Abstract
Periodontitis (PD) is a common source of uncontrolled inflammation in obesity-associated type 2 diabetes (T2D). PD apparently fuels the inflammation of T2D and associates with poor glycemic control and increased T2D morbidity. New therapeutics are critically needed to counter the sources of periodontal infection and inflammation that are accelerated in people with T2D. The precise mechanisms underlying the relationship between PD and T2D remain poorly understood. Every major immune cell subset has been implicated in the unresolved inflammation of PD, regardless of host metabolic health. However, analyses of inflammatory cells in PD with human periodontal tissue have generally focused on mRNA quantification and immunohistochemical analyses, both of which provide limited information on immune cell function. We used a combination of flow cytometry for cell surface markers and enzyme-linked immunospot methods to assess the subset distribution and function of immune cells isolated from gingiva of people who had PD and were systemically healthy, had PD and T2D (PD/T2D), or, for flow cytometry, were systemically and orally healthy. T-cell subsets dominated the cellular immune compartment in gingiva from all groups, and B cells were relatively rare. Although immune cell frequencies were similar among groups, a higher proportion of CD11b+ or CD4+ cells secreted IFNγ/IL-10 or IL-8, respectively, in cells from PD/T2D samples as compared with PD-alone samples. Our data indicate that fundamental differences in gingival immune cell function between PD and T2D-potentiated PD may account for the increased risk and severity of PD in subjects with T2D. Such differences may suggest unexpected therapeutic targets for alleviating periodontal inflammation in people with T2D.
Keywords: cytokines, gingiva, inflammation, periodontal disease, obesity
Introduction
Periodontitis (PD) is one of the most prevalent diseases, with approximately 42% of the US adult population having some form of PD and almost 8% diagnosed with severe PD (Eke et al. 2018). Type 2 diabetes (T2D) is a significant risk factor for PD, with changes in immune system cells shown to potentiate PD in animal models of T2D, and with strong evidence that T2D increases the risk of PD in people (reviewed by Wu et al. 2015). The continuing rise in the proportion of people with T2D (CDC National Diabetes Statistics Report 2020) predicts a parallel rise in PD. Several mechanisms have been proposed to explain the likely bidirectional relationship between PD and T2D. For example, T2D may change the oral microbiota, resulting in an imbalance of pathogenic bacteria such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. The formation of advanced glycation end products, especially in poorly controlled T2D, increases activation of their receptors, which in turn trigger proinflammatory cytokine production and bone resorption. T2D also exacerbates function of multiple immune cells implicated in PD, including the Th17 T-cell subset (Jagannathan-Bogdan et al. 2011; Moutsopoulos et al. 2012; McLaughlin et al. 2014; Ip et al. 2016; Dutzan et al. 2018; Nicholas et al. 2019), B cells that play roles in PD of obese but not lean mice (Zhu et al. 2014), and myeloid cells (Hatanaka et al. 2006; Hand et al. 2007). Overall, the strong correlation between T2D and PD in people and the mechanistic links between the diseases in animal models (Naguib et al. 2004; Andriankaja et al. 2012; Pacios et al. 2012) indicate that a more comprehensive understanding of the differences in the oral immune compartment in PD lesions among individuals with and without relatively well-controlled T2D is required to precisely tailor therapies targeted toward normalizing risk for PD in an increasingly T2D population.
Multiple major factors drive the inflammatory milieu in tissues, including the relative frequencies of immune cell subsets and the magnitude of the pro- and anti-inflammatory functions of these subsets. Although all major proinflammatory immune cell subsets and their secreted cytokines, such as TNFα, IL-1β, IL-6, and IL-17, have been independently implicated in T2D and PD (Assuma et al. 1998; Fujihara et al. 2014; Dutzan et al. 2017), historical analyses of PD in humans have focused on mRNA quantification and immunohistochemical analyses (Reinhardt et al. 1988; Seymour et al. 1988; Fujihashi et al. 1996; Cardoso et al. 2009; Souto et al. 2014), both of which provide limited information on immune cell function. Alternatively, flow cytometry studies of intact cells in suspension allow for a more comprehensive analysis of the immune compartment of a relatively large piece of gingival tissue (>60 mm3). T cells are the most frequent subset in the resident immune population in samples from systemically healthy people with and without PD, and the absolute number of T cells was about 10-fold higher per gram of tissue in PD (Dutzan et al. 2016). The CD4+ T-cell subpopulation had a higher frequency of IL-17-producing cells in samples from subjects with PD as compared with periodontally healthy subjects, although, perhaps surprisingly, the frequency of IFNγ-producing CD4+ and CD8+ T cells was indistinguishable (Dutzan et al. 2016). These analyses showed lower frequencies of granulocytes and dendritic cells/macrophages in PD lesions as compared with T cells and a very low frequency of B cells, with few differences in PD and healthy gingiva, consistent with some but not all data from previous studies (Lappin et al. 1999; Thorbert-Mros et al. 2015). Additional flow-based analyses of PD lesions have not been performed to our knowledge, nor has this platform been applied to T2D-potentiated PD to date.
We used flow cytometric phenotyping and enzyme-linked immunospot assay (ELISpot; Lehmann and Zhang 2012) to quantitate immune cell frequencies, surface phenotypes, and ex vivo effector function in the gingival tissue immune compartment of subjects who have PD or T2D-potentiated PD, including analysis of immune cells in healthy gingiva as a comparator for flow cytometry. Our data show that immune cell subset distribution is similar in gingiva from all cohorts but that the function of CD4+ T cells and CD11b+ myeloid cells differed in PD ± T2D gingiva. These data highlight differences in periodontal immune cell function between PD and T2D-potentiated PD and suggest targets for alleviating periodontal inflammation in people with T2D.
Methods
Human Subjects
Study participants were recruited from the Department of Periodontology, Henry M. Goldman School of Dental Medicine, Boston University, and the Center for Clinical and Translational Research, Forsyth Institute, under Institutional Review Board–approved protocols. Participants gave written informed consent for research use of gingiva samples removed during a standard-of-care treatment. The exclusion criteria included smoking, antibiotics, steroids, drug abuse, alcohol abuse, autoimmune disease, cancer within the past 5 y, seasonal allergy medications, and any underlying oral or systemic health conditions other than PD, obesity, or T2D. PD was defined according to the American Academy of Periodontology criteria based on severity (Armitage 1999). Briefly, healthy periodontal status was defined as having ≤3 mm pocket depth and no bleeding on probing with no signs of clinical inflammation, including redness and edema. PD was defined with at least 2 interproximal sites (on separate teeth) with ≥5 mm pocket depth, ≥3 mm clinical attachment loss, and bleeding on probing. According to standard-of-care periodontal treatment, patients with periodontal disease (PD/T2D and PD alone) received scaling and root planing as the initial therapy, as well as oral hygiene instructions. Three to 6 wk after completion of scaling and root planing, whole mouth periodontal evaluations were done, including pocket depth measurements, and sites with residual pockets≥6 mm received surgical periodontal treatment for pocket elimination/reduction and osseous reconstruction, as needed. Patients with periodontal disease requiring surgery following scaling and root planing were enrolled in the study to obtain the discarded excised tissues. Tissue samples included inflamed gingival and pocket epithelium and underlying connective tissue. Patients with healthy periodontal tissues and no T2D were scheduled for a crown-lengthening procedure prior to prosthetic procedures. Tissue samples included healthy gingival and sulcus epithelium and underlying connective tissue. T2D was defined by a clinical diagnosis of T2D, and all enrolled subjects had a hemoglobin A1c <9.0% with 1 exception (11.3%). The Table lists characteristics of subjects.
Tissue Processing
Gingival tissue samples were collected in 15 mL conical tubes with RPMI complete medium (Gibco Life Technologies) supplemented with 10% fetal bovine serum (Atlanta Biological) and 1% penicillin-streptomycin (Sigma Aldrich). The tissue was rinsed with sterile phosphate-buffered saline (PBS) to remove blood clots, submerged into 1 to 2 mL of Worthington collagenase I (LS004196, 1 mg/mL, in Medium 199 supplemented with 1% bovine serum albumin from Gibco), and minced in a Petri dish. The fragments were transferred to a conical tube with 2 to 5 mL of collagenase solution and digested during incubation in a shaker set at 220 rpm at 37 °C for 1 h. Digested gingival tissue was triturated 5 to 6 times and then filtered through a 70-µm BD filter; 2 to 3 mL of FACS buffer (PBS with 0.5% bovine serum albumin and 2mM EDTA) was added to the tube to remove all remaining fragments. A 3-mL syringe plunger was used to disrupt the remaining tissue fragments collected in the filter to liberate additional cells from the tissue. FACS buffer (3 to 5 mL) was used to wash out the filter to collect maximal numbers of gingival cells. The wash-through liquid was transferred into a new 15-mL conical tube, and FACS buffer was added up to 15 mL. Gingival cells were centrifuged at 1,200 rpm for 10 min; the supernatant was removed before cells were washed a second time with PBS. Cell pellet was resuspended in 0.5 mL of 1:200 Zombie Aqua live-dead discrimination dye (Biolegend), stained on ice for 30 min in the dark, and then washed with 1 mL of FACS buffer. Cells were incubated with human Fc block (Human TruStain, FcX; Biolegend) and then washed prior to specific marker staining. A master mix of antibodies was prepared as indicated in Appendix Table 1, and 50 μL of the master mix was added to pellets of Zombie Aqua–stained gingival cells and incubated on ice in the dark for 30 min. FACS buffer (1 mL) was added to each Eppendorf tube and centrifuged (2,500 rpm, 10 min). The pellet was resuspended in 300 μL of FACS buffer and left on ice in the dark until immune subsets were analyzed and sorted.
Table.
Characteristics of Subjects: Healthy, PD, and PD/T2D.
| Total | Healthy | PD | PD/T2D | |
|---|---|---|---|---|
| Patients, n | 40 | 5 | 26 | 9 |
| Male | 20 | 2 | 10 | 8a |
| Female | 20 | 3 | 16 | 1 |
| Mean ± SD | ||||
| Probing depth, mm | 2.6 ± 0.55b | 6.7 ± 2.4 | 8.2 ± 2.0 | |
| Body mass index | 24 ± 4.0 | 25 ± 3.4 | 30 ± 3.9a | |
| Age, y | 35 ± 6.3b | 49 ± 11 | 53 ± 4.7 |
Differences all achieved significance of P < 0.01 as calculated by 1-way analysis of variance. Cells from healthy subjects were used for flow cytometric analyses only, and all PD and PD/T2D samples were used for enzyme-linked immunospot assay.
PD, periodontitis only; PD/T2D, type 2 diabetes–potentiated periodontitis.
Values differed from healthy and PD.
Values differed from PD and PD/T2D.
Cell Sorting and Plating
Stained gingival cells were sorted with the BD FACSARIA II SORP at the Boston University Flow Cytometry Core Facility. The immune cells were gated as live single CD45+ cells, and subpopulations were sorted as follows into FACS tubes containing 200 μL of complete RPMI media:
1) CD4+ T cells (live/CD45+/CD3+/CD4+) and CD8+ T cells (live/CD45+/CD3+/CD8+)
2) Myeloid cells (live/CD45+/CD11bhi)—including monocytes, dendritic cells, and granulocytes (natural killer cells [CD11blo] were excluded on the basis of CD56 back gating)
3) B cells (live/CD45+/CD19+)
4) NK cells (live/CD45+/CD56+/CD3-)
The majority (>99%) of B cells from gingiva were CD19+CD20+ (not shown); thus, the 2 markers are interchangeable for practical purposes. The cell count for each cell fraction was recorded. Total leukocytes (CD45+), CD4+ T cells, CD8+ T cells, and B cells were stimulated with 50 ng/mL of PMA (Millipore Sigma) and 250 ng/mL of ionomycin (Millipore Sigma). Sorted myeloid cells were stimulated with 100 ng/mL of Escherichia coli lipopolysaccharide O111:B4 (Millipore Sigma). The cells were plated in 200 μL of RPMI medium in wells of ELISpot plates for 20 to 24 h at 37 °C for culture and functional analysis.
Enzyme-Linked Immunospot Assay
Each of 8 antibodies specific for IL-2, IL-4, IL-6, IL-8, IL-10, IL-17A, TNFα, or IFNγ was added to 500 μL of PBS in Eppendorf tubes as indicated in Appendix Table 2. A total of 50 μL of each diluted antibody was used to coat wells of an ELISpot plate (MAIPN4550; Sigma Millipore) for 1 h at 37 °C, washed with PBS, and filled with 50 μL of RPMI/10% fetal bovine serum medium. Approximately 500 sorted cells from each subpopulation and stimulation components or vehicle (PBS) were added to the wells. Cells were cultured for 20 to 24 h at 37 °C, 5% CO2. At the end of the culture period, plates were washed with PBS +0.1% Tween (PBST); then, biotinylated secondary antibodies were diluted with 0.1% Tween 20 and 2% bovine serum albumin (PBSTB) at concentrations listed in Appendix Table 2, and 50 μL of diluted antibody was added to the appropriate wells. The ELISpot plates were wrapped in a damp paper towel, sealed in a plastic bag, and left in a 4 °C refrigerator for 24 h before being washed 3 times with 200 μL of PBST. A total of 50 μL of streptavidin-conjugated alkaline phosphatase dilution (1:1,000, streptavidin in PBSTB; AP-Conjugated Streptavidin, Jackson ImmunoResearch) was then added to each well and incubated at room temperature for 30 to 45 min, when wells were washed with 200 μL of PBSTB. The plastic backing of the ELISpot plate was removed after washing, and the plate was fully submerged in PBST for 1 h at room temperature. The plate was then rinsed with PBS, and 100 μL of the substrate solution (in 5 mL of 100mM Tris HCl; Vector Blue Substrate Mix, AP Substrate Kit III, SK-5300, Vector Labs) was added to each well and left in the dark for 15 min. The plate was washed with distilled water and left to dry for 24 h with minimal exposure to light. Spots were counted on an ImmunoSpot Universal Analyzer (ImmunoSpot) to calculate the frequencies of cytokine-producing cells (spots) per number of plated cells per well.
Statistics
Comparison of 2 groups was analyzed by nonparametric Mann-Whitney U tests. Comparison among 3 groups was based on 1-way analysis of variance with Holm-Sidak’s multiple-comparison tests. Linear regressions compared associations between ELISpot results and age. Significant differences were accepted at P < 0.05, and all analyses were completed on Prism (version 8; GraphPad).
Results
CD4+ and CD8+ T Cells Dominate the Gingival Compartment
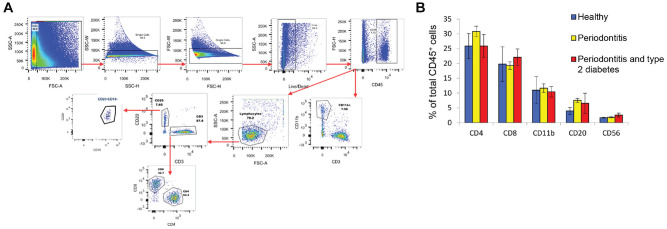
To independently test whether immune cell subset frequencies are similar in gingiva from systemically healthy subjects with or without PD (Dutzan et al. 2016) and to compare these outcomes with gingiva of people with T2D-potentiated PD, we performed immunophenotyping of gingival cells by flow cytometry, gating immune cell subsets as outlined in Figure 1A and quantified in Figure 1B. CD4+ T cells were the most frequent immune cell subset among total CD45+ cells (mean ± SEM: 26% to 31% ± 1.81% to 4.21%) in gingival cell preparations from all subjects. CD8+ T cells were present at 18.5% to 22.5% ± 1.28% to 5.82%, and CD11b+ myeloid cells represented 12.0% ± 1.47% to 4.59% of total CD45+ cells. CD20+ B-cell frequencies were 5% to 7.5% ± 0.69% to 1.19%, and CD56+ natural killer cells represented <3.0% ± 0.11% to 0.66% of the CD45+ population. Frequencies of all immune cell subsets were similar among cohorts (Fig. 1B), extending previous comparisons of immune cell frequencies in gingiva from healthy subjects or those with PD (Dutzan et al. 2016).
Figure 1.
Immunophenotyping of gingival tissue from subjects with periodontitis (PD) or type 2 diabetes with PD (PD/T2D). (A) Flow cytometric strategy to quantify frequency of indicated immune cell subsets and to sort cells for subset-specific functional analyses. Results from 1 representative PD sample are shown. (B) Frequency of immune subsets in total CD45+ cells isolated from gingiva of healthy (blue), PD (yellow), or PD/T2D (red) samples. One-way analysis of variance within each subset showed no statistical difference based on health status. Data are presented as mean ± SEM in analysis of 5 healthy, 19 PD, and 3 PD/T2D samples.
Immune Cell Subset Function Is Selectively Altered in Periodontal Lesions from Subjects with T2D
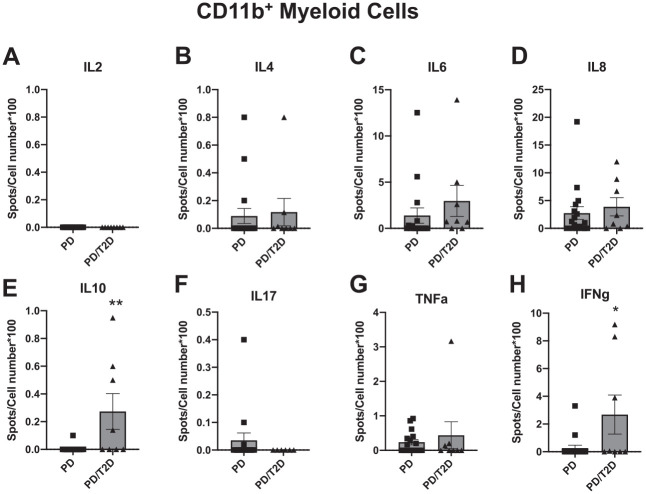
We sorted gingival CD45+ immune cells from healthy, PD, or PD/T2D samples into >95% pure populations of CD11b+ myeloid, CD4+ T cells, or CD8+ T cells and plated the purified cells onto ELISpot wells prepared and then processed as described above. The ELISpot platform quantifies the frequency of the small number of cytokine-producing immune cells from the gingiva and, in our assays, included analysis of IL-2, IL-4, IL-6, IL-8, IL-10, IL-17A, TNFα, or IFNγ producers. As expected, CD11b+ cells produced undetectable amounts of IL-2 (Fig. 2A) in response to lipopolysaccharide stimulation but measurable amounts of a number of cytokines, including IL-4, IL-6, IL-8, IL-10, TNFα, and IFNγ, the last an underappreciated product of myeloid cells (Bogdan and Schleicher 2006). Infrequent IL-17-producing myeloid cells were present in 3 of 26 PD samples but never in PD/T2D samples, consistent with our work on circulating myeloid cells from subjects with T2D (Jagannathan-Bogdan et al. 2011). In contrast, a larger proportion of lipopolysaccharide-stimulated myeloid cells from PD/T2D samples as compared with PD samples produced IL-10 and IFNγ, and IL-6 trended higher in PD/T2D than in PD samples (P = 0.09). The proportion of other myeloid cytokine producers was similar in samples from the PD/T2D and PD cohorts (Fig. 2B–H). PMA/ionomycin-stimulated peripheral blood mononuclear cells generated spots for all cytokines to confirm technical success of the ELISpot (data not shown).
Figure 2.
Frequency of cytokine-producing CD11b+ myeloid cells from gingival tissue of subjects with periodontitis (PD) or type 2 diabetes with PD (PD/T2D) following stimulation of cells with Escherichia coli lipopolysaccharide. (A–H) Spots for the indicated cytokine detected per 100 cells plated into ELISpot well. Sample sizes are as shown in the Table, although not every sample yielded sufficient cells for analysis of all cytokines. Data are presented as mean ± SEM.
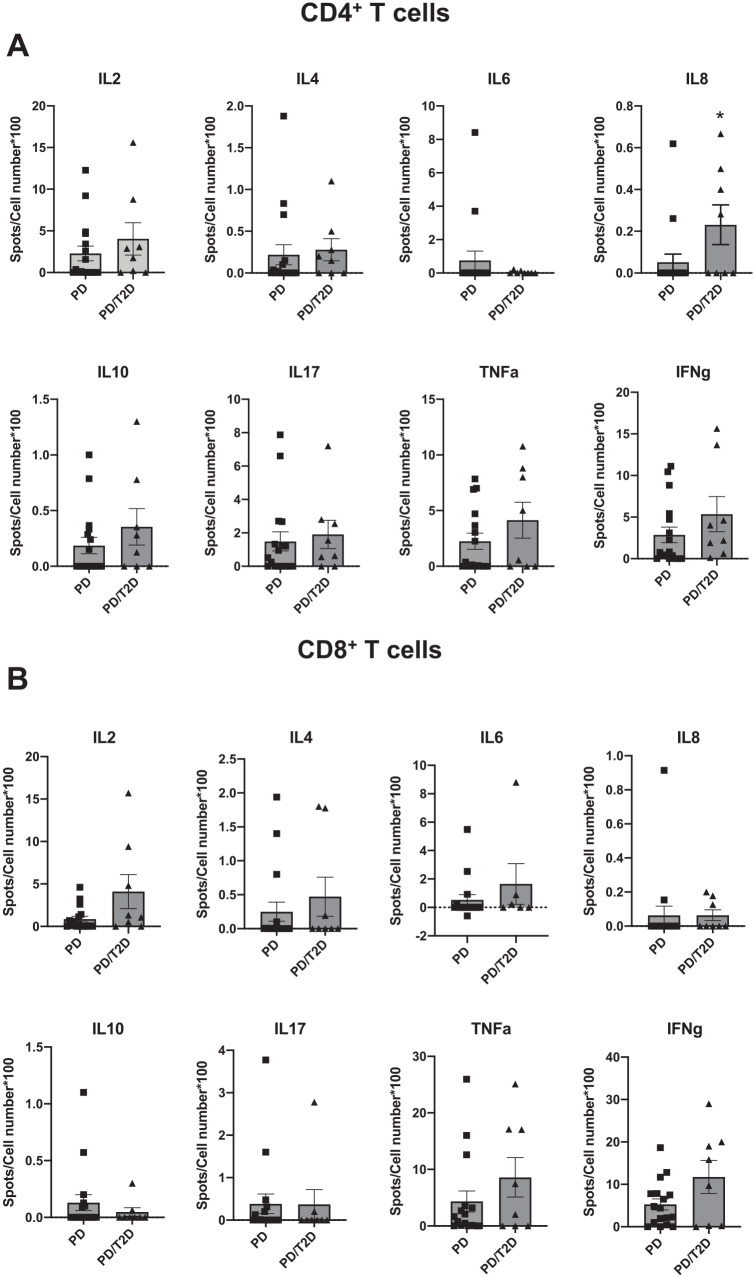
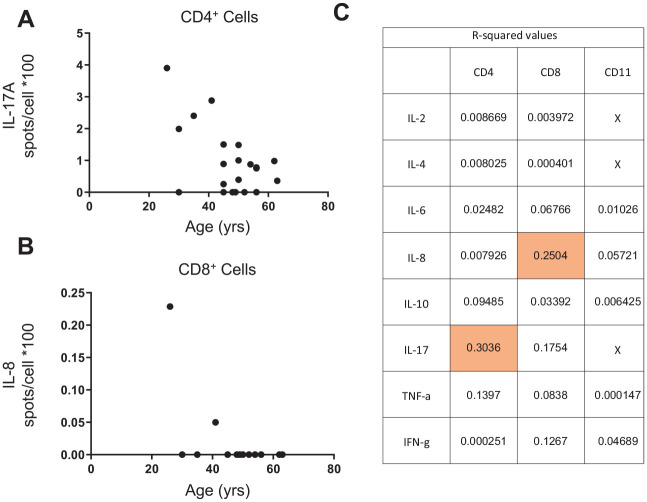
We similarly compared the proportion of cytokine-producing CD4+ T cells. Although the frequency of most cytokine-producing CD4+ T cells was similar for the PD/T2D and PD samples, IL-8 producers were more frequent in gingiva from PD/T2D versus healthy/PD gingiva (Fig. 3A). The frequency of cytokine-producing CD8+ T cells was indistinguishable among cohorts (Fig. 3B), and the number of B cells and natural killer cells purified from most samples was too low to generate reliable results. Linear regression analysis showed an association between age and IL-17+ CD4+ cells from subjects with PD, the frequency of which decreased with age (P = 0.0096; Fig. 4A) but did not differ between PD and PD/T2D samples (Fig. 3A). Other cytokines had no convincing relationship with age; the relationship between CD8+ IL-8 frequency and age was driven by 2 (of 21) data points (P = 0.021; Fig. 4B), and all other associations were statistically nonsignificant (P > 0.05) and had modest predictive value as indicated by R2 values (Fig. 4C). This analysis found that differences in age among cohorts was unlikely to be a determinant of the cytokine differences in cells from PD versus T2D samples. Furthermore, reanalysis of the PD-only outcomes by sex showed no effect of sex on ELISpot outcomes, weakening the possibility that sex differences among cohorts was a driving factor for PD and PD/T2D differences. Taken together, our data demonstrate specific differences in functions of multiple immune cell subsets in gingiva from subjects with PD, with and without T2D.
Figure 3.
Frequency of cytokine-producing (A) CD4+ T cells and (B) CD8+ T cells from gingival tissue of subjects with periodontitis (PD) or type 2 diabetes with PD (PD/T2D) following stimulation of cells with PMA + ionomycin. Spots for the indicated cytokine detected per 100 cells plated into ELISpot well. Sample sizes are as shown in the Table, although not every sample yielded sufficient cells for analysis of all cytokines. Data are presented as mean ± SEM.
Figure 4.
Linear regression analysis between age and (A) IL-17A or (B) IL-8 produced by CD4+ or CD8+ cells, respectively, as measured by ELISpot of cells from subjects with periodontitis only. (C) Coefficient of determination (R2) for all ELISpot outcomes to indicate the proportion of variation explained by age. Shading highlights combinations that show a significant association with age. The IL-8+/CD8+ association was driven by a small number of data points and was less meaningful than the IL-17+/CD4+ association with age.
Discussion
Despite the general variability among individual samples in the number of cytokine-producing immune cells (normalized by the number of cells plated), our data are consistent with previous proposals that subpopulations of CD4+ helper T cells and CD11b+ myeloid cells play roles in T2D-associated PD (Zhu and Nikolajczyk 2014). Analysis of tissues from the PD cohort suggests 1) individual variation in the immune compartment of people with PD and 2) consistent overrepresentation of IFNγ- or IL-10-producing myeloid cells and IL-8-producing CD4+ T cells in PD/T2D samples. Together these data identify previously unappreciated cellular sources of cytokines in diseased gingival tissue. These results are consistent with recent meta-analyses indicating high expression of IFNγ and IL-8 associates with PD, albeit in studies that may or may not include people with T2D (Finoti et al. 2017; Fiorillo et al. 2018). Our work does not rule out the possibility that the higher body mass index of the PD/T2D group versus the PD-only group plays a role in cytokine outcomes, although previous work from our laboratory and many others indicates that T2D has a much stronger effect on inflammation than does obesity in euglycemic people, the latter a condition that is not echoed in typical models of obese/insulin resistance in mice. Although IL-10 has well-documented anti-inflammatory actions, IL-10 can promote inflammation under some circumstances (Rojas et al. 2017). Furthermore, the blunted anti-inflammatory responses to IL-10 in cells exposed to high glucose (to mimic the hyperglycemia of T2D; Barry et al. 2016) and compensatory IL-10 production following perturbations in a different cell type (Kowalski et al. 2011) raise several possible explanations for the inability of higher myeloid IL-10 to neutralize periodontal inflammation.
Variability, as measured by the number of cytokine-positive spots per 100 cells isolated from individuals within a cohort, was a limitation in these studies. We performed power analysis using data here to demonstrate that to identify differences in IL-17A, which appears to trend higher as disease load is increased, >100 subjects per group (PD and PD/T2D) would need to be assayed for conclusions at a power of 0.8; thus, meaning for any single tissue would be limited. As such, bioinformatic rather than statistical tools may be superior for understanding the relative importance of outcomes on an individualized basis. Variability in outcomes could also be introduced by environmental factors, including patients who had previous periodontal treatment (e.g., repeated scaling and root planing) at various times prior to gingival excision, which may unevenly alter different forms of inflammation. Another possible source of variation is the size/condition of the gingival sample received from the periodontal clinic and excision based on standard of care rather than research criteria. Alterations in the gingival resection techniques may decrease variability if the outcome variations are not due to mainly biological considerations, such as genetic background, which can be a major driver of variability in human tissue analyses.
Comparison of outcomes herein with previously published work shows consistencies and differences, although most cellular studies focused on comparison of gingival immune compartments in systemically healthy subjects with PD. Phenotyping confirmed previous demonstrations that CD4+ T cells are the majority T cell in PD lesions (Lappin et al. 1999; Dutzan et al. 2016) and the paucity of B cells in periodontal lesions in cellular studies (Dutzan et al. 2016), which disagreed with mRNA and immunohistochemical studies (Reinhardt et al. 1988; Lappin et al. 1999; Kawai et al. 2006). Studies that discount B cells in PD have largely relied on B cell–specific markers, such as CD19 and CD20, which other studies combine with CD138+ plasma cells, the terminally differentiated cousins of CD19+CD20+ B cells. ELISpot confirmed work by Dutzan et al. (2016), which showed that the frequency of IFNγ-producing CD4+ and CD8+ T cells was indistinguishable in the presence or absence of PD and that myeloid cells were less frequent than T cells in PD lesions. Overall, our data highlight the possibility that modest differences in the immune compartment of people with PD ± T2D as identified by ELISpot may benefit from analysis through independent methods. The highly sensitive immune profiling approaches that we have completed in the peripheral blood mononuclear cells from people with T2D (but no periodontal assessment) to more holistically assess “inflammation” (Ip et al. 2016; Nicholas et al. 2019) are a logical next step. Variability in our outcomes further cautions studies with approaches such as single-cell RNA sequencing, which may require development of new analytic methods that are more refractory to biological variation for robust interpretation.
Author Contributions
A.C. Belkina, J.E. Snyder-Cappione, contributed to conception, design, and data analysis, critically revised the manuscript; M. Azer, J.J. Lee, contributed to conception, design, and data analysis, drafted the manuscript; H.H. Elgaali, R. Pihl, M. Cleveland, J. Carr, C. Habib, contributed to data analysis, critically revised the manuscript; S. Kim, contributed to data analysis, drafted the manuscript; H. Hasturk, contributed to conception and design, critically revised the manuscript; B.S. Nikolajczyk, contributed to conception and design, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520912188 for Single-Cell Analysis of the Periodontal Immune Niche in Type 2 Diabetes by A.C. Belkina, M. Azer, J.J. Lee, H.H. Elgaali, R. Pihl, M. Cleveland, J. Carr, S. Kim, C. Habib, H. Hasturk, J.E. Snyder-Cappione and B.S. Nikolajczyk in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This work was supported by National Institute of Dental and Craniofacial Research (R01DE025383, U01025383), the University of Kentucky College of Medicine, the Boston University Flow Cytometry Core Facility, and the Markey Cancer Center Immune Monitoring Core at the University of Kentucky (National Institutes of Health, P30 CA177558).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: A.C. Belkina  https://orcid.org/0000-0001-7967-6254
https://orcid.org/0000-0001-7967-6254
B.S. Nikolajczyk  https://orcid.org/0000-0001-5647-5740
https://orcid.org/0000-0001-5647-5740
References
- Andriankaja OM, Galicia J, Dong G, Xiao W, Alawi F, Graves DT. 2012. Gene expression dynamics during diabetic periodontitis. J Dent Res. 91(12):1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage GC. 1999. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 4(1):1–6. [DOI] [PubMed] [Google Scholar]
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. 1998. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 160(1):403–409. [PubMed] [Google Scholar]
- Barry JC, Shakibakho S, Durrer C, Simtchouk S, Jawanda KK, Cheung ST, Mui AL, Little JP. 2016. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci Rep. 6:21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C, Schleicher U. 2006. Production of interferon-gamma by myeloid cells—fact or fancy? Trends Immunol. 27(6):282–290. [DOI] [PubMed] [Google Scholar]
- Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS. 2009. Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol. 24(1):1–6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2020. National diabetes statistics report, 2020. Washington (DC): Department of Health and Human Services; [accessed 2020 March]. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. [Google Scholar]
- Dutzan N, Abusleme L, Bridgeman H, Greenwell-Wild T, Zangerle-Murray T, Fife ME, Bouladoux N, Linley H, Brenchley L, Wemyss K, et al. 2017. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity. 46(1):133–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Kajikawa T, Abusleme L, Greenwell-Wild T, Zuazo CE, Ikeuchi T, Brenchley L, Abe T, Hurabielle C, Martin D, et al. 2018. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 10(463):eaat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. 2016. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 9(5):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA, Genco RJ. 2018. Periodontitis in us adults: National Health and Nutrition Examination Survey 2009–2014. J Am Dent Assoc. 149(7):576–588.e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finoti LS, Nepomuceno R, Pigossi SC, Corbi SC, Secolin R, Scarel-Caminaga RM. 2017. Association between interleukin-8 levels and chronic periodontal disease: a PRISMA-compliant systematic review and meta-analysis. Medicine. 96(22):e6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo L, Cervino G, Herford AS, Lauritano F, D’Amico C, Lo Giudice R, Laino L, Troiano G, Crimi S, Cicciu M. 2018. Interferon crevicular fluid profile and correlation with periodontal disease and wound healing: a systemic review of recent data. Int J Mol Sci. 19(7):E1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujihara R, Usui M, Yamamoto G, Nishii K, Tsukamoto Y, Okamatsu Y, Sato T, Asou Y, Nakashima K, Yamamoto M. 2014. Tumor necrosis factor-alpha enhances RANKL expression in gingival epithelial cells via protein kinase A signaling. J Periodontal Res. 49(4):508–517. [DOI] [PubMed] [Google Scholar]
- Fujihashi K, Yamamoto M, Hiroi T, Bamberg TV, McGhee JR, Kiyono H. 1996. Selected Th1 and Th2 cytokine mRNA expression by CD4(+) T cells isolated from inflamed human gingival tissues. Clin Exp Immunol. 103(3):422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand WL, Hand DL, Vasquez Y. 2007. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 76(1):44–50. [DOI] [PubMed] [Google Scholar]
- Hatanaka E, Monteagudo PT, Marrocos MS, Campa A. 2006. Neutrophils and monocytes as potentially important sources of proinflammatory cytokines in diabetes. Clin Exp Immunol. 146(3):443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip B, Cilfone NA, Belkina AC, DeFuria J, Jagannathan-Bogdan M, Zhu M, Kuchibhatla R, McDonnell ME, Xiao Q, Kepler TB, et al. 2016. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFalpha production. Obesity. 24(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, Nikolajczyk BS. 2011. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 186(2):1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, et al. 2006. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 169(3):987–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski GM, Nicholls HT, Risis S, Watson NK, Kanellakis P, Bruce CR, Bobik A, Lancaster GI, Febbraio MA. 2011. Deficiency of haematopoietic-cell-derived IL-10 does not exacerbate high-fat-diet-induced inflammation or insulin resistance in mice. Diabetologia. 54(4):888–899. [DOI] [PubMed] [Google Scholar]
- Lappin DF, Koulouri O, Radvar M, Hodge P, Kinane DF. 1999. Relative proportions of mononuclear cell types in periodontal lesions analyzed by immunohistochemistry. J Clin Periodontol. 26(3):183–189. [DOI] [PubMed] [Google Scholar]
- Lehmann PV, Zhang W. 2012. Unique strengths of elispot for T cell diagnostics. Methods Mol Biol. 792:3–23. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Liu LF, Lamendola C, Shen L, Morton J, Rivas H, Winer D, Tolentino L, Choi O, Zhang H, et al. 2014. T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler Thromb Vasc Biol. 34(12):2637–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsopoulos NM, Kling HM, Angelov N, Jin W, Palmer RJ, Nares S, Osorio M, Wahl SM. 2012. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun. 39(4):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naguib G, Al-Mashat H, Desta T, Graves DT. 2004. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol. 123(1):87–92. [DOI] [PubMed] [Google Scholar]
- Nicholas DA, Proctor EA, Agrawal M, Belkina AC, Van Nostrand SC, Panneerseelan-Bharath L, Jones ARt, Raval F, Ip BC, Zhu M, et al. 2019. Fatty acid metabolites combine with reduced beta oxidation to activate Th17 inflammation in human type 2 diabetes. Cell Metab. 30(3):447–461.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacios S, Kang J, Galicia J, Gluck K, Patel H, Ovaydi-Mandel A, Petrov S, Alawi F, Graves DT. 2012. Diabetes aggravates periodontitis by limiting repair through enhanced inflammation. FASEB J. 26(4):1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RA, Bolton RW, McDonald TL, DuBois LM, Kaldahl WB. 1988. In situ lymphocyte subpopulations from active versus stable periodontal sites. J Periodontol. 59(10):656–670. [DOI] [PubMed] [Google Scholar]
- Rojas JM, Avia M, Martin V, Sevilla N. 2017. IL-10: a multifunctional cytokine in viral infections. J Immunol Res. 2017:6104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GJ, Gemmell E, Walsh LJ, Powell RN. 1988. Immunohistological analysis of experimental gingivitis in humans. Clin Exp Immunol. 71(1):132–137. [PMC free article] [PubMed] [Google Scholar]
- Souto GR, Queiroz-Junior CM, de Abreu MH, Costa FO, Mesquita RA. 2014. Pro-inflammatory, Th1, Th2, Th17 cytokines and dendritic cells: a cross-sectional study in chronic periodontitis. PLoS One. 9(3):e91636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorbert-Mros S, Larsson L, Berglundh T. 2015. Cellular composition of long-standing gingivitis and periodontitis lesions. J Periodontal Res. 50(4):535–543. [DOI] [PubMed] [Google Scholar]
- Wu YY, Xiao E, Graves DT. 2015. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 7(2):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Belkina AC, DeFuria J, Carr JD, Van Dyke TE, Gyurko R, Nikolajczyk BS. 2014. B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation. J Leukoc Biol. 96(2):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Nikolajczyk BS. 2014. Immune cells link obesity-associated type 2 diabetes and periodontitis. J Dent Res. 93(4):346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520912188 for Single-Cell Analysis of the Periodontal Immune Niche in Type 2 Diabetes by A.C. Belkina, M. Azer, J.J. Lee, H.H. Elgaali, R. Pihl, M. Cleveland, J. Carr, S. Kim, C. Habib, H. Hasturk, J.E. Snyder-Cappione and B.S. Nikolajczyk in Journal of Dental Research