Abstract
The pig oocyte maturation protocol differs from other mammalian species due to dependence on follicular fluid (FF) supplementation. One of the most abundant components of the porcine follicular fluid are fatty acids (FAs). Although evidence from other mammalian models revealed a negative impact of saturated fatty acids (SFA) on developmental competence of oocytes, pig has not yet been widely analyzed. Therefore, we aimed to investigate whether supplementation of IVM medium with 150 μM of stearic acid (SA) and oleic acid (OA) affects lipid content and expression of genes related to fatty acid metabolism in porcine cumulus–oocyte complexes and parthenogenetic embryo development. We found significant influence of fatty acids on lipid metabolism in cumulus cells without affecting the oocyte proper. The expression of ACACA, SCD, PLIN2, FADS1, and FADS2 genes was upregulated (P < 0.01) in cumulus cells, while their expression in oocytes did not change. The increase in gene expression was more pronounced in the case of OA (e.g., up to 30-fold increase in PLIN2 transcript level compared to the control). The number of lipid droplets and occupied area increased significantly in the cumulus cells and did not change in oocytes after SA treatment. Oleic acid improved the blastocyst rate (48 vs 32% in control), whereas stearic acid did not affect this parameter (27%). Additionally, we have discovered a phenotypic diversity of LD in cumulus cells in response to FA supplementation, suggesting extensive lipolysis in response to SA. Stearic acid excess in maturation media led to the formation of multiple micro lipid droplets in cumulus cells.
Keywords: cumulus, oocyte, blastocyst, lipid droplet, parthenogenesis, lipid metabolism, gene expression, lipolysis, fatty acids, in vitro maturation
Cumulus cells are the main target of exogenous fatty acids during in vitro maturation of porcine oocytes.
Introduction
The domestic pig is currently considered one of the best animal research models for human medicine due to anatomical and physiological similarities. Moreover, several similarities like the kinetics of early embryo development and the moment of embryo genome activation resemble the early preimplantation development of human embryos [1]. The transgenic pig models had been already developed to study the etiology of human diseases such as diabetes, obesity, and cancer and metabolic disorders [2]. Therefore, pig is extremely interesting for the research in the field of reproduction and developmental biology. The factors crucial for improving the effectiveness of in vitro embryo production as well as noninvasive prediction of successful pregnancy are the most important concern in assisted reproductive techniques (ART). It has been proven, however, that the success in obtaining an embryo depends on the quality of the initial material, that is, the oocyte, while the quality of the resulting embryo strictly depends on the culture conditions [3, 4]. Therefore, obtaining competent oocytes and application of efficient in vitro maturation (IVM) protocol are pivotal to the entire procedure of in vitro embryo production. The protocol of porcine IVM differs from other mammalian species due to the requirement for follicular fluid (FF) supplementation for the entire period of 44 h maturation. At this stage, follicular fluid is essential to support oocyte maturation, fertilization (decrease of polyspermy), and embryonic development (pronuclear formation) [5–7]. Supplementation of IVM medium with 10–20% v/v of FF is a generally accepted approach, regardless of the basal medium composition. However, supplementation of IVM medium with FF makes it chemically undefined, and the majority of studies do not comment on its composition (e.g., the level of hormones, growth factors, amino acids, fatty acids in relation to the outcome of the entire procedure). Meanwhile, a high variability in FF composition may trigger unexpected variation in the experimental setup.
One of the most abundant components of the porcine FF are fatty acids (FAs). It is known from the bovine and the human studies that saturated FA (SFA) can negatively, in a dose-dependent manner, affect the developmental competence of oocytes [8–14]. Considering a long-term effect, the negative impact of SFA during oocyte IVM may be reflected in the metabolism of the developing embryo. On the other hand, unsaturated FA (e.g., oleic, arachidonic) stimulates oocyte maturation and development of early embryos by neutralizing the negative impact of SFA [15–17]. Hence, lipid metabolism is currently one of the most frequently studied areas of reproductive and developmental biology. In particular, it applies to cattle reproduction in the context of negative energy balance but also to animal models assessing lipid metabolism as a factor affecting fertility. Moreover, oocytes of some animal species (including pigs) contain several times more fat than others (human, mouse, cattle) [18, 19]. The qualitative and quantitative composition of the FF can also be used in human reproductive medicine, to predict the success rate of ongoing pregnancy (noninvasive approach) [9, 20].
For the recent years, along with the development of high-throughput analytical techniques, cumulus cells have been experiencing tremendous popularity giving hope for capturing a noninvasive marker of the developmental potential of the oocytes and embryos. Although CCs are crucial for oocyte growth, maturation, and fertilization, they are unnecessary afterward. Taking into account that metabolism of cumulus cells to some extent reflects that of the oocyte proper, it can be subjected to a wide range of molecular analyses [21–24]. It has been shown that cumulus cells massively accumulate lipids due to fatty acid excess in growth environment protecting the oocyte from lipotoxicity [25, 26]. Mammalian cells store neutral lipids in the lipid droplets (LDs) in the form of triglycerides (TAG) and sterol esters. Dynamic changes of LD shape and size suggest their growth by the excess of fatty acids or diminishment/shrinkage if the process of lipolysis is launched according to the metabolic requirements [27–30]. However, based on our best knowledge, no mechanism describing LD shrinkage caused by SFA has been published so far in any cell type. Moreover, the influence of FA supplemented to IVM medium has not been analyzed broadly so far in the pig; hence we investigated the effect of stearic (SA) and oleic (OA) FA on the expression of genes related to FA metabolism in both oocytes and corresponding cumulus cells.
Material and methods
Ethics statement
All procedures were performed in accordance with the guidelines of the National Ethics Commission for Animal Research (Ministry of Science and Higher Education, Poland), which complies with the European Union Legislation for the protection of animals used for scientific purposes. According to these regulations ethics approval was not required, as the biological material (ovaries) was collected upon animal slaughter in abattoir (Sokolow S.A. Robakowo). Unless stated otherwise, all chemicals were purchased from Sigma.
Collection of cumulus–oocyte complexes
The COCs used in experiment were collected postmortem from porcine ovarian follicles of 3–6 mm diameter. Ovaries were transported in thermoisolated flask 2 h after slaughter to the laboratory. Pubertal animals were 5–6 months old and weighted 100–110 kg. Immature COCs were aspirated from ovarian follicles with needle and syringe and categorized morphologically in HEPES-Talp medium. Only COCs with evenly granulated cytoplasm and at least 4–5 layers of cumulus cells were used in the experiment.
In vitro maturation
In vitro maturation of COC was performed in NCSU-23 (North Carolina State University Medium-23). Every COC group was incubated in 500 μl of IVM medium in four-well plates (Nunc, NY, USA) in HeraCell 150 incubator (ThermoFisher Scientific, MA, USA) under conditions: 5% CO2 in atmosphere, 39 °C, and maximum humidity. The first 20 h of IVM included IVM medium supplemented with hormones: 10 U PMSG (pregnant mare serum gonadotropin, Chorulon, MSD Animal Health, NL) and 10 U hCG (Folligon, MSD Animal Health, NL). The next step included transfer of COCs to fresh, equilibrated medium without hormones and incubation for 24 h. The same batch of follicular fluid (10% v/v), collected from 3 to 6 mm follicles of cyclic gilts with known FA profile, was used in the experiment. Whole IVM procedure included supplementation of media with (1) oleic or (2) stearic FA in the concentration of 150 μM. FA stocks were prepared in ethanol (98%) and processed as previously described in order to prepare working solutions [13]. The same FA stocks were used for oocyte IVM and adipocyte and hepatocyte culture. After IVM, COCs were denuded, and all oocytes presenting first polar body and no degenerative changes were activated for parthenogenetic development or transferred to PBS with 0.2% PVP (polyvinylpyrrolidone), frozen or fixed for further analyses.
Embryo culture
Embryo culture conditions were described previously [31]. Briefly, denuded oocytes were activated using 5 μM ionomycin followed by incubation in 2 mM 6DMAP for 4 h in NCSU23 supplemented with BSA (prevention of second polar body extrusion). Embryos were cultured up to 7 days of development (blastocyst). At the 5th day of development, half of medium was exchanged with fresh and supplemented with 20% of FBS. Blastocyst rate was calculated to all cleaved embryos.
Adipocyte and hepatocyte culture
Porcine mesenchymal stem cells were derived from the adipose tissue (AD-MSCs). Cells were grown in Advanced DMEM (Advanced Dulbecco Modified Eagle Medium) (Gibco, ThermoFisher Scientific) supplemented with 10% (v/v) FBS (fetal bovine serum), 5 ng/ml FGF-2 (PromoKine, PromoCell Germany), 2 mM L-glutamine (PAA), 1 mM 2-mercaptoethanol, 1× antibiotic–antimycotic solution, and MEM NEAA (ThermoFisher Scientific) at 37 °C in 5% CO2. When these cells reached confluency, they were cultured in the adipogenic differentiation medium composed of Advanced DMEM (Gibco, ThermoFisher Scientific), with 10% (v/v) FBS, 1× antibiotic–antimycotic solution (MEM NEAA—ThermoFisher Scientific), 5 ng FGF-2 (PromoKine), 1× Linoleic Acid Albumin, 1× ITS, 1 μM Dexamethasone, 100 μM Indomethacin, and 50 mM IBMX. The differentiation process lasted for 7 days. As a model for research on hepatocytes, we used HepG2 (human hepatoma cells), which is the most common model used for this type of research [32, 33]. HepG2 were cultured to 90% of confluency in Eagle Minimum Essential Medium (EMEM) supplemented with 10% of FCS (fetal calf serum) and antibiotics. Then cells were washed in PBS, and the medium was changed to experimental EMEM containing 1% of fatty acid-free bovine serum albumin (Probumin Merck Millipore, USA) supplemented with investigated fatty acids. The experiment was performed using FA conjugated with BSA and unconjugated, dissolved in EtOH at the same concentrations as for the COC maturation and adipocyte culture. Cells were cultured for 48 h.
Lipid droplet staining
Oocytes were fixed in 4% PFA for 30 min at 37 °C in four-well plates (Nunc, NY, USA). PFA was removed by washing the oocytes twice in PBS with 0.2% PVP. Cells were stored at 4 °C no longer than 2 weeks. Oocytes were permeabilized in 0.2% Triton X-100 solution for 30 min at RT and washed 2× times in 0.2% PVP/PBS. Fluorescent dye used to stain lipid droplets was 20 μg/ml BODIPY 493/503 (ThermoFisher Scientific). Incubation was performed in 500 μl of dye solution in PBS at room temperature for 1 h. The nucleus and the polar body were visualized by staining the oocytes with 0.5 μg/ml DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA, USA). Stained and washed oocytes were mounted on glass slide with single concave (Comex, Poland), coverslipped and stored at 4 °C. Oocytes were analyzed using confocal microscope Zeiss LSM 880 using 488 nm filter with bandpass 500–550 nm for Bodipy 493/503 (Laser Argon2) and 420–480 nm for DAPI (Laser Diode 405). Lipid droplets were assessed through several optical sections of 3 μm thickness each (Z-stack) captured every 10 μm to exclude double positioning of the same structures on two stacks. Every oocyte was captured from equatorial section to the top of the cell. Objective LD LCl Plan Apochromat 40×/1.2 Imm Korr DIC 27 (Zeiss, Germany), pinhole, filters, and offset settings were kept constant throughout the experiments. LD were counted using ImageJ software (NIH, USA) and “Events” tool followed by the creation of Excel file with final results for all oocytes. For the estimation of the area occupied by the LD in oocytes, the ImageJ software was used. Firstly, the binary, 8-bit (black and white) photos were created. After setting the threshold for fluorescent signals, the “Watershed” tool was used to separate the overlapping LD. The lipid droplet area (μm2) from all analyzed stacks was dived by the oocyte area (from the corresponding stacks) giving final result in the percentage share of the LD occupied area within the oocyte.
Cumulus cells from denuded oocytes were centrifuged at 1000g for 5 min., resuspended in fresh PBS, and distributed on adhesive slides (SuperFrost Menzel, ThermoFisher Scientific) using Cytospin 4 (ThermoFisher Scientific). Cells were stained in 4% PFA in Coplin jar for 1 h, washed 3× in PBS, and stained with Bodipy 493/503, DAPI (Vectashield), and Phalloidin (R415, ThermoFisher Scientific) to measure CC individually. LD and chromatin were stained with the same protocol as for the oocytes, while Phalloidin according to the manufacturer’s protocol. Confocal imaging included superresolution AiryScan mode on LSM880 system (Zeiss) using LD LCl Plan Apochromat 40×/1.2 Imm Korr DIC 27 (Zeiss) objective and slicing every 0.3 μm. Images were processed with ZEN Black software using AiryScan processing mode set as automatic 3D reconstruction.
Lipid droplets in adipocytes and HepG2 cells were stained with the same protocol as for the oocytes and cumulus. Cells grown on coverslips were directly subjected to staining with 20 μg/ml BODIPY 493/503 for 1 h in dark covered with parafilm. After staining, coverslips were washed 3× with PBS and placed upside down on microscope slide with 10 μl DAPI with antifade (Vectashield diluted 3×). Images were captured with the same filter sets and objective as for the cumulus cells.
mRNA gene expression
Gene expression on mRNA level was performed on oocytes before and after in vitro maturation and embryos at blastocyst stage. Denuded oocytes (30 per sample) and pools of cumulus cells from corresponding 30 oocytes were placed in separate 1.5 ml DNA LoBind tubes (Eppendorf, Poland) in PBS and frozen in liquid nitrogen. All samples were stored at −80 °C. The total RNA was extracted with mirVANA Paris Kit (Ambion, ThemoFisher Scientific) according to the manufacturer protocol. Briefly, the samples were incubated in Cell Disruption Buffer and 2× Denaturing Solution. Afterward the acid phenol chloroform was added to cell lysate and centrifuged for 15 min at 14 000 rpm. The upper, clear phase was mixed with isopropanol (1.25 v/v) and placed in a filter column and centrifuged at 8000g for 1 min. The next steps involved 2× washing to remove contaminants. Finally, the total RNA was eluted into a fresh 1.5 ml LoBind tube with 100 μl of prewarmed Elution Buffer. The RNA samples were further precipitated with NF Pellet Paint Co-Precipitant. 1 μl of Pellet Paint, 10 μl of 3 M sodium acetate, and 200 μl of freshly prepared 96% EtOH were added to RNA sample. After 5 min incubation, the samples were centrifuged at top speed for 10 min. The RNA pellet was washed followed by centrifugation by 75 and 96% EtOH and dried in 40 °C. RNA was resuspended in Molecular Biology Grade water in 10 μl. Next RNA was reverse transcribed using Transcriptor First Strand cDNA synthesis Kit (Roche, Poland) following the manufacturer’s protocol and using total isolated RNA. The protocol included denaturation of RNA and primers (60 μM random hexamer and 2.5 μM oligo-dT) at 65 °C for 10 min, followed by reverse transcription at 25 °C for 5 min and 42 °C for 1 h and inactivation at 80 °C for 10 min. The cDNA samples were stored at −20 °C until further analysis. Genes responsible for fatty acid metabolism (ACACA, SCD, FASN, FADS1, FADS2, PLIN2, SREBF1, PPARG) were analyzed. Additionally, based on single-cell RNA-seq data (unpublished), we decided to analyze ADCY3 and ATGL as one of the most abundantly expressed genes of lipid metabolism family. Each cDNA sample was analyzed in two independent PCR runs, and the mean value was used for the calculations of relative transcript abundance to the most stable reference genes ACTB and GAPDH (data not shown). The lists of analyzed genes, primer, and probe sequences designed by TibMolbiol (Berlin, Germany) are shown in Table 1. qPCR was conducted using the standard curve method. For this purpose, the desired sequences for all analyzed genes were amplified by PCR and visualized on 1.5% agarose gel with the Gene Ruler 100 bp DNA Ladder (Fermentas, Canada). The PCR product was excised from the gel and isolated and purified using the GeneJet Gel Extraction Kit (ThermoFisher Scientific). Based on the DNA concentration measured with a Nanodrop 2000c system (ThermoFisher Scientific), a serial tenfold dilutions of a DNA with a known concentration (standards) were generated. Each standard was used as a separate template for a real-time PCR reaction to produce the appropriate standard curve with the LightCycler 480 II software (Roche). The reaction conditions and efficiency of the reactions for all genes were analyzed separately. All reactions were performed using the LightCycler 480 II system with a set of supplied reagents (LightCycler 480 Probes Master, Roche). The 10-μl reaction mixture consisted of 5 μl of the 2× concentrated LightCycler Probe Master, 0.5 μM primers, 0.3 μM probe, and 1 μl of the cDNA. The reaction conditions were as follows: denaturation at 95 °C for 10 min; amplification of 40 cycles at 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 1 s; and final cooling at 40 °C. The temperature slope was set at 20 °C/s during amplification.
Table 1.
Primer pairs and FRET probes sequences used for real time PCR gene expression analyses.
| Gene | Sequences of primers (F, R) and probes (FRET—FL, LC, hydrolysis probes—TM) | Length (bp) | |
|---|---|---|---|
|
PLIN2
Gene ID: 397402 |
F | AGCTAGAGCCTCAGATTGCCAT | 246 |
| R | GAATGCTTTTTCTACTCCACTGCTC | ||
| FL | AAGACCAAGTCTGTGGTTAATGGAAGCA | ||
| LC | ACACTGTCCTGGGAAGTCGGATGATG | ||
|
FADS1
Gene ID: 444995 |
F | CAAGAGGAAGAAGTGGGTGGACT | 210 |
| R | CATGGAGAACCAGACCACGTT | ||
| FL | CAACAGTGGCACATAAGTGAGGAAGATGC | ||
| LC | CACATAGAAGGACGTCATCCAGGCCA | ||
|
ACACA
Gene ID: 397324 |
F | CCTGGATTCTGAAGCCAAGATAATC | 206 |
| R | CGTAAGCCGTCCACGATGTAA | ||
| FL | CCCTTCGCGGTTGAAGTCCTTGA | ||
| LC | GCCTGATAGGTCTTAAACGCAGAGTCTGGA | ||
|
SCD
Gene ID: 396670 |
F | GGTGATGTTCCAGAGGAGGTACT | 241 |
| R | CCAATGAAACCAGGATATTCTCC | ||
| FL | GCCATCGTGCTCAATGCCACCT | ||
| LC | CTGGTAAACAGTGCTGCCCACCTATACG | ||
|
FASN
Gene ID: 397561 |
F | CCTCACCTCCATCCAGATC | 149 |
| R | CAGTAGCTGGAGAGCACAG | ||
| FL | CCACCTCGCCCAGGGAGT | ||
| LC | CCCGATGATGCCGTCAGGCT | ||
|
ACTB
Gene ID: 414396 |
F | CAAAGCCAACCGTGAGAAGA | 121 |
| R | GTACCCCTCGTAGATGGGCA | ||
| FL | TGTCCCTGTACGCCTCTGGCC | ||
| LC | CACCACTGGCATCGTGATGGACTCCG | ||
|
GAPDH
Gene ID: 396823 |
F | CCCACGAGCACACCTCAGAA | 141 |
| R | TGCAGCCTGTACTCCCGCT | ||
| FL | GGCAGCAGGTGGGACAGCAG | ||
| LC | GGGTGCAGGAGAGGGGCGAC | ||
|
SREBF1
Gene ID: 397308 |
F | CACACCATGGGGAAGTACC | 134 |
| R | GGAGACTGGTCTTGACTCGC | ||
| TM | F-CAGCACGGCCACGGACAGG-Q | ||
|
ATGL
Gene ID: 100049704 |
F | GGGTCTGCCTGGGTGATAC | 236 |
| R | GACGTTGGCCTGGATGAG | ||
| TM | F-CACTGCACCCCTCCTTCAACCTG-Q | ||
|
PPARG
Gene ID: 397671 |
F | CACTGGAATTAGATGACAGCGAC | 87 |
| R | TGTCTTGAATGTCCTCGATGG | ||
| TM | F-CGCCCAGGTTTGCTGAATGT-Q | ||
|
FADS2
Gene ID: 444997 |
F | GACGGCCTTCATCCTTGCTA | 375 |
| R | CTGGTACTGGAAGTACAAGGGGATA | ||
| FL | CTTGGCATGATGCTGGAAGTGGC | ||
| LC | ATGGTTCCACCAGTTGGCAGAGGC | ||
|
ADCY3
Gene ID: 100514402 |
F | GCGATTACCTGGATGAGA | 339 |
| R | CGTTGAGTAGCTGGTTGA | ||
| TM | F-AACCTACCTCATCATTGCGTCCA-Q |
F, R—primers (forward, reverse), FL and LC—FRET probes, TM—hydrolysis probe.
Apoptosis screening and cAMP measurement
The oocytes and cumulus cells prior to TUNEL (Promega, USA) reaction were fixed in 4% PFA for 30 min at RT and stored in 4 °C. Positive control slides were prepared with exactly the same protocol with DNAse treatment prior to TUNEL reaction. The protocol was previously published [31]. cAMP assay was performed using Cyclic AMP ELISA Kit (581001) from Cayman Chemical (USA) according to the instructions provided by the manufacturer.
Statistical analysis
A comparison of the experimental groups was performed using IBM SPSS Statistics 23.0. All data (before computing) were subjected to testing for normal distribution using the Kolmogorov–Smirnov and Shapiro–Wilk tests. The differences in lipid droplet number and occupied area in oocytes of different groups were calculated using the Kruskal–Wallis and two-tailed Mann–Whitney U tests. mRNA gene expression differences were analyzed using nonparametric two-tailed Mann–Whitney U test. All data with P < 0.05 were considered statistically significant.
Results
Fatty acid supplementation affects preimplantation embryo development
Stearic and oleic acids did not inhibit MII rate of oocytes and COC morphology after IVM (Supplementary Figure S1). Maturation rate (polar body extrusion) accounted for 88% (489/556), 86% (550/640), and 86% (410/472) for control, stearic, and oleic FA treatments, respectively. Stearic acid did not inhibit the embryo development to the blastocyst stage (27%; 98/363 vs 32%; 165/515 in control), while oleic acid supplementation improved the blastocyst yield (48%; 201/420) at day 7 of development (rates per activated oocyte, Supplementary Figure S2). Cumulus–oocyte complexes exhibited similar morphology and cumulus expansion rate during IVM at different time points in all groups.
mRNA gene expression in response to fatty acid supplementation
Genes related to lipid and fatty acid metabolism were differentially expressed in cumulus cells and oocytes. In oocytes FADS2, SCD, and FASN showed upregulation in both stearic and oleic groups, however, with only FASN being significantly changed compared to control. In the case of FADS1 (desaturase), its expression increased after supplementation with stearic acid; however, no significant difference was noticed. Conversely, oleic acid supplementation FADS1 resulted in nonsignificant downregulation compared to control. ACACA and PLIN2 expression did not change in experimental groups compared to control (P > 0.05; Figure 1). Comparison of two experimental groups, stearic and oleic FA treatments, showed significant change only in FADS1 gene expression (P < 0.05).
Figure 1.
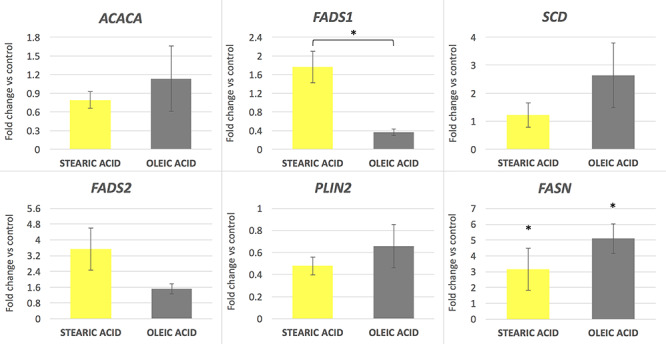
mRNA gene expression level in porcine oocytes after supplementation of maturation media with 150 μM of stearic and oleic fatty acids indicated as fold change relative to the control group (±SEM). *P < 0.05.
Significant changes in gene expression occurred due to the influence of experimental factors in the cumulus cells surrounding the oocytes. All analyzed genes excluding FASN were significantly upregulated in response to both stearic and oleic FA supplementation compared to control (P < 0.05 for ACACA, SCD, FADS1, and FADS2 and P < 0.01 for PLIN2). Moreover, the action of oleic acid resulted in 2–3× fold increase in the expression level in relation to the group supplemented with stearic acid. The highest effect was found for the PLIN2 gene at 12× and 32× fold increase in expression compared to the control group for stearic and oleic FA respectively (Figure 2). The lowest (nonsignificant) increase in expression concerned the FASN gene, at a similar level in response to both experimental factors, around 2.5× fold change compared to the control. SREBF1, ATGL, PPARG, and ADCY3 showed expression at similar level to control group (Figure 3). Comparison of two experimental groups, stearic and oleic FA treatments, showed significant change only in FADS2 gene expression (P < 0.05).
Figure 2.
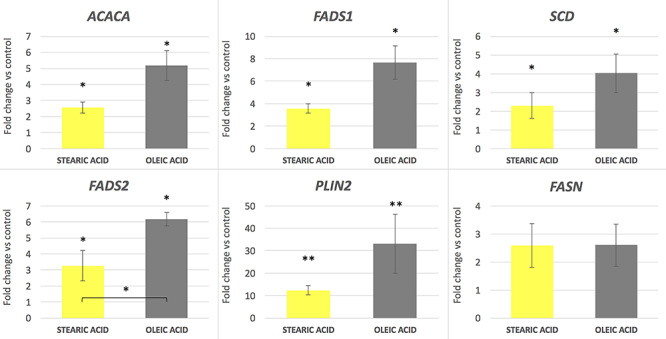
mRNA gene expression level in corresponding cumulus cells after supplementation of maturation media with 150 μM of stearic and oleic fatty acids indicated as fold change relative to the control group (±SEM). *P < 0.05; **P < 0.01.
Figure 3.
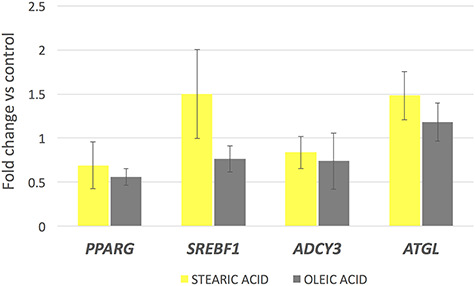
mRNA gene expression level in corresponding cumulus cells after supplementation of maturation media with 150 μM of stearic and oleic fatty acids indicated as fold change relative to the control group (±SEM).
Figure 5.
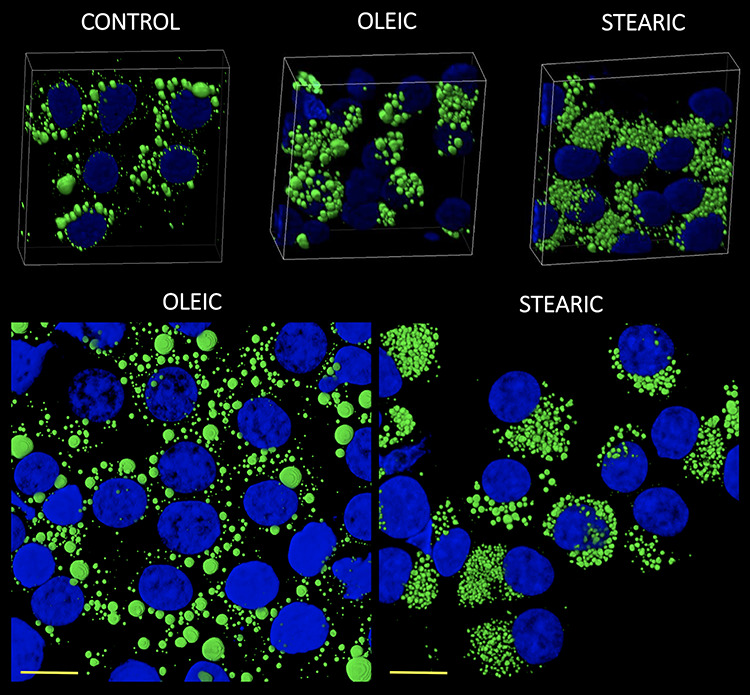
3D reconstruction of confocal images showing morphology and number of lipid droplets and nucleus in cumulus cells after COCs IVM in media supplemented with oleic and stearic FA compared to control. Cells were stained with Bodipy 493/503 and DAPI to visualize lipid droplets (green) and chromatin (blue), respectively.
Fatty acid supplementation and lipid droplet number in oocytes and cumulus cells
No significant change in the number and morphology of LD were noticed in response to stearic and oleic FA in oocytes and blastocyst stage embryos. Mean LD number accounted for 580 (±164), 604 (±135), and 721 (±194) for control, stearic, and oleic FA oocyte groups. A remarkable change in the phenotype, the number, and average size of LD was noticed in cumulus cells. Altogether 1036 cumulus cells were analyzed (SA, 405; OA, 364; control, 267). Response to growth environment was different for stearic and oleic FA. We found similar number of LD between oleic and control groups; however, they increased in size significantly upon media supplementation (Figures 4–6). On the other hand, stearic acid supplementation leads to phenotypic shift into numerous small lipid droplets present in all fixed cells. LDs were significantly smaller compared to control and oleic groups; however, their population increased fourfold. It has to be underlined that, although confocal stacks have been captured using superresolution mode, some of the LD overlapped after processing using ImageJ and thus the final number may be even underestimated. Therefore, as we have previously shown, more precise factor describing area of LDs per cell should be used [31]. We showed that supplementation with stearic acid significantly increased the area occupied by LD. However, the increase in LD number but their smaller size in stearic group does not mean the status quo of lipid content in cumulus cells but their significant increase which was even more evident than in oleic acid group. Moreover, only in the stearic group LD exhibited polar localization in cumulus cell. Additionally, individual cumulus cell culture or in vitro maturation of denuded oocytes showed no change in LD morphology under SA supplementation, meaning the bidirectional regulation of lipid metabolism within COC (Figure 7). No influence of IVM FA supplementation was noticed in the morphology of LD in the blastocyst stage embryos (day 7 of development; Figure 8).
Figure 4.
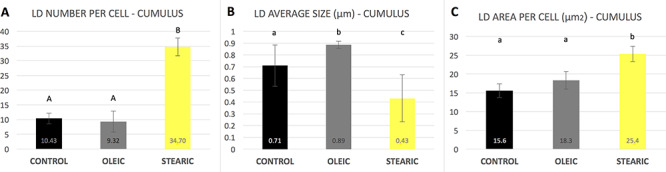
(A) Lipid droplet number estimated per single cumulus cell; (B) average size of LD; (C) the area occupied by LD in response to stearic and oleic fatty acid supplementation during IVM (±SD). Different letters represent statistically significant differences.
Figure 6.
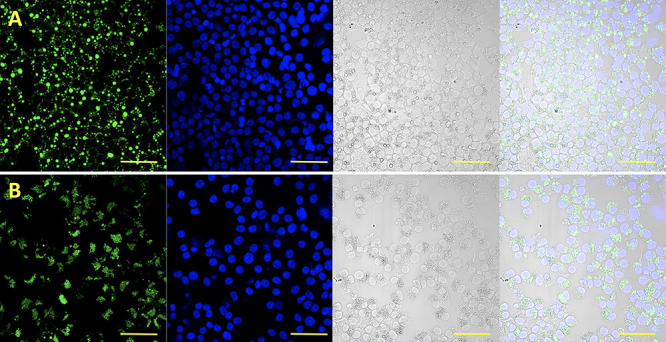
Morphology of lipid droplets (LD) in porcine cumulus cells after COC IVM in media supplemented with (A) oleic and (B) stearic FA. (1) LD stained with Bodipy 493/503 (green); (2) chromatin stained with DAPI (blue); (3) Nomarski, visible light; (4) merged channels. Scale bar represents 50 μm.
Figure 7.
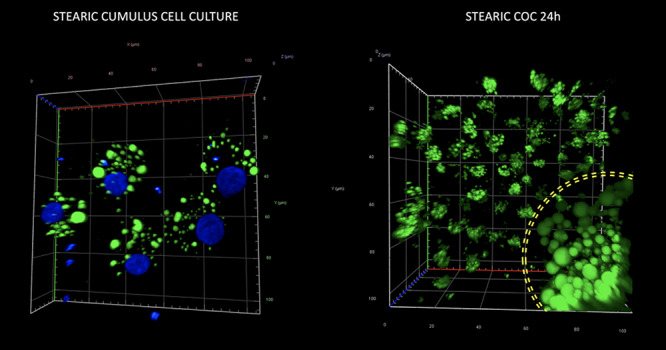
Morphology and quantity of lipid droplets in cultured porcine cumulus cells (left panel) and a cumulus–oocyte complex (right panel) after supplementation of media with stearic FA. The yellow circle on the right panel corresponds to the zona pellucida and indicates LD population of the oocyte surrounded by several, expanded cumulus cells. Staining with Bodipy 493/503 and DAPI to visualize lipid droplets (green) and chromatin (blue), respectively.
Figure 8.
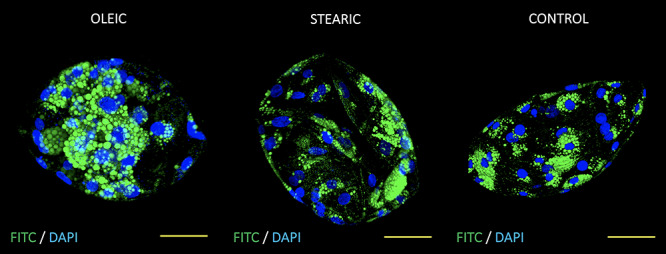
Optical sections of day 7 porcine parthenogenetic blastocysts originating from oocytes matured in media supplemented with oleic, stearic FA, and control. Embryos were stained with Bodipy 493/503 and DAPI to visualize lipid droplets (green) and chromatin (blue), respectively. Scale bar represent 50 μm.
Fatty acid supplementation and lipid droplet number in adipocytes and hepatocytes
To find whether the shrinking LD phenotype in cumulus cells exposed to stearic acid is cell-specific, we decided to examine the LD accumulation and the phenotype during adipocyte differentiation from day 3 to 7 and in human hepatocytes after 3 days of culture. Conversely to cumulus cells, we did not find the correlation of stearic and oleic FA supplementation on LD phenotype with control adipocyte cells during the 7-day culture. The LD area increased significantly compared to control in the 3rd, 5th, and 7th day of culture for OA and at the 3rd and 7th day for SA; however, the action of stearic acid was different than in cumulus cell leading to increased lipid accumulation without presentation of shrinking phenotype. Similarly, massive accumulation of lipid droplets was noted in hepatocytes in groups supplemented with FA; however, no change in phenotypic divergence was noticed (Figures 9 and 10; Supplementary Figure S3).
Figure 9.
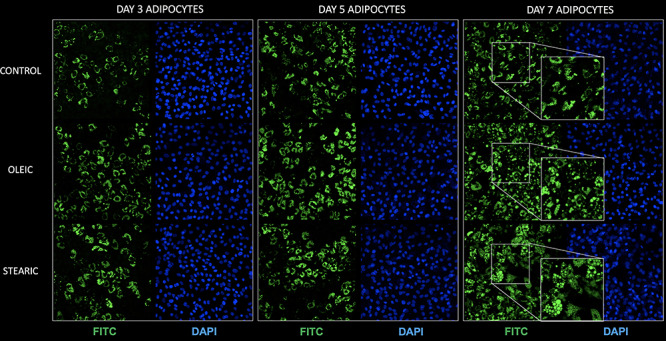
Morphology and quantity of lipid droplets (LD) in porcine adipocytes cultured in control media as well as supplemented with stearic and oleic FA (day 3, 5, and 7 of in vitro culture). Staining with Bodipy 493/503 (green) and DAPI (blue) to visualize lipid droplets and chromatin (blue), respectively.
Figure 10.
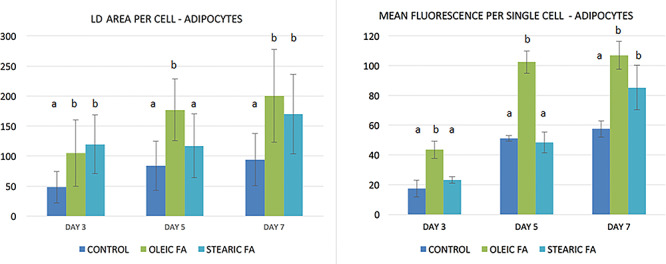
Lipid droplet area and mean fluorescence per single porcine adipocyte (arbitrary units) cultured in media supplemented with stearic and oleic FA (day 3, 5, and 7 of in vitro culture). Different letters represent statistically significant differences.
Fatty acid supplementation and apoptosis occurrence and cAMP level in cumulus
Frequency of apoptotic cumulus cells was relatively low accounting for 2% after 48 h IVM taking together all experimental and control groups. Supplementation of media with stearic acid did not lead to the increase in apoptosis rate in cumulus cells (1.6 vs 1.9% in control). Surprisingly a higher apoptosis rate was observed in oleic group accounting for 3.1% of cells (Supplementary Figure S4). cAMP concentration was increased in the cumulus isolated from stearic FA-supplemented groups (P < 0.05; Figure 11).
Figure 11.
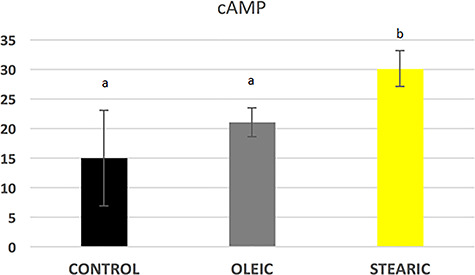
cAMP level in porcine cumulus cells after supplementation of maturation media with 150 μM stearic and oleic fatty acids compared to the control group. Different letters represent statistically significant differences.
Discussion
The last decade of studies in the field of domestic animal reproduction significantly enriched the current state of knowledge on lipid metabolism. It concerns in particular the link between endogenous lipid stores and access to environmental nutrients and developmental competence of oocytes and embryos. Meanwhile, cumulus cells (CC) that accompany the growing oocyte and establish a bidirectional communication are essential for shaping oocyte quality followed by successful fertilization. We show that porcine cumulus cells accumulate significant amounts of lipids to protect the oocytes from lipotoxicity under the excess of FA in culture media. Results of the present experiment revealed a distinct response of the oocyte and corresponding cumulus cells to the exogenous fatty acids—stearic and oleic. Unlike oocytes, cumulus cells were affected by the FA supplementation and responded differently depending on the type of FA used. Cumulus cells exhibited phenotypic divergence of LD so that stearic acid promoted massive shrinkage or division into multiple microLD, while oleic FA exhibited lipid accumulation and LD growth. IVM media supplementation with FA affected expression of genes related to lipid metabolism in cumulus cells only and influenced the development of parthenogenetic embryos to the blastocyst stage.
Leroy et al. proposed a bovine model based on elevated non-esterified fatty acids (NEFA, combination of stearic, palmitic and oleic FA) demonstrating a negative impact on in vitro maturing oocytes and developing embryos [13, 34, 35]. The excessive NEFA impaired gene expression, methylation, and the quality of obtained embryos (morphology; reduced pyruvate, glucose, and oxygen consumption; increased lactate consumption). Moreover, NEFA triggered ER (endoplasmic reticulum) stress in cumulus cells leading to lower blastocyst rate and lower mtDNA copy number in oocytes and blastocyst [36]. The experiments carried out on murine and bovine oocytes and embryos as well as our recent research on porcine parthenogenetic embryos revealed the programming effect of the oocyte microenvironment on the metabolism and gene expression in embryos at blastocyst stage [31, 37–39]. However, it is worth emphasizing that all experiments performed on bovine embryos refer to media supplemented with selected FA or their combination of known concentration, referring to physiological levels and pathological conditions. Such approach is not possible in the pig due to common application of 10% v/v of follicular fluid to IVM media. However, the porcine FF has been characterized by a wide heterogeneity in FA profile affecting the metabolism of the cumulus–oocyte complex [31, 40]. Therefore, it is extremely important to work with a defined batch of FF during the entire experiment. Upon lipotoxic conditions, mitochondria and ER functions in oocytes are disturbed which leads to increased ROS production, depletion of glutathione stores, and induction of apoptotic pathways. Our results showed stable levels of apoptosis in oocytes of different experimental groups. Interestingly a monounsaturated oleic FA caused higher incidence of apoptosis compared to saturated stearic FA and control group but only in cumulus cells after IVM, however, still on very low level (3.1 vs 2% in control). Therefore, the rate of apoptosis at the level of 3% seems to be very safe for developing COCs.
Fatty acids and sterols are stored as neutral lipids in numerous lipid droplets (mostly converted to triacylglycerols—TAG). In adipocytes and other cells, lipid droplets are major energy depot that is being recruited under certain conditions followed by reorganization, fusion, and de novo biogenesis of lipid droplets [41]. In our experiment, only LD in cumulus cells has responded to the supplementary SA, and no changes in this regard were observed in oocytes and day 7 parthenogenetic blastocysts. Most probably, cumulus cells accumulated the excess of SA supplemented to IVM media and reduced its lipotoxic effect on maturing oocytes and parthenogenetic blastocyst quality. Bovine morulae embryos derived from oocytes maturing with exogenous SA (75 μM) displayed reduced quality reflected by the lower number of LD occupying smaller area within the embryo [13]. Moreover, SA added to IVM medium reduced glutathione level (GSH) in oocytes which suggests either a robust ROS neutralization or limited GSH provision by CCs characterized by a reduced expression of the glutathione peroxidase (GPX1) [35]. On the other hand, in our study we did not observe changes in LD morphology in porcine oocytes and embryos in response to OA supplementation. With regard to the developmental potential of parthenogenetic embryos, OA supplementation during IVM positively affected embryo culture and significantly increased the blastocyst yield compared to control and SA groups. Studies on rat embryos also demonstrated that the development to blastocyst stage is promoted upon media supplementation with unsaturated FA (oleic, linoleic) but not after saturated palmitic FA [42]. It is therefore evident that OA as a monounsaturated FA supports maturation of COCs and its action is reflected in subsequent stages by more efficient in vitro development of parthenotes.
The observed changes in LD morphology in cumulus cells induced by the SA persuaded us to investigate this phenomenon in three other types of mammalian cells—porcine in vitro cultured adipocytes, human hepatocytes, and bovine cumulus cells. It turned out that both exogenous FA increased the number of LD due to lipid accumulation, however, without any phenotypic divergence. In human adipocytes, two fractions of small LD have often been described, namely, micro- and nanolipid droplets (microLD; nanoLD), which are >1 μm and <1 μm in diameter, respectively [27, 43]. The nomenclature applied to adipocytes cannot be, however, implemented to LD in cumulus cells, which are usually smaller than 1 micron which contrasts the heterogeneity of LD size in porcine oocyte (from <1 μm up to 10 μm). In adipocytes, some studies showed that under lipolytic stimulation, large LDs disperse into numerous microLDs [43]. However, time-lapse experiments uncovered that microLDs are found in different parts of cytoplasm, also lacking large LD, which indicates a mechanism of de novo formation rather than fragmentation of existing LD [27]. Moreover, lipolysis induced by forskolin or IBMX and isoproterenol did not lead to microLD formation until BSA removal from the medium. Rapid depletion of lipid stores in adipocytes was triggered due to the action of BSA which is known as an effective FA scavenger. Therefore, the lack of BSA in medium during lipolysis forced the cell to reincorporate FA into de novo synthetized numerous microLDs as shown also in our observation. This could also explain observed in our experiments the lack of SA lipolytic effect on bovine cumulus cells due to FA-free BSA supplementation for bovine cumulus–oocyte complex maturation which is omitted for the maturation of porcine COCs. Additionally, Hashimoto et al. showed using CARS microspectroscopy that FA originating from TAG hydrolysis are re-esterified and incorporated in newly formed microLD on ER surface. These microLDs are coated by perilipins (PLIN), ATGL, HSP, and CGI-58 indicating active sites of lipolysis [44]. The well-tuned process of lipolysis involves lipid droplet-associated proteins as well as lipases and other proteins. Unphosphorylated PLIN coating LD binds the CGI-58 preventing activation of ATGL lipase to hydrolyze the TAG into DAG and fatty acids. PLIN may be phosphorylated by cAMP-dependent PKA under lipolytic stimulation, followed by the entrance of ATGL and HSP lipases to LD [45]. Our study showed increased cAMP concentrations in cumulus cells after supplementation of media with SA and OA fatty acids which prove lipolysis-like answer of the cells to culture conditions although the ADCY3, adenylate cyclase 3, which we picked from RNA-seq data as the most abundantly expressed in oocytes among other cAMP enzymes showed similar expression among experimental and control groups. However, only saturated, stearic FA triggered microLD formation with specific polar localization in most of the cells, while the action of OA FA was different and leads to accumulation of lipids in cells exhibited by the LD size.
Interestingly, the in vitro culture of porcine cumulus cells obtained from COCs and individual cumulus depleted oocytes did not result in LD phenotypic change into multiple microLD. Staining of the entire COCs after IVM and during culture illustrated the bidirectional cooperation of oocytes and surrounding cumulus cells in terms of lipid metabolism. At least to some extent the FA uptake and LD biogenesis are regulated by the entire COC rather than independently by the oocyte and cumulus cells. An intensive cross-talk between oocyte and cumulus cells via TZP (transzonal projections) is well known. It is, therefore, very likely that lipid metabolism is orchestrated by the oocyte and surrounding cumulus cells ensuring the homeostasis and acquirement of developmental competence by the oocyte. Our results prove the findings of Del Collado et al. who showed active involvement of FABP3 in lipid metabolism between oocyte and cumulus cells which is present in TZPs [46]. Moreover, FABP3 is expressed and colocalized with TZP only in cumulus-surrounded oocytes or in parts of the zona pellucida having contact with cumulus cells. This finding strongly supports the involvement of the bidirectional talk between the oocyte and cumulus cells in the lipid metabolism regulation.
In our study FA excess in maturation media triggered LD growth and microLD de novo formation depending on FA supplementation. In both experimental conditions, genes engaged in FA metabolism and biogenesis of LD in cumulus cells were upregulated which may indicate the mechanism of FA uptake and incorporation during in vitro maturation. Smirnova et al. found that ATGL plays a role in adiposome degradation not only in adipocytes, as previously described (adipose triglyceride lipase), but also in HeLa and COS-7 cells [47]. Moreover, the depletion of the ATGL gene expression by RNA interference led to significant increase in the size of LD. In our study, ATGL was expressed at similar level among groups together with PPARG and SREBF1, some well-known factors regulating expression of genes engaged in FA synthesis, transport, and modification. However, genes involved in LD biogenesis as well as FA desaturation and synthesis were significantly upregulated in cumulus cells reflecting extensive lipid metabolism. ACACA and FASN are major players in de novo synthesis of FA from acetyl-CoA and malonyl-CoA [48]. FADS1, FADS2, and SCD as well as ELOVLs are responsible for FA elongation and desaturations and, therefore, play a crucial role in lipid metabolism in the cell [49]. Our study indicates that cumulus cells uptake FA, however, store, metabolize, and/or selectively pass them to the oocyte. The PLIN proteins (1–5) are expressed among different tissues with PLIN1 exclusively in adipocytes [45]. It was shown by Zhang that porcine oocytes express PLIN2 at mRNA and protein levels which is consistent with our data [50]. Zhang showed similar expression of PLIN2 in GV and MII porcine oocytes similarly to our observations made for oocytes from prepubertal and cyclic sows where donor FF did not influence mRNA expression [50]. In our study oocytes were not affected by FA supplementation; however, PLIN2 increased dramatically in cumulus cells after IVM which indicate lipid uptake and storage. The available data on the PLIN2 involvement in the process of lipolysis and ATGL activation are scarce and unclear. Some studies indicated that PLIN2 is not phosphorylated by PKA like PLIN1 in adipocytes and therefore does not play a role in lipase activation but rather serves as a gatekeeper of internal lipid stores. It was postulated that in oocytes other proteins may mediate lipolysis signaling and ATGL/HSL activation (like PLIN5) which needs to be elucidated [51, 52]. Recently, however, it was shown that PLIN2 is phosphorylated and therefore released from the LD surface, enabling the lipases entrance and cleavage of TAG. Moreover, the expression and action of PLIN1 and other PAT proteins in cumulus cells need to be analyzed to study the process of lipolysis.
In conclusion, we discovered that fatty acids supplemented to porcine IVM medium significantly affected lipid metabolism in the cumulus cells but not in the proper oocyte. The exogenous FA induced massive lipid storage in cumulus cells accompanied by changes in lipid droplets morphology dependent on the FA type. We demonstrated a stimulating action of the unsaturated oleic acid in shaping the development potential of the oocyte and the subsequent embryo. We also discovered a lipolysis-like answer of cumulus cells to elevated saturated stearic acid concentrations. It is therefore interesting to unravel the importance of LD heterogeneity in oocytes and cumulus cells and the metabolic fate decisions made under the particular culture conditions. The blastocyst yield may arise from the impaired lipid metabolism of COC; however, the action of saturated (SA) and nonsaturated (OA) fatty acids needs to be elucidated. Last but not least, it is interesting to find out the differences and/or similarities in lipid metabolism between cumulus–oocyte complexes and other cell types.
Supplementary Material
Acknowledgments
We acknowledge veterinary doctor, Piotr Kempski, for obtaining the tissues for research, and Joanna Lechtanska for technical support in the laboratory.
Author contributions
PP designed and performed the experiments, analyzed data, and wrote the manuscript; NM performed experiments (immunostaining, lipid droplet staining, data computing); ISz and PK performed experiments (adipocyte and hepatocyte culture).
Conflict of Interest
All authors declare there is no conflict of interest that would prejudice the impartiality of this scientific work.
Conference Presentation: Pawlak P., Malyszka N., Szczerbal I., Warzych E., Madeja Z. E., Lechniak D. (2018). The influence of stearic and oleic fatty acids supplementation on early embryo development, gene expression and phenotypic divergence of lipid droplets in cumulus cells. Visegrad Group Society for Developmental Biology: Inaugural Meeting, 7–9 September 2018, Brno, Czech Republic, P. 49.
Footnotes
† Grant Support: This work was supported by research project from the Ministry of Science and Higher Education in Poland “Iuventus Plus” (0051/IP1/2013/72) and the National Science Centre, Poland (2014/13/D/NZ2/03901). The cost of Open Access publication was covered by the Society for Biology of Reproduction in Poland.
References
- 1. Madeja ZE, Pawlak P, Piliszek A. Beyond the mouse: Non-rodent animal models for study of early mammalian development and biomedical research. Int J Dev Biol 2019; 63:187–201. [DOI] [PubMed] [Google Scholar]
- 2. Niemann H, Petersen B. The production of multi-transgenic pigs: Update and perspectives for xenotransplantation. Transgenic Res 2016; 25:361–374. [DOI] [PubMed] [Google Scholar]
- 3. Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol Reprod Dev 2002; 61:234–248. [DOI] [PubMed] [Google Scholar]
- 4. Lonergan P, Rizos D, Kanka J, Nemcova L, Mbaye AM, Kingston M, Wade M, Duffy P, Boland MP. Temporal sensitivity of bovine embryos to culture environment after fertilization and the implications for blastocyst quality. Reproduction 2003; 126:337–346. [DOI] [PubMed] [Google Scholar]
- 5. Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril 2015; 103:303–316. [DOI] [PubMed] [Google Scholar]
- 6. Ducolomb Y, Gonzalez-Marquez H, Fierro R, Jimenez I, Casas E, Flores D, Bonilla E, Salazar Z, Betancourt M. Effect of porcine follicular fluid proteins and peptides on oocyte maturation and their subsequent effect on in vitro fertilization. Theriogenology 2013; 79:896–904. [DOI] [PubMed] [Google Scholar]
- 7. Grupen CG, Armstrong DT. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: Temporal effects of follicular fluid during IVM. Reprod Fertil Dev 2010; 22:1100–1109. [DOI] [PubMed] [Google Scholar]
- 8. Leroy J, Vanholder T, Mateusen B, Christophe A, Opsomer G, de Kruif A, Genicot G, Van Soom A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005; 130:485–495. [DOI] [PubMed] [Google Scholar]
- 9. O'Gorman A, Wallace M, Cottell E, Gibney MJ, McAuliffe FM, Wingfield M, Brennan L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 2013; 146:389–395. [DOI] [PubMed] [Google Scholar]
- 10. Valckx SDM, Arias-Alvarez M, De Pauw I, Fievez V, Vlaeminck B, Fransen E, Bols PEJ, Leroy J. Fatty acid composition of the follicular fluid of normal weight, overweight and obese women undergoing assisted reproductive treatment: A descriptive cross-sectional study. Reprod Biol Endocrinol 2014; 12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Valckx SDM, De Bie J, Michiels ED, Goovaerts IG, Punjabi U, Ramos-Ibeas P, Gutierrez-Adan A, Bols PE, Leroy JL. The effect of human follicular fluid on bovine oocyte developmental competence and embryo quality. Reprod Biomed Online 2015; 30:203–207. [DOI] [PubMed] [Google Scholar]
- 12. Valckx SDM, De Pauw I, De Neubourg D, Inion I, Berth M, Fransen E, Bols PEJ, Leroy J. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum Reprod 2012; 27:3531–3539. [DOI] [PubMed] [Google Scholar]
- 13. Van Hoeck V, Rizos D, Gutierrez-Adan A, Pintelon I, Jorssen E, Dufort I, Sirard MA, Verlaet A, Hermans N, Bols PEJ, Leroy J. Interaction between differential gene expression profile and phenotype in bovine blastocysts originating from oocytes exposed to elevated non-esterified fatty acid concentrations. Reprod Fertil Dev 2015; 27:372–384. [DOI] [PubMed] [Google Scholar]
- 14. McKeegan PJ, Sturmey RG. The role of fatty acids in oocyte and early embryo development. Reprod Fertil Dev 2012; 24:59–67. [DOI] [PubMed] [Google Scholar]
- 15. Marei WFA, De Bie J, Mohey-Elsaeed O, Wydooghe E, Bols PEJ, Leroy J. Alpha-linolenic acid protects the developmental capacity of bovine cumulus-oocyte complexes matured under lipotoxic conditions in vitro. Biol Reprod 2017; 96:1181–1196. [DOI] [PubMed] [Google Scholar]
- 16. Marques C, Galvao A, Baptista M, Prates E, Horta A, Ferreira-Dias G, Skarzynski D, Pereira R. Effect of trans10, cis12 conjugated linoleic acid on bovine and porcine oocyte prostaglandins (PG) and lipid content during maturation. Reprod Domest Anim 2013; 48:106–106. [Google Scholar]
- 17. Aardema H, Vos PLAM, Lolicato F, Roelen BAJ, Knijn HM, Vaandrager AB, Helms JB, Gadella BM. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol Reprod 2011; 85:62–69. [DOI] [PubMed] [Google Scholar]
- 18. McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JSM, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil 2000; 118:163–170. [PubMed] [Google Scholar]
- 19. Apparicio M, Ferreira CR, Tata A, Santos VG, Alves AE, Mostachio GQ, Pires-Butler EA, Motheo TF, Padilha LC, Pilau EJ, Gozzo FC, Eberlin MN et al. Chemical composition of lipids present in cat and dog oocyte by matrix-assisted desorption ionization mass spectrometry (MALDI-MS). Reprod Domest Anim 2012; 47:113–117. [DOI] [PubMed] [Google Scholar]
- 20. Bayasula IA, Kobayashi H, Goto M, Nakahara T, Nakamura T, Kondo M, Nagatomo Y, Kotani T, Kikkawa F. A proteomic analysis of human follicular fluid: Comparison between fertilized oocytes and non-fertilized oocytes in the same patient. J Assist Reprod Genet 2013; 30:1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Assidi M, Montag M, Van Der Ven K, Sirard M-A. Biomarkers of human oocyte developmental competence expressed in cumulus cells before ICSI: A preliminary study. J Assist Reprod Genet 2011; 28:173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Assidi M, Dufort I, Ali A, Hamel M, Algriany O, Dielemann S, Sirard M-A. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod 2008; 79:209–222. [DOI] [PubMed] [Google Scholar]
- 23. Cillo F, Brevini TAL, Antonini S, Paffoni A, Ragni G, Gandolfi F. Association between human oocyte developmental competence and expression levels of some cumulus genes. Reproduction 2007; 134:645–650. [DOI] [PubMed] [Google Scholar]
- 24. Uyar A, Torrealday S, Seli E. Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril 2013; 99:979–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aardema H, Lolicato F, van de Lest CHA, Brouwers JF, Vaandrager AB, van Tol HTA, Roelen BAJ, Vos PLAM, Helms JB, Gadella BM. Bovine cumulus cells protect maturing oocytes from increased fatty acid levels by massive intracellular lipid storage. Biol Reprod 2013; 88:164. [DOI] [PubMed] [Google Scholar]
- 26. Lolicato F, Brouwers JF, van de Lest CHA, Wubbolts R, Aardema H, Priore P, Roelen BAJ, Helms JB, Gadella BM. The cumulus cell layer protects the bovine maturing oocyte against fatty acid-induced lipotoxicity. Biol Reprod 2015; 92:16. [DOI] [PubMed] [Google Scholar]
- 27. Paar M, Jüngst C, Steiner NA, Magnes C, Sinner F, Kolb D, Lass A, Zimmermann R, Zumbusch A, Kohlwein SD, Wolinski H. Remodeling of lipid droplets during lipolysis and growth in adipocytes. J Biol Chem 2012; 287:11164–11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 2009; 50:3–21. [DOI] [PubMed] [Google Scholar]
- 29. Urrutia RA, Kalinec F. Biology and pathobiology of lipid droplets and their potential role in the protection of the organ of Corti. Hear Res 2015; 330:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Olzmann JA, Carvalho P. Dynamics and functions of lipid droplets. Nature reviews. Mol Cell Biol 2019; 20:137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pawlak P, Warzych E, Cieslak A, Malyszka N, Maciejewska E, Madeja ZE, Lechniak D. The consequences of porcine IVM medium supplementation with follicular fluid become reflected in embryo quality, yield and gene expression patterns. Sci Rep 2018; 8:15306–15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gerets HH, Tilmant K, Gerin B, Chanteux H, Depelchin BO, Dhalluin S, Atienzar FA. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol Toxicol 2012; 28:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sefried S, Häring HU, Weigert C, Eckstein SS. Suitability of hepatocyte cell lines HepG2, AML12 and THLE-2 for investigation of insulin signalling and hepatokine gene expression. Open Biol 2018; 8:pii:180147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Hoeck V, Sturmey RG, Bermejo-Alvarez P, Rizos D, Gutierrez-Adan A, Leese HJ, Bols PEJ, Leroy J. Elevated non-esterified fatty acid concentrations during bovine oocyte maturation compromise early embryo physiology. PLoS One 2011; 6:e23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Hoeck V, Leroy J, Alvarez MA, Rizos D, Gutierrez-Adan A, Schnorbusch K, Bols PEJ, Leese HJ, Sturmey RG. Oocyte developmental failure in response to elevated nonesterified fatty acid concentrations: Mechanistic insights. Reproduction 2013; 145:33–44. [DOI] [PubMed] [Google Scholar]
- 36. Sutton-McDowall ML, Wu LLY, Purdey M, Abell AD, Goldys EM, MacMillan KL, Thompson JG, Robker RL. Nonesterified fatty acid-induced endoplasmic reticulum stress in cattle cumulus oocyte complexes alters cell metabolism and developmental competence. Biol Reprod 2016; 94:23. [DOI] [PubMed] [Google Scholar]
- 37. Warzych E, Wrenzycki C, Peippo J, Lechniak D. Maturation medium supplements affect transcript level of apoptosis and cell survival related genes in bovine blastocysts produced in vitro. Mol Reprod Dev 2007; 74:280–289. [DOI] [PubMed] [Google Scholar]
- 38. Warzych E, Peippo J, Szydlowski M, Lechniak D. Supplements to in vitro maturation media affect the production of bovine blastocysts and their apoptotic index but not the proportions of matured and apoptotic oocytes. Anim Reprod Sci 2007; 97:334–343. [DOI] [PubMed] [Google Scholar]
- 39. Watkins AJ, Lucas ES, Fleming TP. Impact of the periconceptional environment on the programming of adult disease. J Dev Orig Health Dis 2010; 1:87–95. [DOI] [PubMed] [Google Scholar]
- 40. Pawlak P, Cieslak A, Warzych E, Zejden Z, Szumacher-Strabel M, Molinska-Glura M, Lechniak D. No single way to explain cytoplasmic maturation of oocytes from prepubertal and cyclic gilts. Theriogenology 2012; 78:2020–2030. [DOI] [PubMed] [Google Scholar]
- 41. Walther TC, Farese RV Jr. The life of lipid droplets. Biochim Biophys Acta 1791; 2009:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khandoker MAM, Tsujii H. Effect of exogenous fatty acids on in vitro development of rat embryos. Asian Australas J Anim Sci 1999; 12:169–173. [Google Scholar]
- 43. Marcinkiewicz A, Gauthier D, Garcia A, Brasaemle DL. The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem 2006; 281:11901–11909. [DOI] [PubMed] [Google Scholar]
- 44. Hashimoto T, Segawa H, Okuno M, Kano H, Hamaguchi H-o,, Haraguchi T, Hiraoka Y, Hasui S, Yamaguchi T, Hirose F, Osumi T. Active involvement of micro-lipid droplets and lipid-droplet-associated proteins in hormone-stimulated lipolysis in adipocytes. J Cell Sci 2012; 125:6127–6136. [DOI] [PubMed] [Google Scholar]
- 45. Sztalryd C, Brasaemle DL. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim Biophys Acta Mol Cell Biol Lipids 1862; 2017:1221–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Del Collado M, da Silveira JC, Sangalli JR, Andrade GM, LRdS S, Silva LA, Meirelles FV, Perecin F. Fatty acid binding protein 3 and Transzonal projections are involved in lipid accumulation during in vitro maturation of bovine oocytes. Sci Rep 2017; 7:2645–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep 2006; 7:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Valsangkar DS, Downs SM. Acetyl CoA carboxylase inactivation and meiotic maturation in mouse oocytes. Mol Reprod Dev 2015; 82:679–693. [DOI] [PubMed] [Google Scholar]
- 49. Aardema H, van Tol HTA, Wubbolts RW, Brouwers JFHM, Gadella BM, Roelen BAJ. Stearoyl-CoA desaturase activity in bovine cumulus cells protects the oocyte against saturated fatty acid stress. Biol Reprod 2017; 96:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang RN, Fu XW, Jia BY, Liu C, Cheng KR, Zhu SE. Expression of Perilipin 2 (PLIN2) in porcine oocytes during maturation. Reprod Domest Anim 2014; 49:875–880. [DOI] [PubMed] [Google Scholar]
- 51. Gemmink A, Daemen S, Kuijpers HJH, Schaart G, Duimel H, López-Iglesias C, van Zandvoort MAMJ, Knoops K, Hesselink MKC. Super-resolution microscopy localizes perilipin 5 at lipid droplet-mitochondria interaction sites and at lipid droplets juxtaposing to perilipin 2. Biochim Biophys Acta Mol Cell Biol Lipids 1863; 2018:1423–1432. [DOI] [PubMed] [Google Scholar]
- 52. Wang H, Sreenivasan U, Hu H, Saladino A, Polster BM, Lund LM, D-w G, Stanley WC, Sztalryd C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res 2011; 52:2159–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


