Dear Sirs,
Covid-19 infection has been associated with a predominant prothrombotic state causing venous and arterial thrombosis [1]. Although cerebrovascular complications were reported in 0.8% and in 5.7% of the patients with non-severe and severe Covid-19 disease [2], respectively, they were associated with 2.5-fold increased odds of severe disease in patients with Covid-19 infection [3]. Previous case reports or case series described patients with Covid-19 infection who developed acute stroke [4–12]. Here, we describe the clinical features, neuroimaging and laboratory findings of a case series of patients with Covid-19 infection consecutively admitted to our hyper-acute stroke unit (HASU), Charing Cross Hospital, Imperial College Health Care NHS Trust (ICHT) with acute stroke via the acute stroke pathway. From the 1 March to 30 April 2020, eight acute stroke patients who tested positive for Covid-19 were consecutively admitted to our HASU. Seven out of eight patients suffered an ischemic stroke, with one patient with a haemorrhagic stroke. The median age of our patients was 74 years old (IQR 11.8), and the median NIHSS on admission was 8.5 (IQR 6.3) (Table 1). Our patients developed the symptoms of stroke after a median interval time of 7 days (IQR 10.5) after the onset of their Covid-19 infection. Of note, three patients out of eight showed the neurological symptoms of stroke at the same time as the symptoms of the Covid-19 infection. In the seven patients with ischemic stroke, the majority were in the anterior circulation (n = 6). Large vessel occlusion or floating thrombus in a large vessel was seen in three patients (Fig. 1). Multiple ischemic infarcts were documented in 5 cases out of 7 of which three patients had bilateral lesions. The size of the infarct was classified as small in four cases [13]. One patient (no. 7) was treated successfully with intravenous thrombolysis with tissue plasminogen activator (t-PA) at 3 h and 15 min after the onset of his symptoms. After 24 h, his NIHSS dropped from 8 to 3 and he was discharged after three days with no neurological symptoms and being functionally independent (mRs 0). Table 2 shows the laboratory and radiologic findings on admission for our patients. Most of our patients had elevated levels of fibrinogen, D-dimer and C-reactive protein. In three out of the four, the patients with severe Covid-19 lymphocytopenia were present while one patient (no. 3) showed an abnormal elevated lymphocyte count in the context of chronic lymphocytic leukemia.
Table 1.
Clinical characteristics of the eight patients with acute stroke
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Age (years), sex | 63, F | 83, M | 88, M | 77, M | 71, M | 55, M | 79, M | 70, M |
| Onset of Neurologic syndrome | Concomitant with fever and dyspnea | 11 Days after fever and dyspnea | 12 Days after fever, malaise, dyspnea | Concomitant with dyspnea and cough | 1 Day before dyspnea and tachypnea | 10 Days after cough and anosmia | After 7 Days of cough and fever | Concomitant with fever and cough |
| Severity of COVID infection | Severe | Severe | Severe, developed ARDS | Severe | Moderate | Moderate | Moderate | Moderate |
| Type of stroke; TOAST classificationa | Ischemic stroke with small lesions in the anterior circulation; undetermined etiology for incomplete evaluation | Ischemic stroke with small lesions in the anterior circulation; cardioembolic | Ischemic stroke with a small lesion in the posterior circulation; cardioembolic | Ischemic stroke with a medium lesion in the anterior circulation; cardioembolic | Ischemic stroke with a medium lesion in the anterior circulation; undetermined etiology for incomplete evaluations | Haemorragic stroke likely hypertensive | Ischemic stroke with small lesions in the anterior circulation; undetermined etiology for incomplete evaluations | Ischemic stroke with a medium lesion in the anterior circulation; cardioembolic |
| Comorbid conditions | Peripheral vascular disease, ischemic coronary artery disease, hypertension | New onset of Atrial fibrillation | Prostate cancer, known atrial fibrillation, previous intracranial haemorrhage, COPD, hypertension | Neuroendocrine tumor in the colon, diabetes type 2, smoking, hypertension | Hypertension, smoking | Diabetes type 2, Hypercholesterolemia, previous TIAs, hypertension | Diabetes type 2, atrial fibrillation, coronary artery disease | |
| NIHSS on admission and signs/symptoms of stroke | 9; neglect, dysaphasia, left arm paresis | 19; dysphasia, right hemiplegia, sensory deficit, gaze preference, facial droop | 3; facial droop, right hemiparesis | 13; dysarthria, dysphasia, right hemiparesis | 12; right hemianopia, right hemiparesis, dysphasia, dysarthria | 2; right arm paresis and ataxia | 8 and 3 after 24 h; dysphasia, right arm paresis, sensory deficit | 7; dysphasia, inattention and dysarthria |
| Brain scan results | Multiple and Bilateral infarcts | Multiple infarcts; floating thrombus in the left ICA | Single infarct | Multiple and bilateral infarcts | Multiple infarcts with hemorrhagic transformation type PH1; floating thrombus in the left ICA | Small intracranial haemorrhage in the left external capsule | No acute infarct on first CT; 24 h MRI showed multiple and bilateral infarcts with hemorrhagic transformation type HI-1 | Single infarct with M1/M2 junction large vessel occlusion |
| Treatment and outcomes | Received 14 days of Aspirin 300 mg followed by Clopidogrel 75 mg; discharged at home after 7 days with mRs of 3 | Received LMWH; still inpatient with mRs of 5 | Received 14 days of Aspirin 300 mg followed by Apixaban; still inpatient with mRs of 1 | Received Apixaban; discharged at home after 26 days with mRs of 3 | Received LMWH; still inpatient with mRs of 1 | Received antihypertesive medications; discharged at home after 6 days with mRs of 0 | Received iv. Alteplase and 14 days of Aspirin 300 mg followed by Clopidogrel 75 mg; discharged at home after 3 days with mRs of 0; re-admitted after 30 days with a new TIA | Received 14 days of Aspirin 300 mg followed by Clopidogrel 75 mg; still inpatient with mRs of 3 |
F female, M male, NIHSS National Institutes of Health Stroke Scale, mRs modified Rankin Scale, ICA internal carotid artery, LMWH low molecular weight heparin, TIA transient ischemic attack
aTOAST Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Classification applied for ischemic stroke only
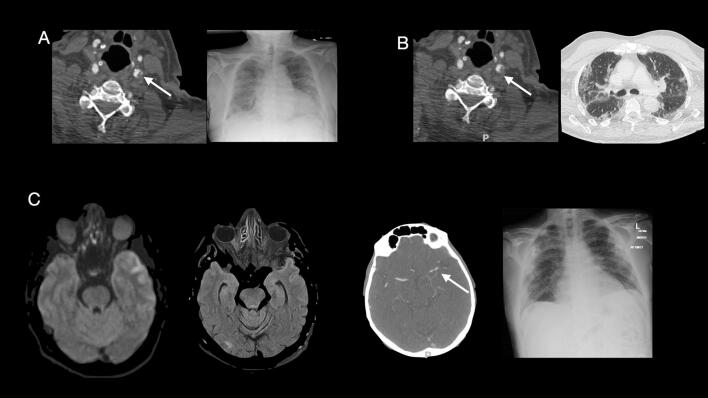
Fig. 1.
Case 5. A CT angiography demonstrates lobulated soft tissued plaque at the left common carotid artery bifurcation which involves the origin of the left internal carotid artery, causing approximately 50% narrowing of the left ICA origin and in keeping with a floating thrombus. The chest XR shows diffuse bilateral air space opacifications. Case 2. B CT angiography illustrates a mixture of soft tissue and calcified mural plaque with an intraluminal tail of thrombus extending into the Internal Carotid Artery. The Chest CT shows bilateral predominantly peripheral interstitial and airspace opacifications as well as bronchocentric opacities predominantly in the lower lobes. Case 8. C CT angiography demonstrates acute left middle cerebral artery M1/M2 junction occlusion, and the MRI brain shows acute infarct of the left anterolateral temporal lobe. The chest XR shows bilateral peripheral predominant multiple opacities
Table 2.
Radiological and laboratory findings of the eight patients with acute stroke
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Chest XR/CT results | Bilateral consolidations with small pleural effusions | Multifocal bilateral GGO | Multifocal bilateral GGO and bilateral pleural effusions | Unilateral consolidation | Multifocal bilateral GGO | No abnormalities | Multifocal, Unilateral GGO | Multifocal, Bilateral GGO |
| White blood cell count (4.2–7.0 × 109/L) | 5.6 | 5.1 | 27.4 | 7.2 | 9.5 | 6.5 | 4.8 | 11.4 |
| Lymphocyte count (1.1–3.6 × 109/L) | 0.6 | 0.7 | 18.9 | 0.7 | 1.8 | 1.2 | 1.1 | 1.8 |
| Platelet count (130–370 × 109/L) | 132 | 376 | 225 | 214 | 590 | 232 | 148 | 634 |
| CRP (< 5.0 mg/L) | 8.4 | 213.4 | 279.2 | 96.2 | 44.3 | 98 | 18.6 | 80.7 |
| Fibrinogen (1.90–4.30 g/L) | 4.21 | 7.59 | 9.82 | 4.96 | 5.26 | 4.89 | 5.17 | 7.8 |
| APTT (seconds) (25–35) | > 180 | 74.8 | 32.3 | 38.8 | 30.3 | 24.7 | 30.2 | 28.6 |
| Prothrombin time (seconds) (12.8–17.4) | 13.0 | 18.1 | 18.7 | 19.2 | 14.8 | 11.8 | 13.9 | 14 |
| D-Dimer level (< 500 ng/mL) | 9709 | 1256 | > 2000 | 3846 | 1557 | – | – | 5952 |
GGO ground-glass-opacity
Our case series provided descriptive data on patients with Covid-19 disease that developed acute stroke and were admitted to our HASU via the acute stroke pathways. A recent WSO survey across multiple countries including UK, Italy, Belgium, Greece, Iran, Chile and Colombia has documented that the Covid-19 pandemic has affected the stroke care with a significant fall in the number of stroke admissions, up to 80%, during the COVID-19 outbreak [14]. Moreover, preliminary data suggested that a smaller proportion of patients with milder stroke symptoms presented to hospital during the COVID-19 pandemic [15] due to fears of infection. The median NIHSS on admission of our patient sample was 8.5 suggesting that these more severe symptoms cannot be ignored by patients or family members.
Previous studies described the clinical characteristics and course of acute stroke in patients with Covid-19 disease in different healthcare systems compared to ours [4, 16]. Compared to the case series of Oxley et al. [4], most of our patients with ischemic stroke had multiple and small ischemic lesions on the brain scans. Interestingly, as also documented in the case series of Avula et al. [17], we showed three patients with acute stroke as a presenting symptom.
The presence of Covid-19 infection has been associated with a predominant prothrombotic state [1] affecting the fibrinolysis and regulated by various pro-inflammatory cytokines [18].
In our case series, the severe Covid-19 patients were commonly associated with markedly elevated D-dimer, high fibrinogen and elevated APTT levels. This is in line with previous studies suggesting a more pronounced microvascular thrombosis associated with Covid-19 than those induced by non-SARS-CoV2 [19].
As the inflammatory processes have fundamental roles in stroke in either the aetiology and pathophysiology of cerebral ischemia [20], the presence of Covid-19 infection could be a factor in the genesis or worsening of stroke in addition to the potential risk of cardioembolic stroke due to ACE-2 expression in the heart and subsequent cardiac dysfunction [21].
Taken together, our case series highlights the importance of investigating the role of the Covid-19 in the aetiology and pathophysiology of the cerebrovascular disease as a complication of the disease. Viral infections can trigger stroke with different mechanisms that depend on the associated pathogen and host characteristics. Varicella zoster virus (VZV) is responsible for a distinctive vasculopathy involving both large and small arteries [22], while human immunodeficiency virus (HIV) can cause brain large vessel vasculopathy [23]. Data acquisition of larger case series is urgently needed to investigate the potential causative association between Covid-19 and stroke. We believe that, if proven, this would emphasize the importance of early detection of stroke symptoms in Covid-19 patients to allow better identification of those patients who could benefit from reperfusion therapy. In conclusion, we believe that our case description provides further evidence of the heterogeneous neurological complications associated with SARS-CoV-2. Future researches and data acquisition are needed to characterize the casual association and the clinical pattern of new cases of acute stroke observed in the context of Covid-19 pandemic.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank the entire medical and nurse staff of the HASU at Charing Cross Hospital, Imperial College London NHS Healthcare Trust for their contribution.
Funding
No funding supported this research.
Availability of data and material
The data that support the findings of this study are available from the corresponding author, LD, upon reasonable request.
Compliance with ethical standards
Conflicts of interest
Lucio D’Anna, Joseph Kwan, Zoe Brown, Omid Halse, Sohaa Jamil, Dheeraj Kalladka, Marius Venter, Soma Banerjee have no conflict of interest.
Ethics approval
This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent of subjects was not needed as the data collected for the study were information collected as part of the routine care, and only de-identified data were used in the research.
References
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China: JAMA Neurol; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus Disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020 doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 4.Oxley TJ. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020 doi: 10.1056/nejmc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tunç A, Ünlübaş Y, Alemdar M, Akyüz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg MF, Goldberg MF, Cerejo R, Tayal AH. Cerebrovascular disease in COVID-19. Am J Neuroradiol. 2020 doi: 10.3174/ajnr.a6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Co COC, Yu JRT, Laxamana LC, David-Ona DIA. Intravenous thrombolysis for stroke in a COVID-19 positive filipino patient, a case report. J Clin Neurosci. 2020 doi: 10.1016/j.jocn.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bnà C, Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020 doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang A, Mandigo GK, Yim PD, Meyers PM, Lavine SD. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerv Surg. 2020 doi: 10.1136/neurintsurg-2020-016220. [DOI] [PubMed] [Google Scholar]
- 11.Zayet S, Klopfenstein T, Kovẚcs R, Stancescu S, Hagenkötter B. Acute cerebral stroke with multiple infarctions and COVID-19, France, 2020. Emerg Infect Dis. 2020 doi: 10.3201/eid2609.201791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escalard S, Maïer B, Redjem H, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19. Stroke. 2020 doi: 10.1161/strokeaha.120.030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain cerebral hemispheres. Neurology. 1998 doi: 10.1212/WNL.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 14.Brainin M (2020) Stroke care and the COVID19 pandemic words from our President
- 15.Teo K-C, Leung WCY, Wong Y-K, et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke. 2020 doi: 10.1161/STROKEAHA.120.030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaghi S, Ishida K, Torres J, et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. 2020 doi: 10.1161/strokeaha.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020 doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003 doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 21.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 22.Gilden DH, Kleinschmidt-DeMasters BK, Wellish M, Hedley-Whyte ET, Rentier B, Mahalingam R. Varicella zoster virus, a cause of waxing and waning vasculitis: the New England Journal of Medicine case 5-1995 revisited. Neurology. 1996 doi: 10.1212/WNL.47.6.1441. [DOI] [PubMed] [Google Scholar]
- 23.Chetty R, Batitang S, Nair R. Large artery vasculopathy in HIV-positive patients: another vasculitic enigma. Hum Pathol. 2000 doi: 10.1016/S0046-8177(00)80253-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, LD, upon reasonable request.