Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease with increasing incidence and mortality. More than half of PDAC patients develop metastases, with the liver being the most common site. Patients with pancreatic ductal adenocarcinoma with liver metastases (PCLM) have a very limited scope for surgery due to aggressive tumor behavior and poor prognosis. However, with the improvements in preoperative systemic therapy and perioperative outcomes, an increasing number of patients are being considered for surgical management. However, the best choice of surgical treatment and criteria for selecting suitable PCLM patients who may benefit from surgical treatment remains controversial. Palliative local treatments, such as ablation, locoregional chemotherapy, and brachytherapy, which are less invasive and have fewer contraindications and complications, are the preferred alternatives to surgery. The present study reviews the advances in the management of PCLM, with focus on resection and local therapies.
Keywords: hepatectomy, liver metastases, local therapy, pancreatic ductal adenocarcinoma, surgery
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most lethal cancer, with increasing incidence and mortality.1 PDAC is highly malignant, has an insidious onset and aggressive tumor biology, and leads to early distant metastases, with a reported 5-year survival rate of less than 5%.2 Radical surgical resection has been considered as the most effective curative treatment for PDAC. However, less than 20% of patients undergo curative surgery due to locally advanced or metastatic disease at the time of diagnosis.3 Liver metastases (LM) occur in 40–50% patients, and are associated with very poor prognosis, with a median overall survival (mOS) of 3–5 months.4 Traditional guidelines do not recommend the resection of primary lesions for patients with pancreatic ductal adenocarcinoma with liver metastases (PCLM).4 Chemotherapy is presently the main treatment for PCLM. Nonetheless, with the advancements in surgical devices, techniques, and postoperative care, many centers have performed either synchronous, or metachronous resections of primary and metastatic lesions in selected patients.5,6 In the past decades, with the significant progress in the development of new cytotoxic drugs and combined chemotherapy regimens, the overall survival (OS) of patients with PCLM has been prolonged to 6–10 months.7 Furthermore, with the development of neoadjuvant chemotherapy and vascular resections, more patients with unresectable PDAC are able to undergo curative resections. Interventional and/or local destructive therapies have been used for the palliation of symptoms and improvement of quality of life. Furthermore, recent studies have reported that these minimal invasive treatments are effective in dealing with metastatic liver lesions and prolonging survival.8–10 The present study reviews the recent advances in the surgical management and local treatment of PCLM.
Materials and methods
Search strategy
A literature search was performed using the PubMed database to identify studies that reported on surgical and/or local treatment for patients with PCLM. The search keywords used were as follows: (“pancreatic cancer” OR “pancreatic ductal adenocarcinoma”) AND (“liver metastases” OR “hepatic metastases”) AND [“hepatic resection” OR “hepatectomy” OR “metastasectomy” OR “ablation” OR “selective internal radiation therapy (SIRT)” OR “transarterial chemoembolization (TACE)”]. Furthermore, similar articles listed on the PubMed homepage and the references of the searched articles were also reviewed.
Inclusion and exclusion criteria
Studies published in the English language from 1995 to 2019 and who reported on patients with PCLM undergoing hepatectomy or local regional therapy for liver metastases were considered eligible. The exclusion criteria were as follows: (a) studies that reported on non-surgical and/or systemic treatments, (b) uncertain pathological types, (c) studies without description of survival data, (d) editorials, letters to the editor and expert opinions, (e) animal and cell line studies, and (f) systematic reviews and meta-analyses.
Results and discussion
A total of 22 studies were identified. Among these studies, 14 studies focused on hepatic resection for PCLM, while the remaining eight studies reported non-surgical local therapies, such as TACE (n = 2), thermal ablation (n = 2), and SIRT (n = 4) for PCLM.
Resection of PCLM
Liver resection for colorectal LM has become a well-established treatment, and has been considered to be a safe procedure with good oncological outcomes.11 However, data on resection of PCLM remains sparse, and are mostly limited to case series or case reports.12 Hence, it remains controversial whether patients with PCLM can benefit from resection. The main concerns are the postoperative mortality, morbidity, and most importantly, long-term survival. The survival data of patients who underwent resection for PCLM is listed in Tables 1 and 2. A total of 14 studies described the survival data of resection for synchronous and/or metachronous liver metastases. Three studies revealed that PCLM patients benefited from resection, and had longer mOS (11.4–39.0 months), when compared with patients who received chemotherapy or other palliative therapy (5.9–11.0 months).5,13,14 However, six studies failed to identify a significant difference in mOS.6,15–19 The comparison of survival data between the surgical and non-surgical groups is listed in Table 3.
Table 1.
Characteristics of studies that included patients who underwent surgery for pancreatic cancer with synchronous liver metastasis.
| Author | Time period | Patient number | Age | Female/male | No. of LM | Size of LM | Neoadjuvant therapy | Primary lesion | Operation for liver metastases | Mortality | DFS | OS | Positive prognostic factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dunschede et al.6 | 1996–2008 | 9 | 55 (39–72) | 4/5 | 3 (1–5) | 3.5 (1–9) | NR | PD (67%) DP (33%) |
Trisegmentectomy (11%) Hemihepatectomies (11%) Atypical liver resections (67%) |
0% | NR | 8 | NR |
| Zanini et al.20 | 2003–2014 | 11 | 61 (52–78) | 7/4 | 3 (1–3) | 2.2 (1.8–2.5) | 0% | PD (55%) DP (27%) TP (18%) |
Wedge resection (91%) Bisegmental resection (9%) |
0% | 4 | 8.3 | Metachronous, Single LM |
| Yamada et al.18 | 1981–2007 | 11 | NR | NR | NR | NR | 0% | PD (55%) DP (36%) TP (9%) |
NR | NR | NR | 10.1 | NR |
| Andreou et al.21 | 1993–2015 | 76 | 64 (31–85) | 30/46 | 1 (1–5) | 1 (1–13) | 5% | PD (51%) DP (19%) TP (6%) |
Major hepatectomy (4%) Minor hepatectomy (96%) |
5% | NR | NR | G1/G2 grade PDAC Perioperative chemotherapy R0 resection of LM |
| Hackert et al.7 | 2001–2014 | 62 | NR | NR | NR | NR | 16% | PD (43%) DP (41%) TP (17%) |
Minor hepatectomy (98%) Major hepatectomy (2%) |
2.9% | NR | 10.6 | NR |
| Gleisneret al.15 | 1995–2005 | 17 | NR | NR | NR | NR | NR | PD (68%) DP (32%) | Minor hepatectomy (95%) Major hepatectomy (5%) |
NR | NR | 5.9 | Failed to reveal any positive prognostic factor |
| Klein et al.22 | 2004–2009 | 22 | 57.5 (31–78) | 8/14 | NR | NR | NR | PD (78%) DP (5%) TP (18%) |
Enucleation (68%) Segmentectomy (32%) |
0% | NR | 7.6 | NR |
| Shi et al.23 | 2007–2015 | 30 | 62.2 ± 10.0 | 20/10 | NR | 4.0 (2.5–5.0) | NR | PD (37%) DP (60%) TP (3%) | NR | 0% | NR | 15.7 | Age ⩽ 62 CA-125 ⩽62 U/ml |
| Shrikhande et al.13 | 2001–2005 | 11 | NR | NR | NR | NR | 3% | PD (36%) DP (55%) TP (9%) |
NR | 0% | NR | 11.4 | NR |
| Tachezy et al.5 | 1994–2014 | 69 | <65 (50%) ⩾65 (50%) |
30/39 | 2 (1–11) | NR | 13% | PD (60%) DP (36%) TP (3%) |
Minor hepatectomy (100%) | 1% | NR | 14.5 | PDAC localized in the pancreatic head |
| Seelig et al.16 | 2004–2007 | 14 | NR | NR | NR | NR | 10% | NR | Minor hepatectomy (100%) | 0% | NR | 11 | NR |
| Takada et al.19 | 1981–1995 | 11 | NR | NR | NR | NR | NR | PD (100%) | Minor hepatectomy (100%) | NR | NR | 6 | NR |
| Yang et al.17 | 2012–2017 | 48 | NR | 20/28 | NR | NR | 23% | PD (42%) DP (58%) |
Wedge resection (90%) Segmentectomy (8%) Hemihepatectomy (2%) |
4% | NR | 7.8 | PDAC with liver oligometastases |
CA, carbohydrate antigen; DFS, disease free survival; DP, distal pancreatectomy; LM, liver metastases; NR, not reported; OS, overall survival; PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma; TP, total pancreatectomy.
Table 2.
Characteristics of studies that included patients who underwent surgery for pancreatic with metachronous liver metastasis.
| Author | Time period | Patient number | Age | Female/male | No. of LM | Size of LM | Neoadjuvant therapy | Primary lesion | Operation for liver metastases | Mortality | Interval between pancreatectomy and LM | OS | Positive prognostic factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zanini et al.20 | 2003–2014 | 4 | 48 (39–56) | 0/4 | 1 | 2.2 (1.8–3.5) | 0% | PD (55%) | Wedge resection (100%) | 0% | 9 | 11.4 | Metachronous, Single LM |
| Dünschede et al.6 | 1996–2008 | 4 | 42 (41–81) | 2/2 | 1.75 (1–2) | 2.2 (1–3) | NR | NR | Minor hepatectomy (100%) | 0% | 9 | 31 | NR |
| Hackert et al.7 | 2001–2014 | 23 | NR | NR | NR | NR | 23% | PD (43%) DP (41%) TP (17%) |
Minor hepatectomy (74%) Major hepatectomy (26%) |
4.3% | 18.4 | 14.8 | NR |
DP, distal pancreatectomy; LM, liver metastases; NR, not reported; OS, overall survival; PD, pancreatoduodenectomy; TP, total pancreatectomy.
Table 3.
Comparison of outcomes of combined hepatic and pancreatic resection for PCLM and palliative therapies.
| Reference | No-resection |
Resection |
Significance |
||
|---|---|---|---|---|---|
| Patient number | mOS (months) | Patient number | mOS (months) | p-value | |
| Positive studies | |||||
| Shrikhande et al.13 | 118 | 5.9 | 11 | 11.4 | 0.04 |
| Tachezy et al.5 | 69 | 8 | 69 | 14 | <0.01 |
| Crippa et al.14 | 116 | 11 | 11 | 39 | <0.0001 |
| Other studies | |||||
| Gleisner et al.15 | 66 | 5.6 | 22 | 5.9 | 0.46 |
| Seelig et al.16 | 20 | 15.6 | 20 | 10.7 | 0.11 |
| Yang et al.17 | 31 | 7.6 | 48 | 7.8 | 0.37 |
| Yamada et al.18 | 28 | 6.8 | 11 | 10.1 | NS |
| Takada et al.19 | 22 | 3 | 11 | 6 | NR |
| Duenschede et al.6 | 5 | 11 | 8 | 9 | NR |
mOS, median overall survival; PCLM, Pancreatic ductal adenocarcinoma with liver metastases.
Surgery for PDAC with synchronous LM
The mOS of PDAC with synchronous LM remains very limited. Furthermore, it remains debatable whether surgery should be performed, and which procedure should be chosen (synchronous pancreas and liver resection, or staged procedure), because the outcomes of surgical treatment among different centers widely vary. A study with the largest number of patients with PCLM reported the synchronous resection of pancreatic and liver lesions in 62 of 85 patients. It was reported that the postoperative mOS was 10.6 months and the 5-year survival rate was 8.1%, which were better than the reported survival with palliative treatment.7 In a recent meta-analysis, 1147 patients from 11 cohort studies were divided into two groups: surgical group (n = 217) and non-surgical group (n = 930). The results revealed that the surgical resection of LM was associated with a significantly improved overall 1-year (52.8% versus 27.1%, p < 0.01) and 3-year (17.2% versus 3.7%, p < 0.01) survival.2 In a multicentric retrospective cohort study from six European pancreatic centers, the OS of 69 PCLM patients who received synchronous liver resection were compared with 69 PCLM patients who underwent exploration without tumor resection. It was found that the mOS was significantly prolonged in the synchronous resection group (14 months versus 8 months, p < 0.01). On the subgroup analysis, patients were divided according to the location of the primary tumor, and each group was compared with non-surgical treatment. It was found that patients with pancreatic head cancer (mOS 13.6 versus 7 months, p < 0.001) had a significant survival benefit, unlike patients with body/tail PDAC (mOS 14 versus 15 months, p = 0.312).5
However, there were several studies with comparable or even worse prognosis following surgical resection.15–18 In a retrospective study conducted by Gleisner et al.,15 the authors reported that the OS of 22 patients who underwent simultaneous liver and pancreas resection reached 5.6 months, and that the 1-year and 3-year survival rate was 13.3% and 6.7%, respectively. Although the majority of these 22 patients had low metastatic tumor burden, the simultaneous resection failed to bring survival benefits, when compared with the 60 patients who underwent a palliative bypass operation (mOS 5.9 versus 5.6 months, p = 0.46). Another retrospective study revealed that the mOS of the combined surgical resection in 20 PCLM patients was shorter than the matched 20 patients who received a purely PDAC resection (10.7 months versus 15.6 months), but the difference was not statistically significant due to the small sample size (p = 0.11).16 Therefore, there is insufficient evidence to conclude that synchronous resection can significantly improve survival. Hence, surgery may be suitable for highly selected cases.
Metachronous liver resection after previous pancreatectomy
Patients who undergo primary pancreatic resection and postoperative chemotherapy often have poor performance status, and often cannot tolerate a second surgery for metachronous liver lesions. Thus, postoperative mortality and morbidity limits the metastasectomy for metachronous liver lesions. However, with the advancements in surgical techniques and postoperative rehabilitation, a notable decline has occurred in mortality after liver metastasectomy for PDAC during the past two decades.2 The only concern that remains unanswered is whether metachronous liver resection can prolong survival. According to the present literature, it appears that for selected patients who can tolerate a second surgery, and when R0 resection is possible, metachronous liver metastasectomy may prolong the mOS.6,7,20 The study conducted by Hackert et al.7 on 23 PDAC cases revealed that the resection of LM is a safe procedure, with a mOS of 14.8 months. The interval between the initial pancreatectomy and occurrence of LM reflects the tumor biology and potential prognosis of the patient.6,7 The longer the interval of the pancreatectomy and appearance of the metachronous lesion, the less aggressive the tumor biology is, and such patient can receive maximum benefits from the liver metastasectomy. It is noteworthy that huge or multiple (>5 or bilobar metastases) metastases may require major hepatectomy. Major hepatectomy for PCLM is rarely performed due to the risks and complications associated with such aggressive approach, which outweighs the long-term survival benefits. In conclusion, liver resection may be considered for patients with PDAC with postoperative metachronous LM, when the general condition of the patient is good, and the LM is amenable for R0 resection.
Resection following effective neoadjuvant therapy
The application of new chemotherapy regimens, such as FOLFIRINOX (oxaliplatin, irinotecan, fluorouracil, and leucovorin), nab-paclitaxel plus gemcitabine, and other gemcitabine-based regimens, have achieved dramatic survival prolongation for locally advanced or metastatic PDAC.14,24 The use of these chemotherapy regimens for PCLM in the neoadjuvant setting has been found to be associated with better results.14,20,25–28 With the good response to neoadjuvant chemotherapy, a reduction in size, or even the disappearance of liver metastases can often be observed. Through this approach, the downstaging of an initially unresectable tumor may help regain the surgical opportunity for R0 resection.26
It is often difficult and inaccurate to determine the response to neoadjuvant therapy using computed tomography (CT). Thus, a combination of serum CA19-9 level, CT and 18positron emission tomography-computer tomography (PET-CT) should be used for evaluation.7,14,26 Downstaging was considered to be achieved when there is a major biochemical response (reduction of CA19-9 >50%) and/or a good radiological response, as assessed by Response Evaluation Criteria in Solid Tumors (RECIST) criteria.14 Schneitler et al.28 reported two cases of PCLM with the complete disappearance of LM after FOLFIRINOX chemotherapy. Both patients received surgical resection and were still alive after 1.5 years. Another retrospective analysis enrolled 24 patients who received resection after the downstaging of PCLM, and R0 resection was achieved in 88% of cases, with a median OS and progression free survival (PFS) reaching 56 and 27 months, respectively.26 However, even with the good response to chemotherapy and R0 resection, local recurrence in the liver or any other site can still occur due to the undetectable micro-metastasis.14
Prognostic factors and patient selection
In a nutshell, highly selected patients may benefit from either the synchronous or metachronous resection of LM, where the accurate selection of surgical candidates with PCLM appears to be crucial.7,20 Unfortunately, no standard selection criteria are available due to lack of data, and the dependence on the experience of the surgical team and treating oncologist. Some researchers have investigated the potential prognostic factors that can guide in the patient selection.5,14,17,23 For example, the study conducted by Shi et al.23 demonstrated that patients with PCLM with serum CA125 levels between 38 U/ml and 62 U/ml benefited the most through the synchronous resection of LM. The number of LM >5, [hazard ratio (HR): 3.515] and the reduction of CA19-9 <50% from the baseline value after neoadjuvant chemotherapy (HR: 3.515) were risk factors for survival.14 In the multicentric retrospective study conducted by Tachezy et al.,5 patients with single metastasis (n = 65) had longer mOS, when compared with 73 patients with ⩾2 metastases (11.6 months versus 5.6 months, p = 0.005). A recent retrospective control study conducted in China reported that PDAC patients with liver oligometastases (n = 23) benefited from synchronous liver resection, when compared with systemic chemotherapy (n = 31) and palliative therapies (n = 10) (16.1 versus 7.6 months, p = 0.02; 16.1 versus 4.3 months, p < 0.0001; respectively).17 However, these reports were retrospective studies with a small sample size. Hence, it remains difficult to determine the valid cut-off level for tumor markers, and the tumor location, size, and number in the selection of patients. The investigators considered that the following criteria may help in the selection of appropriate candidates for surgery: (a) good general condition (performance status 1 or 2) for surgery; (b) no extrahepatic metastasis;6,20 (c) single or small burden of LM (diameter < 3 cm), which allows a high possibility of R0 resection with low risk;5–7,20 (d) resectable or borderline resectable primary PDAC;14 (e) long interval between pancreatectomy and the appearance of a metachronous lesion;6,7 (f) stable disease/partial response/complete response after neoadjuvant therapy.25,26 Further multicentric and prospective clinical trials are needed to precisely define the selection criteria.5,7
Local therapies for PCLM
At the time of diagnosis of PCLM, the majority of patients are not suitable candidates for surgery. Therefore, alternative minimally invasive treatment options that could achieve a similar local control, but with lower morbidity and mortality, are highly desirable. The local therapies for PCLM, including local thermal ablation, transarterial chemoembolization, and selective internal radiation therapy, provide a meaningful control of LM and a significant relief of symptoms. The studies that reported on patients who underwent non-surgical local treatment for PCLM are listed in Table 4.
Table 4.
Studies that reported on patients who underwent local treatment for PCLM.
| Author | Treatment | Patient number | Age | Female/male | Number of LM | Size of LM | Effectiveness | PFS | OS | Positive prognostic factors |
|---|---|---|---|---|---|---|---|---|---|---|
| Park et al.29 | RFA | 34 | 58.5 (38–78) | 15/19 | 1–4 | 0.8–3.2 | Complete ablation (100%) | NR | 14 | Single LM, LM < 2 cm, good or moderate differentiation pathological type |
| Hua et al.9 | RFA | 102 | 55.3 (38–78) | 36/66 | 2.0 (1–3) | 2.65 (0.8–5.0) | Complete ablation (94.5%) | 9 | 11.4 | Primary tumor in body/tail, LM ⩽3 cm, |
| Azizi et al.30 | TACE | 32 | 60 (49.5–67.5) | 16/16 | <5 (40.6%) ⩾5 (59.4%) |
NR | SD (71.87%) PR (9.37%) |
NR | 16 | Male patients |
| Vogl et al.31 | TACE | 69 | NR | NR | <5 (26.1%) ⩾5 (73.9%) |
NR | SD (78.26%) PR (11.59%) |
NR | 19 | NR |
| Kim et al.32 | SIRT | 33 | 64 (47–83) | 8/25 | NR | NR | SD (37%) PR (42%) |
NR | 8.1 | NR |
| Michl et al.8 | SIRT | 19 | 63 (43–77) | 9/10 | NR | NR | Objective response (47%) | 3.4 | 9.0 | NR |
| Gibbs et al.33 | SIRT | 14 | 62 (48–76) | 8/6 | NR | NR | SD (71%) PR (21%) |
5.2 | 5.5 | Resected primary PDAC |
| Kim et al.34 | SIRT | 16 | 63 (50–73) | 5/11 | NR | NR | SD (38%) PR (31%) |
NR | 12.5 | NR |
LM, liver metastases; NR, not reported; OS, overall survival; PCLM, pancreatic ductal adenocarcinoma with liver metastases; PDAC, pancreatic ductal adenocarcinoma; PFS, progression free survival; PR, partial response; RFA, radiofrequency ablation; SD, stable disease; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolization.
Local thermal ablation for LM
Thermal ablation therapies mainly include radiofrequency ablation (RFA) and microwave ablation. These minimal invasive approaches are associated with less trauma, mild disturbances in liver function, and quick recovery. Furthermore, these can be used in patients who cannot tolerate the surgical resection of LM. In the management of hepatocellular carcinoma and colorectal LM, thermal ablation has comparable oncological outcomes and less postoperative morbidity.35,36 However, the role of local thermal ablation for PCLM has not been well-established at present. Park et al.29 retrospectively analyzed 34 cases of patients with PCLM, in which all patients received ultrasound-guided RFA for liver metastasis simultaneously with pancreatic resection, or after curative resection. The postoperative complications were mild and only one patient developed liver abscess, which was treated by percutaneous drainage. The mOS was 14 months from the time of appearance of LM. The multivariate analysis revealed that single and small metastatic lesions, and well or moderately differentiated pathological types of primary tumors were good prognostic factors for survival after RFA. However, 18 (58.1%) patients had intrahepatic recurrence after the first RFA and underwent additional RFA. Furthermore, one patient required a total of seven sessions of RFA. Another retrospective study that included 102 patients with 254 LMs investigated the effect of RFA alone for synchronous liver oligometastasis without resection of the primary PDAC. They reported a complete ablation rate of 96.1%. The postoperative complications were mainly self-limiting, with an incidence of approximately 9.8%. Furthermore, the 1-year survival rate reached 47.1%, and the mOS was 11.4 months, which was longer than the reported survival following the FOLFIRINOX regimen or gemcitabine plus nab-paclitaxel. Nonetheless, the difference was not statistically significant.9 In summary, RFA alone provides reasonable short-term results, but not long-term survival benefits, due to the high recurrence rate.
It is noteworthy that the overall efficacy of thermal ablation is not good for large (>5 cm) and multiple (>3) LMs.9,37 Furthermore, it is risky to perform RFA for LMs adjacent to the main vessels, diaphragm, gastrointestinal tract, or gallbladder due to the risk of potential injury. Furthermore, the extent of ablation in these regions is also insufficient and may increase the local recurrence rate.
With the development in imaging technologies, novel guidance methods are emerging which have improved the accuracy and real-time performance of thermal ablation. Traditional ultrasound guidance procedures can be easily affected by the gas bubbles that appear after ablation, which may mislead the assessment of the postoperative ablation zone. Minami et al.38 advocated the use of ultrasound–ultrasound fusion imaging to guide RFA. This novel technique can display the ablation zone and edges in real time, allowing for the timely discovery of poor ablation margins and re-ablation in the same sitting.
Selective internal radiation therapy
SIRT is a type of liver-directed brachytherapy, where radioactive yttrium 90 microspheres are selectively delivered to the tumor-supplying vessels. Michl et al.8 reported their experience of 19 patients who received SIRT for PCLM. Among these patients, 47% of these patients had an objective response after treatment. The median local PFS and mOS were 3.4 months and 9.0 months, respectively. The 1-year survival rate was 24%. Some patients had short-term nausea, vomiting, fatigue, fever, abdominal pain, and other minor side effects. In a phase II trial conducted by Gibbs et al.,33 14 eligible patients were enrolled, and the control rate of LM reached up to 93% [two cases with partial response (PR), one case with uncertain PR, and 10 cases with stable disease (SD)]. The mOS of patients with sole LM was 12.2 months. The results of the study conducted by Kim et al.32 achieved a PR of 42% and a SD of 37%, with a mOS of 8.1 months in 33 patients.
In general, SIRT provides an encouraging local control of PCLM, but its long-term adverse events still need further evaluation. Significant toxicity was observed in the trial conducted by Gibbs et al.,33 where one patient died of liver failure at seven months after SIRT, and other severe complications such as liver abscess, gastroduodenal perforation and splenic infarction also occurred.
Transarterial chemoembolization
TACE is a well-established modality for the palliative treatment and symptomatic relief for metastatic liver tumors. It is a minimal invasive procedure with short hospital stay and mild side effects. Vogl et al.31 retrospectively investigated the results of TACE for 69 patients with PCLM. After treatment, 78.26% of patients had SD and 11.59% of patients had PR. In terms of the tumor response, the number of liver metastases was not a statistically significant factor. The mOS of all patients was 19 months, while the mOS for patients with SD was 26 months. The role of repetitive TACE cycles was evaluated by Azizi et al.30 A total of 32 patients underwent a mean of 3.2 sessions of TACE for liver lesions. Among these patients, 71.87% of patients had SD, 9.37% of patients had PR, and 18.75% of patients had progressive disease (PD). Furthermore, an improved mOS of 20 months was observed in the SD group, while the mOS in the PD group was only 5 months. No significant survival difference was observed in the oligonodular (n < 5) and multinodular (n ⩾ 5) groups. These results indicate that TACE may be a promising treatment with respectable survival, especially for patients with multiple liver metastases.
Conclusion and prospect
To date, it remains controversial whether patients with PCLM should undergo surgical treatment. Highly selected patients appear to benefit from operative management and achieve longer OS, but there are no established criteria for selection. Effective neoadjuvant therapy can lead to a reduction in tumor burden and increase the R0 resection rate. Furthermore, preoperative systemic therapy can also help in understanding the aggressiveness of the tumor, and in selecting patients with a less invasive disease. However, it remains to be determined which neoadjuvant chemotherapy regimen should be used and when to perform surgery, since these are not well defined and needs further studies. With the aim to determine patients who can benefit from the synchronous resection of primary PDACs and liver oligometastasis after induction chemotherapy, a phase III trial (Clinical Trials.gov identifier: NCT03398291) called CSPAC-1 has been initiated in China.39 The results of this trial would be worth looking forward to.
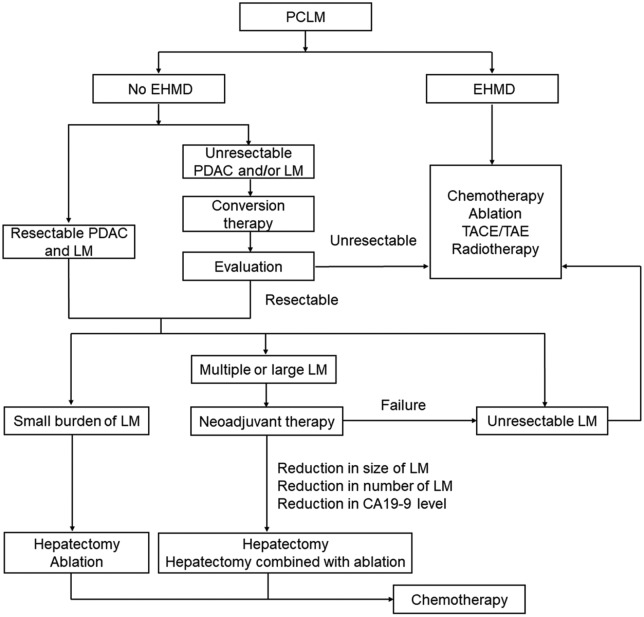
The local thermal ablation of LM appears to be an effective alternative to surgical resection since this can reduce the tumor load and prolong the OS. For LMs that are not treatable by surgery due to their location or patients with comorbidities, ablation procedures can be used. Liver resection with a simultaneous ablation procedure is also a safe and feasible strategy for multiple and diffuse LMs. Unfortunately, the high recurrence rate remains as the major limiting factor. In addition, thermal ablation is not suitable for large sized LMs or LMs located near major vessels. Non-thermal ablation, such as irreversible electroporation (IRE), has already been tested for the treatment of locally advanced PDAC. It has been reported that IRE after induction chemotherapy can help in achieving a substantially prolonged survival.40 Local brachytherapy such as SIRT may be applied for selected patients. However, it remains technically challenging, with a reported increased potential of injury to the surrounding organs. Furthermore, the optimal radiation dose and patient selection needs further investigation. Transarterial therapies, including TACE and transarterial embolization, can provide good control of the tumor progression and prolong survival, especially for patients with multiple LMs. The management of PCLM is summarized in Figure 1 based on the literature review and our own experience. However, future prospective studies are required to explore the appropriate treatment strategy for PCLM.
Figure 1.
Flowchart of the PCLM treatment.
EHMD, extra-hepatic metastatic disease; LM, liver metastases; PCLM, pancreatic ductal adenocarcinoma with liver metastases; PDAC, pancreatic ductal adenocarcinoma; TACE, transarterial chemoembolization; TAE, transarterial embolization.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was in part supported by grants from the National Natural Science Foundation of China (No. 81400659 and 81974377), the Natural Science Fund of Liaoning Province (No. 2017225032 and 20180551193) and the 345 Talent Project.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Tianqiang Jin, Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang, China.
Chaoliu Dai, Department of General Surgery, Shengjing Hospital of China Medical University, Shenyang, China.
Feng Xu, Department of General Surgery, Shengjing Hospital of China Medical University, 36 Sanhao Street, Heping District, Shenyang, Liaoning Province 110004, China.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Yu X, Gu J, Fu D, et al. Dose surgical resection of hepatic metastases bring benefits to pancreatic ductal adenocarcinoma? A systematic review and meta-analysis. Int J Surg 2017; 48: 149–154. [DOI] [PubMed] [Google Scholar]
- 3. Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004; 363: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 4. Ghaneh P, Costello E, Neoptolemos JP. Biology and management of pancreatic cancer. Postgrad Med J 2008; 84: 478–497. [DOI] [PubMed] [Google Scholar]
- 5. Tachezy M, Gebauer F, Janot M, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery 2016; 160: 136–144. [DOI] [PubMed] [Google Scholar]
- 6. Dünschede F, Will L, von Langsdorf C, et al. Treatment of metachronous and simultaneous liver metastases of pancreatic cancer. Eur Surg Res 2010; 44: 209–213. [DOI] [PubMed] [Google Scholar]
- 7. Hackert T, Niesen W, Hinz U, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol 2017; 43: 358–363. [DOI] [PubMed] [Google Scholar]
- 8. Michl M, Haug AR, Jakobs TF, et al. Radioembolization with yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases: efficacy, safety and prognostic factors. Oncology 2014; 86: 24–32. [DOI] [PubMed] [Google Scholar]
- 9. Hua YQ, Wang P, Zhu XY, et al. Radiofrequency ablation for hepatic oligometastatic pancreatic cancer: an analysis of safety and efficacy. Pancreatology 2017; 17: 967–973. [DOI] [PubMed] [Google Scholar]
- 10. Sun JH, Zhou TY, Zhang YL, et al. Efficacy of transcatheter arterial chemoembolization for liver metastases arising from pancreatic cancer. Oncotarget 2017; 8: 39746–39755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Akgül Ö, Çetinkaya E, Ersöz Ş, et al. Role of surgery in colorectal cancer liver metastases. World J Gastroenterol 2014; 20: 6113–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bellon E, Gebauer F, Tachezy M, et al. Pancreatic cancer and liver metastases: state of the art. Updates Surg 2016; 68: 247–251. [DOI] [PubMed] [Google Scholar]
- 13. Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 2007; 14: 118–127. [DOI] [PubMed] [Google Scholar]
- 14. Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol 2016; 42: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 15. Gleisner AL, Assumpcao L, Cameron JL, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 2007; 110: 2484–2492. [DOI] [PubMed] [Google Scholar]
- 16. Seelig SK, Burkert B, Chromik AM, et al. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg 2010; 2010: 579672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang J, Zhang J, Lui W, et al. Patients with hepatic oligometastatic pancreatic body/tail ductal adenocarcinoma may benefit from synchronous resection. HPB (Oxford) 2020; 22: 91–101. [DOI] [PubMed] [Google Scholar]
- 18. Yamada S, Fujii T, Sugimoto H, et al. Pancreatic cancer with distant metastases: a contraindication for radical surgery? Hepatogastroenterology 2009; 56: 881–885. [PubMed] [Google Scholar]
- 19. Takada T, Yasuda H, Amano H, et al. Simultaneous hepatic resection with pancreato-duodenectomy for metastatic pancreatic head carcinoma: does it improve survival? Hepatogastroenterology 1997; 44: 567–573. [PubMed] [Google Scholar]
- 20. Zanini N, Lombardi R, Masetti M, et al. Surgery for isolated liver metastases from pancreatic cancer. Updates Surg 2015; 67: 19–25. [DOI] [PubMed] [Google Scholar]
- 21. Andreou A, Knitter S, Klein F, et al. The role of hepatectomy for synchronous liver metastases from pancreatic adenocarcinoma. Surg Oncol 2018; 27: 688–694. [DOI] [PubMed] [Google Scholar]
- 22. Klein F, Puhl G, Guckelberger O, et al. The impact of simultaneous liver resection for occult liver metastases of pancreatic adenocarcinoma. Gastroenterol Res Pract 2012; 2012: 939350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shi HJ, Jin C, Fu DL. Preoperative evaluation of pancreatic ductal adenocarcinoma with synchronous liver metastasis: diagnosis and assessment of unresectability. World J Gastroenterol 2016; 22: 10024–10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–1825. [DOI] [PubMed] [Google Scholar]
- 25. Wright GP, Poruk KE, Zenati MS, et al. Primary tumor resection following favorable response to systemic chemotherapy in stage IV pancreatic adenocarcinoma with synchronous metastases: a bi-institutional analysis. J Gastrointest Surg 2016; 20: 1830–1835. [DOI] [PubMed] [Google Scholar]
- 26. Frigerio I, Regi P, Giardino A, et al. Downstaging in stage IV pancreatic cancer: a new population eligible for surgery? Ann Surg Oncol 2017; 24: 2397–2403. [DOI] [PubMed] [Google Scholar]
- 27. Neofytou K, Giakoustidis A, Smyth EC, et al. A case of metastatic pancreatic adenocarcinoma with prolonged survival after combination of neoadjuvant FOLFIRINOX therapy and synchronous distal pancreatectomy and hepatectomy. J Surg Oncol 2015; 111: 768–770. [DOI] [PubMed] [Google Scholar]
- 28. Schneitler S, Kröpil P, Riemer J, et al. Metastasized pancreatic carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection. World J Gastroenterol 2015; 21: 6384–6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park JB, Kim YH, Kim J, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol 2012; 23: 635–641. [DOI] [PubMed] [Google Scholar]
- 30. Azizi A, Naguib NN, Mbalisike E, et al. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas 2011; 40: 1271–1275. [DOI] [PubMed] [Google Scholar]
- 31. Vogl TJ, Mohamed SA, Albrecht MH, et al. Transarterial chemoembolization in pancreatic adenocarcinoma with liver metastases: MR-based tumor response evaluation, apparent diffusion coefficient (ADC) patterns, and survival rates. Pancreatology 2018; 18: 94–99. [DOI] [PubMed] [Google Scholar]
- 32. Kim AY, Frantz S, Brower J, et al. Radioembolization with yttrium-90 microspheres for the treatment of liver metastases of pancreatic adenocarcinoma: a multicenter analysis. J Vasc Interv Radiol 2019; 30: 298–304.e2. [DOI] [PubMed] [Google Scholar]
- 33. Gibbs P, Do C, Lipton L, et al. Phase II trial of selective internal radiation therapy and systemic chemotherapy for liver-predominant metastases from pancreatic adenocarcinoma. BMC cancer 2015; 15: 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kim AY, Unger K, Wang H, et al. Incorporating Yttrium-90 trans-arterial radioembolization (TARE) in the treatment of metastatic pancreatic adenocarcioma: a single center experience. BMC Cancer 2016; 16: 492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang J, Hernandez-Alejandro R, Croome KP, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma in Childs A cirrhotics- a retrospective study of 1,061 cases. J Gastrointest Surg 2011; 15: 311–320. [DOI] [PubMed] [Google Scholar]
- 36. Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes–a 10-year experience at a single center. Radiology 2016; 278: 601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Renz BW, Boeck S, Roeder F, et al. Oligometastatic disease in pancreatic cancer - how to proceed? Visc Med 2017; 33: 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Minami Y, Minami T, Chishina H, et al. US-US fusion imaging in radiofrequency ablation for liver metastases. Dig Dis 2016; 34: 687–691. [DOI] [PubMed] [Google Scholar]
- 39. Shi S, Yu XJ. Time to think: selecting patients who may benefit from synchronous resection of primary pancreatic cancer and liver metastases. World J Gastroenterol 2018; 24: 3677–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin RC, 2nd, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 2015; 262: 486–494. [DOI] [PubMed] [Google Scholar]