Abstract
Background: Reduced heart rate recovery (HRR) in coronary artery disease (CAD) is predictive of increased cardiovascular mortality and is related to reduced parasympathetic tonus.
Objective: To investigate HRR and heart rate variability (HRV) measured at steady state condition and the relationship between these two parameters in CAD.
Materials and Methods: In our study, we enrolled 33 (28 males, mean age 52.4 ± 9.6 years) patients with CAD who did not have heart failure, atrial fibrillation, pacemaker, and any disease state that could affect the autonomic functions and 38 age‐matched healthy subjects (21 males, mean age 48.3 ± 7.8 years). All the patients underwent submaximal treadmill exercise testing (Bruce protocol). HRR was calculated by subtracting the heart rate values at the 1st, 2nd, and 3rd minutes of the recovery phase from the peak heart rate (HRR1, HRR2, HRR3). Before exercise testing, short‐term steady state HRV analyses of all subjects were obtained with the time‐ and frequency‐domain methods and were correlated to HRR. For frequency‐domain analysis, low‐frequency HRV (LF, 0.004–0.15 Hz), high‐frequency HRV (HF, 0.15–0.5 Hz), and LF/HF ratio were measured for 5 minutes in the morning. For time‐domain analysis, standard deviation of the normal‐to‐normal NN intervals (SDNN), square root of the mean squared differences of successive N‐N intervals (RMSSD), and proportion derived by dividing the number of interval differences of successive N‐N intervals greater than 50 ms by the total number of N‐N intervals (pNN50) were obtained. Only HRR3 was used for the correlation analysis.
Results: In CAD groups, the HF, an indicator of parasympathetic activation, was significantly reduced, whereas the LF and LF/HF values, which are indicators of sympathetic activity, were increased (P = 0.0001 for each parameter). The time‐domain parameters SDNN, RMSSD, and pNN50 were significantly reduced in the patient group (P = 0.0001, P = 0.009, and P = 0.0001, respectively). Similar to the HRV parameters, the HRR1, HRR2, and HRR3 values were significantly reduced in the patient group (P = 0.0001 for each parameter). We observed a significant negative correlation between HRR3 and LF (r =−0.67, P = 0.0001) and between HRR3 and LF/HF (r =−0.62, P < 0.0001), while there was a significant positive correlation between HRR3 and HF, SDNN, RMSSD, and pNN50 (r = 0.69, P = 0.0001; r = 0.41, P = 0.0001; r = 0.31, P = 0.008; and r = 0.44, P = 0.0001).
Conclusions: HRR and HRV are significantly reduced in CAD. The reduction in HRR is parallel to the changes in HRV parameters. HRR, which can be measured easily in the recovery phase of exercise testing, can be used to detect the depression of parasympathetic tonus and to evaluate the basal autonomic balance in this patient group.
Keywords: heart rate recovery, heart rate variability, coronary artery disease
Despite impressive advances during the past three decades, ischemic heart disease remains a major health problem in Western societies. Impairment of autonomic cardiovascular regulation has been observed in many disease states including ischemic coronary artery disease (CAD). The relevance of this impairment for clinical cardiology was realized in the late 1980s, when the results of impaired autonomic function were found to predict mortality among patients with CAD who have experienced acute myocardial infarction. 1 Heart rate variability (HRV), a measure of sympathetic and parasympathetic influences on the heart, is reduced in the presence of autonomic dysfunction. The presence of subclinical or clinical autonomic dysfunction is associated with increased mortality. 1 , 2 , 3 Another measure of parasympathetic function is heart rate recovery (HRR); an easily obtained measure derived from routinely graded exercise testing. HRV analysis is very useful in evaluation of sympathovagal modulation of cardiovascular functions when compared with the classical methods, and it has a wide range of usage area. 4 , 5 During exercise, an increase of sympathetic activity and a decrease of vagal discharge lead to an increase of heart rate, stroke volume, and myocardial contractility to satisfy energy demands of working muscles. Exercise cardioacceleration results from release of parasympathetic inhibition at low exercise intensities and from both parasympathetic inhibition and sympathetic activation at moderate intensities. 6 Autonomic contribution to cardiodeceleration after exercise (HRR) is less well understood. Inactive recovery from dynamic exercise is associated with cessation of primary exercise stimulus from the brain (cerebral cortex—central command), which is responsible for the initial rapid drop of heart rate. 2 Slower changes in the stimuli to metaboreceptors and baroreceptors accompanying clearance of metabolites and delayed elimination of body heat and catecholamines are thought to be other factors contributing to HRR after physical activity. Nevertheless, parasympathetic activation is considered to be the main mechanism underlying exponential cardiodeceleration after exercise. 6 , 7 , 8 , 9 The rate of decrease in heart beat frequency and the length of time to recovery after moderate‐to‐heavy exercise is commonly used as indicators of cardiovascular fitness. 5 Recently, a delayed decrease in heart rate during the 1st minute after exercise has been suggested to be a powerful and independent predictor of all‐cause mortality. 8 , 10 , 11 , 12
Although the relationship between HRR and parasympathetic activity during the recovery phase after exercise is very well‐known, its relation with the autonomic system functions during resting period is not understood exactly. For this reason, in our study, we aimed to examine the changes in HRR and basal HRV measurements in ischemic CAD, as well as the relationship between HRR and basal HRV parameters.
MATERIALS AND METHODS
Thirty‐three patients with CAD (28 males, mean age 52.4 ± 9.6 years), who were admitted to our clinic with stable angina pectoris between January 2003 and June 2004, were enrolled in this study. All patients underwent submaximal exercise (treadmill) testing and their coronary angiograms were obtained. Thirty‐eight healthy subjects (21 males, mean age 48.3 ± 7.8 years) with atypical angina pectoris, negative exercise testing, and normal coronary angiograms were enrolled in the control group. Routine physical examination, 12‐lead electrocardiography, chest x‐rays, routine biochemical analyses, and transthoracic echocardiography were obtained from all subjects. Patients with documented heart failure or left ventricular systolic dysfunction (left ventricular ejection fraction (LVEF) ≤ 40%), valvular heart disease, pulmonary disease, atrial fibrillation, history of acute myocardial infarction, any disease state that could affect autonomic functions, implanted pacemakers, and those using beta‐blockers were excluded from this study. The local ethics committee approved the protocol and all participants gave informed consent.
HRV Measurement
A standard ambulatory Holter recording system (Biomedical System Century 2000/3000 Holter monitoring system, version 1.32, St. Louis, Missouri) was used. A 3‐channel recorder was used to record the electrocardiographic traces. All recordings were analyzed by Biomedical Systems Century 2000/3000 HRV package system. The Holter recordings were obtained for an hour between 9.30 and 12.30 a.m. in a dark room, the patients had fasted for 12 hours, and were kept in recumbent position throughout the recordings. The subjects were asked to remain awake, but the depth and rate of breathing were not controlled. Only the beats that had normal morphological characteristics were used for analysis. Intervals between ectopic beats, between normal and ectopic beats, and between the beats that could not be measured properly because of the artifacts were excluded from the analysis. HRV was assessed by two ways: time‐ and frequency‐domain analyses.
Time‐domain HRV indexes: standard deviation of the normal‐to‐normal QRS intervals (SDNN), square root of the mean squared differences of successive N‐N intervals (RMSSD), and proportion derived by dividing the number of interval differences of successive N‐N intervals greater than 50 ms by the total number of N‐N intervals (pNN50). SDNN and RMSSD were calculated after detrending the heart rate series.
The mean heart rate, the standard deviation of all R‐R intervals (SDNN), the standard deviation of mean R‐R intervals in 5‐minute recordings (SDANN), the square root of the sum of squares of the successive R‐R intervals between which there was a difference of greater than 50 ms divided by the number of all R‐R intervals (pNN50) were used for the time‐domain analysis. The Fourier transform method was used for the spectral measurements and the heart rate spectrum between 0.003 and 0.40 Hz was defined as total energy (ms2). This power was divided into two components: low frequency (LF, 0.004–0.15 Hz) and high frequency (HF, 0.15–0.5 Hz). It was assumed that HF is a marker of parasympathetic activity, LF is a measure of sympathetic activity, LF is a measure of sympathetic activity, and LF/HF ratio is an indicator of sympathovagal balance. 13 , 14
Exercise Testing Protocol
Subjects underwent a submaximal exercise test according to the standard Bruce protocol. 15 Exercise was terminated when subjects achieved their target HR, defined as 85% of the age and sex‐predicted maximal HR. 16 After reaching peak exercise, patients immediately got off the treadmill and rested in a supine position. The 12‐lead electrocardiogram, HR, and blood pressure were monitored during exercise and for 4 minutes of recovery. Exercise testing was terminated prematurely for the following reasons: symptoms such as limiting chest or leg discomfort, or dyspnea; a decrease in systolic pressure between consecutive stages; exercise systolic pressure ≥ 250 mmHg; development of significant electrocardiographic abnormalities, including ≥2 mm ST‐segment depression; or complex ventricular ectopy. An ischemic ST‐segment response was defined as a ≥ 1 mm horizontal or down sloping ST‐segment depression (measured 80 ms after the J‐point) during exercise or recovery when compared with the electrocardiogram at rest.
HRR was calculated by subtracting the heart rate values at the 1st, 2nd, and 3rd minutes of the recovery phase from the peak heart rate (HRR1, HRR2, HRR3). Only HRR3 was used for the correlation analysis.
The coronary angiographies of all the subjects enrolled in this study were performed and analyzed by experienced cardiologists who were not informed about the patients' data. The lesions resulting in narrowing of the artery lumen > 70% were evaluated as severe coronary artery stenosis. Severe stenosis of more than one major epicardial coronary artery or > 50% lesion in main coronary artery were accepted as a multivessel disease.
STATISTICS
All statistical analyses were performed by computer pack software SPSS 11.5 (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, U.S.A.). Results are given as mean ± standard deviation. Student t‐test was used for comparison of groups. Chi‐square and Student unpaired t‐tests were used for analysis of nonparametric data and Fisher test was used for proportional data. The relationship between HRR and HRV parameters was evaluated by Pearson correlation analysis. A P value of <0.05 was considered statistically significant.
RESULTS
Thirty‐three patients with positive exercise test and established CAD in their coronary angiographies and 38 subjects with normal coronary arteries and negative exercise test were enrolled in the study. As summarized in Table 1, there was no statistically significant difference between the patient and control groups according to cardiac risk factors, LVEF, and mean age. Male gender and nitrate usage were significantly higher in the patient group with CAD as compared to the control group (Table 1). There was no significant difference between the systolic and diastolic blood pressure of the groups, measured at the beginning of the exercise test. However, the systolic blood pressure increased significantly with exercise in the patient group (P = 0.0001). Exercise time was found to be significantly longer in the control group, although their basal heart rates were higher (P = 0.0001, Table 2). The HRR values measured during the recovery phase (HRR1, HRR2, HRR3) were significantly lower in the patient group (P = 0.0001, for each parameter, Table 2, Fig. 1). There was no significant difference between the groups according to their mean heart rate values measured during the Holter recordings. However, SDNN, which is a rough measure of HRV (P = 0.0001) and RMSDD (P = 0.009) and pNN50 (P = 0.0001) values, which are indicators of parasympathetic activity, were significantly higher in the control group (Fig. 1, Table 3). Similarly, HF, an indicator of parasympathetic activity, was higher (P = 0.0001), whereas LF and LF/HF values, indicators of sympathetic activity, were significantly lower (P = 0.0001, for each parameter) in the control group. The correlation between HRR and HRV parameters is summarized in Table 4 and Fig. 2. HRR3 was negatively correlated to LF and LF/HF, which are indicators of sympathetic activity. However, HRR3 was significantly and positively correlated to HF, RMSSD, pNN50, and SDNN.
Table 1.
Clinical Characteristics of the Patient and Control Groups
| CAD (+) | CAD (−) | P Value | |
|---|---|---|---|
| Number | 33 | 38 | |
| Age (years, mean ± SD) | 52.4 ± 9.6 | 48.3 ± 7.8 | ns |
| Sex (males/females) | 28/5 | 21/17 | 0.007 |
| Cardiac risk factors, n (%) | |||
| History of familial CAD | 10 (30.3%) | 13 (34.2%) | ns |
| Hypertension | 15 (45.5%) | 18 (47.3%) | ns |
| Diabetes mellitus | 4 (12.2%) | 3 (7.8%) | ns |
| Smoking | 19 (57.5%) | 25 (65.7%) | ns |
| Hyperlipidemia | 6 (17.1%) | 8 (21%) | ns |
| Obesity (BMI > 30 kg/m2) | 5 (15.1%) | 6 (15.7%) | ns |
| Drug usage | |||
| Aspirin | 16 (48.8%) | 10 (26.3%) | ns |
| Beta‐blocker | 0 (0%) | 0 (0%) | ns |
| Ca antagonist | 0 (0%) | 0 (0%) | ns |
| Antiarrhythmics | 0 (0%) | 0 (0%) | ns |
| ACEI | 13 (39.3%) | 15 (39.4%) | ns |
| Nitrates | 16 (48.8%) | 12 (31.5%) | 0.004 |
| Single vessel disease, n(%) | 12 (36.3%) | 0 (0%) | |
| Multiple vessel disease, n (%) | 21 (63.7%) | 0 (0%) | |
| LV ejection fraction (%, mean ± SD) | 52.9 ± 5.4 | 54.4 ± 8.1 | ns |
BMI = body mass index; CAD = coronary artery disease; LV = left ventricle; ACEI = angiotensin converting enzyme inhibitors; SD = standard deviation; ns = nonsignificant.
Table 2.
Exercise Characteristics and Heart Rate Recovery Indexes in the Patient and Control Groups (mean ± SD)
| CAD (+) | Control Group | P Value | |
|---|---|---|---|
| Basal heart rate | 82.4 ± 12.5 | 100.4 ± 4.4 | 0.0001 |
| Basal systolic blood pressure | 130.9 ± 15.5 | 125.4 ± 10.5 | 0.08 |
| Basal diastolic blood pressure | 85.4 ± 6.9 | 84.2 ± 7.3 | 0.7 |
| Peak systolic arterial pressure | 180.0 ± 11.1 | 155.4 ± 14.4 | 0.0001 |
| Peak diastolic arterial pressure | 83.3 ± 8.8 | 74.0 ± 10.0 | 0.0001 |
| Exercise duration | 6.9 ±2.7 | 10.6 ± 2.1 | 0.001 |
| Peak heart rate | 145.0 ± 9.4 | 154.3 ± 9.7 | 0.003 |
| HRR1 | 23.6 ± 4.8 | 31.9 ± 9.4 | 0.0001 |
| HRR2 | 40.4 ± 10.0 | 54.4 ± 13.7 | 0.0001 |
| HRR3 | 49.8 ± 9.8 | 70.4 ± 16.6 | 0.0001 |
HRR = heart rate recovery index; CAD = coronary artery disease.
Figure 1.
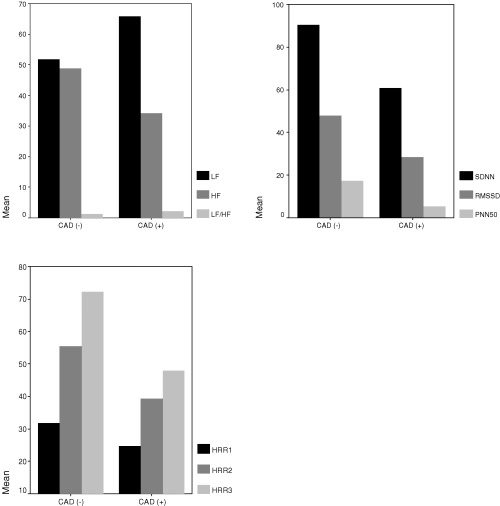
Heart rate recovery and heart rate variability parameters in patients and controls.
Table 3.
Time‐ and Frequency‐Domain HRV Parameters in the Patient and the Control Groups
| CAD (+) Group | Control Group | P Value | |
|---|---|---|---|
| Heart rate (beats/min) | 72.7 | 70.5 | 0.14 |
| SDNN (ms) | 60.9 ± 24.7 | 90.4 ± 35.4 | 0.0001 |
| SDANN (ms) | 37.8 ± 26.4 | 47.7 ± 28.5 | 0.13 |
| RMSSD (ms) | 28.5 ± 25.5 | 47.8 ± 35.0 | 0.009 |
| PNN50 (%) | 5.4 ± 6.0 | 17.3 ± 15.1 | 0.0001 |
| LF | 65.7 ± 6.5 | 51.7 ± 16.9 | 0.0001 |
| HF | 34.1 ± 6.4 | 48.8 ± 16.7 | 0.0001 |
| LF/HF | 2.0 ± 0.7 | 1.2 ± 0.7 | 0.0001 |
HRV = heart rate variability; CAD = coronary artery disease; SDNN = standard deviation of the normal‐to‐normal N‐N intervals; SDANN = standard deviation of mean R‐R intervals in 5‐minute recordings; RMSSD = square root of the mean squared differences of successive N‐N intervals; pNN50 = proportion derived by dividing the number of interval differences of successive N‐N intervals greater than 50 ms by the total number of N‐N intervals; LF = low frequency; HF = high frequency.
Table 4.
Correlation Analysis Between HRV Parameters and HRR3
| Correlation Coefficient | P Value | |
|---|---|---|
| SDNN (ms) | 0.41 | 0.0001 |
| SDANN | 0.15 | 0.15 |
| RMSSD (ms) | 0.31 | 0.008 |
| PNN50 (%) | 0.44 | 0.0001 |
| LF | −0.67 | 0.0001 |
| HF | 0.69 | 0.0001 |
| LF/HF | −0.62 | 0.0001 |
Figure 2.
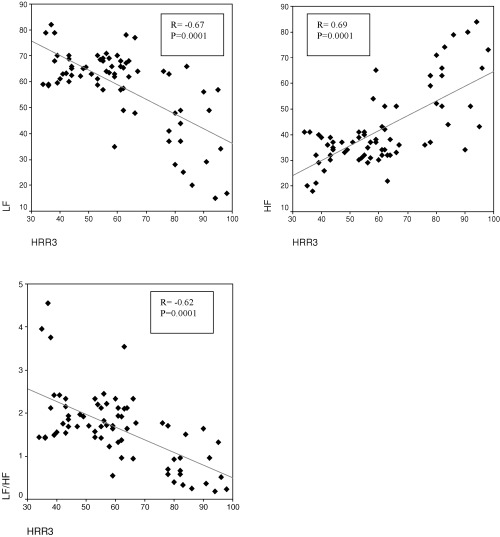
Correlation analysis between HRR3 and frequency‐domain HRV parameters.
DISCUSSION
HRV analysis is a convenient and safe method used for evaluation of the autonomic nervous system functions in various cardiovascular and noncardiovascular disorders. 5 HRV analysis is made by two methods: time‐ and frequency‐domain analyses. In the time‐domain analysis, SDNN, the standard deviation of R‐R intervals, represents a general measurement of autonomic nervous system balance, whereas the pNN50, which is calculated by division of the number of adjacent R‐R intervals differing by more than 50 ms by the number of total R‐R intervals, predominantly reflects the parasympathetic activity. The spectral analysis of R‐R intervals in the frequency‐domain analysis is made in two ways: high frequency (HF, the spectral component between 0.16 and 0.5 Hz)) and low frequency (LF, the spectral component between 0.04 and 0.15 Hz). HF is modulated predominantly by the parasympathetic nervous system, whereas LF is under the influence of both parasympathetic and sympathetic nervous systems. The HF/LF ratio is a marker of the sympathovagal balance. 5 , 13
Abnormal autonomic function is known to predispose to arrhythmias in various clinical and experimental conditions. Sympathovagal imbalance (either increased sympathetic activity or reduced vagal activity) has been shown to be a strong and independent predictor of mortality in patients with myocardial infarction, heart failure, or diabetic neuropathy. 17 , 18 , 19 , 20 Conversely, predominance of vagal activity has protective and antifibrillatory effects. 17 HRV has been shown to be altered among patients with stable CAD and reduced even before the development of symptoms. In 1987, Airaksinen et al. were the first to report the reduced vagal activity among patients with CAD. 21 The HF component of the heart rate is impaired in uncomplicated CAD, particularly during sleep, and the reduction in HRV is correlated with the angiographic severity of the CAD. 22 , 23
The patient population of our study consists of patients without a history of AMI and with relatively preserved LVEFs. The ratio of diabetes mellitus, which is known to affect autonomic functions, is very low in both the patient and the control groups (Table 1). Our findings show that the spectral component of HF is significantly reduced in uncomplicated CAD, and thus support the results of previous studies. The reduction of HF spectral band points to the dominance of LF control mechanism in CAD. Fractal scaling properties of R‐R intervals have also shown to be altered among patients with uncomplicated CAD. 24 In our study, we observed significant reductions in all the time‐domain parameters except SDANN in the CAD group, and these findings were concordant with the changes in the frequency‐domain parameters. The spectral and time‐domain HRV parameters were obtained from short recordings in our study, during which all patients were kept supine to provide stable conditions. Similar to our findings, previous studies have pointed out that frequency‐domain analysis obtained from short recordings are more useful in the prediction of mortality and that time‐domain analysis should be reserved for longer recording. 25 , 26 Thus, the insignificance of the changes in SDANN and the inconsistency of these changes with the parameters in frequency‐domain analysis reported in our study were not evaluated as contradictions.
Although it has been shown that time‐ and frequency‐domain HRV parameters deteriorate in ischemic heart disease, the exact mechanism of these changes is not known. The clinical significance of postexercise HRR and its specificity as an indicator of parasympathetic activity in the recovery phase has been noted by many investigators. 27 , 28 , 29 Cole et al. have identified reduced HRR as a strong and independent predictor of mortality, while Imai et al. have found increased HRR in athletes, but decreased HRR in heart failure. 30 , 31 HRR index is also a strong indicator of risk in asymptomatic and symptomatic CAD, and it is independent from the severity of CAD, Duke treadmill score, and LVEF. 32 , 33 In our study, HRR, like HRV, was reduced significantly in the patient population, similar to the findings of previous studies. CAD was considered responsible for this reduction, because the risk factors, clinical properties, and left ventricular functions were similar in both the patient and the control groups.
In healthy persons, the parasympathetic system is dominant at rest; exercise is associated with parasympathetic withdrawal and, as the intensity of exercise is increased, with sympathetic activity. 6 The autonomic changes associated with cessation of exercise are not yet fully understood. Savin et al. have observed significantly reduced HRR values in individuals whose parasympathetic systems were blocked with atropine after exercise and suggested that parasympathetic reactivation, not sympathetic withdrawal, was responsible for the reduced heart rates in the recovery phase. 34 Crouse et al., on the other hand, have found that sympathetic blockade has very little influence on HRR. 35 These findings support that the parasympathetic system is responsible for the reduced heart rates during the early stages of the recovery phase. Similar results have been found in studies on the relationship between the HRV and the HRR; however, there are few studies that investigate the relationship between HRR and HRV at rest, with conflicting results. 27 , 28 , 29 Javorka et al., in their study where they studied autonomic functions with the HRV and complexity methods, have reported that reduced postexercise HRR is not related to pre‐exercise HRV, but is strongly and significantly correlated with the HRV in the early recovery phase. 29 Ohuchi et al., on the other hand, have found that HRR was correlated with the HRV parameters measured at rest and during early recovery, in their study on children using the baroreflex sensitivity and spectral HRV analysis methods. 28 Pierpont et al. have found no relationship between HRR and the HRV parameters indicating parasympathetic activity measured after maximal exercise, but observed a significant correlation between these two parameters measured after submaximal exercise. 27 Although the results of the studies suggest a significant correlation between HRV and HRR in the recovery phase, there are conflicting results about the HRV and HRR relationship at rest. The reasons for these differences could be the differences between patient populations, the relatively small number of cases, and the methods used for HRV analysis. The purpose of our study was to establish the relationship between HRR and the HRV parameters at rest; thus the patient population and the HRV calculation method were chosen accordingly and the postexercise HRV parameters were not sought for. In our study, we found that HRR and HRV were significantly reduced in uncomplicated CAD and that HRR was significantly and positively correlated with the basal HRV parameters indicating parasympathetic activity, and was significantly and negatively correlated with the basal HRV parameters indicating sympathetic activity. Our findings are similar to the results of Ohiki et al., although we used different case selection criteria and different methods of HRV analysis. 28
The number of our patient population is limited because of the abundance of enrollment criteria we used. Although our patient population is homogeneous, it represents a very small percentage of patients with CAD; thus, its adaptability to clinical usage is relatively limited. HRR is closely related to the intensity of exercise, body temperature, and cardiovascular conditioning. 36 , 37 Another limitation of our study is in not having evaluated the VO2 cardiovascular conditions of our patients. However, Imai et al. have shown that HRR measured 30 seconds after exercise indicated parasympathetic activity independently of the intensity of exercise and cardiovascular condition. 31 In our study, HRR was measured 60, 120, and 180 seconds after exercise; for this reason, the effects of the exercise intensity and condition on HRR can relatively be excluded. HRV parameters (especially HF) are influenced by respiratory rate and depth, and it is usually recommended to control the respiratory rate during recording. 38 We did not control the rate of breathing to avoid hypo‐ or hyperventilation that could result in metabolic and blood gas changes.
CONCLUSIONS
In uncomplicated CAD, HRR and HRV parameters indicative of parasympathetic activity measured in basal conditions are significantly depressed, but the HRV parameters indicating sympathetic activity are augmented. HRR, which is accepted as an indicator of the parasympathetic activity just after cessation of exercise, is also positively correlated with the basal parasympathetic activity. HRR, which can be measured easily in the recovery phase of exercise testing, can be used for evaluation of basal autonomic functions in patients with CAD. However, this issue requires further studies, conducted in larger patient populations.
REFERENCES
- 1. Kleiger RE, Miller JP, Bigger JT, et al Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 1987;59: 256 – 261. [DOI] [PubMed] [Google Scholar]
- 2. Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med 1980;193: 95 – 108. [PubMed] [Google Scholar]
- 3. Malliani A, Lombardi F, Pagani F, et al Power spectral analysis of cardiovascular variability in patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol 1994;5: 274 – 286. [DOI] [PubMed] [Google Scholar]
- 4. Malpas SC, Gordon LP. Circadian variation of heart rate variability. Cardiovasc Res 1990;24: 210 – 213. [DOI] [PubMed] [Google Scholar]
- 5. Stein PK, Bosner MS, Kleiger RF, et al Heart rate variability: A measure of cardiac autonomic tone. Am Heart J 1994;127: 420 – 424. [DOI] [PubMed] [Google Scholar]
- 6. Shephard R. Exercise Physiology. Philadelphia , PA , B.C. Decker Inc., 1987. [Google Scholar]
- 7. Carter R III, Watenpaugh DE, Wasmund WL, et al Muscle pump and central command during recovery from exercise in humans. J Appl Physiol 1999;87: 1463 – 1469. [DOI] [PubMed] [Google Scholar]
- 8. Nishime EO, Cole CR, Blackstone EH, et al Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. J Am Med Assoc 2000;284: 1392 – 1398. [DOI] [PubMed] [Google Scholar]
- 9. Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Phys 1982;53: 1572 – 1575. [DOI] [PubMed] [Google Scholar]
- 10. Chorbajian T. Nomographic approach for the estimation of heart rate recovery time after exercise. J Appl Phys 1971;31: 962 – 964. [DOI] [PubMed] [Google Scholar]
- 11. Ashley EA, Myers J, Froelicher V. Exercise testing in medicine. Lancet 2000;356: 1592 – 1597. [DOI] [PubMed] [Google Scholar]
- 12. Cole CR, Blackstone EH, Pashkow FJ, et al Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med 1999;341: 1351 – 1357. [DOI] [PubMed] [Google Scholar]
- 13. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability, standards of measurement, physiological interpretation, and clinical use. Circulation 1996;93: 1043 – 1065. [PubMed] [Google Scholar]
- 14. Pagani M, Lombardi F, Guzzetti S, et al Power spectral analysis of heart rate and arterial pressure variability as a marker of sympatho‐vagal interaction in man and conscious dog. Circ Res 1986;59: 178 – 193. [DOI] [PubMed] [Google Scholar]
- 15. Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 1973;85: 546 – 562. [DOI] [PubMed] [Google Scholar]
- 16. Sheffield LT. Graded Exercise Tests for Ischemic Heart Disease in Exercise Testing and Training of Apparently Healthy Individuals: A Handbook for Physicians. Dallas , TX , American Heart Association, 1975. [Google Scholar]
- 17. Pumprla J, Howarka K, Groves D, et al Functional assessment of heart rate variability: Physiological basis and practical applications. Int J Cardiol 2002;84: 1 – 14. [DOI] [PubMed] [Google Scholar]
- 18. Kao T, Hsiao HC, Chiu HW, et al The relationship of late potentials to assessment of heart rate variability in post‐infarction patients. Int J Cardiol 2000;74: 207 – 214. [DOI] [PubMed] [Google Scholar]
- 19. Nolan J, Batin PD, Andrews R, et al Prospective study of heart rate variability and mortality in chronic heart failure: Results of the United Kingdom Heart Failure Evaluation and Assessment of Risk Trial (UK‐HEART). Circulation 1998;98(15):1510 – 1516. [DOI] [PubMed] [Google Scholar]
- 20. O`Brien IA, Mcfadden JP, Corrall RJM. The influence of autonomic neuropathy on mortality in insulin‐dependent diabetes. Q J Med 1991;79: 495 – 502. [PubMed] [Google Scholar]
- 21. Airaksinen KEJ, Ikaheimo MJ, Linnaluoto MK, et al Impaired vagal heart rate control in coronary artery disease. Br Heart J 1987;58: 592 – 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huikuri HV, Niemela MJ, Ojala S, et al Circadian rhythms of frequency domain measures of heart rate variability in healthy subjects and patients with coronary artery disease. Effects of arousal and upright posture. Circulation 1994;90: 121 – 126. [DOI] [PubMed] [Google Scholar]
- 23. Hayano J, Sakakibara Y, Yamada A, et al Decreased magnitude of heart rate spectral components in coronary artery disease. Its relation to angiographic severity. Circulation 1990;81: 1217 – 1224. [DOI] [PubMed] [Google Scholar]
- 24. Makikallio TH, Ristimae T, Airaksinen KEJ, et al Heart rate dynamics in patients with stable angina pectoris and utility of fractal and complexity measures. Am J Cardiol 1998;81: 27 – 31. [DOI] [PubMed] [Google Scholar]
- 25. Fei L, Copie X, Malik M, et al Short‐ and long‐term assessment of heart rate variability for risk stratification after acute myocardial infarction. Am J Cardiol 1996;77(9):681 – 684. [DOI] [PubMed] [Google Scholar]
- 26. Howarka K, Pumprla J, Schabmann A. Optimal parameters of short‐term heart rate spectrogram for routine evaluation of diabetic cardiovascular autonomic neuropathy. J Auton Nerv Syst 1998;69: 164 – 172. [DOI] [PubMed] [Google Scholar]
- 27. Pierpont GL, Stolpman DR, Gornick CC. Heart rate recovery post‐exercise as an index of parasympathetic activity. J Auton Nerv Syst 2000;80: 169 – 174. [DOI] [PubMed] [Google Scholar]
- 28. Ohuchi H, Suziki H, Yasuda K, et al Heart rate recovery after exercise and cardiac autonomic nervous activity in children. Pediatr Res 2000;47: 329 – 335. [DOI] [PubMed] [Google Scholar]
- 29. Javorka M, Zila I, Balhárek T, et al Heart rate recovery after exercise: Relations to heart rate variability and complexity. Heart rate variability and post‐exercise recovery. Braz J Med Biol Res 2002;35: 991 – 1000. [DOI] [PubMed] [Google Scholar]
- 30. Cole CR, Blackstone EH, Pashkow FJ, et al Heart‐rate recovery immediately after exercise as a predictor of mortality. New Engl J Med 1999;341: 1351 – 1357. [DOI] [PubMed] [Google Scholar]
- 31. Imai K, Sato H, Hori M, et al Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol 1994;24: 1529 – 1535. [DOI] [PubMed] [Google Scholar]
- 32. Nishime EO, Cole CR, Blackstone EH, et al Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. J Am Med Assoc 2000;284: 1392 – 1398. [DOI] [PubMed] [Google Scholar]
- 33. Shetler K, Marcus R, Froelicher VF, et al Heart rate recovery: Validation and methodologic issues. J Am Coll Cardiol 2001;38: 1980 – 1987. [DOI] [PubMed] [Google Scholar]
- 34. Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol 1982;53: 1572 – 1575. [DOI] [PubMed] [Google Scholar]
- 35. Crouse SF, Sterling J, Tolson H, et al The effect of beta‐adrenergic blockade on heart rate recovery from exercise. J Cardiopulm Rehabil 1989;9: 202 – 206. [Google Scholar]
- 36. Darr KC, Bassett DR, Morgan BJ, et al Effect of age and training status on heart rate recovery after peak exercise. Am J Physiol 1998;254: H340 – H343. [DOI] [PubMed] [Google Scholar]
- 37. Baraldi E, Cooper DM, Zanconato S, et al Heart rate recovery from 1 minute exercise in children and adults. Pediatr Res 1991;29: 575 – 579. [DOI] [PubMed] [Google Scholar]
- 38. Brown TE, Beightol LA, Koh J, et al Important influence of respiration on human R‐R interval power spectra is largely ignored. J Appl Phys 1993;75: 2310 – 2317. [DOI] [PubMed] [Google Scholar]


