Abstract
Background:
The prospect for targeted therapies in advanced hepatocellular carcinoma (HCC) has dramatically changed since several recent clinical trials have yielded promising results. The number of second-line therapies is increasing, though the consequent challenge is to consider differences between these interventions. This is a comparative investigation of presently approved second-line drugs for HCC based on findings from phase III randomized controlled trials.
Methods:
Data related to treatment efficacy including overall survival (OS), progression-free survival (PFS) and objective response rate (ORR) were extracted and compared using a Bayesian approach. Adverse events (AEs) and the rate of discontinuation due to AEs were assessed and compared with provide a more complete understanding. OS and PFS in patients with alpha fetoprotein (AFP) values greater than 400 were compared and ranked as a subgroup.
Results:
A total of five trials involving 2571 patients were included. The comparison suggests that regorafenib and cabozantinib significantly prolong OS compared with placebo. The rate of AEs and treatment discontinuation did not significantly differ, although the types of AEs varied substantially. Subgroup analysis did not highlight a significant OS difference between regorafenib [hazard ratio (HR) 0.68; 95% confidence interval (CI) 0.50–0.92], cabozantinib (HR 0.71; CI 0.54–0.94) and ramucirumab (HR 0.69; CI 0.57–0.84). Conclusion: Among the four second-line HCC therapies compared, regorafenib and cabozantinib appear to be better choices in terms of OS. Cabozantinib, regorafenib and ramucirumab have similar levels of efficacy for those with AFP >400, although ramucirumab has fewer side effects. No significant difference was observed in AEs, but some AEs related to each of these interventions should be given further consideration.
Keywords: comparison, hepatocellular carcinoma, network meta-analysis, second-line therapy
Introduction
Hepatocellular carcinoma (HCC) accounts for approximately 85% of all liver cancers and remains one of the leading causes of cancer-related deaths.1,2 In the early stages, HCC can be cured by radical surgical resection, liver transplantation and/or local ablation, although most are diagnosed in the latter stages when there is no curative intervention. Current treatments for advanced HCC mainly include hepatic arterial chemoembolization and systemic targeted drug therapies which can extend survival.3,4 During the decade prior to 2017, progress in HCC systemic treatment was abysmal; however, fortunately, the systemic treatment of HCC has changed substantially over the past 2 years.
Of the first-line treatments, lenvatinib and a regimen of atezolizumab plus bevacizumab have successfully obtained positive results compared with sorafenib. These reliable findings have changed treatment guidelines in the field of hepatocellular carcinoma.5 Of the second-line treatments, several have been approved recently. For example, in April 2017, regorafenib was approved by the FDA for clinical practice as a first second-line treatment for HCC.6 This was soon followed by cabozantinib and ramucirumab, which were approved in January and May of 2019. Likewise, in 2019, immunotherapeutic drugs nivolumab and pembrolizumab were introduced as second-line treatments.7 Therefore, while these new interventions become readily available in clinical practice, researchers are beginning to consider how to maximize the curative effect while avoiding more serious side effects. Of course, the ultimate goal is to develop individualized care and precise medical regimens although there are a number of unknowns.
For example, ramucirumab, which specifically blocks VEGFR-2, can only be prescribed to patients with an AFP greater than 400. However, for this particular group it is not known whether the alternative drugs have similar levels of efficacy. The objective response rate associated with pembrolizumab can reach 18%, which is far higher than other targeted drugs. Although whether pembrolizumab can provide equal benefit in terms of efficacy is yet to be determined. Up until now, there has not been a comparison of targeted drugs and immune checkpoint inhibitors because each of these drugs, which have only recently been approved, were developed by competing companies. This makes it difficult to obtain comparative information acquired through standard randomized controlled trials.8
This study is a novel attempt to overcome this comparative problem and to hopefully fill the gap within this evidence base. As such, we included a number of large, phase III clinical trials, and looked to indirectly compare overall survival (OS), progression-free survival (PFS), objective response rate (ORR), adverse events (AEs) as well as treatment discontinuation related to all currently approved second-line treatments. We adopt Bayesian analytical methods in order to provide a more sophisticated understanding of the safety and efficacy related to these interventions. It is hoped that the findings will be used for shared decision-making and support clinicians adjusting treatment regimens according to patient responses.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analysis (PRISMA-NMA) was used and the checklist is provided as Supplemental Material Table-S1 online. The protocol was not published prior to conducting this research.
Study selection and eligibility criteria
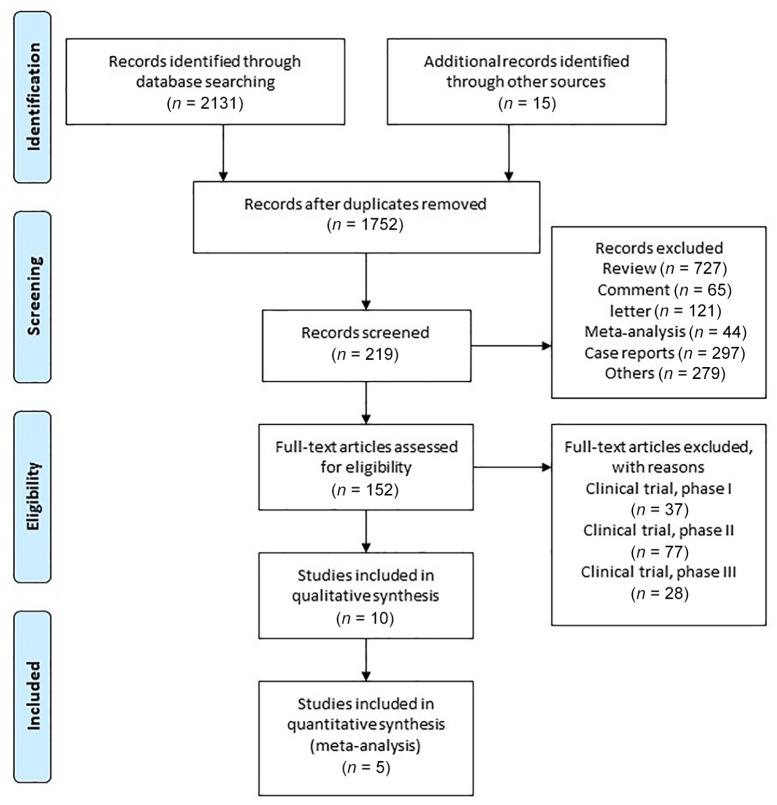
A systematic search was conducted in Embase, PubMed and the Cochrane Central Register of Controlled Trials from 1 January 2005 up until 1 January 2020. A detailed example search has been provided as Supplemental Material S-2. After removing duplicates, further screening took place to exclude reviews, case reports, letters, non-English-language articles and other studies which were non-randomized clinical trials. Finally, we assessed full texts to identify pertinent studies.
All treatment regimens were defined according to EASL/AASLD guidelines, which were tested through phase III randomized controlled trials (RCTs). Eligibility criteria were as follows: (a) Population – pathologically or radiographically confirmed HCC patients with progression on or intolerance to sorafenib; (b) intervention – chemotherapy, targeted therapy, immune therapy, or other agents; (c) comparator – systemic therapy, placebo or best supportive therapy; (d) outcomes – OS, PFS, ORR, the rate of all grade and grade 3–4 adverse events, and the rate of treatment discontinuation due to AEs; (e) design – only phase III RCTs.
Data extraction
All data were independently extracted from relevant trials by two investigators, and entered into a predefined spreadsheet. Efficacy and safety factors extracted from each study included OS, PFS, ORR, AEs and the rate of discontinuation of therapy. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for OS and PFS were used for further comparative analysis. Extracted study and participant characteristics include year of publication, study identification code, sample size, numbers randomized to each arm, level of alpha-fetoprotein (AFP), HCC stage, Child–Pugh scores and previous treatments et cetera.
Methodological quality and risk of bias assessment
Cochrane Collaboration risk of bias tool and publication bias were used to evaluate the quality of evidence. Risk of bias within randomized trials was assessed by two independent reviewers. Risk of publication bias was considered using standard funnel plots.
Data synthesis and statistical analysis
We utilized the Gemtc package v0.8-2 in R version 3.5.3 to perform a Bayesian analysis. The random effects model and consistency models were used to calculate ORs and 95% credibility intervals. OS and PFS data are expressed as HR, and AE rates using relative risk (RR), with corresponding 95% CIs. We used non-informative prior distributions and over-dispersed initial values with a scale of 0–5, in four chains to fit the model. This yielded 100,000 iterations, including 20,000 tuning iterations and a thinning interval of 10 for each chain.
This method was also used to generate distribution parameters for the model. Convergence of iterations was assessed using the Gelman–Rubin–Brooks statistic.9 According to posterior probabilities, we were able to rank probabilities for each intervention. Due to the absence of head-to-head clinical trials, it was not possible to conduct consistency testing. The apparent heterogeneity within the study population meant that we should not combine the two ramucirumab studies for pooled analysis, and therefore we opted to analyse each study separately. Indirect comparisons were performed for different drugs, such as regorafenib versus pembrolizumab. The adjusted indirect comparison was calculated using Bayesian methods embedded in the following formula: ln(HR)=[ln(UL – HR) + ln(LL – HR)]/2; seln(HR)=[ln(UL–HR) – ln(LL–HR)]/(1.96×2); RR was calculated as follows; log(HR)=[log(UL – HR) + log(LL – HR)]/2; selog(HR)=[log(UL – HR) – log(LL – HR)]/(1.96×2); HR <1 or RR <1 was used to identify treatment superiority.
Results
Study selection and patient characteristics
A total of five trials involving 2571 patients were included,6,10–13 with sample sizes ranging from 413 to 843. The complete trial selection process is provided in Figure 1. All five trials provided complete OS, PFS, ORR and AE data. Detailed study and participant characteristics are also provided. Please see Table 1 for details. Subgroup characteristics are also summarized in Table 2.
Figure 1.
Flowchart of study identification and selection process.
Table 1.
Clinical baseline characteristics of the included studies.
| First author | Study ID | Trial phase | Inclusion patients | Total number | Arm (drug/control) | Number in each arm |
|---|---|---|---|---|---|---|
| Finn13 | KEYNOTE 240 | III | Progression on/intolerance of sorafenib | 413 | Pembrolizumab/placebo | 278/135 |
| Zhu11 | REACH 2 | III | Progression on/intolerance of sorafenib; AFP >400 | 292 | Ramucirumab/placebo | 197/95 |
| Abou-Alfa12 | CELESTIAL | III | Previous treated with sorafenib/progression | 707 | Cabozantinib/placebo | 470/237 |
| Bruix6 | RESORCE | III | Progression on/tolerance of sorafenib | 591 | Regorafenib/placebo | 397/194 |
| Zhu10 | REACH | III | Progression on/intolerance of sorafenib | 568 | Ramucirumab/placebo | 283/285 |
AFP, alpha-fetoprotein
Table 2.
Subgroup characteristics of the included studies.
| Study ID | KEYNOTE 240 | RESORCE | CELESTIAL | REACH | REACH 2 |
|---|---|---|---|---|---|
| Drug | Pembrolizumab | Regorafenib | Cabozantinib | Ramucirumab | Ramucirumab |
| Progression on first line treatment/sorafenib | 242 (87.1%) | 379 (100%) | 335 (71%) | 244 (86%) | 166 (84%) |
| Rate of intolerant patients | 36 (12.9%) | 0 | – | 37 (13%) | 31 (16%) |
| EHS | 195 (70.1%) | 265 (70%) | 369 (79%) | 207 (73%) | 141 (72%) |
| MVI | 36 (12.9%) | 110 (29%) | 129 (27%) | 82 (29%) | 70 (36%) |
| ECOG PS1 | 116 (41.7%) | 132 (35%) | 224 (48%) | 124 (44%) | 84 (43%) |
| Median duration of treatment | 3.5 months | 3.6 months | 3.8 months | 3.0 months | 3.0 months |
| Discontinuation due to AEs | 48 (17.2%) | 39 (10%) | 76 (16%) | 28 (10%) | 21 (11%) |
| Median time from stopping previous treatment until study treatment | 1.2 months | 0.9 months | 1.4 months | – | 1.2 months |
Data are presented as number (percentage).
AE, adverse event; ECOG PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic spread; MVI, macrovascular invasion
Structure of network meta-analysis (NMA) and risk of bias
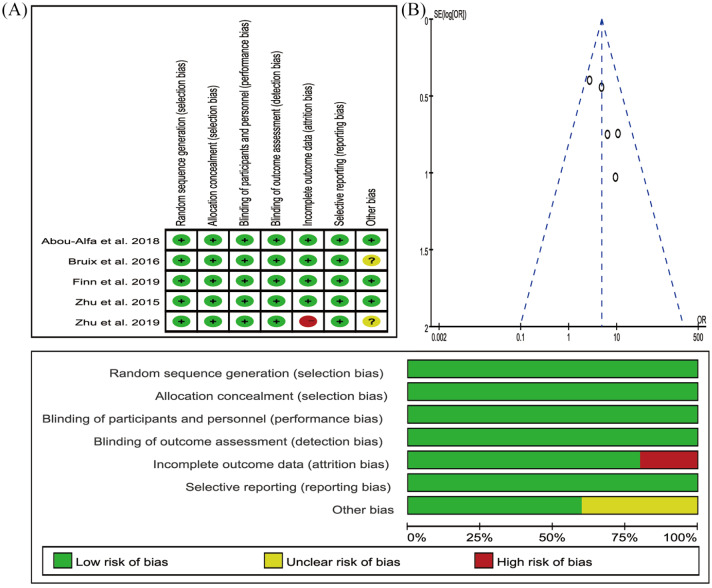
The network plot of treatment regimens used in the analysis is provided as Figure 2. We compared four treatment regimens, that is, cabozantinib, ramucirumab, regorafenib, pembrolizumab, and placebo, which was used as the control. All five studies were randomized, double-blind, multicentre, phase III trials. The included populations were not discernibly different. The results of the risk of bias are provided in Figure 3.
Figure 2.
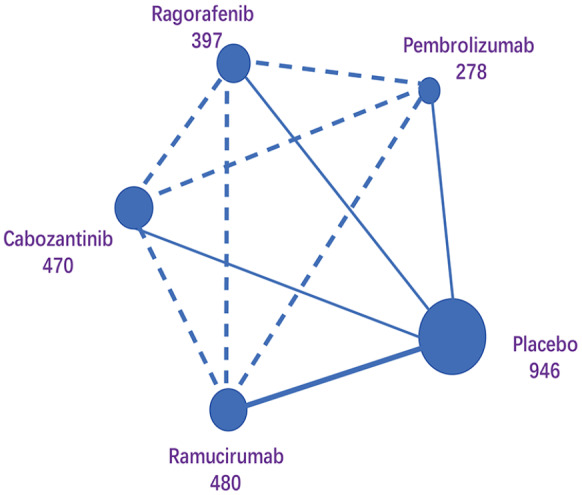
Network maps of comparing interventions.
Each circular node represents a type of treatment. The circle size is proportional to the total number of patients (under the drug name). The width of lines is proportional to the number of studies performing head-to-head comparisons in the same study, and the dotted line is the indirect comparison shown in this NMA.
Figure 3.
The risk of bias of included studies.
SE(log[OR]), standard error of the log odds ratio
NMA results for OS, PFS
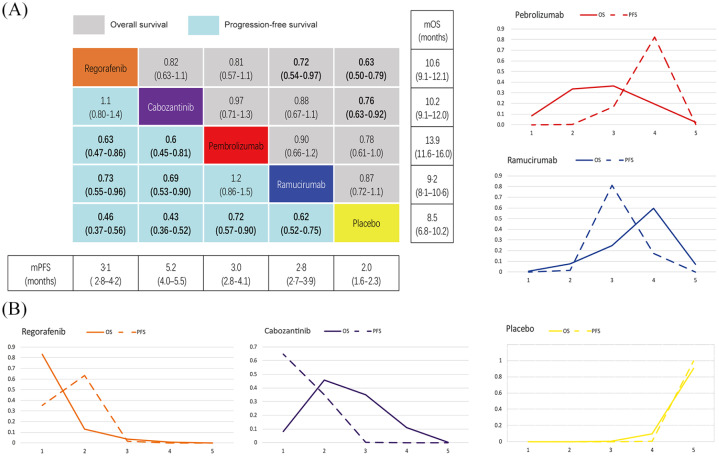
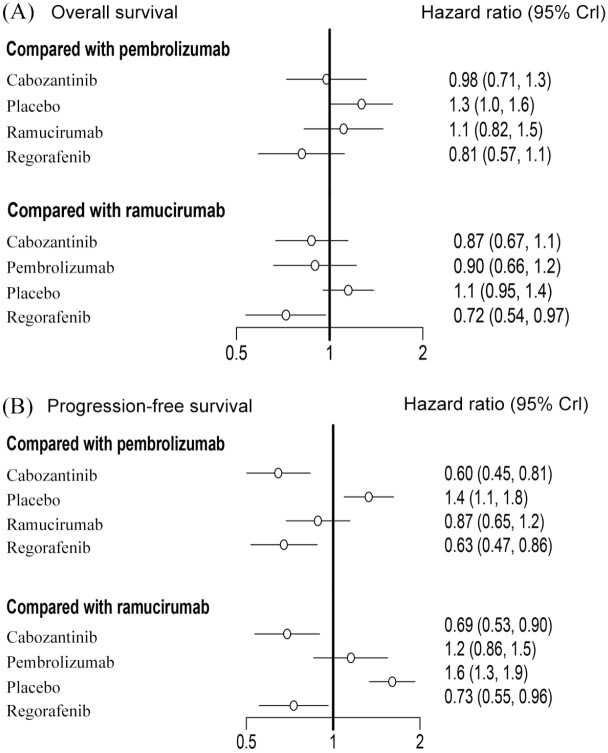
When compared with placebo, the results suggest that both regorafenib and cabozantinib significantly prolong OS, and regorafenib significantly decreases the risk of death when compared with ramucirumab (HR 0.72; CI 0.54–0.97). Each of the included interventions was significantly superior to placebo in terms of PFS. Further comparisons of the active interventions suggest regorafenib (HR 0.73; CI 0.55–0.96) and cabozantinib (HR 0.69; CI 0.53–0.90) are superior to ramucirumab in PFS. Regorafenib (HR 0.63; CI 0.47–0.86) and cabozantinib (HR 0.6; CI 0.45–0.81) also appear to yield more benefit when compared with pembrolizumab (Figure 4A). OS and PFS associated with each of the tested interventions were analysed using forest plots and compared with either pembrolizumab or ramucirumab (Figure 5).
Figure 4.
OS and PFS comparisons and ranking curves of efficacy.
A. Each cell of the block contains the pooled HR and 95% credibility intervals for OS and PFS; significant results are in bold. B. Ranking curves indicate the probability of the highest benefit of OS and PFS, the second highest, the third highest, and so on.
HR, hazard ratio; mOS, median overall survival; mPFS, median progression-free survival; OS, overall survival; PFS, progression-free survival
Figure 5.
Forest plots depicting the direct and indirect results of head-to-head comparisons.
CrI, credible intervals
The order of these four interventions was determined for OS using percentages which were ranked from high to low, as follows: regorafenib (probability 83%), cabozantinib (probability 46%), pembrolizumab (36%) and ramucirumab (probability 60%). Likewise, associated PFS measures were ranked from high to low, as follows: cabozantinib (probability 64%), regorafenib (probability 63%), ramucirumab (probability 81%) and pembrolizumab (probability 82%). Please see Figure 4B for further details.
Indirect comparisons and descriptive analysis of ORR, treatment discontinuation and AEs
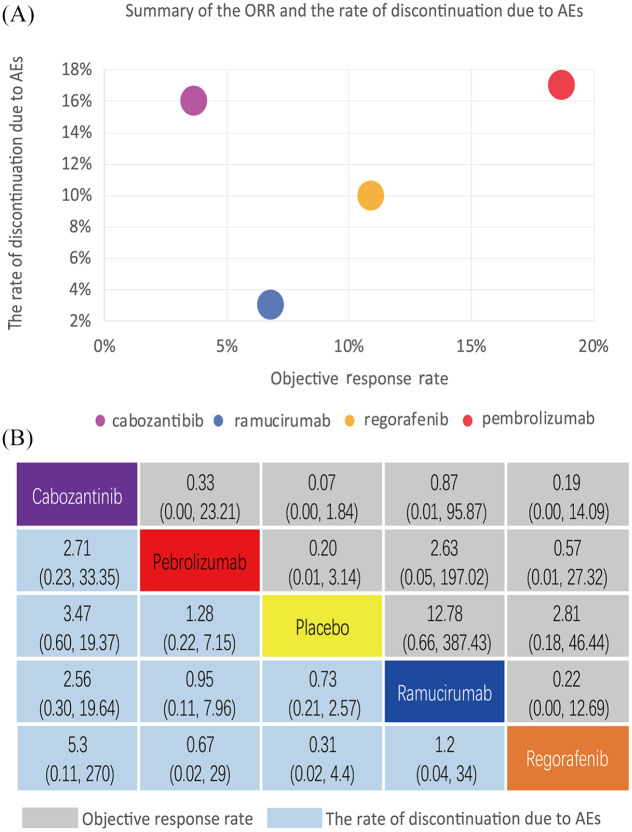
The ORR of pembrolizumab was 18%, which is the highest among the drugs tested, although the rate of discontinuation due to AEs also reached 17%. For cabozantinib, the ORR was only 4% with a discontinuation rate of 16%. The rate of discontinuation and ORR in ramucirumab is 4% and 7%, respectively. For regorafenib, it was 10% and 11%, respectively. The results of this indirect comparison showed no significant differences in terms of ORR or the rate of discontinuation between these interventions. Please see Figure 6 for full details.
Figure 6.
(A) Summary and (B) NMA analysis of ORR and the rate of discontinuation due to AEs.
The circle represents each drug, and the horizontal axis of block shows the ORR of each drug. The longitudinal axis shows the rate of treatment discontinuation. AE, adverse event; NMA, network meta-analysis; ORR, objective response rate
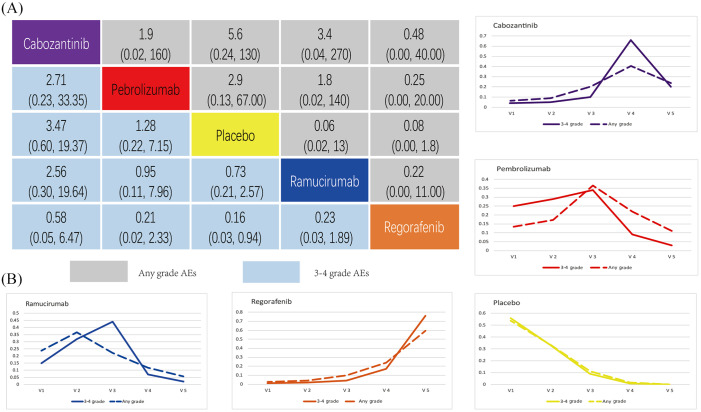
Among AEs with incidence >10%, diarrhoea, fatigue, nausea and decreased appetite occurred in all the trials of these four drugs. Diarrhoea was the most common side effect of cabozantinib. Whereas regorafenib commonly manifests with a hand–foot skin reaction. Only ramucirmuab is associated with peripheral oedema, which is also the most probable side effect. Increased aspartate aminotransferase occurred in nearly one-quarter of all patients (i.e. 23%) receiving pembrolizumab. A detailed overview of treatment-related AEs is provided in Table 3.
Table 3.
Toxicity spectrum for every intervention based on any grade and grade 3–4 adverse events. The rate of adverse events in each drug.
| Adverse events | Any grade adverse events | 3–4 grade adverse events | ||||||
|---|---|---|---|---|---|---|---|---|
| Diarrhoea | Cab(54) | Reg(33) | Ram(18) | Peb(17) | Cab(10) | Reg(2) | Ram(1) | Peb(1) |
| Hand–foot skin reaction | Reg(52) | Cab(46) | Cab(17) | Reg(13) | ||||
| Fatigue | Cab(45) | Reg(29) | Ram(23) | Peb(19) | Cab(10) | Reg(6) | Ram(2) | Peb(3) |
| Peripheral oedema | Ram(36) | |||||||
| Nausea | Cab(31) | Ram(19) | Reg(11) | Peb(11) | Cab(2) | Peb(1) | ||
| Hypertension | Cab(29) | Reg(23) | Cab(16) | Reg(13) | ||||
| Ascites | Ram(27) | Ram(5) | ||||||
| Vomiting | Cab(26) | |||||||
| Aspartate aminotransferase increased | Peb(23) | Cab(22) | Reg(13) | Peb(13) | Cab(12) | Reg(5) | ||
| Asthenia | Cab(22) | Cab(7) | ||||||
| Constipation | Cab(19) | |||||||
| Dysphonia | Cab(19) | Cab(1) | ||||||
| Headache | Ram(19) | Ram(1) | ||||||
| Blood bilirubin increased | Reg(19) | Peb(19) | Peb(8) | Reg(7) | ||||
| Pruritus | Peb(18) | |||||||
| Abdominal pain | Cab(18) | Ram(17) | Cab(2) | Ram(2) | ||||
| Alanine aminotransferase increased | Peb(18) | Cab(17) | Peb(6) | Cab(5) | ||||
| Decreased appetite | Cab(18) | Reg(24) | Ram(22) | Peb(17) | Cab(6) | Reg(3) | Ram(2) | Peb(1) |
| Pyrexia | Ram(17) | |||||||
| Weight loss | Cab(17) | Cab(1) | ||||||
| Proteinuria | Ram(16) | Ram(2) | ||||||
| Cough | Ram(15) | Peb(9) | ||||||
| Oral mucositis | Reg(11) | Reg(1) | ||||||
The number in parentheses represents the incidence of each adverse event for the drug.
Cab, cabozantinib; Pem, pembrolizumab; Ram, ramucirumab; Reg, regorafenib.
In terms of the all grade AEs, the rate of cabozantinib was 99%, and 68% in grade 3–4 AEs, which is the highest among the four drugs analysed. Ramucirumab is associated with relatively mild AEs, and all grade AEs at 44%, with grade 3–4 AEs hitting 36%. The ORR of pembrolizumab was 18%, but for grade 3–4 AEs pembrolizumab manifested at 53%.
The results from indirect comparisons suggest there is no significant difference with regard to all grade AEs or 3–4 grade AEs among the interventions analysed (see Figure 7A). Figure 7B also shows that ramucirumab can be considered the best in terms of safety ranking for all grade AEs, followed by pembrolizumab, cabozantinib and regorafenib. For 3–4 grade AEs, safety ranking found pembrolizumab to be superior followed by ramucirumab, cabozantinib, and regorafenib.
Figure 7.
(A) Comparisons and (B) ranking curves of any grade and 3–4 grade AEs.
AE, adverse event
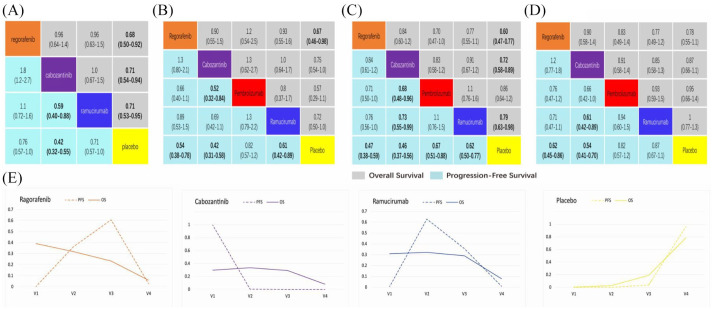
NMA results of subgroup in patients with AFP >400, macrovascular invasion (MVI), extrahepatic spread (EHS) and Eastern Cooperative Oncology Group performance status (ECOG PS1)
According to the predetermined AFP value, stratification analysis was conducted and included four phase III RCTs involving 1324 patients. The output provided as Figure 8A demonstrates that regorafenib (HR 0.68; CI 0.50–0.92), cabozantinib (HR 0.71; CI 0.54–0.94) and ramucirumab (HR 0.69; CI 0.57–0.84) are significantly superior to placebo in terms of OS, although no significant difference was observed between these three interventions. Comparative analysis also demonstrates that the PFS associated with cabozantinib (HR 0.59; CI 0.40–0.88; HR 0.42; CI 0.32–0.55) was significantly better than that of ramucirumab and placebo. PFS associated with each of the three drugs ranked from high to low, was as follows: cabozantinib (probability 99.3%), ramucirumab (probability 62.9%), regorafenib (probability 60.7%). See Figure 8E for further comparative details. Except for the main AFP comparisons, subgroup analysis which included MVI, EHS and ECOG PS1 demonstrated there is no statistically significant difference between these four drugs in terms of PFS. See Figure 8 for further details.
Figure 8.
Comparisons and ranking curves of efficacy in the subgroups.
Each cell of the block contains the pooled odds ratios and 95% credibility intervals for OS and PFS. Significant results are in bold. (a) AFP >400 subgroup, (b) MVI subgroup, (c) EHS subgroup, (d) ECOG PS1 subgroup, (e) ranking curves indicate the probability of the benefit of OS and PFS in AFP >400 subgroup. AFP, alpha-fetoprotein; ECOG PS, Eastern Cooperative Oncology Group performance status; EHS, extrahepatic spread; MVI, macrovascular invasion; PFS, progression-free survival; OS, overall survival
Discussion
In this network meta-analysis of phase III RCTs, we comprehensively synthesized and compared the efficacy and safety of targeted drugs and immunotherapy approved as second-line treatments for advanced HCC. The results suggest that regorafenib and cabozantinib are better options for HCC patients in terms of overall survival. There was no significant difference between the remaining interventions analysed here. Under subgroup analysis involving AFP >400, MVI, EHS or ECOG PS1, we found that cabozantinib, regorafenib and ramucirmuab significantly prolong survival for patients with APF >400 compared with placebo. However, there was no statistical significance in overall survival when these drugs were compared with one another. In terms of AEs during the treatment period, the rate of all grade or grade 3–4 AEs and the rate of discontinuation due to AEs were not significantly different between the four drugs, although the fewest was associated with ramucirumab.
The systematic treatment for hepatocellular carcinoma has developed rapidly over the past 3 years. For second-line treatments, five targeted drugs (i.e. cabozantinib, regorafenib, lenvatinib, ramucirumab and sorafenib) and three kinds of immunotherapy (nivolumab, pembrolizumab and nivolumab plus ipilimumab) have been recommended by NCCN guidelines (version 2020) based on findings from several recent clinical trials.14 The current challenge is to better understand differences in efficacy and safety between these drugs in order to provide personalized treatments according to the individual conditions, while minimizing toxicity.15
In 2017, a meta-analysis of several interventions was conducted for HCC. Kim et al. demonstrated that targeted agents can prolong overall survival in patients with advanced HCC, but the authors did not perform a comparison of each targeted drug.16 More recently, Ziad et al. included a number of comparative trials which explored targeted therapies after receiving sorafenib. This study found that ragorafenib and cabozantinib are potentially the best second-line targeted drugs in terms of overall survival, which was consistent with our results.17 However, this study predominantly compared a variety of unapproved targeted drugs, as well including several phase II studies. In addition, there was an absence of analysis regarding immunotherapy and AFP levels, which reduces the reliability and validity of the results.
The clinical application of tyrosine kinase inhibitors (TKIs) is also challenging because of the different and complex pharmacokinetics of these drugs. Usually better efficacy brings yield both active liver metabolites damage and common toxicities.18 In this analysis, regorafenib and cabozantinib resulted in a higher number of all grade adverse events and grade 3–4 AEs compared with both pembrolizumab and ramucirumab. Of note, in the Keynote 240, pembrolizumab appeared to reduce the risk of death by 22% and improve PFS compared with placebo.13 Additionally, the ORR associated with pembrolizumab in the Keynote 240 was 18.3% while the OS for the pembrolizumab and placebo groups reached 13.9 and 10.6 months, respectively, which is higher than any other targeted drugs. However, in the KEYNOTE 240, pembrolizumab did not reach pre-specified efficacy boundaries and in this NMA it also does not provide the same benefit in terms of overall survival compared with both regorafenib and cabozantinib, although it appears better than ramucirumab. The occurrence of these conditions does not relate solely to the efficacy of the drug itself, but may be related to other factors related to trial conduct including the impact of the unanticipated availability of effective post-study therapies or excessive non-neoplastic deaths in the experimental group. Perhaps more accurate screening of people suitable for immunotherapies and the development of effective combination therapy are required in the future.19
The patients who progressed despite receiving the first-line treatment is of particular concern because the physical condition of these patients was almost the same as those in the comparatively normal population and a significant number of these patients were assessed with a Child–Pugh score of 5–6.20 The exploratory analysis of sequential treatments, with sorafenib followed by regorafenib, shows that median time from the start of sorafenib to death is approximately 26.0 months for regorafenib and 19.2 months (CI, 16.3–22.8) for placebo.21 Moreover, according to the research standards established in the RESORCE study, approximately 30.6% of all patients are treated with ragorafenib after Sorafenib.22 All these factors demonstrate there is an unmet need which could potentially guide prescriptions of second-line interventions. In this NMA, we found there is no significant difference in the number of patients with progression on sorafenib. Unfortunately, we could not include pembrolizumab data where the hierarchical factor was AFP >200 which means the efficacy of pembrolizumab for patients with AFP >400 remains unknown.
It has been 12 years since Bayesian analytical techniques were formally presented, and the basic framework of this methodology has become more widely accepted and implemented by researchers.23 In general, network meta-analysis based on strictly global phase III RCTs can provide more convincing results, and given the rigour with which we designed this study there is only a small risk of bias. The results therefore enabled us to provide a comprehensive report focusing on second-line therapies for HCC and provide a reference for clinical decision-making by comparing and ranking different interventions. In addition, the results of this study may also serve as a reference for optimizing the design of future trials.
Despite the clear benefits of implementing this analytical strategy, this research has certain limitations. First, the inclusion criteria for each of the included studies will result in bias, perhaps skewing NMA results in some way. The RESORCE trial included the patients who progressed after previously being prescribed sorafenib but excluded those who were intolerant. The other trials included patients who were intolerant of sorafenib, which was reported to be between 7% and 15%. The population involved in the CELESTIAL trial included patients who had not only received previous first-line interventions but also second-line and perhaps other alternatives, equating to approximately 27%. Second, lesions were evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST; version 1.1) in the study of cabozantinib, ramucirumab and pembrolizumab, which is different from the evaluation of mRECIST used in the regorafenib study. Use of different evaluation tools is likely to have created some bias in treatment efficacy, not only limited to ORR but also in terms of progression-free survival. Finally, current research lacks head-to-head comparisons between drugs, therefore direct evidence for comparisons between these interventions cannot be obtained for in-depth statistical analysis.
Conclusion
Among the four second-line HCC therapies analysed, all reduced the risk of death compared with placebo although regorafenib and cabozantinib had significant advantages in overall survival. For patients with AFP >400, cabozantinib, regorafenib and ramucirumab appear to have similar efficacy, but ramucirumab has the fewest side effects. Pembrolizumab monotherapy has the highest ORR, but does not show obvious advantages in terms of efficacy and safety. Despite rigorous analysis of the included studies, these results still need to be verified through large prospective RCTs.
Supplemental Material
Supplemental material, Supplement for Comparing the efficacy and safety of second-line therapies for advanced hepatocellular carcinoma: a network meta-analysis of phase III trials by Dongxu Wang, Xu Yang, Jianzhen Lin, Yi Bai, Junyu Long, Xiaobo Yang, Samuel Seery and Haitao Zhao in Therapeutic Advances in Gastroenterology
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Ethics statement: All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and the Helsinki Declaration. There was no interaction with patients directly, as we acquired data from already published articles.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the International Science and Technology Cooperation Projects (2016YFE0107100), the Capital Special Research Project for Health Development (2014-2-4012), the Beijing Natural Science Foundation (L172055 and 7192158), the National Ten-thousand Talent Program, the Fundamental Research Funds for the Central Universities (3332018032), and the CAMS Innovation Fund for Medical Science (CIFMS) (2017-I2M-4-003 and 2018-I2M-3-001).
ORCID iD: Haitao Zhao  https://orcid.org/0000-0002-3444-8044
https://orcid.org/0000-0002-3444-8044
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Dongxu Wang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xu Yang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Jianzhen Lin, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Yi Bai, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Junyu Long, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xiaobo Yang, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Samuel Seery, Department of Humanities, Peking Union Medical College, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Haitao Zhao, Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
References
- 1. Villanueva A. Hepatocellular carcinoma. N Engl J Med 2019; 380: 1450–1462. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3. Schlachterman A, Craft WW, Jr, Hilgenfeldt E, et al. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol 2015; 21: 8478–8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018; 391: 1301–1314. [DOI] [PubMed] [Google Scholar]
- 5. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018; 391: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 6. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 7. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017; 389: 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marquardt JU, Saborowski A, Czauderna C, et al. The changing landscape of systemic treatment of advanced hepatocellular carcinoma: new targeted agents and immunotherapies. Target Oncol 2019; 14: 115–123. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015; 16: 859–870. [DOI] [PubMed] [Google Scholar]
- 11. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20: 282–296. [DOI] [PubMed] [Google Scholar]
- 12. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018; 379: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finn RS, Ryoo BY, Merle P, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2019; 37: 4004–4004. [Google Scholar]
- 14. Vitale A, Trevisani F, Farinati F, et al. Treatment of hepatocellular carcinoma in the precision medicine era: from treatment stage migration to therapeutic hierarchy. Hepatology. Epub ahead of print 18 February 2020. DOI: 10.1002/hep.31187. [DOI] [PubMed] [Google Scholar]
- 15. Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018; 15: 599–616. [DOI] [PubMed] [Google Scholar]
- 16. Kim JH, Kim BJ, Jang HJ, et al. Molecular targeted agents as second-line treatment for hepatocellular carcinoma: a meta-analysis and review. Oncotarget 2017; 8: 102321–102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bakouny Z, Assi T, El Rassy E, et al. Second-line treatments of advanced hepatocellular carcinoma: systematic review and network meta-analysis of randomized controlled trials. J Clin Gastroenterol 2019; 53: 251–261. [DOI] [PubMed] [Google Scholar]
- 18. Hulin A, Stocco J, Bouattour M. Clinical pharmacokinetics and pharmacodynamics of transarterial chemoembolization and targeted therapies in hepatocellular carcinoma. Clin Pharmacokinet 2019; 58: 983–1014. [DOI] [PubMed] [Google Scholar]
- 19. Wang D, Lin J, Yang X, et al. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J Hematol Oncol 2019; 12: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obi S, Sato T, Sato S, et al. The efficacy and safety of lenvatinib for advanced hepatocellular carcinoma in a real-world setting. Hepatol Int 2019; 13: 199–204. [DOI] [PubMed] [Google Scholar]
- 21. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol 2018; 69: 353–358. [DOI] [PubMed] [Google Scholar]
- 22. Uchikawa S, Kawaoka T, Aikata H, et al. Clinical outcomes of sorafenib treatment failure for advanced hepatocellular carcinoma and candidates for regorafenib treatment in real-world practice. Hepatol Res 2018; 48: 814–820. [DOI] [PubMed] [Google Scholar]
- 23. Li T, Puhan MA, Vedula SS, et al. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med 2011; 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplement for Comparing the efficacy and safety of second-line therapies for advanced hepatocellular carcinoma: a network meta-analysis of phase III trials by Dongxu Wang, Xu Yang, Jianzhen Lin, Yi Bai, Junyu Long, Xiaobo Yang, Samuel Seery and Haitao Zhao in Therapeutic Advances in Gastroenterology