detailed phenotypes of the first two patients have been previously published (Rossenbacker et al., 2004). The first patient was diagnosed with presyncopal atrial fibrillation at age 16. Acute treatment with flecainide did not induce a type 1 ECG. Resting ECG showed a first degree AV block and type 2 Brugada pattern. During EPS, typical atrial flutter was provoked while a ventricular stimulation protocol was negative. Posterior isthmus ablation was performed. The AH (115 ms) and HV (50 ms) intervals were borderline. He died suddenly 5 years later during exercise at age 21. He was carrier of the p.(Arg376His) mutation in SCN5A.
The second patient was the index patient and brother of the first patient carrying the same SCN5A mutation. He was diagnosed with atrial flutter at age 22. His resting ECG (Figure 3, panel a) showed first degree AV block (PR 206 ms), alternating aspecific intraventricular and right bundle branch block (QRS 122 ms) and no signs of any Brugada pattern. Procainamide test was negative (Figure 3, panel b). EPS showed evidence for conduction disease (AH interval 125 ms, HV interval 80 ms). A ventricular stimulation protocol was negative. Ablation of the posterior isthmus was performed. Four years later, an ICD was implanted after the sudden death of his brother. During a follow‐up of 11 years, he received two adequate ICD shocks for rapid (270 BPM) sustained monomorphic ventricular tachycardia.
Figure 3.
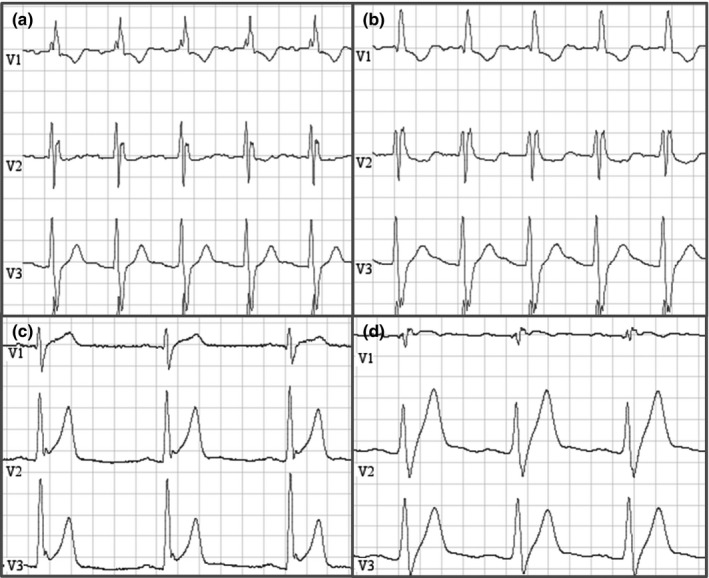
This figure illustrates ECG tracings of two patients with SCN5A mutation but without Brugada phenotype that reached the composite endpoint during follow‐up. Panel (a) illustrates baseline ECG in a patient with the p.(Arg376His) SCN5A mutation. Panel (b) shows his tracing at maximal procainamide dose. Progressive QRS and PR lengthening is noted, but not induction of a type 1 ECG. Panel (c) shows baseline ECG of a patient with the p.(His886Gln) mutation in SCN5A. Early repolarization pattern with a notch at the end of the QRS can be seen. During infusion of ajmaline (panel d), progressive PR and QRS lengthening, with disappearance of the notch at the end of the QRS is observed. There was no induction of a type 1 ECG
The third patient suffered from an out of hospital cardiac arrest during rest at age 38. His resting ECG (Figure 3, panel c) shows prominent early repolarization in the precordial and inferior leads, besides a first degree AV block and an aspecific intraventricular conduction delay (QRS 118 ms). During ajmaline infusion, the ERP pattern attenuates, with progressive QRS (+ 38 ms) and PR (+ 72 ms) prolongation without induction of a type 1 Brugada pattern (Figure 3, panel d). Two years after implantation, he received an ICD shock for ventricular fibrillation. He is carrier of the p.(His886Gln) mutation.
4. DISCUSSION
In the present study, we demonstrate in a large cohort of SCN5A mutation carriers that LP were more prevalent in SCN5A mutation carriers compared to SCN5A‐negative BrS patients, while fQRS was not. However, multivariate analysis indicated that PR interval was the only predictor of carrying a SCN5A mutation in BrS patients. The annual rate of SD or ICD shock in SCN5A mutation carriers was relatively high (1.37%), emphasizing the need for proper risk stratification and follow‐up in SCN5A mutation carriers even without the presence of a diagnostic type 1 ECG. These findings add to the complex genotype–phenotype relationship in BrS and SCN5A disease.
4.1. Established genotype–phenotype relation in SCN5A mutation carriers
Already in 2002, it was reported that BrS patients with SCN5A mutation (N = 23) differed from those without SCN5A mutation (N = 54) in regard to their baseline ECG (Smits et al., 2002). The mutation carriers showed a prolonged PR interval and HV interval. These findings were confirmed in a larger French multicenter study that included 42 BrS patients with SCN5A mutation and 147 BrS patients without SCN5A mutation (Maury et al., 2013). As in these previous reports, in our cohort consisting of 79 SCN5A mutation carriers, PR interval was significantly prolonged in mutation carriers independent of the expression of BrS. Furthermore, QRS interval was 9 ms longer in the SCN5A mutation carriers, similar to an earlier report on 138 BrS patients of whom 40 were SCN5A‐positive (van Hoorn et al., 2012). In fact, since SCN5A is expressed throughout the atria and ventricles, LoF mutations are expected to cause both atrial and ventricular conduction slowing and thus PR and QRS prolongation (van Weerd & Christoffels, 2016). Apart from these electrocardiographic differences, MRI studies have shown that SCN5A mutation carriers also have subtle morphological differences compared to SCN5A‐negative BrS patients (van Hoorn et al., 2012; Rudic et al., 2016).
4.2. LP and fQRS in SCN5A mutation carriers
Publications on LP and fQRS in BrS have mainly focused on risk stratification for SCD. Conflicting data have been published regarding the value of LP in risk stratification (Ajiro et al., 2005; Huang et al., 2009; Ikeda et al., 2001, 2005; Take et al., 2012; Tokioka et al., 2014). In all these reports, there was either no information on SCN5A mutation status (Ikeda et al., 2001, 2005) or the number of mutation carriers were low hindering evaluation of the relation between mutation status and LP: 8 in the Ajiro study (Ajiro et al., 2005), 5 in the Chinese study (Huang et al., 2009), 20 in the Take study (Take et al., 2012) and 17 in the Tokioka study (Tokioka et al., 2014). Our study included 79 SCN5A mutation carriers and LP were more prevalent in SCN5A mutation carriers compared to non‐SCN5A‐related BrS. In univariate analysis, LP was a predictor of carrying a SCN5A mutation; however in multivariate analysis, only PR prolongation was predictive. In line with the more recent reports on LP and risk stratification for SCD, LP was not a predictor of events in our cohort. Furthermore, the high prevalence of LP in our cohort combined with the relatively low number of arrhythmic events argues against an important role of LP in risk stratification using current cut‐off criteria. The observation that LP are more prevalent in SCN5A‐negative Brugada syndrome patients (44%) compared to controls (13%) suggests that in these patients conduction is also impaired. This is in line with the recent GWAS findings and subsequent functional studies, which suggest that in SCN5A‐negative Brugada syndrome patients, genetic variants in HEY2 and SCN10A affect SCN5A expression (Bezzina et al., 2013; van Weerd & Christoffels, 2016).
Compared to LP, fQRS has consistently been shown to be a strong predictor of arrhythmic events in BrS (de Asmundis et al., 2017; Maury et al., 2013; Morita et al., 2008; Priori et al., 2012; Take et al., 2012; Tokioka et al., 2014). Morita was the first to describe this association and also provided guidelines on scoring fQRS in BrS patients, which is complicated by the presence of right bundle branch block on the resting ECG of a substantial proportion of patients (Morita et al., 2008). In the largest prospective evaluation of BrS patients, the PRELUDE study including 308 patients, fQRS was a strong predictor of cardiac events, but again like in the other reports there was only a low number of SCN5A mutation carriers (N = 24) (Priori et al., 2012). The Tokioka study reported no association of fQRS and SCN5A mutation status in 17 carriers. Also in a report on the prevalence of fQRS in Brugada syndrome, no association with SCN5A mutation status was observed. (Conte et al., 2016) In contrast with LP, fQRS was less frequently observed in our cohort. However, we could not identify a single predictor of SCD probably due to the low number of events.
4.3. Methodological issues with LP and fQRS measurement and interpretation
There are some methodological problems regarding the different publications on LP and fQRS. First, there are different systems for acquisition of LP. These produce different results necessitating adapted normal values (Marcus et al., 2007). In most publications on LP in BrS, the arrhythmia research technology (ART) 1200 EPX system (Fitchburg, MA, USA) was used, while none of them used the Marquette system as in our study (Ajiro et al., 2005; Huang et al., 2009; Ikeda et al., 2001, 2005; Morita et al., 2008; Take et al., 2012; Tokioka et al., 2014). According to these reports, LP were considered positive when two criteria were met: RMS40 < 20 μV and LAS40 > 38 ms. All these articles cite the first publication on LP in BrS concerning normal values; however, a reference or rationale on how these normal values were obtained is lacking (Ikeda et al., 2001). Already in 2007, updated, more stringent normal values were published for the Marquette and the ART system after analysis of the available data in normal individuals (Marcus et al., 2007). Whether using these updated normal values would change results in these initial reports is unclear, but it seems worthwhile to reanalyze the data. Our results point out that even these more stringent cut‐off criteria are too lenient to have any value in risk stratification. Finally, it is also well known that LP have important circadian variability in Brugada syndrome patients, especially in those with documented arrhythmias (Yoshioka et al., 2013). Furthermore, this circadian variability is absent in ARVC, another arrhythmogenic heart disease characterized by the presence of LP (Abe et al., 2012). Regarding fQRS, most studies used the definition proposed by Morita (Morita et al., 2008). Only the PRELUDE study defined fQRS in a different manner as ≥2 spikes (apart from an R or R’ wave) in the QRS in leads V1, V2, or V3. Compared to the automated evaluation of LP, evaluation of fQRS is hindered by interobserver and intraobserver variability (Vandenberk et al., 2017).
4.4. Risk of sudden death in LoF SCN5A mutation carriers without Brugada syndrome phenotype
Out of the five patients that reached the composite endpoint of sudden death in our cohort, three were carrier of a LoF SCN5A mutation, but did not show a type 1 ECG either at rest or after provocation with a sodium channel blocker. This is remarkable, since this group of patients has traditionally been categorized as low risk. The group of Silvia Priori reported in 2002 on 13 patients with SCN5A mutation but negative flecainide test (Priori et al., 2002). These were all identified through family screening and none of them had a history of syncope or sudden death. Similarly, Probst et al. reported no events during a mean follow‐up of 93 months in 22 SCN5A mutation carriers without BrS phenotype that were identified through family screening (Probst et al., 2006). In a multicenter European cohort on the incidence of sudden death and life‐threatening arrhythmias in children with SCN5A LoF mutations, only six out of 33 children had a spontaneous type 1 ECG, while a sodium channel blocker test was only performed in two (Chockalingam et al., 2012). The data indicate that several symptomatic infants did not show a spontaneous type 1 ECG; however since a sodium channel blocker test was only performed in a minority of children, BrS was not definitely excluded in these patients. Furthermore, there was an important selection bias. A Japanese multicenter study reported on the incidence of LoF SCN5A mutations in a cohort of 50 idiopathic ventricular fibrillation patients with signs of early repolarization on resting ECG and negative sodium channel blocker test and identified three LoF SCN5A mutations (6%) (Watanabe et al., 2011). This last Japanese study, together with our findings, suggests that reassurance of carriers of a LoF SCN5A mutation solely based on the absence of a type 1 ECG (either spontaneously or provoked) is not possible. Larger studies are required to confirm these findings and to identify risk factors for sudden death in LoF SCN5A mutation carriers.
In fact, the presence of a SCN5A mutation in BrS has not been identified as risk marker for sudden death in most of the large studies and registries (Priori et al., 2012; Probst et al., 2010). It was only very recently that the presence of a SCN5A mutation was first found to be a strong predictor of events in BrS in a large Japanese multicenter registry (Yamagata et al., 2017). The strength of this study was that it only included probands, thereby avoiding selection bias of including more family members of severely affected families. Furthermore, familial history of SCD at young age was included in a recent risk score to predict SCD in BrS suggesting genetic background plays an important role in risk stratification (Sieira et al., 2017).
4.5. Limitations
Some of the drug provocation tests with sodium channel blockers were performed before the introduction of routine placement of ECG leads in the higher precordial leads (2th or 3th intercostal space). Therefore, some of the patients that were classified as SCN5A‐positive but BrS‐negative might actually belong to the BrS‐positive group.
Not all missense mutations included in this cohort were formally functionally evaluated. For these three mutations, we assumed LoF based on occurrence of a phenotype compatible with a LoF SCN5A mutation either in our cohort or in literature. Furthermore, they were all classified as (likely) pathogenic based on the applied variant interpretation scheme (Kapplinger et al., 2015).
The prevalence of SCN5A mutations in our cohort was high (42%) compared to the usual yield of SCN5A in BrS (25%) (Le Scouarnec et al., 2015). This was probably due to the retrospective nature of the study with exclusion of patients that did not undergo saECG, which was probably more frequently performed in individuals with subtle conduction disturbances on their resting ECG.
Finally, in 2016 a novel diagnostic score system regarding BrS including clinical and familial variables has been proposed by an international group of experts, called ‘Shanghai criteria’ (Antzelevitch et al., 2017). In the SCN5A+BrS+ group, 43 out of 44 patients had a definite diagnosis of BrS according to these criteria, while this was only the case for 25 out of 39 patients in the SCN5A−BrS+ group. This was probably due to the fact that in index patients with SCN5A mutation, family screening was more frequently accepted by family members and thus the number of family members screened was higher in the SCN5A positive group (54 from 79 were relatives) compared to the SCN5A‐negative group (four from 39 were relatives).
5. CONCLUSIONS
Late potentials were observed more frequently in SCN5A mutation carriers compared to SCN5A‐negative Brugada syndrome patients; however, fQRS was not. In patients with a Brugada syndrome phenotype, PR interval was the only predictor of carrying a SCN5A mutation. The annual incidence rate of a combined endpoint of SCD in SCN5A mutation carriers was 1.37%, not restricted to patients with a diagnostic type 1 ECG. Proper follow‐up and risk stratification in SCN5A mutation carriers seem warranted independent of the presence of a type 1 ECG.
CONFLICT OF INTEREST
No relevant conflicts of interest regarding this manuscript for any of the authors.
Robyns T, Nuyens D, Vandenberk B, et al. Genotype–phenotype relationship and risk stratification in loss‐of‐function SCN5A mutation carriers. Ann Noninvasive Electrocardiol. 2018;23:e12548 10.1111/anec.12548
REFERENCES
- Abe, A. , Kobayashi, K. , Yuzawa, H. , Sato, H. , Fukunaga, S. , Fujino, T. , … Ikeda, T. (2012). Comparison of late potentials for 24 hours between Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy using a novel signal‐averaging system based on Holter ECG. Circulation. Arrhythmia and Electrophysiology, 5(4), 789–795. 10.1161/CIRCEP.111.969865 [DOI] [PubMed] [Google Scholar]
- Ajiro, Y. , Hagiwara, N. , & Kasanuki, H. (2005). Assessment of markers for identifying patients at risk for life‐threatening arrhythmic events in Brugada syndrome. Journal of Cardiovascular Electrophysiology, 16(1), 45–51. 10.1046/j.1540-8167.2005.04313.x [DOI] [PubMed] [Google Scholar]
- Antzelevitch, C. , Yan, G. X. , Ackerman, M. J. , Borggrefe, M. , Corrado, D. , Guo, J. , … Wilde, A. A. (2017). J‐Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), and the Latin American Society of Cardiac Pacing and Electrophysiology (Sociedad Latinoamericana de Estimulacifin Cardíaca y Electrofisiología [SOLAECE]). Europace, 19(4), 665–694. 10.1093/europace/euw235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Asmundis, C. , Mugnai, G. , Chierchia, G. B. , Sieira, J. , Conte, G. , Rodriguez‐Mañero, M. , … Brugada, P. (2017). Long‐term follow‐up of probands with Brugada syndrome. American Journal of Cardiology, 119(9), 1392–1400. 10.1016/j.amjcard.2017.01.039 [DOI] [PubMed] [Google Scholar]
- Bezzina, C. R. , Barc, J. , Mizusawa, Y. , Remme, C. A. , Gourraud, J. B. , Simonet, F. , … Redon, R. (2013). Common variants at SCN5A‐SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nature Genetics, 45(9), 1044–1049. 10.1038/ng.2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugada, P. , & Brugada, J. (1992). Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. Journal of the American College of Cardiology, 20(6), 1391–1396. https://doi.org/0735-1097(92)90253-J[pii] [DOI] [PubMed] [Google Scholar]
- Chockalingam, P. , Clur, S. A. , Breur, J. M. , Kriebel, T. , Paul, T. , Rammeloo, L. A. , … Blom, N. A. (2012). The diagnostic and therapeutic aspects of loss‐of‐function cardiac sodium channelopathies in children. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 9(12), 1986–1992. 10.1016/j.hrthm.2012.08.011 [DOI] [PubMed] [Google Scholar]
- Conte, G. , de Asmundis, C. , Sieira, J. , Ciconte, G. , Di Giovanni, G. , Chierchia, G. B. , … Brugada, P. (2016). Prevalence and clinical impact of early repolarization pattern and QRS‐fragmentation in high‐risk patients with Brugada syndrome. Circulation Journal, 80(10), 2109–2116. 10.1253/circj.CJ-16-0370 [DOI] [PubMed] [Google Scholar]
- van Hoorn, F. , Campian, M. E. , Spijkerboer, A. , Blom, M. T. , Planken, R. N. , van Rossum, A. C. , … Tan, H. L. (2012). SCN5A mutations in Brugada syndrome are associated with increased cardiac dimensions and reduced contractility. PLoS ONE, 7(8), e42037 10.1371/journal.pone.0042037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z. , Patel, C. , Li, W. , Xie, Q. , Wu, R. , Zhang, L. , … Yan, G. X. (2009). Role of signal‐averaged electrocardiograms in arrhythmic risk stratification of patients with Brugada syndrome: A prospective study. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 6(8), 1156–1162. 10.1016/j.hrthm.2009.05.007 [DOI] [PubMed] [Google Scholar]
- Ikeda, T. , Sakurada, H. , Sakabe, K. , Sakata, T. , Takami, M. , Tezuka, N. , … Yamaguchi, T. (2001). Assessment of noninvasive markers in identifying patients at risk in the Brugada syndrome: Insight into risk stratification. Journal of the American College of Cardiology, 37(6), 1628–1634. https://doi.org/S0735-1097(01)01197-4[pii] [DOI] [PubMed] [Google Scholar]
- Ikeda, T. , Takami, M. , Sugi, K. , Mizusawa, Y. , Sakurada, H. , & Yoshino, H. (2005). Noninvasive risk stratification of subjects with a Brugada‐type electrocardiogram and no history of cardiac arrest. Annals of Noninvasive Electrocardiology, 10(4), 396–403. 10.1111/j.1542-474X.2005.00055.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapplinger, J. D. , Giudicessi, J. R. , Ye, D. , Tester, D. J. , Callis, T. E. , Valdivia, C. R. , & Ackerman, M. J. (2015). Enhanced classification of Brugada syndrome‐associated and long‐QT syndrome‐associated genetic variants in the SCN5A‐encoded Na(v)1.5 cardiac sodium channel. Circulation: Cardiovascular Genetics, 8(4), 582–595. 10.1161/circgenetics.114.000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Scouarnec, S. , Karakachoff, M. , Gourraud, J. B. , Lindenbaum, P. , Bonnaud, S. , Portero, V. , … Redon, R. (2015). Testing the burden of rare variation in arrhythmia‐susceptibility genes provides new insights into molecular diagnosis for Brugada syndrome. Human Molecular Genetics, 24(10), 2757–2763. 10.1093/hmg/ddv036 [DOI] [PubMed] [Google Scholar]
- Marcus, F. I. , Zareba, W. , & Sherrill, D. (2007). Evaluation of the normal values for signal‐averaged electrocardiogram. Journal of Cardiovascular Electrophysiology, 18(2), 231–233. 10.1111/j.1540-8167.2006.00685.x [DOI] [PubMed] [Google Scholar]
- Maury, P. , Rollin, A. , Sacher, F. , Gourraud, J. B. , Raczka, F. , Pasquié, J. L. , … Probst, V. (2013). Prevalence and prognostic role of various conduction disturbances in patients with the Brugada syndrome. American Journal of Cardiology, 112(9), 1384–1389. 10.1016/j.amjcard.2013.06.033 [DOI] [PubMed] [Google Scholar]
- Meregalli, P. G. , Tan, H. L. , Probst, V. , Koopmann, T. T. , Tanck, M. W. , Bhuiyan, Z. A. , … Wilde, A. A. (2009). Type of SCN5A mutation determines clinical severity and degree of conduction slowing in loss‐of‐function sodium channelopathies. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 6(3), 341–348. 10.1016/j.hrthm.2008.11.009 [DOI] [PubMed] [Google Scholar]
- Morita, H. , Kusano, K. F. , Miura, D. , Nagase, S. , Nakamura, K. , Morita, S. T. , … Wu, J. (2008). Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation, 118(17), 1697–1704. 10.1161/CIRCULATIONAHA.108.770917 [DOI] [PubMed] [Google Scholar]
- Priori, S. G. , Gasparini, M. , Napolitano, C. , Della Bella, P. , Ottonelli, A. G. , Sassone, B. , … Colombo, M. (2012). Risk stratification in Brugada syndrome: Results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. Journal of the American College of Cardiology, 59(1), 37–45. 10.1016/j.jacc.2011.08.064 [DOI] [PubMed] [Google Scholar]
- Priori, S. G. , Napolitano, C. , Gasparini, M. , Pappone, C. , Della Bella, P. , Giordano, U. , … Nastoli, J. (2002). Natural history of Brugada syndrome: Insights for risk stratification and management. Circulation, 105(11), 1342–1347. 10.1161/hc1102.105288 [DOI] [PubMed] [Google Scholar]
- Priori, S. G. , Wilde, A. A. , Horie, M. , Cho, Y. , Behr, E. R. , Berul, C. , … Tracy, C. (2013). HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 10(12), 1932–1963. 10.1016/j.hrthm.2013.05.014 [DOI] [PubMed] [Google Scholar]
- Probst, V. , Allouis, M. , Sacher, F. , Pattier, S. , Babuty, D. , Mabo, P. , … Le Marec, H. (2006). Progressive cardiac conduction defect is the prevailing phenotype in carriers of a Brugada syndrome SCN5A mutation. Journal of Cardiovascular Electrophysiology, 17(3), 270–275. 10.1111/j.1540-8167.2006.00349.x [DOI] [PubMed] [Google Scholar]
- Probst, V. , Veltmann, C. , Eckardt, L. , Meregalli, P. G. , Gaita, F. , Tan, H. L. , … Wilde, A. A. (2010). Long‐term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation, 121(5), 635–643. 10.1161/CIRCULATIONAHA.109.887026 [DOI] [PubMed] [Google Scholar]
- Richards, S. , Aziz, N. , Bale, S. , Bick, D. , Das, S. , Gastier‐Foster, J. , … Committee, A. L. Q. A. (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17(5), 405–424. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf, S. , Bruns, H. J. , Wichter, T. , Kirchhof, P. , Ribbing, M. , Wasmer, K. , … Eckardt, L. (2003). The ajmaline challenge in Brugada syndrome: Diagnostic impact, safety, and recommended protocol. European Heart Journal, 24(12), 1104–1112. 10.1016/S0195-668X(03)00195-7 [DOI] [PubMed] [Google Scholar]
- Rossenbacker, T. , Carroll, S. J. , Liu, H. , Kuipéri, C. , de Ravel, T. J. , Devriendt, K. , … Heidbüchel, H. (2004). Novel pore mutation in SCN5A manifests as a spectrum of phenotypes ranging from atrial flutter, conduction disease, and Brugada syndrome to sudden cardiac death. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 1(5), 610–615. 10.1016/j.hrthm.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Rudic, B. , Schimpf, R. , Veltmann, C. , Doesch, C. , Tülümen, E. , Schoenberg, S. O. , … Papavassiliu, T. (2016). Brugada syndrome: Clinical presentation and genotype‐correlation with magnetic resonance imaging parameters. Europace, 18(9), 1411–1419. 10.1093/europace/euv300 [DOI] [PubMed] [Google Scholar]
- Sieira, J. , Conte, G. , Ciconte, G. , Chierchia, G. B. , Casado‐Arroyo, R. , Baltogiannis, G. , … Brugada, P. (2017). A score model to predict risk of events in patients with Brugada Syndrome. European Heart Journal, 38(22), 1756–1763. 10.1093/eurheartj/ehx119 [DOI] [PubMed] [Google Scholar]
- Simson, M. B. (1981). Use of signals in the terminal QRS complex to identify patients with ventricular tachycardia after myocardial infarction. Circulation, 64(2), 235–242. 10.1161/01.CIR.64.2.235 [DOI] [PubMed] [Google Scholar]
- Smits, J. P. , Eckardt, L. , Probst, V. , Bezzina, C. R. , Schott, J. J. , Remme, C. A. , … Wilde, A. A. (2002). Genotype‐phenotype relationship in Brugada syndrome: Electrocardiographic features differentiate SCN5A‐related patients from non‐SCN5A‐related patients. Journal of the American College of Cardiology, 40(2), 350–356. 10.1016/S0735-1097(02)01962-9 [DOI] [PubMed] [Google Scholar]
- Take, Y. , Morita, H. , Toh, N. , Nishii, N. , Nagase, S. , Nakamura, K. , … Ito, H. (2012). Identification of high‐risk syncope related to ventricular fibrillation in patients with Brugada syndrome. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 9(5), 752–759. 10.1016/j.hrthm.2011.11.045 [DOI] [PubMed] [Google Scholar]
- Timmermans, C. , Ector, H. , Haisty, K. W. , Hammill, S. C. , Kienzle, M. G. , Ozawa, Y. , … Underwood, D. A. (1994). Signal‐averaged ECG parameters in cardiac normals using Frank lead system and Fourier transform filter and gender specific differences: A multicenter study. Pacing and Clinical Electrophysiology, 17(3 Pt 1), 303–311. 10.1111/j.1540-8159.1994.tb01392.x [DOI] [PubMed] [Google Scholar]
- Tokioka, K. , Kusano, K. F. , Morita, H. , Miura, D. , Nishii, N. , Nagase, S. , … Ohe, T. (2014). Electrocardiographic parameters and fatal arrhythmic events in patients with Brugada syndrome: Combination of depolarization and repolarization abnormalities. Journal of the American College of Cardiology, 63(20), 2131–2138. 10.1016/j.jacc.2014.01.072 [DOI] [PubMed] [Google Scholar]
- Vandenberk, B. , Robyns, T. , Goovaerts, G. , Claeys, M. , Helsen, F. , Van Soest, S. , … Willems, R. (2017). Inter‐ and intra‐observer variability of visual fragmented QRS scoring in ischemic and non‐ischemic cardiomyopathy. Journal of Electrocardiology, in press. 10.1016/j.jelectrocard.2017.12.002 [DOI] [PubMed] [Google Scholar]
- van Weerd, J. H. , & Christoffels, V. M. (2016). The formation and function of the cardiac conduction system. Development, 143(2), 197–210. 10.1242/dev.124883 [DOI] [PubMed] [Google Scholar]
- Watanabe, H. , Nogami, A. , Ohkubo, K. , Kawata, H. , Hayashi, Y. , Ishikawa, T. , … Makita, N. (2011). Electrocardiographic characteristics and SCN5A mutations in idiopathic ventricular fibrillation associated with early repolarization. Circulation. Arrhythmia and Electrophysiology, 4(6), 874–881. 10.1161/CIRCEP.111.963983 [DOI] [PubMed] [Google Scholar]
- Yamagata, K. , Horie, M. , Aiba, T. , Ogawa, S. , Aizawa, Y. , Ohe, T. , … Shimizu, W. (2017). Genotype‐phenotype correlation of SCN5A mutation for the clinical and electrocardiographic characteristics of probands with Brugada syndrome: A Japanese multicenter registry. Circulation, 135(23), 2255–2270. 10.1161/CIRCULATIONAHA.117.027983 [DOI] [PubMed] [Google Scholar]
- Yoshioka, K. , Amino, M. , Zareba, W. , Shima, M. , Matsuzaki, A. , Fujii, T. , … Tanabe, T. (2013). Identification of high‐risk Brugada syndrome patients by combined analysis of late potential and T‐wave amplitude variability on ambulatory electrocardiograms. Circulation Journal, 77(3), 610–618. [DOI] [PubMed] [Google Scholar]


