Abstract
Postsurgical dental pain is mainly driven by inflammation, particularly through the generation of prostaglandins via the cyclooxygenase system. Thus, it is no surprise that numerous randomized placebo-controlled trials studying acute pain following the surgical extraction of impacted third molars have demonstrated the remarkable efficacy of nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, naproxen sodium, etodolac, diclofenac, and ketorolac in this prototypic condition of acute inflammatory pain. Combining an optimal dose of an NSAID with an appropriate dose of acetaminophen appears to further enhance analgesic efficacy and potentially reduce the need for opioids. In addition to being on average inferior to NSAIDs as analgesics in postsurgical dental pain, opioids produce a higher incidence of side effects in dental outpatients, including dizziness, drowsiness, psychomotor impairment, nausea/vomiting, and constipation. Unused opioids are also subject to misuse and diversion, and they may cause addiction. Despite these risks, some dental surgical outpatients may benefit from a 1- or 2-d course of opioids added to their NSAID regimen. NSAID use may carry significant risks in certain patient populations, in which a short course of an acetaminophen/opioid combination may provide a more favorable benefit versus risk ratio than an NSAID regimen.
Keywords: inflammation, randomized controlled clinical trials, opioid abuse, acute pain, prostaglandins, analgesics
Introduction—We Have a Problem
The prescription opioid misuse and abuse crisis put a bullseye on organized dentistry, with dentists previously having the dubious rank of #2 of all health care professionals prescribing these potentially addicting drugs (Denisco et al. 2011). More recently, our rank has fallen to the fifth most frequent prescribing group, which, while an improvement, remains a significant concern for the profession (Hersh et al. 2018).
A young adult’s first exposure to immediate-release opioid combinations containing acetaminophen plus codeine (i.e., Tylenol #3), hydrocodone (i.e., Vicodin), or oxycodone (i.e., Percocet) often occurs following the surgical removal of their impacted third molar teeth (Schroeder et al. 2019). Approximately 60-70% of these prescriptions are written by oral and maxillofacial surgeons (Gupta et al. 2018), while patients are still numb, and usually prescribed for the worst-case scenario (Moore et al. 2016), even when concomitant nonsteroidal anti-inflammatory drugs (NSAIDs) are the primary analgesic prescribed (Maughan et al. 2016). A significant number of these immediate-release opioid pills remain unused, setting the stage for diversion and misuse (Denisco et al. 2011; Maughan et al. 2016). One recent study reported a significantly higher rate of opioid abuse diagnosis 3 to 12 mo after dental surgical patients were given prescriptions for immediate-release opioid formulations (5.8%) compared to those that were not (0.4%) (Schroeder et al. 2019). Shockingly, a survey of nearly 50,000 high school students revealed that between 2001 and 2005, 10% to 11% of 12th graders admitted to experimenting with Vicodin (Friedman 2006).
This article will review the distinct mechanisms of action of NSAIDs and opioids, the evidence-based data clearly indicating that NSAIDs should be “the first-line analgesics” for acute postprocedural dental pain, strategies to reduce the use of potentially addicting opioid analgesics, and emerging research that may help predict an individual’s analgesic response to NSAIDs before the surgical procedure.
Mechanisms of Postsurgical Dental Pain and Its Relationship to Analgesic Efficacy
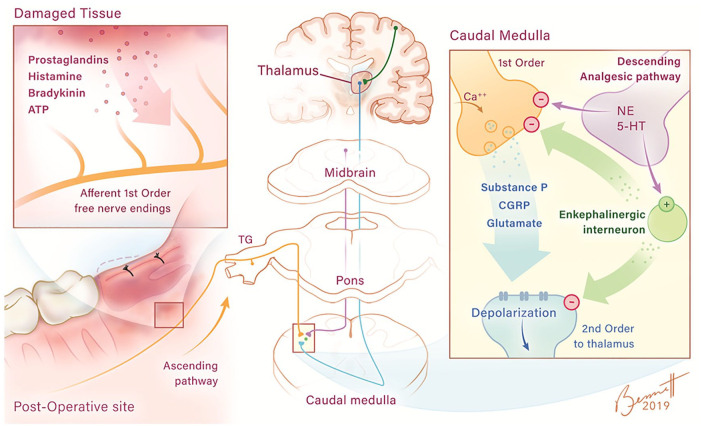
Dental postsurgical pain is mainly driven by inflammation, with cyclooxygenase-derived prostaglandins being key sensitizers of free nerve ending to other mediators of pain such as histamine, bradykinin, adenosine triphosphate (ATP), and low pH (Chen et al. 2013; Ji et al. 2016; Fig. 1). These free nerve endings also express other receptors such as transient receptor potential vallinoid (TRPV) ion channels, voltage-gated sodium (Nav) and calcium (VGCC) channels, and acid-sensing ion channels (ASICs) besides those specific for sensitizing or pain-provoking mediators. Activation of these receptors generates action potentials that travel along thinly myelinated A delta or unmyelinated C fibers, which make synaptic connections to second-order neurons in the dorsal horn of the spinal cord (Fig. 1). Chemicals released from the central terminals of free nerve endings include substance P and the excitatory amino acid glutamate, which cause the second-order neurons to depolarize and generate their own action potentials (Chen et al. 2013; Grosser et al. 2017; Raffa et al. 2017). Eventually, synaptic connections with supraspinal third-order and fourth-order neurons that reside in the thalamus, hippocampus, and cerebral cortex result in the physiologic aspects and emotional components of the pain response.
Figure 1.
Peripheral and central pain and pain control neuronal pathways. Pain-sensitizing (prostaglandins) and pain-provoking (histamine, bradykinin, and adenosine triphosphate [ATP]) molecules generated or released from damaged tissue from the impacted third molar surgical site induce depolarization and action potentials of A delta and C fiber free nerve endings (damaged tissue panel). These action potentials travel through these nerve fibers (shown in orange) toward the central nervous system (CNS) through the trigeminal ganglion (TG) entering the CNS at the level of the pons. The fibers then descend on the ipsilateral side to the medulla where they make their first synapse with the second-order neuron (shown in blue). The influx of calcium at the central terminal of the first-order neuron causes the release of peptides, including substance P, calcitonin gene-related peptide (CGRP), and the amino acid glutamate (caudal medulla panel), which depolarizes the second-order neuron, which crosses to the contralateral side of the medulla. These nerve fibers ascend to higher levels of the CNS, including the thalamus, where they synapse with third-order neurons. These third-order neurons then send projections to the cerebral cortex, where the pain suffering is perceived, and to other regions of the CNS such as the hippocampus (not shown in diagram). Pain-reducing neuronal pathways (shown in purple) originate in midbrain and descend and synapse, releasing norepinephrine (NE) or serotonin (5-HT) with enkephalinergic interneurons. The enkephalinergic interneurons subsequently synapse on the central terminal of the primary afferent neuron and the dendrites of the second-order neuron with the neuropeptides met and leu enkephalin being released. It is thought that enkephalins induce a reduction of calcium influx in the first-order neuron and an enhancement of potassium efflux at the second-order site, resulting in a reduction of substance P, CGRP, and glutamate release in the former and hyperpolarization of the latter. Illustration by Brittany C. Bennett, MA.
While the surgical trauma of third molar removal involves both soft tissue and bone, the wound surface is relatively small compared to other medical surgical procedures. However, a marked increase in prostaglandins can be measured both locally (Roszkowski et al. 1997; Gordon, Brahim, Rowan, et al. 2002) and systemically (Theken et al. 2019). The generation of prostaglandins and other pain-sensitizing or pain-provoking mediators in the central nervous system (CNS) also augments pain transmission (Chen et al. 2013; Ji et al. 2016; Grosser et al. 2017; Fig. 1). Since traditional NSAIDs block both cyclooxygenase isoforms (COX-1 and COX-2) and the ultimate generation of prostaglandins (Vane and Botting 1995), the remarkable efficacy and safety of currently marketed NSAIDs from a mechanistic standpoint in treating postsurgical dental pain should not be surprising.
While it is now appreciated as overly simplistic to classify COX-1 as solely the constitutive COX isoform responsible for producing prostaglandin products involved in many homeostatic processes such as gastrointestinal (GI) cytoprotection (i.e., PGE2) and platelet aggregation (i.e., thromboxane A2), and COX-2 as solely the upregulated isoform producing prostaglandin products involved in pain and inflammation (i.e., PGE2 and prostacyclin), it is still a good way to “pigeonhole” NSAIDs with regard to physiological activity and potential adverse effects (Hersh et al. 2005; Fig. 2). For example, ketorolac, being approximately 300-fold more selective at blocking COX-1 versus COX-2, is the most ulcerogenic of all marketed NSAIDs, and this explains why it is only employed for acute pain of no more than a 5-d duration. By contrast, celecoxib is approximately 8-fold selective for blocking the COX-2 isoform and has often been employed for chronic inflammatory conditions such as osteoarthritis because of its lower risk of GI ulcerations and bleeds compared to traditional NSAIDs such as naproxen. However, celecoxib with chronic use carries a higher risk of precipitating myocardial infarctions and strokes, particularly in patients with preexisting cardiovascular morbidities. Its relatively unopposed COX-2 blockade dampens the vasodilatory and antiplatelet effects of prostacyclin while allowing the COX-1 product thromboxane A2 to exert its proaggregatory and vasoconstrictive effects (Fig. 2). More detailed descriptions of NSAID mechanisms and COX isoforms can be found in other publications (Chen et al. 2013; Ji et al. 2016; Grosser et al. 2017).
Figure 2.
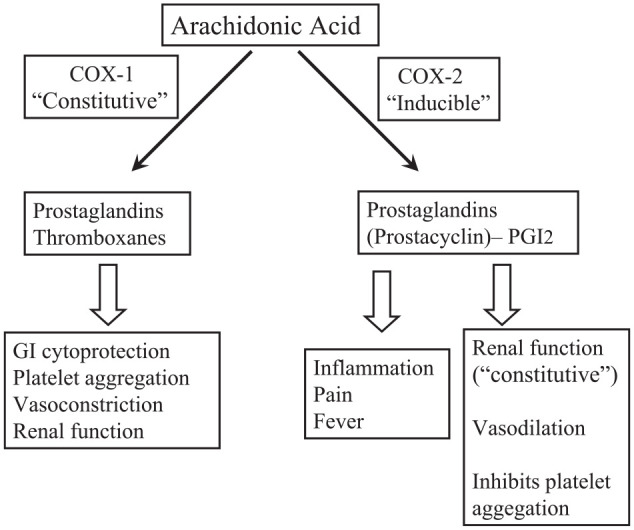
Some physiologic roles of cyclooxygenase (COX) isoforms and their products. Note the opposing cardiovascular effects of COX-1 and COX-2 products. Ketorolac, being 300-fold more selective for blocking COX-1, has a higher potential than other nonsteroidal anti-inflammatory drugs (NSAIDs) of inducing gastrointestinal (GI) bleeding and ulceration. Celecoxib, by being 8-fold selective for blocking COX-2, produces less GI toxicity but greater cardiovascular risk than other NSAIDs. With permission from Hersh et al. (2005).
Acetaminophen is a relatively weak inhibitor of both cyclooxygenase enzymes that, unlike ibuprofen, naproxen, or diclofenac, does not block the substrate binding channel of the enzymes but disrupts electron transfer within the catalytic center (Aronoff et al. 2006). This feature may allow acetaminophen to work more downstream than traditional NSAIDs in the arachidonic acid/cyclooxygenase cascade. It is this mechanistic difference in biochemical inhibition of the cyclooxygenase enzyme that provides the rationale for combining ibuprofen with acetaminophen to produce a synergistic effect (Mehlisch et al. 2010; Daniels et al. 2011). There may also be additional central mechanisms contributing to the actions of this drug, including the activation of cannabinoid or descending serotonergic systems (Moore and Hersh 2013).
The actions of opioid analgesic medications resemble those of endogenous opioid peptides—namely, endorphins, enkephalins, dynorphins, and endomorphins (Raffa et al. 2017)—and these peptides show some preferential binding to specific opioid receptors: µ, β-endorphin; δ, enkephalins; and κ, dynorphin (Benarroch 2012; Stein 2016). They are located throughout the CNS, on peripheral nociceptors, and in intestinal smooth muscle (Benarroch 2012). A fourth receptor has recently been described and termed the ORL-1 or “opioid receptor-like” orphanin receptor (Dietis et al. 2011), where its upregulation may play an important role in the recently described phenomena of opioid-induced hyperalgesia, when patients legitimately or abusively on chronic opioids exhibit lower thresholds to pain stimuli (Colvin et al. 2019). Major mechanisms of action of both endogenous and exogenous opioids include an inhibition of substance P and excitatory amino acid release from the primary afferent neuron in the dorsal horn of the spinal cord and the medulla, hyperpolarization of central postsynaptic neurons via enhanced potassium efflux, and activation of descending analgesic pathways that release endogenous opiates, norepinephrine, and serotonin in various locations in the CNS (Fig. 1). Peripheral opioid receptors are also transiently upregulated in the presence of inflammation. This process depends on both neuronal activity and the production of proinflammatory cytokines, nerve growth factor, and bradykinin (Benarroch 2012; Stein 2016). Thus, the free nerve endings themselves may represent an additional site of both endogenous and exogenous opioid analgesic action, although their expression in the periphery is probably more clinically relevant with chronic inflammatory pain and not acute postsurgical dental pain.
A Look at the Data
The fact that NSAIDs should be highly effective in treating pain after the surgical removal of impacted third molars because of the inflammatory nature of the pain is supported by the analysis of 460 randomized clinical trials involving about 50,000 patients undergoing third molar extractions (Moore et al. 2015b). Many of these studies can be considered landmark investigations in the evaluation of analgesics for acute inflammatory pain (Cooper et al. 1977, 1982, 1989; Dionne and Cooper 1978; Forbes et al. 1983; Cooper 1984, 1988; Giglio and Campbell 1986; Hersh et al. 1993, 2000, 2004; Dionne et al. 1994; Kiersch et al. 1994). However, the translation of this information to clinical practice has been exceedingly slow and fraught with resistance to relinquishing traditional practices (Moore and Hersh 2013). Unfortunately, it is often state regulations, most recently the mandatory use of prescription drug monitoring programs (PDMPs), that ultimately drive dental professionals to more rational pharmacotherapeutic decisions with regard to acute pain control (Rasubala et al. 2015) and not evidence-based data, the majority of which has been in the literature for decades.
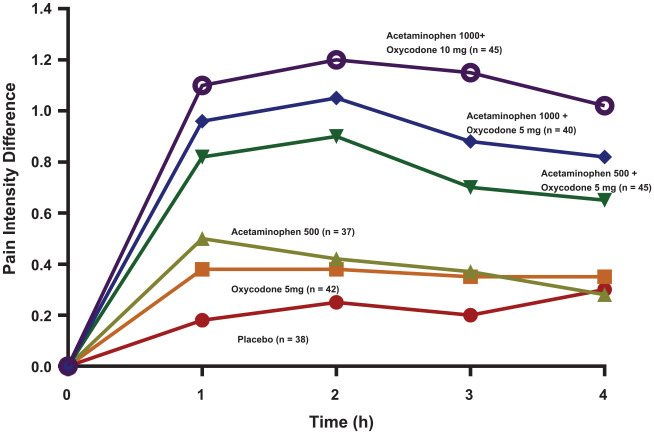
Until recently, immediate-release opioid combinations also containing acetaminophen were “the reflexive choice” by a majority of practitioners to control pain after the removal of impacted third molar teeth (Moore et al. 2006). The most commonly employed opioids were oxycodone, hydrocodone, codeine, and tramadol, with 85% of oral surgeons responding that they “almost always” prescribed opioids following this procedure (Moore et al. 2006). While recent research suggests that these drugs may possess a peripheral anti-inflammatory effect that is distinct from COX inhibition (Benarroch 2012; Stein 2016), as single entities (not combined with acetaminophen or ibuprofen), they perform miserably in the control of postsurgical dental impaction pain (Cooper et al. 1980, 1982; Mehlisch 1998), with 5 mg oxycodone being equianalgesic to an over-the-counter (OTC) dose of acetaminophen 500 mg (Cooper et al. 1980; Fig. 3) or no better than placebo (Van Dyke et al. 2004), 60 mg of codeine being inferior to aspirin 650 mg (Cooper et al. 1982; Fig. 4), and tramadol 100 mg only marginally more effective than placebo (Mehlisch 1998). In fact, even an orally administered 60-mg dose of immediate-release morphine (which is twice the typical dose employed for break-through cancer pain in an opioid-naive patient) is inferior to ibuprofen 400 mg in this acute pain model (Kleinert et al. 2008).
Figure 3.
Time-action curves of mean pain intensity difference scores following dental impaction surgery. Pain intensity was scored as 0 = none, 1 = slight, 2 = moderate, and 3 = severe. Pain intensity difference scores are calculated as baseline pain intensity minus the pain intensity at that time point following study drug administration. Research patients were not allowed to ingest study medication until their pain had reached a moderate (2) or severe intensity (3). Adapted with permission from Cooper et al. (1980).
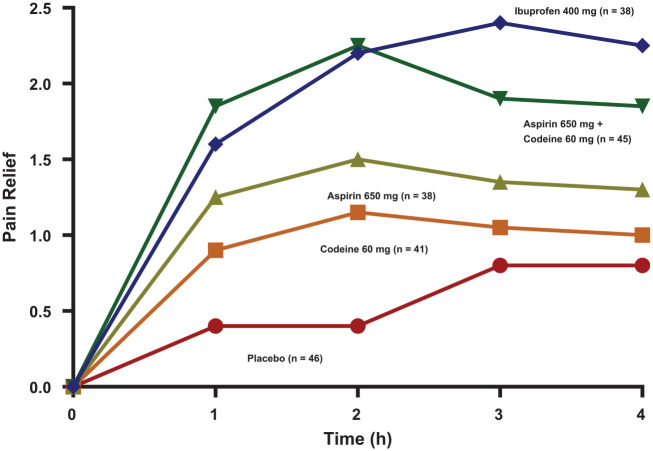
Figure 4.
Time-action curves of mean pain relief scores of various treatments following dental impaction surgery. Research patients were not allowed to ingest study medication until their pain had reached a moderate or severe intensity. Pain relief after study drug intake was scored as 0 = none, 1 = a little, 2 = some, 3 = a lot, and 4 = complete. Adapted with permission from Cooper et al. (1982).
While it is beyond the scope of this article to speak about each individual study in detail, a few will be highlighted here. Cooper et al. (1982) were first to demonstrate that ibuprofen 400 mg was at least as efficacious as aspirin 650 mg plus codeine 60 mg, which is a full therapeutic dose of this combination (Fig. 4). The term full therapeutic dose is an important concept because clinicians often prescribe suboptimal doses of these opioid combination drugs. For example, common prescriptions read “take one or two Tylenol #3s (acetaminophen 300 mg plus codeine 30 mg) as needed for pain.” Clinical research reveals that in postsurgical dental pain, 1 Tylenol #3 is actually slightly inferior to an OTC dose of 600 mg acetaminophen (Cooper 1984). So, the clinician may be compounding the problem by prescribing a dose of this opioid combination that is potentially addicting but not sufficient to relieve the pain in a majority of patients. Meta-analysis data of randomized, placebo-controlled dental impaction postsurgical pain trials reveal a number needed to treat to achieve benefit (NNTB) of between 4 and 10 (95% confidence intervals) for acetaminophen 300 mg plus codeine 30 mg. This means between 4 and 10 patients would need to be treated with this drug to obtain 1 patient with at least a 50% maximum total pain relief (TOTPAR) score. TOTPAR is simply the sum of the individual time-weighted pain relief scores at each observation point where 0 = no pain relief, 1 = a little pain relief, 2 = some pain relief, 3 = a lot of pain relief, and 4 = complete pain relief. In a 4-h study, this theoretical maximum for an individual patient would be a 16 assuming that he or she reported complete pain relief at each observation point. Therefore, in a 4-h study, a TOTPAR score of at least 8 would need to be obtained to declare that the individual benefited from the treatment (Barden et al. 2004; Fig. 5). Mean NNTB represents a treatment-specific effect and can be calculated as
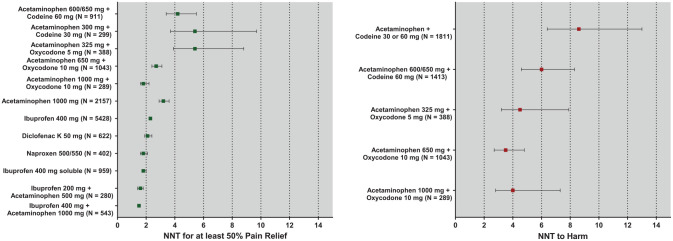
Figure 5.
Numbers needed to treat (NNT) to obtain benefit and harm of selected analgesics. Benefit is defined as a patient reaching at least 50% of the maximum theoretic total pain relief (TOTPAR) score, which is 16 (50% max = 8) or 24 (50% max = 12) in a 4- or 6-h study, respectively. Harm is defined as a reported or observed side effect. Adapted from data presented by Barden et al. (2004), Derry et al. (2011), and Moore et al. (2015a, 2015b).
The best theoretical NNTB would be a 1, which never occurs because 100% of research subjects would have to obtain benefit from the active treatment, with 0% showing benefit from the placebo treatment. Probably a maximum theoretical NNTB of 1.25 is more realistic in dental postsurgical pain trials because roughly 20% of placebo patients do obtain this benefit (Hersh et al. 2004). Still, the lower the NNTB in these meta-analyses, the more efficacious the analgesic. A number need to treat to do harm (NNTH) is calculated by substituting the percentage being harmed (experiencing a side effect) by the active treatment or placebo or
Moore et al. 2018). Figure 5 clearly shows that full therapeutic doses of NSAIDs (ibuprofen 400 mg, naproxen sodium 550 mg, diclofenac 50 mg) are more efficacious than acetaminophen/codeine combinations, and combining ibuprofen with acetaminophen appears to provide additional benefit beyond the NSAID alone (Barden et al. 2004; Moore et al. 2015b). In addition, Figure 5B demonstrates that the drugs with significantly lower NNTHs than placebo are all immediate-release opioid preparations (Moore et al. 2015a; Moore et al. 2018). With these opioid preparations, the NNTB and NNTH are very close to one other, meaning that for every patient experiencing substantial pain relief, there is also likely to be one that also experiences an adverse effect, certainly not indicative of a favorable benefit to risk ratio.
When treating postsurgical dental pain, opioids only differ in potency and not efficacy, where oxycodone 5 mg = hydrocodone 10 mg = codeine 60 mg or tramadol 75 mg (Hersh et al. 2007), and none of these opioids, when used as the sole analgesic, demonstrate substantial efficacy in treating pain following impacted wisdom teeth surgery (Cooper et al. 1980, 1982; Mehlisch 1998), again indicative of the inflammatory nature of postsurgical dental pain. Only when these opioids are combined with aspirin, acetaminophen, or ibuprofen and other NSAIDs is substantial efficacy demonstrated and only when a full therapeutic dose of acetaminophen or the NSAID is employed (Cooper et al. 1980, 1982; Moore et al. 1998; Van Dyke et al. 2004). Rapid dispersible or solubilized formulations of NSAIDs provide greater blood levels and a more rapid time to peak blood levels than equivalent doses of tablet formulations (Hersh et al. 2000, 2004; Brain et al. 2015), which may translate into enhanced efficacy (Fig. 5). However, the marketed formulations of these entities have not been compared to opioid combination drugs, one reason being that 2 of the widely selling faster-acting ibuprofen formulations described above (liquid gels and sodium film tabs) are solely distributed for OTC use. OTC drug manufacturers are prohibited from making marketing claims against prescription analgesics, so the appropriate studies comparing these rapid-release formulations of NSAIDs to acetaminophen/opioid combination drugs are never initiated or funded by the drug manufacturers.
Patterns of Use, Drug Interactions, and Side Effects
Dental impaction surgery patients are almost always young healthy adults, and analgesics are only needed for a maximum of 3 to 5 d (Hersh et al. 1993). In fact, the clinical research studies on the effectiveness and tolerability of analgesic agents typically exclude patients with a variety of medical conditions and drug intake (with the exception of contraceptive agents and sometimes prophylactic antibiotics). However, young healthy adults are not the only patient population taking NSAIDs for postsurgical dental pain. These drugs should be avoided in patients with aspirin- or NSAID-sensitive asthma because the blockade of COX can shift the arachidonic acid pathway toward the production of bronchoconstrictive and proinflammatory lipoxygenase products (leukotrienes) in these individuals, patients with true allergies to these drugs, patients with a history of gastrointestinal ulcers, patients on oral anticoagulants including warfarin and the non–vitamin K–dependent novel anticoagulant drugs because of the additive antiplatelet effects of NSAIDs, and patients taking lithium because NSAIDs inhibit the active secretion of this widely employed bipolar depression drug (Hersh et al. 2007; Hersh and Moore 2015). While working by completely different mechanisms, a commonality between oral anticoagulants and lithium is their low therapeutic index (Hersh et al. 2007; Hersh and Moore 2015). So NSAIDs that can increase free blood levels of these drugs (i.e., warfarin and lithium) or have additive effects on their pharmacodynamic action (i.e., anticoagulants) pose a danger of inducing a serious adverse drug-drug interaction. Thus, for some patients, acetaminophen/opioid combination drugs are better choices than NSAIDs from a benefit versus risk ratio.
While meta-analysis data reveal that short-term use of NSAIDs (10 d or less), especially in the OTC dosage range, is usually very well tolerated with patient-reported side effect profiles no different from placebo (Kellstein et al. 1999; Bansal et al. 2001), the same cannot be said for drugs containing opioids. Drowsiness, dizziness, psychomotor impairment, nausea, and vomiting are prominent with short-term use of these drugs (Cooper et al. 1980; Zuniga et al. 2019). One recent study reported a 21% incidence of vomiting with a 24-h course of acetaminophen 325 mg plus hydrocodone 7.5 mg (Zuniga et al. 2019). Stimulation of the chemoreceptor trigger zone is responsible for the emetic activity of opioids while most other adverse effects can be explained by their generalized CNS depressant effects. The well-described constipating effects of opioids are mediated via stimulation of mu opioid receptors in the intestinal tract, resulting in decreased peristalsis.
There are additional pharmacogenetic issues with codeine and tramadol. Codeine is fully a prodrug or an inactive parent molecule, while tramadol is partly one with regard to its pharmacologic activity, and their demethylation (codeine to morphine and tramadol to desmethyltramadol) via CYP2D6 is important for their therapeutic and adverse event profile (Hersh et al. 2007). So-called poor metabolizers of these drugs could exhibit a reduced therapeutic effect while extensive metabolizers may exhibit an increased incidence of adverse events, including respiratory depression reported in children, which has resulted in the strongest US Food and Drug Administration (FDA) warnings to avoid codeine and tramadol in patients younger than 12 y and 18 y, respectively (US Food and Drug Administration 2017).
A Few Words about Opioid Abuse and Addiction
As stated early in this article, the first experience many patients have with prescription opioids is following dental impaction surgery, and they are often prescribed many excess pills, which increases the risk of misuse, abuse, and addiction (Denisco et al. 2011; Moore et al. 2016; Maughan et al. 2016). It has been estimated that 85% to 90% of opioid-naive individuals find the intake of these drugs to be unpleasant (Hersh et al. 2018) because of their side effects (Cooper et al. 1980; Zuniga et al. 2019). However, up to 15% are at risk for misuse, abuse, and addiction because of the euphoria they create. While the major cause of death from opioid overdose is respiratory depression, those recreationally consuming excessive amounts of immediate-release acetaminophen/opioid combinations are also at risk for permanent liver damage from the acetaminophen component—one reason why prescription opioid abusers will transition to heroin (Hersh et al. 2018). One study showed that those individuals who abuse prescription opioids are 40-fold more likely than the general public to be using injectable heroin the following year (Compton et al. 2016). Injectable and inhaled heroin, while lacking the potential hepatoxic acetaminophen component, possesses an even greater risk than oral opioids of unconsciousness, respiratory depression, and death because of the higher peak blood levels of opioid they typically produce. Heroin also is frequently laced with more potent synthetic opioids of the fentanyl class, which increases the risk of severe adverse effects and death incalculably.
Opioid tolerance refers to the need of increasing quantities of drug over time to get the same pharmacological effects, whether they be therapeutic (analgesia) or toxic (respiratory depression). In the case of opioids, tolerance is thought to be pharmacodynamic in nature, involving a downregulation of various opioid receptor subtypes. Interestingly, 2 physiologic events produced by opioids where tolerance does not occur are ability of these drugs to cause pupillary constriction (miosis) and constipation (Raffa et al. 2017). Physical dependence implies that upon abrupt discontinuation of the drug or exposure to an antagonist of that drug (in this case naloxone or naltrexone), an abstinence or withdrawal syndrome will occur. Withdrawal syndromes are always in the opposite direction of the drug’s effect. Since opioids are CNS depressants, analgesic, and constipating and produce miosis, withdrawal is often manifested by anxiety, elevated blood pressure, joint and muscle pain, diarrhea, and pupillary dilation (mydriasis). The term opioid addiction or psychological dependence has been replaced by opioid misuse disorder by many in the field. Individuals will knowingly behave in a way that is averse to themselves or others to obtain the drug (i.e., criminal behavior to obtain money to purchase the drug) or knowingly put themselves and others at risk after self-administrating the drug (i.e., sharing needles to inject heroin with the well-known risks of contracting hepatitis B, hepatitis C, and human immunodeficiency virus or, in our area of expertise, practicing dentistry while impaired by alcohol, benzodiazepines, or opioids) (Denisco et al. 2011). While it is well known that many drugs of abuse induce euphoria via enhanced dopaminergic transmission in the brain (Raffa et al. 2017), the exact mechanisms resulting in the tragic behavior patterns of opioid misuse remain elusive, and a thorough discussion of this topic is beyond the scope of the current article.
Beyond NSAIDs as Needed for Pain: Additional Opioid-Sparing Strategies
Pivotal FDA phase 2 or phase 3 analgesic clinical trials typically explore the action of drugs during the worst-case scenario—that is, waiting for the local anesthetic to dissipate and then dose patients only when their pain intensity reaches a moderate to severe level. “Chasing pain” is not the best way to employ NSAIDs for more invasive surgical procedures (Moore and Hersh 2013). Well-designed studies published more than 30 y ago have demonstrated that presurgical ibuprofen 400 mg and flurbiprofen 100 mg significantly delayed the onset of postoperative pain compared to placebo and acetaminophen 650 mg plus oxycodone 10 mg (2 Percocet), respectively (Dionne and Cooper 1978; Dionne 1986). The rationale behind this approach is to reach therapeutic blood levels of the NSAID before the surgical trauma generates various prostaglandins. NSAIDs inhibit prostaglandin synthesis but do not attenuate the response to prostaglandins once they have been formed. Their preemptive use either an hour before surgery or immediately after surgery when local anesthesia has yet to dissipate, then prescribing around-the-clock for up to 3 d, represents an optimal strategy to employ these drugs (Dionne and Cooper 1978; Moore and Hersh 2013). Combining ibuprofen 200 mg and 400 mg with acetaminophen 500 mg or 1,000 mg, respectively, enhances analgesic effectiveness compared to either drug alone (Mehlisch et al. 2010; Daniels et al. 2011) and could attenuate the need for an additional short course of an opioid combination drug (Moore and Hersh 2013). However, we suggest that the combination of ibuprofen 400 mg plus acetaminophen 1,000 mg be used with caution. While average pain relief curves produced by this combination are in fact exceptional (Mehlisch et al. 2010; Daniels et al. 2011), with durations of effect in the 6- to 8-h range, individual patients within these mean pain relief curves require rescue analgesic intake within 4 h. With multidosing typical after impacted third molar surgery (Hersh et al. 1993), it is likely that supratherapeutic dosing of acetaminophen would occur in some patients. Therefore, it has been recommended that a maximum acetaminophen dose of 500 mg be employed to limit potential hepatotoxicity when combined with 400 mg to 600 mg ibuprofen (Moore and Hersh 2013).
The use of injectable 0.5% bupivacaine plus 1:200,000 epinephrine at the completion of third molar surgery appears to have a prolonged analgesic effect well beyond its duration of action, possibly by reducing central sensitization caused by afferent barrages of pain fibers (Gordon, Brahim, Dubner, et al. 2002). In this scenario, the clinician could commence analgesic dosing while the actions of this long-acting local anesthetic are still in effect. A liposomal formulation of bupivacaine (Exparel) is now clinically available and is indicated solely for infiltration use around the surgical wound site. The FDA considers a third molar extraction site to fall within this realm. Well-designed and controlled dental impaction studies are needed to justify the expense (30-fold higher on a volume basis than an equivalent amount of 0.5% bupivacaine plus 1:200,000 epinephrine) of this new strategy (Lieblich and Danesi 2017).
Personalized Medicine: Predicting an Individual’s Analgesic Response before Surgery
The preceding comments have mainly considered average pain relief or average pain intensity difference scores generated in pivotal randomized placebo-controlled analgesic trials. However, it has already been emphasized that individual patient variation should be considered when dosing with the combination of NSAIDs plus acetaminophen. Knowledge of variation in individual patient responses in clinical practice has stimulated research in the expanding field of personalized medicine (Theken et al. 2019). Even with the very best analgesic therapies, there are always nonresponders or partial responders (Theken 2018; Theken et al. 2019). Identifying biomarkers such as pain coping skills, previous experience with analgesics, cytochrome P450 isomers important in NSAID metabolism, the oral and GI microbiome that may affect the metabolic disposition of the drug and inflammation itself, inherent COX activity, and genomics prior to surgery may help us identify those 80% to 85% of individuals who will have their postsurgical pain adequately managed by just an NSAID or an NSAID/acetaminophen combination and those 15% to 20% of individuals who legitimately require an opioid combination drug in addition to their NSAID regimen. For example, it has recently been reported that in patients with moderate to severe pain following dental impaction surgery, those individuals exhibiting the greatest levels of urinary prostaglandin metabolites postsurgery were also the very same patients who were classified as complete analgesic responders to ibuprofen sodium film tabs 400 mg, defined as their lack of need for immediate-release acetaminophen plus hydrocodone within 4 h of ibuprofen dosing (Theken et al. 2019). This enhanced efficacy of ibuprofen in patients with greater prostaglandin levels is consistent with an enhanced role of COX-mediated inflammation in this subgroup of patients. While these urinary prostaglandin metabolites represent a postsurgical biomarker of future NSAID analgesic response, research in the presurgical expression of biomarkers, whether they be behavioral, functional (functional magnetic resonance imaging), microbial, or genetic, remains a continuing focus of orofacial pain research.
Conclusion
Dental postsurgical pain is primarily driven by inflammation, with the generation of prostaglandins in the periphery and CNS being key components in initiating and propagating the pain experience. NSAIDs including ibuprofen, naproxen sodium, and diclofenac, when prescribed at full therapeutic doses, are more effective analgesics than single-entity immediate-release opioid formulations of codeine, oxycodone, or morphine for treating pain after the surgical removal of impacted third molar teeth. In addition, meta-analysis data demonstrate that in oral surgery pain, ibuprofen 400 mg is more effective than acetaminophen 600 mg plus codeine 60 mg and at least as effective as acetaminophen 650 mg plus oxycodone 10 mg. Drugs containing opioids induce a much higher incidence of acute side effects related to the CNS and gastrointestinal motility than do NSAIDs. There is no doubt that the overuse of prescription drugs containing opioids has been a major contributor to the opioid abuse crisis, and more often than not, a potentially vulnerable young patient’s first exposure to an opioid is a prescription following the removal of impacted third molar teeth. While strategies to further reduce opioid prescribing, such as preemptive NSAIDs, the administration of the long-acting local anesthetic solution 0.5% bupivacaine plus 1:200,000 epinephrine, and combining an NSAID with acetaminophen 500 mg, appear to accomplish this goal, there are patients, because of inadequate pain relief or with a contraindication to NSAIDs, who will legitimately require a short course of an acetaminophen/opioid combination product. A potential holy grail that needs to be further explored is the personalization of analgesic therapy for each patient. The identification of key biomarkers prior to surgery that will predict analgesic response in the individual patient will not only improve a patient’s experience postoperatively but can become another strategy of reducing prescriptions for opioids.
Author Contributions
E.V. Hersh, K.N. Theken, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; P.A. Moore, T. Grosser, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript; R.C. Polomano, contributed to design, data analysis, and interpretation, critically revised the manuscript; J.T. Farrar, contributed to design, data acquisition, and interpretation, critically revised the manuscript; M. Saraghi, C.H. Mitchell, contributed to conception, design, data analysis, and interpretation, critically revised the manuscript; S.A. Juska, contributed to design and data analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
This work was supported by a Precision Medicine Accelerator Grant from the University of Pennsylvania School of Medicine (T. Grosser, K.N. Theken, E.V. Hersh and J.T Farrar), a National Institutes of Health (NIH)/National Institute on Drug Abuse Pain Consortium Grant (N01DA-15-4429, J.T Farrar, R. Polomano and E.V. Hersh), and a Health Resources and Services Administration Grant (D88HP28508, P.A. Moore).
Over the past 15 years, E.V. Hersh has received funding from Charleston Laboratories, Pfizer Consumer Healthcare, and AAI International and consulting fees from Johnson & Johnson and Bayer Pharmaceuticals. In the last 20 years P.A. Moore has served as a research consultant for several pharmaceutical companies, including Dentsply Pharmaceutical, Kodak Dental Systems, Septodont USA, St Renatus, Novalar Inc, and Novocol of Canada Inc. T. Grosser reports receiving consulting fees from Bayer Healthcare, Novartis, Plx Pharma and Aralez Pharmaceuticals and has received funding from the National Heart, Lung and Blood Institute (HL117798). J.T. Farrar has received research grants and contracts from the US Food and Drug Administration and the NIH; consulting fees from Analgesic Solutions, Aptinyx, Biogen, Opioid Post-marketing Consortium, Daiichi Sankyo, DepoMed, Evadera, Jansen, Lilly, Novartis, Vertex, and Pfizer; and DSMB services from NIH-NIA and Cara Therapeutics. R.C. Polomano has received research grants and contracts from the NIH and an honorarium from AcelRx Pharmaceuticals for an educational program. M. Saraghi is the Dental Prescribing Practices Subject Matter Expert to the NYS Dept of Health, the latter is the recipient of a HRSA Grant to States to Support Oral Health Workforce Activities (T12HP30337). C.H. Mitchell is currently funded by the NIH (EY013434 and EY015537) and Astra Zeneca. S.A. Juska and K.N. Theken have no potential conflicts to report. The authors declare no further potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aronoff DM, Oates JA, Boutaud O. 2006. New insights into the mechanism of action of acetaminophen: its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clin Pharmacol Ther. 79(1):9–19. [DOI] [PubMed] [Google Scholar]
- Bansal V, Dex T, Proskin H, Garreffa S. 2001. A look at the safety profile of over-the-counter naproxen sodium: a meta-analysis. J Clin Pharmacol. 41(2):127–138. [DOI] [PubMed] [Google Scholar]
- Barden J, Edwards JE, McQuay HJ, Wiffen PJ, Moore RA. 2004. Relative efficacy of oral analgesics after third molar extraction. Br Dent J. 97(7):407–411. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. 2012. Endogenous opioid systems: current concepts and clinical correlations. Neurology. 79(8):807–814. [DOI] [PubMed] [Google Scholar]
- Brain P, Leyva R, Doyle G, Kellstein D. 2015. Onset of analgesia and efficacy of ibuprofen sodium in postsurgical dental pain: a randomized, placebo-controlled study versus standard ibuprofen. Clin J Pain. 31(5):444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang G, Grosser T. 2013. Prostanoids and inflammatory pain. Prostaglandins Other Lipid Mediat. 104–105:58–66. [DOI] [PubMed] [Google Scholar]
- Colvin LA, Bull F, Hales TG. 2019. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 393(10180):1558–1568. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT. 2016. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 374(2):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SA. 1984. Five studies on ibuprofen for postsurgical dental pain. Am J Med. 77(1A):70–77. [DOI] [PubMed] [Google Scholar]
- Cooper SA. 1988. Ketoprofen in oral surgery pain: a review. J Clin Pharmacol. 28(Suppl 1):S40–S46. [DOI] [PubMed] [Google Scholar]
- Cooper SA, Engel J, Ladov M, Precheur H, Rosenheck A, Rauch D. 1982. Analgesic efficacy of an ibuprofen-codeine combination. Pharmacotherapy. 2(3):162–167. [DOI] [PubMed] [Google Scholar]
- Cooper SA, Needle SE, Kruger GO. 1977. Comparative analgesic potency of aspirin and ibuprofen. J Oral Surg. 35(11):898–903. [PubMed] [Google Scholar]
- Cooper SA, Precheur H, Rauch D, Rosenheck A, Ladov M, Engel J. 1980. Evaluation of oxycodone and acetaminophen in treatment of postoperative dental pain. Oral Surg Oral Med Oral Pathol. 50(6):496–501. [DOI] [PubMed] [Google Scholar]
- Cooper SA, Schachtel BP, Goldman E, Gelb S, Cohn P. 1989. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 29(11):1026–1030. [DOI] [PubMed] [Google Scholar]
- Daniels SE, Goulder MA, Aspley S, Reader S. 2011. A randomised, five-parallel-group, placebo-controlled trial comparing the efficacy and tolerability of analgesic combinations including a novel single-tablet combination of ibuprofen/paracetamol for postoperative dental pain. Pain. 152(3):632–642. [DOI] [PubMed] [Google Scholar]
- Denisco RC, Kenna GA, O’Neil MG, Kulich RJ, Moore PA, Kane WT, Mehta NR, Hersh EV, Katz NP. 2011. Prevention of prescription opioid abuse: the role of the dentist. J Am Dent Assoc. 142(7):800–810. [DOI] [PubMed] [Google Scholar]
- Derry S, Wiffen PJ, Moore RA. 2011. Relative efficacy of oral analgesics after third molar extraction—a 2011 update. Br Dent J. 211(9):419–420. [DOI] [PubMed] [Google Scholar]
- Dietis N, Rowbotham DJ, Lambert DG. 2011. Opioid receptor subtypes: fact or artifact? Br J Anaesth. 107(1):8–18. [DOI] [PubMed] [Google Scholar]
- Dionne RA. 1986. Suppression of dental pain by the preoperative administration of flurbiprofen. Am J Med. 80(3A):41–49. [DOI] [PubMed] [Google Scholar]
- Dionne RA, Cooper SA. 1978. Evaluation of preoperative ibuprofen for postoperative pain after removal of third molars. Oral Surg Oral Med Oral Pathol. 45(6):851–856. [DOI] [PubMed] [Google Scholar]
- Dionne RA, Snyder J, Hargreaves KM. 1994. Analgesic efficacy of flurbiprofen in comparison with acetaminophen, acetaminophen plus codeine, and placebo after impacted third molar removal. J Oral Maxillofac Surg. 52(9):919–924. [DOI] [PubMed] [Google Scholar]
- Forbes JA, Kolodny AL, Beaver WT, Shackleford RW, Scarlett VR. 1983. A 12-hour evaluation of the analgesic efficacy of diflunisal, acetaminophen, and acetaminophen-codeine combination, and placebo in postoperative pain. Pharmacotherapy. 3(2 Pt 2):47S–54S. [PubMed] [Google Scholar]
- Friedman RA. 2006. The changing face of teenage drug abuse—the trend toward prescription drugs. N Engl J Med. 354(14):1448–1450. [DOI] [PubMed] [Google Scholar]
- Giglio JA, Campbell RL. 1986. Comparison of etodolac, zomepirac, and placebo for relief of pain after oral surgery. J Oral Maxillofac Surg. 44(10):765–770. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Brahim JS, Dubner R, McCullagh LM, Sang C, Dionne RA. 2002. Attenuation of pain in a randomized trial by suppression of peripheral nociceptive activity in the immediate postoperative period. Anesth Analg. 95(5):1351–1357. [DOI] [PubMed] [Google Scholar]
- Gordon SM, Brahim JS, Rowan J, Kent A, Dionne RA. 2002. Peripheral prostanoid levels and nonsteroidal anti-inflammatory drug analgesia: replicate clinical trials in a tissue injury model. Clin Pharmacol Ther. 72(2):175–183. [DOI] [PubMed] [Google Scholar]
- Grosser T, Theken KN, FitzGerald GA. 2017. Cyclooxygenase inhibition: pain, inflammation, and the cardiovascular system. Clin Pharmacol Ther. 102(4):611–622. [DOI] [PubMed] [Google Scholar]
- Gupta N, Vujicic M, Blatz A. 2018. Opioid prescribing practices from 2010 through 2015 among dentists in the United States: what do claims data tell us? J Am Dent Assoc. 149(7):619–627. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Cooper S, Betts N, Wedell D, MacAfee K, Quinn P, Lamp C, Gaston G, Bergman S, Henry E. 1993. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surg Oral Med Oral Pathol. 76(6):680–687. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Lally ET, Moore PA. 2005. Update of cyclooxygenase inhibitors: has a third COX isoform entered the fray? Curr Med Res Opin. 21(9):1217–1226. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Levin LM, Adamson D, Christensen S, Kiersch TA, Noveck R, Watson G, II, Lyon JA. 2004. Dose-ranging analgesic study of Prosorb diclofenac potassium in postsurgical dental pain. Clin Ther. 26(8):1215–1227. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Levin LM, Cooper SA, Doyle G, Waksman J, Wedell D, Hong D, Secreto SA. 2000. Ibuprofen liquigel for oral surgery pain. Clin Ther. 22(11):1306–1318. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Moore PA. 2015. Three serious drug interactions that every dentist should know about. Compend Contin Educ Dent. 36(6):408–413. [PubMed] [Google Scholar]
- Hersh EV, Pinto A, Moore PA. 2007. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin Ther. 29(Suppl):2477–2497. [DOI] [PubMed] [Google Scholar]
- Hersh EV, Saraghi M, Moore PA. 2018. The prescription opioid abuse crisis and our role in it. Gen Dent. 66(4):10–13. [PubMed] [Google Scholar]
- Ji RR, Chamessian A, Zhang YQ. 2016. Pain regulation by non-neuronal cells and inflammation. Science. 354(6152):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellstein DE, Waksman JA, Furey SA, Binstok G, Cooper SA. 1999. J Clin Pharmacol. 39(5):520–532. [PubMed] [Google Scholar]
- Kiersch TA, Halladay SC, Hormel PC. 1994. A single-dose, double-blind comparison of naproxen sodium, acetaminophen, and placebo in postoperative dental pain. Clin Ther. 16(3):394–404. [PubMed] [Google Scholar]
- Kleinert R, Lange C, Steup A, Black P, Goldberg J, Desjardins P. 2008. Single dose analgesic efficacy of tapentadol in postsurgical dental pain: the results of a randomized, double-blind, placebo-controlled study. Anesth Analg. 107(6):2048–2055. [DOI] [PubMed] [Google Scholar]
- Lieblich SE, Danesi H. 2017. Liposomal bupivacaine use in third molar impaction surgery: INNOVATE Study. Anesth Prog. 64(3):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan BC, Hersh EV, Shofer FS, Wanner KJ, Archer E, Carrasco LR, Rhodes KV. 2016. Unused opioid analgesics and drug disposal following outpatient dental surgery: a randomized controlled trial. Drug Alcohol Depend. 168:328–334. [DOI] [PubMed] [Google Scholar]
- Mehlisch DR. 1998. Double-blind, single-dose comparison of bromfenac sodium, tramadol, and placebo after oral surgery. J Clin Pharmacol. 38(5):455–462. [DOI] [PubMed] [Google Scholar]
- Mehlisch DR, Aspley S, Daniels SE, Bandy DP. 2010. Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: a randomized, double-blind, placebo-controlled, parallel-group, single-dose, two-center, modified factorial study. Clin Ther. 32(5):882–895. [DOI] [PubMed] [Google Scholar]
- Moore PA, Crout RJ, Jackson DL, Schneider LG, Graves RW, Bakos L. 1998. Tramadol hydrochloride: analgesic efficacy compared with codeine, aspirin with codeine, and placebo after dental extraction. J Clin Pharmacol. 38(6):554–560. [DOI] [PubMed] [Google Scholar]
- Moore PA, Dionne RA, Cooper SA, Hersh EV. 2016. Why do we prescribe Vicodin? J Am Dent Assoc. 147(7):530–533. [DOI] [PubMed] [Google Scholar]
- Moore PA, Hersh EV. 2013. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: translating clinical research to dental practice J Am Dent Assoc. 144(8):898–908. [DOI] [PubMed] [Google Scholar]
- Moore PA, Nahouraii HS, Zovko JG, Wisniewski SR. 2006. Dental therapeutic practice patterns in the U.S.: II. Analgesics, corticosteroids, and antibiotics. Gen Dent. 54(3):201–207. [PubMed] [Google Scholar]
- Moore PA, Ziegler KM, Lipman RD, Aminoshariae A, Carrasco-Labra A, Mariotti A. 2018. Benefits and harms associated with analgesic medications used in the management of acute dental pain: an overview of systematic reviews. J Am Dent Assoc. 149(4):256–265. [DOI] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Wiffen PJ. 2015. a. Adverse events associated with single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev. 10:CD011407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Wiffen PJ. 2015. b. Single dose oral analgesics for acute postoperative pain in adults—an overview of Cochrane reviews. Cochrane Database Syst Rev. 9:CD008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Ossipov MH, Porreca F. 2017. Opioid analgesics and antagonists. In: Dowd FJ, Johnson BS, Mariotti AJ, editors. Pharmacology and therapeutics for dentistry. 7th ed. St. Louis (MO): Elsevier. p. 241–256. [Google Scholar]
- Rasubala L, Pernapati L, Velasquez X, Burk J, Ren YF. 2015. Impact of a mandatory prescription drug monitoring program on prescription of opioid analgesics by dentists. PLoS One. 10(8):e0135957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszkowski MT, Swift JQ, Hargreaves KM. 1997. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 73(3):339–345. [DOI] [PubMed] [Google Scholar]
- Schroeder AR, Dehghan M, Newman TB, Bentley JP, Park KT. 2019. Association of opioid prescriptions from dental clinicians for US adolescents and young adults with subsequent opioid use and abuse. JAMA Intern Med. 179(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C. 2016. Opioid receptors. Annu Rev Med. 67:433–451. [DOI] [PubMed] [Google Scholar]
- Theken KN. 2018. Variability in analgesic response to non-steroidal anti-inflammatory drugs. Prostaglandins Other Lipid Mediat. 139:63–70. [DOI] [PubMed] [Google Scholar]
- Theken KN, Hersh EV, Lahens NF, Lee HM, Li X, Granquist EJ, Giannakopoulos HE, Levin LM, Secreto SA, Grant GR, et al. 2019. Variability in the analgesic response to ibuprofen is associated with cyclooxygenase activation in inflammatory pain. Clin Pharmacol Ther. 106(3):632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration. 2017. FDA Drug Safety Communication: FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women [accessed 28 February 2020]. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-restricts-use-prescription-codeine-pain-and-cough-medicines-and.
- Van Dyke T, Litkowski LJ, Kiersch TA, Zarringhalam NM, Zheng H, Newman K. 2004. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: a double-blind, placebo- and active-controlled parallel-group study. Clin Ther. 26(12):2003–2014. [DOI] [PubMed] [Google Scholar]
- Vane JR, Botting RM. 1995. A better understanding of anti-inflammatory drugs based on isoforms of cyclooxygenase (COX-1 and COX-2). Adv Prostaglandin Thromboxane Leukot Res. 23:41–48. [PubMed] [Google Scholar]
- Zuniga JR, Papas AS, Daniels SE, Patrick K, Muse DD, Oreadi D, Giannakopoulos HE, Granquist EJ, Levin LM, Chou JC, et al. 2019. Prevention of opioid-induced nausea and vomiting during treatment of moderate to severe acute pain: a randomized placebo-controlled trial comparing CL-108 (hydrocodone 7.5 mg/acetaminophen 325 mg/rapid-release, low-dose promethazine 12.5 mg) with conventional hydrocodone 7.5 mg/acetaminophen 325 mg. Pain Med. 20(12):2528–2538. [DOI] [PubMed] [Google Scholar]