Abstract
Atopic dermatitis (AD) is a chronic inflammatory disease of the skin that substantially affects a patient’s quality of life. While steroids are the most common therapy used to temporally alleviate the symptoms of AD, effective and nontoxic alternatives are urgently needed. In this study, we utilized a natural, plant-derived phenolic compound, phloretin, to treat allergic contact dermatitis (ACD) on the dorsal skin of mice. In addition, the effectiveness of phloretin was evaluated using a mouse model of ACD triggered by 2,4-dinitrochlorobenzene (DNCB). In our experimental setting, phloretin was orally administered to BALB/c mice for 21 consecutive days, and then, the lesions were examined histologically. Our data revealed that phloretin reduced the process of epidermal thickening and decreased the infiltration of mast cells into the lesion regions, subsequently reducing the levels of histamine and the pro-inflammatory cytokines interleukin (IL)-6, IL-4, thymic stromal lymphopoietin (TSLP), interferon-γ (IFN-γ) and IL-17A in the serum. These changes were associated with lower serum levels after phloretin treatment. In addition, we observed that the mitogen-activated protein kinase (MAPK) and NF-κB pathways in the dermal tissues of the phloretin-treated rodents were suppressed compared to those in the AD-like skin regions. Furthermore, phloretin appeared to limit the overproliferation of splenocytes in response to DNCB stimulation, reducing the number of IFN-γ-, IL-4-, and IL-17A-producing CD4+ T cells in the spleen back to their normal ranges. Taken together, we discovered a new therapeutic role of phloretin using a mouse model of DNCB-induced ACD, as shown by the alleviated AD-like symptoms and the reversed immunopathological effects. Therefore, we believe that phloretin has the potential to be utilized as an alternative therapeutic agent for treating AD.
Keywords: 2,4-dinitrochlorobenzene (DNCB); allergic contact dermatitis; topic dermatitis; IgE; mast cells; phloretin; T cells
Introduction
Atopic dermatitis (AD), a common chronic inflammatory skin disease, affects up to 20% of children and 1% of adults worldwide and has a complex etiology that involves immunological and inflammatory pathways.1,2 AD is characterized by relapsing eczema with intense pruritus of xerotic skin. Immunosuppressive drugs, such as corticosteroids, tacrolimus, pimecrolimus, cyclosporine, and methotrexate, have been widely used to temporally control the symptoms of AD.3,4 However, these immunotherapeutic approaches often lead to adverse side effects, such as skin atrophy, osteoporosis, skin cancer, and metabolic disorders. Therefore, it is critical to identify alternative therapies that can mitigate atopic disease. Recently, increasing evidence has shown that plant-derived flavonoids can modulate and equilibrate the overreactive immune system in various skin diseases via anti-inflammatory effects.5–7 Among the flavonoids, phloretin is unique due to its well-known antioxidant and anti-inflammatory pharmacological effects.8
Phloretin, a natural dihydrochalcone flavonoid, can be abundantly extracted from apples and apricots (Prunus mandshurica). Although it was among the first flavonoids to be discovered, phloretin has recently drawn more attention due to its immunoregulatory functions, which were also demonstrated in our previous works.9,10 Specifically, phloretin has been found to reduce the levels of inflammatory cytokine induced by TLR2/1 and TLR4 agonists, Pam3CSK4 and LPS, respectively, in bone marrow–derived dendritic cells and RAW264.7 macrophages.9,11,12 The TLR2-mediated nuclear translocation of NF-κB and phosphorylation of mitogen-activated protein kinases (MAPKs) were also suppressed when phloretin was present.13,14 In addition to innate immunity, phloretin appeared to influence T-cell-mediated cytokine production to attenuate oxidative stress and allergic airway inflammation in asthmatic mice by inhibiting Th2 cytokines.15 In our previous work, we found that phloretin could suppress autoantibody production, which plays a crucial role in the pathogenesis of collagen-induced arthritis in an animal model.10
Given the complicated and considerable factors associated with the pathogenesis of AD, epicutaneous sensitization with irritants, such as 2,4-dinitrochlorobenzene (DNCB), is a common approach used to establish AD to test drug candidates.16 We utilized the DNCB-triggered allergic contact dermatitis (ACD) mouse model to recapitulate AD-like symptoms to test phloretin, aiming to explore the pharmacological benefits of phloretin in alleviating the symptoms of AD.
Materials and methods
Animals
Eight-week-old male BALB/c mice (weight, 20–22 g) were obtained from the National Laboratory Animal Center (Taipei, Taiwan). A total of 20 mice were randomly divided into four groups (n = 5), and this sample size was determined according to previous published information to avoid underpowered experiments and ensure adequately minimalizing the number of animals.17–19 All mice were acclimatized to standard laboratory conditions for a week before ACD experiments and procedures, and the ethical approval for this study was obtained from Institutional Animal Care and Use Committee (IACUC) of National Chung Hsing University (NCHU) (approval number: IACUC109-040). This study abode by relevant guidelines for humane animal treatment and complied with regulations.
DNCB-induced AD-like skin lesions in mice
The dorsal skin of BALB/c mice was sensitized with DNCB (Sigma-Aldrich, St. Louis, MO, USA) to induce AD-like symptoms as previously described.20 Briefly, following dorsal hair removal (approximately 4 cm2), 150 μL of 2% DNCB solution (dissolved in a 3:1 mixture of acetone and olive oil) was applied to the dorsal skin. Four days later, 150 μL of 0.5% DNCB was applied to the dorsal skin every 3 days (Figure 1) throughout the experiment to induce AD-like symptoms.
Figure 1.
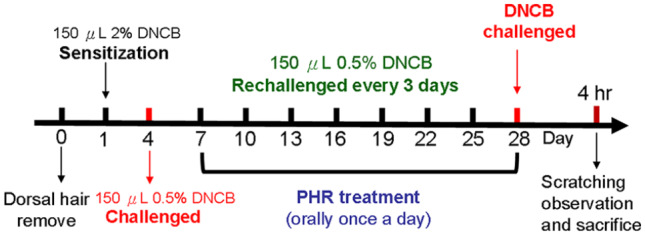
Schematic illustration of allergic contact dermatitis (ACD) induction and phloretin treatment. The dorsal skin regions of the BALB/c mice (n = 5) from which the hair was removed were sensitized with 2% DNCB on day 1, followed by a series of repetitive challenges with 0.5% DNCB every third day thereafter. In addition, 50 and 100 mg/kg of phloretin were orally administered to the BALB/c mice daily from day 7 to day 28 before the mice were sacrificed 4 h after the last DNCB challenge. The pathological conditions caused by DNCB irritation with or without phloretin treatment were examined.
Treatment
Phloretin was purchased directly from Sigma-Aldrich and dissolved in dimethyl sulfoxide. Two groups of ACD mice (n = 5 in each group) were orally administered 50 and 100 mg/kg of phloretin (PHR50 and PHR100, respectively) once a day beginning on day 6 postsensitization and continuing until day 27. The mice in the normal group (referred to as NOR) were sensitized with solvent alone without DNCB, whereas the mice in the treatment vehicle control group (referred to as CON) were sensitized with DNCB, but no phloretin treatments were administered. The clinical features were then observed 4 h after the last DNCB challenge. The symptomatic development of erythema, edema, exfoliation, and scaling of the skin lesions was scored from 0 to 3 depending on the severity by blinded assessment as previously described.21 At the same time, the frequency of scratching behaviors was observed for a duration of 10 min, and serum, dorsal skin, and spleen samples were collected.
Histological analysis
Dorsal dermal tissue specimens of the mice were excised 4 h after the last DNCB challenge, fixed with 10% phosphate-buffered formalin, and embedded in paraffin. The thickness of the epidermis and dermis from five randomly selected tissues was evaluated after staining the skin sections (5 μm) with hematoxylin and eosin (Merck Millipore, Billerica, MA, USA). The infiltration of mast cells was visualized by toluidine blue staining (Sigma-Aldrich) and assessed under a light microscope (Olympus, Kensington, London, England) at a magnification of 200×. The epidermal thickness is measured using ImageJ software (National Institutes of Health (NIH), Bethesda, MD, USA).
Measurement of serum immunoglobulin E, histamine, and skin cytokine levels and spleen sizes
Blood samples were collected from the hearts of CO2-asphyxiated mice and were centrifuged (10,000g, 10 min) to obtain serum. The total protein was isolated from 100 mg of dorsal skin tissue by homogenization in 1 mL of a tissue protein extraction reagent (Pierce, Rockford, IL, USA) with protease inhibitors (Roche, Indianapolis, IN, USA) and was quantified by a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA). The levels of total immunoglobulin E (IgE) (BD Pharmingen, San Diego, CA, USA), histamine (BioVision, Milpitas, CA, USA), interleukin (IL)-6, IL-17A (eBioscience, San Diego, CA, USA), IL-4, interferon-γ (IFN-γ) (BioLegend, San Diego, CA, USA), and thymic stromal lymphopoietin (TSLP; Abcam, Cambridge, MA, USA) were analyzed by sandwich ELISA (enzyme-linked immunosorbent assay) according to the manufacturer’s instructions.
The spleens were weighed. The splenic weight index was defined as the spleen weight divided by the whole body weight.
Western blot analysis
Murine skin homogenates were prepared in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich) containing 1% protease inhibitor cocktail (Sigma-Aldrich) and 2% phenylmethanesulfonyl fluoride (PMSF; Sigma-Aldrich). To detect the NF-κB activity, nuclear extracts were prepared from the skin homogenates with the NE-PER Nuclear and Cytoplasmic Extraction system (Pierce) according to the manufacturer’s instructions, and the protein in the extracts was quantified with a BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of skin homogenate were loaded onto a 12% SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) gel for electrophoresis and then transferred onto an Immobilon-P Transfer Membrane (Merck Millipore). The membranes were blocked with phosphate-buffered saline (PBS) containing 0.1% (v/v) Tween 20 and 5% (w/v) nonfat dry milk for 3 h at room temperature and were hybridized with anti-p38 (clone Y122; Epitomics, Burlingame, CA, USA), phospho-p38 (clone D3F9; Cell Signaling Technology, Denver, CO, USA), extracellular signal-regulated kinase (ERK) 1/2 (clone H-72; Santa Cruz Biotechnology, Santa Clara, CA, USA), phospho-ERK 1/2 (clone E-4, Santa Cruz Biotechnology), c-JUN N-terminal kinase (JNK) (clone EP1597Y; Epitomics), phospho-JNK (clone 81E11; Gene Tex, Denver, CO, USA), phopho-IκB-α (ser32) (clone 14D4; Cell Signaling Technology), IκB-α (Polyclonal, Gene Tex), phopho-NF-κBp65 (ser 536) (clone 7F1; Cell Signaling Technology), NF-κB p65 (clone GTX107678; Gene Tex), or glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (14C10; Cell Signaling Technology) at 4°C overnight. After three washes, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) at 4°C overnight followed by visualization using an enhanced chemiluminescence detection reagent (Amersham Pharmacia, Piscataway, NJ, USA). All the bands on the blots were quantified and normalized to the levels of GAPDH in each lane using ImageJ v1.47 software from the NIH (Bethesda, MD, USA).
T-cell activation stimulated by DNCB in vitro
A single-cell suspension was made from each spleen through a 70-mm nylon mesh. Following treatment with red blood cell lysis buffer (Merck), the splenocytes were cultured at a density of 5 × 105 cells/well in round-bottomed 96-well plates (Thermo Fisher Scientific) with 200 μL/well RPMI-1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal calf serum (Gibco), 50 μg/mL streptomycin, 2 mM glutamine, and 50 μM 2-mercaptoethanol (Sigma-Aldrich). The splenocytes were stimulated with 0.5 μg/mL of DNCB for 72 h at 37°C. Proliferation assays were subsequently conducted. Briefly, 18 h before harvesting the cultured cells, [3H] thymidine (1 μL Ci/well; Amersham Pharmacia Biotech) was added to each well. The radioactivity of the incorporated [3H]-TdR was counted using a Matrix 9600 direct ionization beta counter (Packard Instrument, Meridian, CT, USA). The amount of radioactivity is expressed as the mean counts per minute (cpm). To measure intracellular cytokines, the splenocytes were incubated with 0.5 μg/mL of DNCB for 48 h and brefeldin A (5 μg/mL; eBioscience) for 6 h. The cells were then equilibrated in FACScan buffer and stained for the surface marker CD4 using phycoerythrin-conjugated anti-mouse CD4 antibodies (BD Pharmingen). Staining was used to identify the various T-cell populations. The cells were fixed and permeabilized using the Cytofix/Cytoperm Plus Kit (BD Biosciences) according to the manufacturer’s instructions before intracellular cytokine staining. Intracellular staining was performed using fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) against murine IFN-γ (clone XMG1.2, BioLegend), IL-4 (clone 11B11; BD Biosciences), and IL-17A (clone TC11-18H10.1; BioLegend). All the samples were analyzed on a BD Accuri C5 flow cytometer using C6 Accuri system software (Accuri Cytometers Inc., Ann Arbor, MI, USA).
Statistical analysis
The results are expressed as means ± standard deviations (SDs) (n = 5), and one-way ANOVA with post hoc Tukey’s t-test was used to compare multiple experimental groups throughout the study unless otherwise indicated. Statistical significance was considered when the value of P was <0.05.
Results
Phloretin alleviated the clinical symptoms of AD-like skin lesions in mice
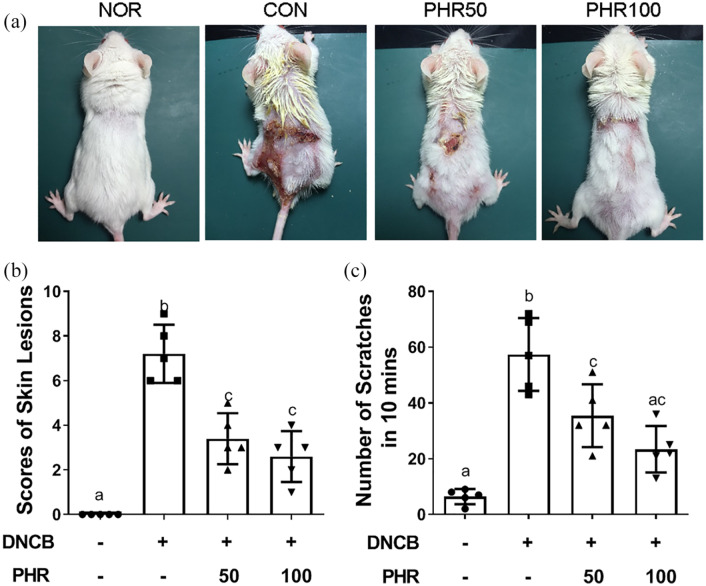
First, the therapeutic effects of phloretin on the symptoms of ACD were evaluated. AD-like lesions were induced on the mice by a series of DNCB re-exposures, as schematically illustrated in Figure 1. As shown in Figure 2(a), daily oral treatment with phloretin remarkably alleviated the AD-like skin lesions in a dose-dependent manner. The mice that were subjected to DNCB irritation but did not receive phloretin treatment exhibited severe inflammatory symptoms. The severity of the lesions, including erythema, edema, exfoliation, skin scaling, and scratching behavior frequency, was significantly decreased in the phloretin-treated mice compared to vehicle control–treated mice. This result indicates that phloretin can alleviate the symptoms associated with DNCB-triggered skin lesions (Figure 2(b) and (c)).
Figure 2.
Inhibitory effects of phloretin on the AD-like skin symptoms and behaviors of mice. (a) The clinical appearance of the normal (NOR), DNCB-sensitized (CON), and DNCB/phloretin-treated (PHR50/100) mice after 28 days of the experiment. (b) and (c) Scoring indexes of the severity of the skin erythema, edema, exfoliation, lesioned skin scaling, and scratching frequencies were recorded as described in the “Materials and Methods” section. Bars display the mean ± SD of one of three independent experiments with similar results and with five mice per group. Different letters indicate significant differences between the groups (P < 0.05) according to one-way ANOVA.
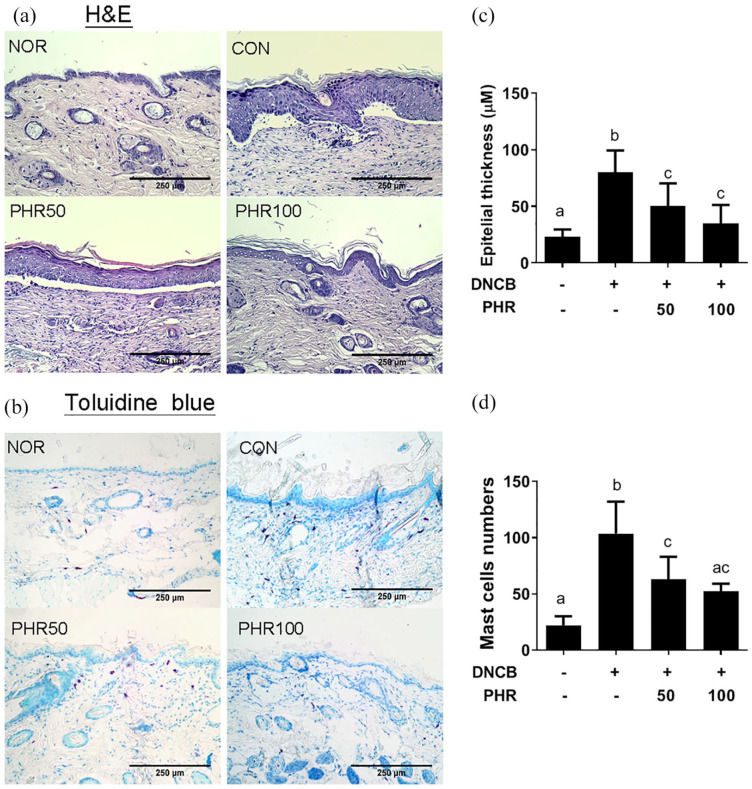
Phloretin reduced epidermal hyperplasia and mast cell infiltration in AD-like skin lesions
A thickened epidermal barrier and epithelial cell hypertrophy are characteristic of the infiltration of mast cells and lymphocytes in the ACD mouse model.22 Therefore, the thickness of the epidermis and the infiltration of mast cells into the dermis of the mice treated with and without phloretin were examined. As shown in Figure 3, the mice exposed to repetitive sensitization with DNCB exhibited increased epidermal hyperplasia, epidermal thickening, and mast cell infiltration compared to the normal mice (NOR group). However, administration of 50 or 100 mg/kg of phloretin decreased the thickness of the epidermal hyperplasia and the number of mast cells in the dermis compared to administration of the vehicle treatment (Figure 3(c) and (d)), supporting the conclusion that phloretin exerts therapeutic effects in alleviating AD-like skin lesions.
Figure 3.
Histological examination of pathological changes in the stratum corneum of mice. (a) Epidermal hyperplasia observed by H&E staining and (b) mast cell hypertrophy observed by toluidine blue staining of the dermis. Quantitative measurement of (c) the epidermal thickness and (d) mast cell numbers. Slides were examined at 100× magnification. Data are presented as mean ± SD of one representative of three independent experiments with five mice per group. Different letters indicate significant differences between the groups (P < 0.05).
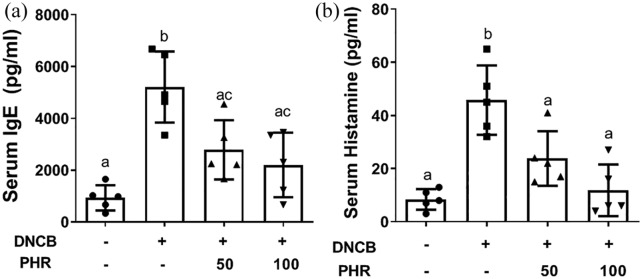
Phloretin decreased serum IgE, histamine, and dermic pro-inflammatory cytokines
Excessive IgE production and histamine release from activated mast cells are clinical indicators of AD and have also been observed in the mouse model.23,24 Thus, we measured the levels of IgE and histamine in the serum and the levels of pro-inflammatory cytokines in the homogenized dermal tissues from the mice treated with or without phloretin. The ELISA results summarized in Figure 4 and Table 1 demonstrate that the serum IgE (Figure 4(a)) and histamine (Figure 4(b)) levels induced by DNCB treatment were significantly decreased after oral phloretin administration (PHR groups). Similarly, the levels of the cytokines relevant to AD, such as IL-6, IL-4, TSLP, IFN-γ, and IL-17A, which contribute to the pathogenesis of AD were suppressed in the skin tissue homogenates from phloretin-treated ACD mice (Table 1). Our findings indicate that phloretin can reduce not only dermal hyperplasia and cell hypertrophy but also IgE, histamine, and proinflammatory cytokine overproduction, which are characteristic of ACD.
Figure 4.
Concentrations of (a) IgE and (b) histamine levels in the serum of the normal and DNCB-induced ACD mice. Data are shown as mean ± SD of one of three independent experiments with similar results. Letters indicate significant differences between the groups (P < 0.05).
Table 1.
Cytokine profiles of atopic inflammation in AD-like skin lesions.
| NOR | Vehicle | DNCB |
||
|---|---|---|---|---|
| PHR |
||||
| 50 | 100 | |||
| TSLP | 23.2 ± 12.4a | 81.3 ± 12.3b | 55.2 ± 32.4a | 39.0 ± 19.7a |
| IL-6 | l62.0 ± 105.1a | 1421.6 ± 553.4b | 556.6 ± 146.6a | 345.6 ± 111.5a |
| IFN-γ | 15.8 ± 4.6a | 81.6 ± 19.2b | 46.6 ± 12.8a | 38.4 ± 16.3a |
| IL-4 | 39.4 ± 18.4a | 239.0 ± 76.2b | 74.5 ± 23.6a | 58.8 ± 44.4a |
| IL-17A | 15.2 ± 4.8a | 67.2 ± 18.4b | 35.8 ± 10.8a | 28.6 ± 11.8a |
AD: atopic dermatitis; NOR: mice in the normal group; DNCB: 2,4-dinitrochlorobenzene; PHR: phloretin; TSLP: thymic stromal lymphopoietin; IL: interleukin; IFN-γ: interferon-γ.
The levels of cytokines (pg/mL) in the skin lesions were analyzed by enzyme-linked immunosorbent assay. The numerical results are reported as mean ± SEM. Different letters indicate significant differences between the groups (P < 0.05).
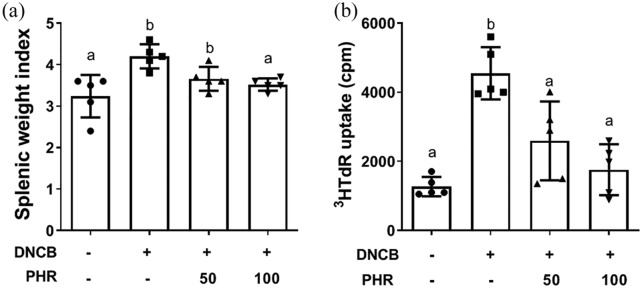
Phloretin ameliorated the DNCB-induced CD4 T-cell overproliferation in the spleen
Acute AD skin lesions exhibit Th2-dominant inflammation. This type of inflammation is mainly characterized by the dermal infiltration of CD4+ T cells and eosinophils and by the increased expression of Th2 cytokines.25,26 Furthermore, recent studies have noted that Th17 skewing and Th2-driven inflammation are more prominent in clinical AD patients.27,28 To elucidate the T-cell composition of the mice irritated with DNCB and treated with or without phloretin, the spleen size, presented as the spleen index, was compared, and the results are summarized in Figure 5(a). The spleen index of the ACD mice was significantly increased compared to that of the normal mice. However, oral phloretin administration decreased the spleen size in the ACD mice back to values within the normal range. The proliferation ability of the splenocytes was then determined by the uptake of radioactive [3H] thymidine. The substantial proliferation triggered by DNCB in the ACD mice was remarkably suppressed after phloretin treatment (Figure 5(b)).
Figure 5.
(a) Spleen size presented as the splenic weight index and (b) splenocyte proliferation determined by [3H] thymidine incorporation. Data (n = 5) are presented as mean ± SD of one of three independent experiments with similar results. Different letters indicate significant differences between the groups (P < 0.05).
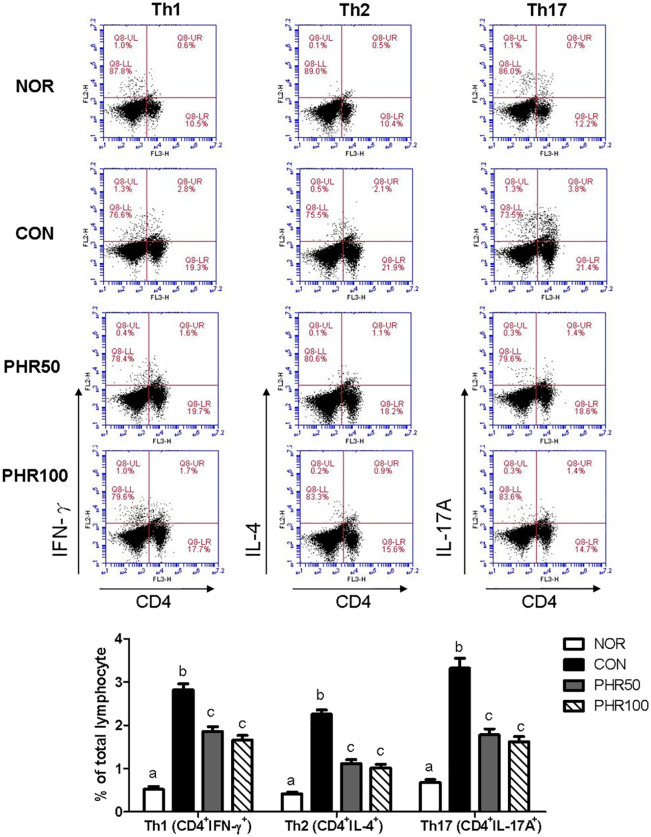
The subsets of CD4+ T-cell populations before and after DNCB and/or phloretin treatment were then analyzed. Our data showed that the frequencies of Th1 (CD4+IFN-γ+), Th2 (CD4+IL-4+), and Th17 (CD4+IL-17A+) cells were elevated after DNCB exposure. However, the elevated frequencies of Th1, Th2, and Th17 cells were reversed and substantially decreased after phloretin treatment (Figure 6), suggesting that overresponsive CD4 T cells could account for the exacerbated ACD symptoms after DNCB exposure. Therefore, phloretin could be utilized to ameliorate this response.
Figure 6.
Frequencies of T-cell subsets in the murine spleens. The splenocytes were obtained from the normal, DNCB-, and DNCB/phloretin-treated mice and cultured with 0.5 μg/mL DNCB for 3 days prior to staining with anti-CD4-FITC, anti-IFN-γ-PE, anti-IL-4-PE, anti-IL-17A-PE, and anti-Foxp3-PE antibodies to identify the specific subsets by flow cytometry. Bar graphs display the percentage of a given T-cell subset of the total analyzed splenocytes and are presented as mean ± SD of one of three independent experiments with five mice per group. Different letters indicate significant differences between the groups (P < 0.05).
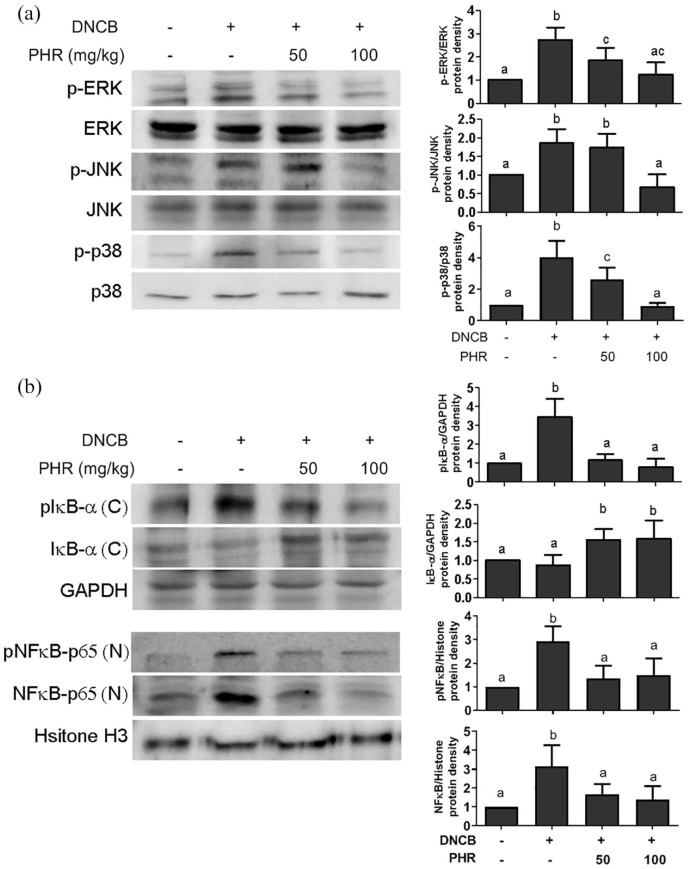
Phloretin inhibited the activation of MAPKs and NF-κB in AD-like skin lesions
Activation of the MAPK/NF-κB pathway is associated with the inflammatory pathogenesis of allergic reactions.29,30 To further explore how phloretin might affect these pathways and subsequently mitigate AD-like symptoms, the phosphorylation status of MAPK-associated kinases, namely, ERK, JNK, and p38, was first examined. The Western blot data in Figure 7(a) show that phloretin inhibited the DNCB-induced phosphorylation of all three of the kinases.
Figure 7.
Molecular signal transduction of mice treated with or without phloretin. (a) The phosphorylation of ERK, p38, and JNK in the MAPK pathway and (b) the phosphorylation and degradation of IκBα and the translocation of NF-κB were evaluated by Western blotting analysis. Quantitative results after normalizing by individual GAPDH expression are presented as mean ± SD of one of three independent experiments with five mice per group. Different letters indicate significant differences between the groups (P < 0.05).
Furthermore, given that the IκB/NF-κB pathway can alternatively activate the inflammation observed in AD,31 the protein and phosphorylation levels of IκBα and NF-κB in the cytoplasm and nucleus of dermal tissues, respectively, were also examined; in this experiment, we attempted to elucidate whether the reduced cytokine production observed above is the result of changes in the expression of proteins in the IκB/NF-κB pathway. Figure 7(b) reveals that phosphorylated IκBα and NFκB-p65 were drastically increased in the skin tissues of the vehicle-treated control mice. However, upon phloretin treatment, the phosphorylation of IκBα in the cytoplasm was decreased in a dose-dependent manner. Similarly, the DNCB-stimulated levels of pNFκB p65 and translocation into the nucleus were significantly reduced upon phloretin treatment, revealing that the phosphorylation status of the MAPK pathway and the nuclear translocation of NF-κB could be involved in the regulatory mechanism of phloretin.
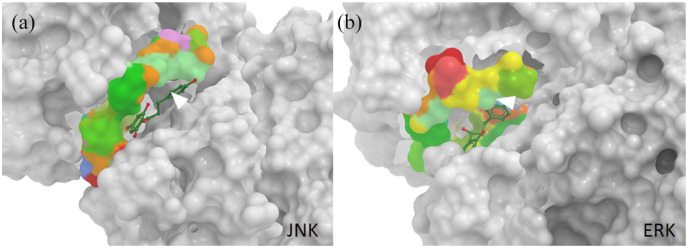
Computational simulation of the interaction between phloretin and the JNK and ERK kinases
To further support our results of the Western blot analyses that the phosphorylation of JNK (PDB entry: 3v3v) and ERK (PDB entry: 5lck) were suppressed by phloretin in a dose-dependent manner, we performed ligand docking prediction via SwissDock to determine the changes in the delta Gibbs free energy (ΔG).32,33 The predicted ΔG values for phloretin bound to JNK and ERK were as low as −7.88305 and −8.00873, respectively, suggesting a strong binding interaction between phloretin and JNK and ERK. In addition, the secondary sequences of JNK and ERK were analyzed through TargetATPsite to identify all the potential ATP-binding sites.34 The predicted ATP-binding residues are shown in Supplementary Figure 1. Surprisingly, the potential ATP-binding residues were located near the same docking pockets of JNK and ERK with which phloretin interacted (Figure 8). These data confirm that the phosphorylation of JNK and ERK could be greatly interrupted by phloretin, supporting our speculation that phloretin modulates kinases in the MAPK pathway to reduce the symptoms of ACD.
Figure 8.
Computational docking prediction of phloretin with ERK and JNK. Phloretin in green (adjacent to the white arrows) is illustrated with a stick-and-ball model, and the predicted ATP-binding residues of (a) JNK and (b) ERK are illustrated with a space-filling model and colored to be distinguished from the rest of the peptides of JNK and ERK in gray. The 3D docking models are visualized via MolSoft ICM Browser software. The numbers are the changes in Gibbs free energy.
Discussion
The DNCB-sensitization mouse model is commonly utilized to evaluate the pharmaceutical benefits of potential compounds, since the physiological symptoms of this mouse model resemble those of the symptoms of ACD in patients. In this study, the potential anti-ACD activity of phloretin was assessed, and the molecular pathways by which phloretin could exert its therapeutic effects in ameliorating humoral and cellular hypersensitivities were explored. We observed that following phloretin treatment, the epidermal hyperplasia and mast cell hypertrophy in the dermis were alleviated, the abnormally elevated circulating levels of IgE and histamine were reduced, and the exacerbated overproliferation of CD4-lineage T-cell subsets and activation of the MAPK/NF-κB pathway were mitigated in the DNCB-induced ACD mouse model. All these findings demonstrate that phloretin could be a valuable therapeutic candidate.
As a natural flavonoid-derived phytochemical compound, phloretin has been recognized due to its low cytotoxicity in mammalian cells14 and has been identified as an effective antioxidant and anti-inflammatory agent.35 These factors make phloretin a good candidate for use against AD. However, it is important to note that there is always a concern regarding the feasibility of a phytochemical product being manufactured as a clinical drug. Phlorizin was used as a prodrug, and its analogs, such as sergliflozin and dapagliflozin, have been clinically tested for the treatment of diabetes mellitus (DM) due to their inhibitory effects on cellular sodium-glucose cotransporter 2 (SGLT2).36 The former is currently undergoing a phase III clinical trial, and the latter was approved by the US Food and Drug Administration (FDA) in 2014.37,38 Hence, given the success of other phloretin-derived drugs, we believe that the development and modification of phloretin to develop a suitable prodrug is worthwhile. Another concern is the dosage of phloretin that should be used to treat ACD. In this study, phloretin was orally administered to mice at dosages of 50 and 100 mg/kg daily, which is equivalent to 1–2 mg per mouse (20–22 g). The bioavailability of phloretin in rodents through the gastrointestinal system has been investigated, and through its rapid excretion in the urine, the concentration of phloretin returned to baseline 24 h after ingesting 22 mg of phloretin in a meal.39 While these dosages in mice appeared to be reasonable due to the quick excretion kinetics of phloretin and based on comparisons of other similar studies using flavonoids to alleviate allergic dermatitis,40,41 the dosages of phloretin require further optimization in subsequent clinical studies.
Similar to phlorizin, which is an SGLT inhibitor, phloretin is also known as a potent glucose transporter (GLUT) inhibitor, and it competitively inhibits glucose uptake via GLUT.42 In addition to the various biological effects of phloretin, we speculate that the ability of phloretin to modulate glucose metabolism might play a role in the alleviation of ACD in mice, although we did not investigate the correlation of glucose metabolism and ACD in this study. However, it has recently been observed that there is an association between high blood sugar and ACD. Specifically, there is a higher prevalence rate of ACD in type 2 DM patients.43 Similarly, a previous clinical observation revealed that sweat from AD patients with acute inflammation contained higher concentrations of glucose than the sweat from normal individuals. The same research team also topically applied a glucose solution to the damaged stratum corneum and found that the recovery of the dermal lesions was delayed.44 In contrast, type 1 diabetes (insulin-dependent diabetes mellitus (IDDM)) appears to be negatively related to AD due to the distinct Th1 and Th2 responses.45 It is inferred that glucose metabolism is associated with the complicated causes of AD or ACD. Therefore, the ability of phloretin to regulate glucose could contribute to the anti-ACD effects shown in this study.
Despite the data indicating that the Th1, Th2, and Th17 cell populations were dramatically increased for approximately 4 weeks after DNCB sensitization in our study, the progression of T-cell populations is not entirely discernible. In fact, elevated levels of Th2 and Th17 cells are observed in the peripheral blood mononuclear cells (PBMCs) of AD patients at an acute stage, whereas at the chronic state, Th1 and regulatory T cells subsequently increased.27 This phenomenon has been determined to be one of the limitations that arises with the use of the ACD animal model to investigate changes in the T-cell subsets at different stages of ACD progression and to determine how phloretin can regulate both innate and adaptive immunity. Alternatively, a hapten, oxazolone, can induce acute erythematous and edematous dermatitis as early as 6–12 h after a single challenge in murine skin, and this approach might provide another suitable model for understanding the distinct allergic profiles of AD progression.46 On the contrary, which cell type in dermal tissues responds to the effects of phloretin remains unclear. Putatively, three cell types, keratinocytes,14,47 dendritic cells,9 and mast cells,48 might account for the alleviation of symptoms after phloretin treatment, but further investigations are required.
Conclusion
Our results demonstrate that phloretin is able to exert various immunomodulatory functions. These immunomodulatory functions are shown by the lower allergic IgE, histamine, and cytokine levels, which can alleviate immune activation in lymphocytic populations. The regulation occurs via the suppression of MAPK/NF-κB pathways, which indicates that phloretin is a potent agent for controlling ACD progression in the rodent model. Taken together, we believe that phloretin and its derivatives should be further investigated for clinical use. The use of phloretin could provide a more effective and safer therapeutic option for AD patients.
Supplemental Material
Supplemental material, Suppl._Fig_1_1 for Phloretin alleviates dinitrochlorobenzene-induced dermatitis in BALB/c mice by Chieh-Shan Wu, Shih-Chao Lin, Shiming Li, Yu-Chih Chiang, Nicole Bracci, Caitlin W Lehman, Kuo-Tung Tang and Chi-Chien Lin in International Journal of Immunopathology and Pharmacology
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants by VGHKS107-179 from the Kaohsiung Veterans General Hospital, TCVGH-1087313C from the Taichung Veterans General Hospital, 107-2320-B-005-007-MY3 from the Ministry of Science and Technology, and Animal Biotechnology Center from the Feature Areas Research Center Program of Taiwan Ministry of Education (MOE-107-S-0023-E).
ORCID iD: Chi-Chien Lin  https://orcid.org/0000-0002-1464-0231
https://orcid.org/0000-0002-1464-0231
Supplemental material: Supplemental material for this article is available online.
References
- 1. Weidinger S, Novak N. (2016) Atopic dermatitis. Lancet 387(10023): 1109–1122. [DOI] [PubMed] [Google Scholar]
- 2. Novak N, Bieber T, Leung DY. (2003) Immune mechanisms leading to atopic dermatitis. The Journal of Allergy and Clinical Immunology 112(6 Supp. l): S128–S139. [DOI] [PubMed] [Google Scholar]
- 3. Eichenfield LF, Tom WL, Berger TG, et al. (2014) Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. Journal of the American Academy of Dermatology 71(1): 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sidbury R, Davis DM, Cohen DE, et al. (2014) Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. Journal of the American Academy of Dermatology 71(2): 327–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoon JY, Kwon HH, Min SU, et al. (2013) Epigallocatechin-3-gallate improves acne in humans by modulating intracellular molecular targets and inhibiting P.acnes. The Journal of Investigative Dermatology 133(2): 429–440. [DOI] [PubMed] [Google Scholar]
- 6. Kim M, Lim SJ, Kang SW, et al. (2014) Aceriphyllum rossii extract and its active compounds, quercetin and kaempferol inhibit IgE-mediated mast cell activation and passive cutaneous anaphylaxis. Journal of Agricultural and Food Chemistry 62(17): 3750–3758. [DOI] [PubMed] [Google Scholar]
- 7. Perez-Cano FJ, Castell M. (2016) Flavonoids, inflammation and immune system. Nutrients 8(10): 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mariadoss AVA, Vinyagam R, Rajamanickam V, et al. (2019) Pharmacological aspects and potential use of phloretin: A systemic review. Mini-Reviews in Medicinal Chemistry 19(13): 1060–1067. [DOI] [PubMed] [Google Scholar]
- 9. Lin CC, Chu CL, Ng CS, et al. (2014) Immunomod-ulation of phloretin by impairing dendritic cell activation and function. Food & Function 5(5): 997–1006. [DOI] [PubMed] [Google Scholar]
- 10. Wang SP, Lin SC, Li S, et al. (2016) Potent antiarthritic properties of phloretin in murine collagen-induced arthritis. Evidence-Based Complementary and Alternative Medicine: eCAM 2016: 9831263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J, Durai P, Jeon D, et al. (2018) Phloretin as a potent natural TLR2/1 inhibitor suppresses TLR2-induced inflammation. Nutrients 10(7): 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang WT, Huang WC, Liou CJ. (2012) Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages. Food Chemistry 134(2): 972–979. [DOI] [PubMed] [Google Scholar]
- 13. Aliomrani M, Sepand MR, Mirzaei HR, et al. (2016) Effects of phloretin on oxidative and inflammatory reaction in rat model of cecal ligation and puncture induced sepsis. DARU Journal of Pharmaceutical Sciences 24(1): 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheon D, Kim J, Jeon D, et al. (2019) Target proteins of phloretin for its anti-inflammatory and antibacterial activities against propionibacterium acnes-induced skin infection. Molecules 24(7): 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang WC, Fang LW, Liou CJ. (2017) Phloretin attenuates allergic airway inflammation and oxidative stress in asthmatic mice. Frontiers in Immunology 8: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin H, He R, Oyoshi M, et al. (2009) Animal models of atopic dermatitis. The Journal of Investigative Dermatology 129(1): 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang HR, Lee H, Kim JH, et al. (2019) Therapeutic effect of Rumex japonicus Houtt. On DNCB-induced atopic dermatitis-like skin lesions in Balb/c mice and human keratinocyte HaCaT cells. Nutrients 11(3): 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choo GS, Lim DP, Kim SM, et al. (2019) Anti-inflammatory effects of D morbifera in lipopolysaccharide-stimulated RAW264.7 macrophages and in an animal model of atopic dermatitis. Molecular Medicine Reports 19(3): 2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chung TH, Kang TJ, Cho WK, et al. (2012) Effec-tiveness of the novel herbal medicine, KIOM-MA, and its bioconversion product, KIOM-MA128, on the treatment of atopic dermatitis. Evidence-Based Complementary and Alternative Medicine 2012: 762918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park HJ, Choi WS, Lee WY, et al. (2018) A novel mouse model of atopic dermatitis that is T helper 2 (Th2)-polarized by an epicutaneous allergen. Environ Toxicol Pharmacol 58: 122–130. [DOI] [PubMed] [Google Scholar]
- 21. Matsuoka H, Maki N, Yoshida S, et al. (2003) A mouse model of the atopic eczema/dermatitis syndrome by repeated application of a crude extract of house-dust mite Dermatophagoides farinae. Allergy 58(2): 139–145. [DOI] [PubMed] [Google Scholar]
- 22. Kitamura A, Takata R, Aizawa S, et al. (2018) A murine model of atopic dermatitis can be generated by painting the dorsal skin with hapten twice 14 days apart. Scientific Reports 8(1): 5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leung DY, Guttman-Yassky E. (2014) Deciphering the complexities of atopic dermatitis: Shifting paradigms in treatment approaches. The Journal of Allergy and Clinical Immunology 134(4): 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thangam EB, Jemima EA, Singh H, et al. (2018) The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Frontiers in Immunology 9: 1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laberge S, Ghaffar O, Boguniewicz M, et al. (1998) Association of increased CD4+ T-cell infiltration with increased IL-16 gene expression in atopic dermatitis. The Journal of Allergy and Clinical Immunology 102(4 Pt 1): 645–650. [DOI] [PubMed] [Google Scholar]
- 26. Brandt EB, Sivaprasad U. (2011) Th2 cytokines and atopic dermatitis. Journal of Clinical and Cellular Immunology 2(3): 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Su C, Yang T, Wu Z, et al. (2017) Differentiation of T-helper cells in distinct phases of atopic dermatitis involves Th1/Th2 and Th17/Treg. European Journal of Inflammation 15(1): 46–52 [Google Scholar]
- 28. Brunner PM, Israel A, Zhang N, et al. (2018) Early-onset pediatric atopic dermatitis is characterized by TH2/TH17/TH22-centered inflammation and lipid alterations. The Journal of Allergy and Clinical Immunology 141(6): 2094–2106. [DOI] [PubMed] [Google Scholar]
- 29. Yang L, Cohn L, Zhang DH, et al. (1998) Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. Journal of Experimental Medicine 188(9): 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roux PP, Blenis J. (2004) ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiology and Molecular Biology Reviews 68(2): 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dajee M, Muchamuel T, Schryver B, et al. (2006) Blockade of experimental atopic dermatitis via topical NF-kappaB decoy oligonucleotide. The Journal of Investigative Dermatology 126(8): 1792–1803. [DOI] [PubMed] [Google Scholar]
- 32. Gilson MK, Zhou HX. (2007) Calculation of protein-ligand binding affinities. Annual Review of Biophysics and Biomolecular Structure 36: 21–42. [DOI] [PubMed] [Google Scholar]
- 33. Grosdidier A, Zoete V, Michielin O. (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Research 39(Suppl. 2): W270–W277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu DJ, Hu J, Huang Y, et al. (2013) TargetATPsite: A template-free method for ATP-binding sites prediction with residue evolution image sparse representation and classifier ensemble. Journal of Computational Chemistry 34(11): 974–985. [DOI] [PubMed] [Google Scholar]
- 35. Zielinska D, Laparra-Llopis JM, Zielinski H, et al. (2019) Role of apple phytochemicals, phloretin and phloridzin, in modulating processes related to intestinal inflammation. Nutrients 11(5): 1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rieg T, Vallon V. (2018) Development of SGLT1 and SGLT2 inhibitors. Diabetologia 61(10): 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhillon S. (2019) Dapagliflozin: A review in type 2 diabetes. Drugs 79(10): 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cefalo CMA, Cinti F, Moffa S, et al. (2019) Sotagliflozin, the first dual SGLT inhibitor: Current outlook and perspectives. Cardiovascular Diabetology 18(1): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crespy V, Aprikian O, Morand C, et al. (2001) Bioavailability of phloretin and phloridzin in rats. The Journal of Nutrition 131(12): 3227–3230. [DOI] [PubMed] [Google Scholar]
- 40. Shen Y, Xu J. (2019) Resveratrol exerts therapeutic effects on mice with atopic dermatitis. Wounds 31(11): 279–284. [PubMed] [Google Scholar]
- 41. Lee HN, Shin SA, Choo GS, et al. (2018) Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB‑induced atopic dermatitis animal models. International Journal of Molecular Medicine 41(2): 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lefevre PG, Marshall JK. (1959) The attachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. Journal of Biological Chemistry 234: 3022–3026. [PubMed] [Google Scholar]
- 43. Silverberg JI, Greenland P. (2015) Eczema and cardiovascular risk factors in 2 US adult population studies. The Journal of Allergy and Clinical Immunology 135(3): 721–728.e6. [DOI] [PubMed] [Google Scholar]
- 44. Ono E, Murota H, Mori Y, et al. (2018) Sweat glucose and GLUT2 expression in atopic dermatitis: Implication for clinical manifestation and treatment. PLoS ONE 13(4): e0195960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Olesen AB, Juul S, Birkebaek N, Thestrup-Pedersen K. (2001) Association between atopic dermatitis and insulin-dependent diabetes mellitus: A case-control study. Lancet 357(9270): 1749–1752. [DOI] [PubMed] [Google Scholar]
- 46. Man MQ, Hatano Y, Lee SH, et al. (2008) Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: Structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. The Journal of investigative dermatology 128(1): 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huang WC, Dai YW, Peng HL, et al. (2015) Phloretin ameliorates chemokines and ICAM-1 expression via blocking of the NF-κB pathway in the TNF-α-induced HaCaT human keratinocytes. International Immunopharmacology 44(5): 480–485. [DOI] [PubMed] [Google Scholar]
- 48. Grosman N. (1998) Inhibitory effect of phloretin on histamine release from isolated rat mast cells. Agents Actions 25(3–4): 284–290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Suppl._Fig_1_1 for Phloretin alleviates dinitrochlorobenzene-induced dermatitis in BALB/c mice by Chieh-Shan Wu, Shih-Chao Lin, Shiming Li, Yu-Chih Chiang, Nicole Bracci, Caitlin W Lehman, Kuo-Tung Tang and Chi-Chien Lin in International Journal of Immunopathology and Pharmacology