Abstract
Rationale
Working memory deficits are present in schizophrenia (SZ) but remain insufficiently resolved by medications. Similar cognitive dysfunctions can be produced acutely in animals by elevating brain levels of kynurenic acid (KYNA). KYNA’s effects may reflect interference with the function of both the a7 nicotinic acetylcholine receptor (α7nAChR) and the glycineB site of the NMDA receptor.
Objectives
The aim of the present study was to examine, using pharmacological tools, the respective roles of these two receptor sites on performance in a delayed non-match-to-position working memory (WM) task (DNMTP).
Methods
DNMTP consisted of 120 trials/session (5, 10, and 15 s delays). Rats received two doses (25 or 100 mg/kg, i.p.) of L-kynurenine (KYN; bioprecursor of KYNA) or L-4-chlorokynurenine (4-Cl-KYN; bioprecursor of the selective glycineB site antagonist 7-Cl-kynurenic acid). Attenuation of KYN- or 4-Cl-KYN-induced deficits was assessed by co-administration of galantamine (GAL, 3 mg/kg) or PAM-2 (1 mg/kg), two positive modulators of a7nAChR function. Reversal of 4-Cl-KYN- induced deficits was examined using D-cycloserine (DCS; 30 mg/kg), a partial agonist at the glycineB site.
Results
Both KYN and 4-Cl-KYN administration produced dose-related deficits in DNMTP accuracy that were more severe at the longer delays. In KYN-treated rats, these deficits were reversed to control levels by GAL or PAM-2 but not by DCS. In contrast, DCS eliminated performance deficits in 4-Cl-KYN-treated animals.
Conclusions
These experiments reveal that both a7nAChR and NMDAR activity are necessary for normal WM accuracy. They provide substantive new support for the therapeutic potential of positive modulators at these two receptor sites in SZ and other major brain diseases.
Keywords: Delayed non-matching to position task, Kynurenic acid, Working memory, Galantamine, PAM-2, D-cycloserine, 4-Cl-kynurenic acid
The construct of working memory (WM) occupies a critical position in the series of operations that support normal executive function or cognitive control and has been shown to be impaired in patients with schizophrenia (SZ; Elvevag and Goldberg 2000; Goldman-Rakic 1999; Park and Holzman 1992), major depressive disorder (MDD; Mohn and Rund 2016), and Alzheimer’s disease (AD; Castel et al. 2009). Cognitive deficits, including impairments in attention (Luck and Gold 2008; Nuechterlein et al. 2009), WM (Abi-Dargham et al. 2002; Perlstein et al. 2001), and cognitive control (Everett et al. 2001; Thoma et al. 2007), are in fact core symptoms of SZ, as they often present prior to the initial clinical episode and are also observed in first-degree relatives who do not display the respective phenotype (Snitz et al. 2006). Unfortunately, while the severity of these deficits is predictive of the patient’s functional outcome (Green 1996; Green et al. 2004), these symptoms are not effectively relieved by current medications or behavioral interventions (Bosia et al. 2017; Nielsen et al. 2015). Thus, additional information on the neurochemical events involved in these cognitive limitations, particularly when obtained from validated animal models and by using cognitive tasks that reliably measure the relevant behavioral constructs, is highly desirable and may lead to innovative treatment strategies in SZ as well as other disorders.
Impaired executive function in SZ includes dysregulations of multiple brain circuits and neurotransmitter systems. Of special relevance in this regard, dysfunctions in attention, WM, and cognitive flexibility critically involve impairments of cholinergic (Berman et al. 2007) and glutamatergic (Merritt et al. 2013; Paz et al. 2008; Thoma and Daum 2013)transmission in the prefrontal cortex (PFC), an area involved in the mediation/regulation of higher order cognition. Cholinergic abnormalities include reduced expression of the alpha 7 nicotinic acetylcholine receptor (α7nAChR) in the dorsolateral PFC (Mathew et al. 2007), as well as dysfunction of genes encoding the α7nAChR (Araud et al. 2011; Stefansson et al. 2008; Zammit et al. 2007). With regard to glutamatergic transmission, a considerable number of imaging and other studies in patients indicate hypofunctional NMDAR signaling within the PFC (Bickel and Javitt 2009; Poels et al. 2014), though AMPA/kainate or metabotropic glutamate receptors also appear to play distinct roles in the dysregulation of glutamatergic signaling (Javitt 2012; Howes et al. 2015 for review).
An additional pathophysiological feature reported in patients with SZ and MDD that may contribute significantly to both the cholinergic and glutamatergic dysfunctions discussed above is a marked elevation in brain levels of the tryptophan metabolite kynurenic acid (KYNA) (Erhardt et al. 2001; Miller et al. 2006; Sathyasaikumar et al. 2011; Schwarcz et al. 2001). KYNA is derived from its immediate bioprecursor L-kynurenine (KYN), a pivotal product of tryptophan metabolism, and is synthesized in astrocytes (Guillemin et al. 2001). Upon release, nanomolar concentrations of KYNA negatively modulate α7nAChR function in vivo (Alexander et al. 2013; Pocivavsek etal. 2012), though the underlying mechanism(s) may be indirect and remain to be clarified (Albuquerque and Schwarcz 2013; Flores-Barrera et al. 2017; Hilmas et al. 2001; Lopes et al. 2007; Stone 2019). In turn, antagonism of presynaptic α7nAChRs decreases the release and tone of several major neurotransmitters, including ACh (Zmarowski et al. 2009), glutamate (Beggiato et al. 2013; Konradsson-Geuken et al. 2010; Potter et al. 2010; Wu et al. 2010), GABA (Beggiato et al. 2014), and dopamine (Livingstone et al. 2010). At higher (low micromolar) concentrations, KYNA competitively antagonizes the glycine co-agonist site of the NMDAR (the “glycineB” receptor; Birch et al. 1988; Kessler et al. 1989; Stone and Darlington 2013). Not surprisingly, therefore, acute elevations of brain KYNA in adult rodents result in impairments that mimic several of the cognitive dysfunctions seen in SZ, including deficits in sensorimotor gating (Erhardt et al. 2004), WM (Chess et al. 2007), contextual learning (Chess et al. 2009; Pocivavsek et al. 2011), and cognitive flexibility (Alexander et al. 2012).
The aims of the present study were twofold. First, we examined the impact of acute elevations in brain KYNA on performance in a well-studied operant WM task by administering KYN systemically to adult rats, using the delayed nonmatching to position task (DNMTP) developed by Dunnett (1985) as an outcome measure. We then assessed the respective roles of the α7nAChR and the glycineB receptor by pharmacological means. To this end, we compared the cognition-impairing effects of KYN with those of 4-Cl-kynurenine (4-Cl-KYN), the bioprecursor of the selective glycineB receptor antagonist 7-Cl-kynurenic acid (7-Cl-KYNA; Kemp et al. 1988; Hilmas et al. 2001) and used positive allosteric modulators (PAMs) of α7 nAChRs, including galantamine (GAL; Lopes et al. 2007; Wadenberg et al. 2017) and PAM-2 (3-furan-2-yl-N-p-tolyl-acrylamide) (Arias et al. 2011, 2016; Potasiewicz et al. 2015, 2017; Targowska-Duda et al. 2016), and the partial glycineB receptor agonist D-cycloserine (DCS; Goff 2012) as tools to reverse performance deficits.
Methods
Animals
Adult male Wistar rats (Charles River Laboratory, Wilmington, MA) weighing 225–250 g at the start of training were singly housed under a 12-h light/dark cycle (lights on 06:00–18:00 h). Food was available ad libitum, but water intake was progressively restricted over the course of 1 week (water deprived successively 2, 4, 6, 12, 16, 20, and 22 h per day). Access was then maintained at 2 h/day throughout the duration of the experiment (including the test session). During this week, animals were also habituated to being handled, and weighed. Animal care and experimentation was performed in accordance with protocols approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee and was consistent with the NIH Guide for the Care and Use of Laboratory Animals.
Drugs and treatment groups
Four groups were used in these experiments and are identified in Table 1 according to the between- and within-group treatment differences. Three of the four groups were tested four times in the task; the other (PAM-2 group) was tested three times. Table 1 also illustrates the dosing schemes used. The first three groups tested the acute effects of KYN (Sai Advantium, Hyderabad, India) on DNMTP performance as well as the effects of three putative cognition enhancers (GAL, Sigma St. Louis, MO, USA; PAM-2, synthesized as in Arias et al., (2011); DCS, Bioexpress, Kaysville, UT, USA) on affecting KYN-induced deficits. The fourth group was used to determine if administration of 4-Cl-KYN (VistaGen Therapeutics Inc., Burlingame, CA, USA) impairs working memory and whether the co-agonist DCS influences performance deficits in this paradigm. All drugs were dissolved in 0.9% saline and administered (i.p.) in a volume of 1 ml/kg.
Table 1.
Summary of treatment groups and within subject injections
| Treatment group | Within subject injections | |||
|---|---|---|---|---|
| Kynurenine + galantamine | Vehicle + vehicle | 25 mg/kg KYN + vehicle | 100 mg/kg KYN + vehicle | 100 mg/kg KYN + 3 mg/kg GAL |
| Kynurenine + PAM-2 | Vehicle + vehicle | n/a | 100 mg/kg KYN + vehicle | 100 mg/kg KYN + 1 mg/kg PAM-2 |
| Kynurenine + D-cycloserine | Vehicle + vehicle | 25 mg/kg KYN + vehicle | 100 mg/kg KYN + vehicle | 100 mg/kg KYN + 30 mg/kg DCS |
| 4-Chlorokynurenine + D-cycloserine | Vehicle + vehicle | 25 mg/kg 4-Cl-KYN + vehicle | 100 mg/kg 4-Cl-KYN + vehicle | 100 mg/kg 4-Cl-KYN + 30 mg/kg DCS |
Four separate treatment groups of rats are represented in these experiments. The first column lists the drug used, as a between subject factor, to impair task performance (KYN kynurenine, 4-Cl-KYN 4-chlorokynurenine). The next four columns represent the individual injections given to all animals within each treatment group prior to each DNMTP drug session to reinstate performance (vehicle, GAL galantamine, PAM-2 3-furan-2-yl-N-p-tolyl-acrylamide, DCS D-cycloserine). The order of injections was randomized with a Latin Square to prevent order effects
As the optimal doses, injection routes, and time intervals used for testing the central effects of KYN and 4-Cl-KYN are well- established, the brain levels of newly produced KYNA and 7-Cl- KYNA were not measured in the present study. Specifically, the doses of KYN used were based on previous work showing the detrimental effects of an acute administration of this KYNA precursor on cognition (Alexander et al. 2012; Chess et al. 2007), and the doses of 4-Cl-KYN were guided by the affinity of 7-Cl-KYNA to the NMDA/glycineB receptor (IC50: 560 nM; Kemp et al. 1988). Thus, our i.p. administration of KYN rapidly raises the concentration of extracellular KYNA (Alexander et al. 2012), and an i.p. injection of 4-Cl-KYN leads to the presence of extracellular 7-Cl-KYNA (Wu et al. 1997) in the brain. Both these effects are dose-dependent, resulting in peak concentrations of ~ 40 nM KYNA (Alexander et al. 2012; Bortz et al. 2017) and ~ 50 nM 7-Cl-KYNA (Linderholm et al. 2010; Wu et al. 2000), respectively, approximately 2 h after the application of 100 mg/kg of the precursor.
Testing the ability of GAL and PAM-2 to reverse deficits was guided by the assumption that the doses used restricted these compounds to their abilities to selectively affect α7nAChR function (Arias et al. 2011; Choueiry et al. 2019; Kita et al. 2013). The GAL dose was based on recovery from deficits in an attentional set shifting task induced by acute administration of KYN (Alexander et al. 2012), and the dose of PAM-2 was selected based on its ability to effect promnesic and pro-cognitive activity through the potentiation of α7 nAChR function (Potasiewicz et al. 2015; Targowska-Duda et al. 2016; Potasiewicz et al. 2017). The dose of DCS was chosen based on rodent studies using a range of informative memory tasks (Gabriele and Packard 2007; Leslie et al. 2012; Shaw et al. 2009; Vurbic et al. 2011).
Training and testing procedure
All rats were trained in a DNMTP using a procedure that was modified from the original protocol. A standard rat operant system (10 chambers; Med Associates, St Albans, VT, USA) was used for training and testing. Each chamber was fitted with two fixed levers positioned on either side of a central water dispenser. Two white stimulus lights were located above each lever. Reinforcement was provided by water dispensed from a central reward port after the appropriate lever response. A house light was positioned in the top center of the back panel, opposite the levers and water dispenser. The training procedure involved 7 phases: lever pressing, sample phase, choice phase (subdivided into 5, 10, and 15 s delays sequentially added), a baseline performance acquisition phase testing that basal accuracy had returned prior to drug challenges, and, finally, a WM testing phase during which drugs were administered. Details regarding the criterion necessary for advancement to the next phase are outlined below.
Habituation to operant chambers and lever pressing
On day one of training, rats were placed in the operant chambers for 100 trials or a 60-min duration (whichever came first) and trained with a continuous FR1 reinforcement procedure. Each trial began when the house light was illuminated and levers were extended. If the rat pressed either lever, a droplet of water (0.02 ml) was dispensed at the water port. The development of a side bias was minimized by making each lever inactive after five consecutive presses, reactivating it only after the opposite lever was pressed. Criterion for advancement to sample phase training was met when both the left and right levers were pressed a minimum of 50 times within 1 h.
Sample phase
During sample phase training, a signal light illumination was added as a condition for reinforcement. Presentation of the left and right signals was balanced overall. Pressing the lever signaled at the start of sample phase (i.e., match) resulted in a water reward (0.02 ml), ending illumination of the signal light and the trial. In later stages of task training, for example when choice phase is introduced, the position of the signal light during the sample phase would determine the correct choice (i.e., non-matched) after the delay.
Delayed non-matching to position training and testing
Subjects were trained with the addition of a DNMTP rule where a delay (5 s) was added after the sample phase response. Both signal lights were then illuminated (choice phase). If the rat pressed the lever opposite to the sample (i.e., non-matching position), a correct response was recorded, both lights extinguished, and the rat was rewarded with a water droplet (0.05 ml). If the rat pressed the same lever as the sample (i.e., matching), an incorrect response was recorded, and the animal was not rewarded. The sample phase continued to be rewarded with 0.02 ml of water, while the choice phase was rewarded with 0.05 ml of water. The sample phase was continually reinforced to ensure that the animal re-oriented itself toward the reward dispenser, thus reducing the development of mediating strategies (Dudchenko 2004; Dunnett 1985). The larger water reward for the choice phase was provided to motivate completion of the full trial. Each trial was followed by a fixed inter-trial interval (ITI) of 10 s during which the house light was not illuminated; each session consisted of 120 trials. Advancement criterion for the 5 s delay length required > 70% correct responses and completion of at least 75% of the 120 trials for one session.
Once the advancement criterion was met, delay conditions (first 10 s, then 15 s) were added. Delays were randomly presented and interspersed throughout the session with approximately equal frequency. The final criteria for all three combined delays were: (a) > 80% overall correct responses, (b) > 80% correct in both 5 and 10 s delays, (c) > 75% correct in 15 s delay, and (d) > 75% of trials completed. Once these criteria were met on three consecutive days, drug testing days were initiated, and rats were required to meet final criteria for two consecutive training days before each successive drug test. This ensured that animals maintained baseline performance, and that a minimum of 48 h passed between each test. Approximately every 2 weeks animals were given at least a 24-h period of ad libitum access to water to help maintain body fluid balance and renal health.
Omissions
During each trial, the rat had 3 s to respond to the sample lever. If the lever was not pressed during this period, the signal and house lights were extinguished, and the event was recorded (“sample omission”). This was followed by an ITI of 5 s with the house light off. Failure to respond to the choice phase presentation after responding to a sample lever (“delay omission”) was recorded separately for each delay (5,10, and 15 s).
Statistical analysis
Drug effects were evaluated using two-way repeated measures ANOVAs. Both delay and drug conditions were within- subject factors, with performance determined at each delay level and drug dose (n = 7–8 rats/group). The first set of ANOVAs was designed to isolate the dose- and delay-dependent effects of KYN and 4-Cl-KYN. Following significant main and interaction effects, a second set of ANOVAs was used to compare the effects of putative cognition enhancers with the drug effects (including the vehicle/vehicle condition). Multiple post hoc comparisons were conducted in order to compare correct trial percentage (accuracy) at three different levels of delay and three different drug doses against vehicle performance. In order to minimize the probability of Type II errors, comparisons were made using a Bonferroni-corrected t test for dependent measures (Abdi 2007). Omission data were compiled and analyzed separately for selected treatment groups using drug dose as the independent variable (dose = 4 levels). The four groups were combined for acquisition (means ± SEM). All comparisons were made using commercial software (Prism 5.0c, GraphPad Software, Inc., 2004). P < 0.05 was considered statistically significant.
Results
Acquisition of DNMTP
Rats in all treatment groups and conditions readily acquired each stage of the DNMTP task as follows (expressed in days; mean ± SEM of all groups): Lever Press (1.2 ±0.1), Sample Phase (2.7 ±0.2), Choice Phases (5 s, 8.2 ±0.5; 10 s, 8.7 ± 0.7; 15 s, 15.7 ±2.3), and Stabilization of Baseline (13.7 ± 0.6). Total training time from lever press to initiation of testing averaged 50.6±3.0 days. One rat was excluded from the KYN + GAL condition after losing weight below our removal criterion of 15% loss from baseline.
Task performance
Acute KYN administration and the effect of galantamine
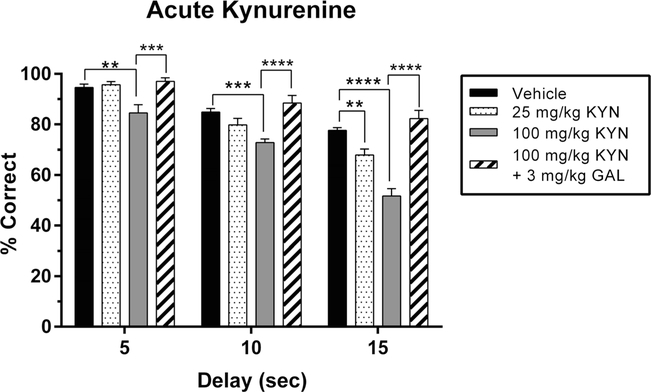
Initial testing revealed that acute administration of KYN produced significant overall reductions in performance accuracy on the DNMTP task when compared to the vehicle/vehicle condition (Fig. 1). As outlined in the data analysis portion of the “Methods” section, the dose- and delay-impairing effects of KYN were first calculated in the absence of the GAL treatment condition. The negative effects of KYN on performance became more pronounced as the dose of KYN increased from 25 to 100 mg/kg (main effect of dose: F2, 36 = 54.40, P < 0.0001). There was also an overall main effect of delay (F2,18 = 85.75, P < 0.0001). Thus, both vehicle- and KYN-treated rats exhibited poorer task performance as the delay length between sample and choice phases increased. Finally, we observed a greater sensitivity to the disrupting effects of 10 and 15 s delays in KYN-treated than in vehicle-treated animals (interaction effect: F4, 36 = 5.57, P = 0.001). The 5 s (P = 0.002) and 10 s (P = 0.0003) delays were affected only by 100 mg/kg KYN, whereas the longest delay was affected by both 25 mg/kg (P = 0.0036) and 100 mg/kg KYN (P <0.0001).
Fig. 1.
Effects of acute kynurenine (KYN) administration, with and without galantamine (GAL), on working memory performance in the delay non-match to position (DNMTP) task. Data represent the percentage of correctly completed trials (mean ± SEM), in a single group of rats (n = 7), during four drug sessions (order randomized: vehicle, 25 mg/kg KYN, 100 mg/kg KYN, 100 mg/kg KYN + 3 mg/kg GAL). Choice accuracy is depicted for all three delay intervals (5, 10, 15 s). Acute administration of KYN resulted in a delay-dependent reduction in accuracy that also interacted with drug dose. The low dose of KYN (25 mg/kg) reduced choice accuracy relative to vehicle controls during 15s delay trials (but not during the two shorter delays). The high dose of KYN (100 mg/kg) reduced choice accuracy during all three delays. Co-administration of GAL (3 mg/kg) fully prevented the performance deficit caused by KYN (100 mg/kg) during all three delays. Significance is denoted as P < 0.01 (**), P < 0.001 (***), and P <0.0001 (****)
Figure 1 also illustrates that co-administration of GAL (3 mg/kg) reversed the impairing effects of KYN on performance in the DNMTP task to vehicle control levels at all delays. As described in the data analysis section, a second set of ANOVAs was calculated to reveal the pro-cognitive effects of GAL treatment. With the GAL treatment condition included, the previously described main and interaction effects were preserved (delay: F2,18 = 65.21, P <0.0001; dose: F3,54 = 52.72, P <0.0001; interaction: F6,54 = 5.29, P =0.0002). Post hoc analyses revealed that GAL was most effective when tested under conditions that led to the greatest deficits, i.e., at the higher dose of KYN (100 mg/kg) and at the longest delay (15 s) (P < 0.0001 compared to KYN alone, P = 0.68 compared to vehicle).
Acute KYN administration and the effect of PAM-2
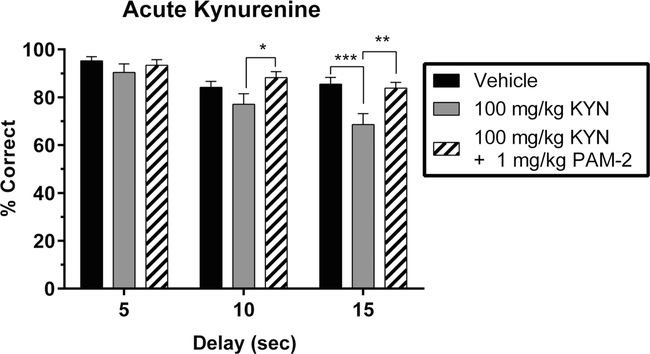
A separate group of KYN-treated animals was used to test the effectiveness of PAM-2, a highly selective α7nAChR PAM (Arias et al. 2011; Arias et al. 2016). To relate the results to those shown in Fig. 1, only rats receiving 100 mg/kg KYN or vehicle were compared. An initial analysis excluding the PAM-2 condition revealed main effects of KYN dose (F1,21 = 19.50; P = 0.0002), delay (F2,21 = 8.26; P = 0.002), and a trend toward an interaction between dose and delay (F2,21 = 2.91; P = 0.07). An additional analysis including the PAM-2 condition revealed main effects of group (F2,42= 13.97; P <0.0001), delay (F2,21 = 9.41; P = 0.001), and a trend toward an interaction between group and delay (F4,42 = 2.25; P = 0.07). As shown in Fig. 2, the KYN- induced deficit at the 15-s delay interval was completely abolished by the administration of PAM-2 (1 mg/kg, P = 0.001), reaching an accuracy level that was indistinguishable from vehicle-treated controls (P = 0.99).
Fig.2.
Effects of acute kynurenine (KYN) administration, with and without PAM-2, on working memory performance in the delay non-match to position (DNMTP) task. Data represent the percentage of correctly completed trials (mean ± SEM), in a single group of rats (n = 8), during three drug sessions (order randomized: vehicle, 100 mg/kg KYN, 100 mg/kg KYN + 1 mg/kg PAM-2). Choice accuracy is depicted for all three delay intervals (5, 10, 15 s). Acute administration of KYN resulted in a reduction in accuracy that interacts with delay length. KYN (100 mg/kg) reduced choice accuracy during the 10 and 15s delay trials but not during 5 s delay trials. Co-administration of PAM-2 (1 mg/kg) prevented the performance deficit caused by KYN (100 mg/kg) during the 10 and 15 s delay trials. Significance is denoted as P < 0.05 (*), P <0.01 (**), and P <0.001 (***)
KYN-induced deficits: no reversal by the NMDA/glycineB agonist D-cycloserine with higher cognitive load
The next experiment was designed to assess whether the impairing effects of KYN on task performance included, in addition to α7nAChR-based mechanisms, antagonism of NMDA-mediated transmission. To this end, we determined the ability of DCS, an NMDA/glycineB receptor agonist that does not bind to α7nAChRs (Hood et al. 1989), to counteract the impairing effects of KYN (see Fig. 3).
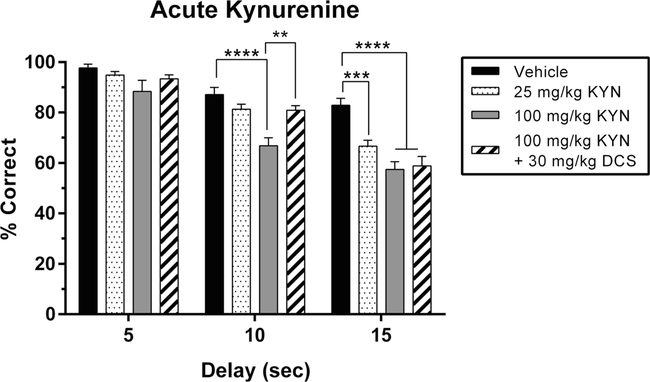
Fig.3.
Effects of acute kynurenine (KYN) administration, with and without D-cycloserine (DCS), on working memory performance in the delay non-match to position (DNMTP) task. Data represent the percentage of correctly completed trials (mean ± SEM), in a single group of rats (n = 7), during four drug sessions (order randomized: vehicle, 25 mg/kg KYN, 100 mg/kg KYN, 100 mg/kg KYN + 30 mg/kg DCS). Choice accuracy is depicted for all three delay intervals (5, 10, 15 s). Acute administration of KYN resulted in a delay-dependent reduction in accuracy that also interacted with drug dose. Compared to vehicle, the low dose of KYN (25 mg/kg) resulted in a deficit during 15 s delay trials but not during shorter delay trials. The high dose of KYN (100 mg/kg) reduced choice accuracy during the 10 and 15 s delay trials but not during 5 s delay trials. Co-administration of DCS (30 mg/kg) prevented the performance deficit caused by KYN (100 mg/kg) during the 10 s delay trials but did not significantly restore performance for 15 s delay trials. Significance is denoted as P <0.01 (**), P <0.001 (***) and P <0.0001 (****)
We first confirmed that administration of KYN resulted in significant effects of dose (F2,36 = 38.06; P < 0.0001), delay (F2,18 = 49.84; P <0.0001), and an interaction between the two (F4,36 = 3.34; P = 0.02). Including the DCS condition, we again found significant effects of dose (F3,54 = 22.56; P< 0.0001), delay (F2,18 = 118.6; P < 0.0001), and an interaction between the two (F6,54 = 3.34; P =0.007). However, unlike the case with GAL and PAM-2, administration of DCS (30 mg/kg) did not counteract the deficits produced by KYN (100 mg/kg; P = 0.99; Fig. 3) at the longest delay length (15 s). There was, however, a DCS-mediated recovery of KYN-induced deficits (100 mg/kg; P =0.0046) at the medium delay length (10 s).
Acute blockade of NMDA/glycineB receptors: reversal of 4-Cl-KYN-induced deficits
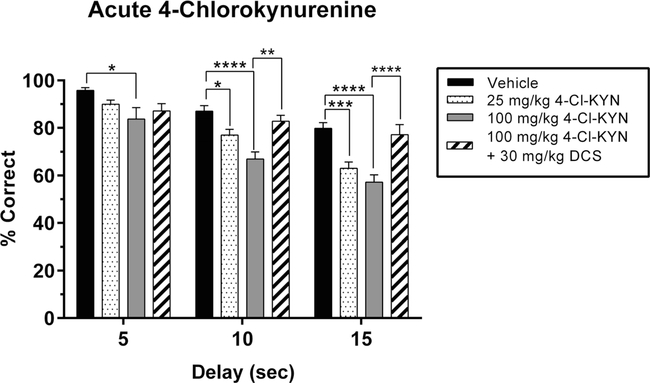
Previous studies have shown that administration of NMDA receptor antagonists causes deficits in WM (Didriksen et al. 2007; Galizio et al. 2012). Here we determined whether 4-Cl-KYN administration, resulting in the formation of the specific NMDA/glycineB receptor antagonist 7-Cl-KYNA (Wu et al. 1997), would be sufficient to produce deficits in WM. Acute administration of 4-Cl-KYN caused significant reductions in accuracy in the DNMTP task compared to vehicle treatment (main effect of dose: F2,36 = 26.5, P <0.0001; Fig. 4), and the magnitude of the reduction increased with the length of the delay (main effect of delay: F2,18 = 84.02, P < 0.0001). Notably, the lower dose of 4-Cl-KYN (25 mg/kg) affected only the intermediate and longest delays, whereas 100 mg/kg reduced performance even at the shortest delay. For 4-Cl-KYN, there was no interaction between delay and dose (F4,36= 1.142, P =0.35).
Fig. 4.
Effects of acute 4-chlorokynurenine (4-Cl-KYN) administration, with and without D-cycloserine (DCS), on working memory performance in the delay non-match to position (DNMTP) task. Data represent the percentage of correctly completed trials (mean ± SEM), in a single group of rats (n = 7), during four drug sessions (order randomized: vehicle, 25 mg/kg 4-Cl-KYN, 100 mg/kg 4-Cl-KYN, 100 mg/kg 4-Cl-KYN + 30 mg/kg DCS). Choice accuracy is depicted for all three delay intervals (5, 10, 15 s). Acute administration of 4-Cl-KYN resulted in a delay- dependent reduction in accuracy that did not show a dose × delay interaction. The low dose of 4-Cl-KYN (25 mg/kg) resulted in a deficit during 10 and the 15s delay trials but not during 5 s delay trials. The high dose of 4-Cl-KYN (100 mg/kg) reduced choice accuracy during all three delays. Co-administration of DCS (30 mg/kg) prevented the performance deficit caused by 4-Cl-KYN (100 mg/kg) during the 10 and 15 s delay trials but did not significantly restore performance for 5 s delay trials. Significance is denoted as P <0.05 (*), P <0.01 (**), P <0.001 (***), and P <0.0001 (****)
Regardless of delay length, co-administration of DCS (30 mg/kg) with 4-Cl-KYN (100 mg/kg) restored performance to levels that did not differ from vehicle-treated controls (all P values >0.05; Fig. 4). With the DCS treatment condition included, the previously described main effects were maintained (delay: F2,18 = 42.16, P <0.0001; dose: F3,54 = 21.17, P < 0.0001) while there was an interaction effect that trended toward statistical significance (F6,54 = 2.116, P = 0.066).
Trials completed and task omissions
The performance data were compiled from completed trials only, i.e., any trial that the animal did not complete (by either not responding to the sample or choice phase of a trial) was not incorporated into accuracy data. This prevented trials that were not completed (i.e., omissions) from influencing the accuracy results. We did, however, analyze the omission data as a means of determining whether drug affects could reflect declines in motivational state (i.e., satiety) or sensorimotor capacity that were then interpreted as a specific cognitive effect on WM.
Acute KYN administration and the effect of galantamine on task omissions
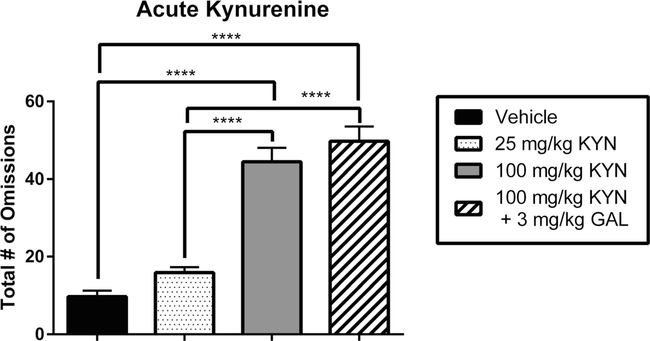
Initial testing revealed that acute administration of KYN produced significant increases in the number of omissions when compared to the vehicle/vehicle condition (main effect of dose: F2,18 = 59.40 P < 0.0001; Fig. 5). This effect was seen after administration of 100 mg/kg (P <0.0001) but not 25 mg/kg (P = 0.26) KYN. The increase in omissions occurred due to reduced sample phase responding (sample omissions 39.86 ±2.34 vs. delay omissions 4.57 ±1.42). Additional analysis revealed that KYN significantly increased omissions, relative to the vehicle condition, during the sample phase (F1,6 = 90.86, P <0.001) but not the choice phase (F1,9 = 2.82, P = 0.12).
Fig. 5.
Effects of acute kynurenine (KYN) administration, with and without galantamine (GAL), on task omissions in the delay non-match to position (DNMTP) task. Data represent the number of omissions (mean ± SEM), in a single group of rats (n = 7), during four drug sessions (order randomized: vehicle, 25 mg/kg KYN, 100 mg/kg KYN, 100 mg/kg KYN + 3 mg/kg GAL). Acute administration of KYN resulted in a dose-dependent increase in the number of omissions. The high dose (100 mg/kg) but not the low dose (25 mg/kg) of KYN significantly increased omissions compared to vehicle. Treatment with KYN (100 mg/kg) induced an increase in omissions. Co-administration of GAL (3 mg/kg) did not return the number of omissions to control levels despite the fact that accuracy values were normalized (cf. Fig. 1). Significance is denoted as P < 0.0001 (****)
Notably, the restoration of performance accuracy by GAL in KYN-treated animals was not accompanied by a reduction in the number of omissions. Co-administration of KYN and GAL was indistinguishable from the high dose KYN condition (P = 0.99) and remained higher than the number of omissions compared to the vehicle/vehicle condition (F3,24 = 50.67, P <0.0001).
Acute 4-Cl-KYN administration and the effect of D-cycloserine on task omissions
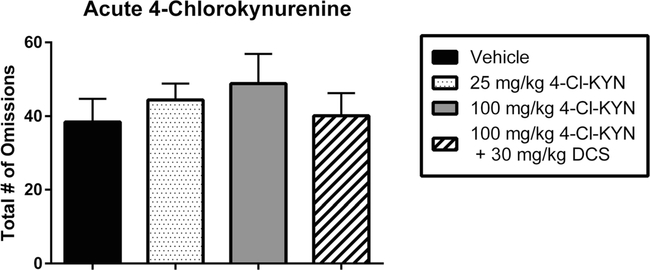
Acute administration of 4-Cl-KYN did not significantly change the number of omissions when compared to the vehicle/vehicle condition (main effect of dose: F2,18 = 0.66 P = 0.52; Fig. 6). The restoration of performance accuracy by DCS also did not alter the number of omissions compared to the vehicle/vehicle condition (F3,24 = 0.54, P <0.66). Co-administration of 4-Cl-KYN and DCS condition was indistinguishable from all other dose conditions (P = 0.99).
Fig. 6.
Effects of acute 4-chlorokynurenine (4-Cl-KYN) administration, with and without D-cycloserine (DCS), on task omissions in the delay non-match to position (DNMTP) task. Data represent the number of omissions (mean ± SEM), in a single group of rats (n = 7), during four drug sessions (order randomized: vehicle, 25 mg/kg 4-Cl-KYN, 100 mg/kg 4-Cl-KYN, 100 mg/kg 4-Cl-KYN+ 30 mg/kg DCS). Acute administration of 4-Cl-KYN did not significantly increase the number of omissions at either the high dose (100 mg/kg) or the low dose (25 mg/kg). Co-administration of 4-Cl-KYN and DCS also had no significant effect on the number of omissions despite the fact that DCS returned choice accuracy to control levels (cf. Fig. 4)
Discussion
The present study led to several novel and significant observations. First, an acute single injection of KYN (the immediate bioprecursor of KYNA) reduced accuracy of adult rats in the DNMTP WM task in both a dose- and delay-dependent fashion. Second, co-administration of GAL, an acetylcholinesterase inhibitor with positive allosteric modulatory (PAM) activity on nAChRs (Lopes et al. 2007), or PAM-2, a highly selective PAM of α7nAChRs (Arias et al. 2011, 2016; Targowska-Duda et al. 2016), eliminated KYN-induced impairments and restored task performance to normal levels. Third, performance deficits in KYN-treated rats were not reversed by addition of the glycineB/NMDA receptor agonist DCS. Finally, acute de novo synthesis of the selective glycineB/NMDA antagonist 7-Cl-KYNA (via systemic injection of its precursor 4-Cl-KYN) also produced deficits in performance accuracy which were similar in magnitude to those seen following KYN administration. These detrimental effects of 4-Cl-KYN were prevented by co-administration of DCS, a compound that was ineffective in alleviating deficits induced by KYN at the long delay (i.e., high cognitive load). Collectively, these observations indicate that both α7nACh and NMDA receptor function are required for normal WM. Moreover, they support the conclusion that the cognitive impairments seen following acute elevations of KYNA, through administration of up to 100 mg/kg of its precursor KYN (Alexander et al. 2012; Chess et al. 2009; Pocivavsek et al. 2011), are mediated by reductions in α7nACh function and not by NMDA receptors—a mechanism that may become operative, however, following greater and more prolonged disruptions to the kynurenine pathway (Forrest et al. 2015). The discussion that follows focuses on the mechanisms underlying the performance deficits produced by KYN and 4-Cl-KYN.
In our experiments, the magnitude of the impairments was dose- and delay-dependent—a profile consistent with a deficit in the construct of WM (Goldman-Rakic 1994). As expected, the greatest deficits were observed at the higher dose and at a longer delay interval (i.e., 15 s). Use of putative cognition enhancers such as GAL, PAM-2, and DCS as pharmacological probes indicated that the impairments in WM induced by KYN, as well as those seen after the administration of 4-Cl-KYN, were caused by identifiable and readily dissociable neurochemical mechanisms. Specifically, though they cannot distinguish between direct and indirect inhibition of the receptor (Albuquerque and Schwarcz 2013; Stone 2019), our results support a critical role of α7nAChRs in the deleterious effects of KYN described in the present study. Thus, elimination of the KYN-induced WM deficits by GAL and PAM-2 indicates that the WM impairment was triggered by KYNA’s action as an effective inhibitory modulator of α7nAChR function in vivo. This interpretation is in line with the fact that α7nAChR inhibition also plays a central role in other cognitive impairments which are seen in rodents after a systemic injection of 100 mg/kg KYN (Alexander et al. 2012; Pocivavsek et al. 2011) and, more generally, and that both GAL and PAM-2 produce a plethora of pro-cognitive and promnesic effects in rodents through their action on α7nAChRs (Ludwig et al. 2010; Potasiewicz et al. 2015, 2017; Targowska-Duda et al. 2016; Wadenberg et al. 2017). These effects were antagonized by methyllycaconitine (a selective α7nAChR antagonist) and showed synergistic efficacy when co-administered with α7 nAChR-selective agonists, supporting the notion that α7* nAChR potentiation is involved in these processes. Of special note in this context, though GAL acts as a cholinesterase inhibitor at higher concentrations (Bickel et al. 1991), the drug’s ability to neutralize the neurochemical consequences of increased KYNA levels (Beggiato et al. 2013, 2014; Wu et al. 2010), as well as several other biochemical and electrophysiological effects of GAL (Kita et al. 2013; Kroker et al. 2013; Schilström et al. 2007), are not duplicated by the cholinesterase inhibitor donepezil, another established cognition enhancer. This evidence supports the view that the effect elicited by GAL is mediated by its PAM activity on endogenous α7* nAChRs. Although our results support an important role of α7* nAChRs in WM, there is ample experimental evidence indicating that both α7nACh and NMDA receptors interact in the modulation of circuits devoted to WM, including those involving the hippocampus and PFC. For example, during early development, α7* nAChRs are needed for glutamatergic synapse formation (Lozada et al. 2012), whereas α7nAChR gene deletion decreases synaptic NMDAR levels and glutamate-related synaptic formation in cortical neurons with concomitant cognitive deficits (Lin et al. 2014). On the other hand, α7nAChR activation upregulates NMDARs (Li et al. 2013) and induces long-term potentiation (LTP) (Mann and Greenfield 2003) in hippocampal neurons, and these effects, important for learning and memory, are abolished by NMDAR blockers (Mann and Greenfield 2003) or when the structural interaction between α7nAChRs and NMDARs is disrupted (Li et al. 2013).
In marked contrast to GAL and PAM-2, DCS, a pro-cognitive partial agonist at the glycineB site of the NMDA receptor (Gabriele and Packard 2007; Goff 2012; Leslie et al. 2012), failed to counter the most detrimental effect of KYN on WM in our study. This provides further support for the argument that NMDARs are not a primary target of KYNA in mediating its adverse effects on cognition. This is of relevance since NMDAR blockade is clearly linked to a number of cognitive dysfunctions including WM impairment (Gonzalez-Burgos and Lewis 2012; Neill et al. 2010; Rowland et al. 2005), and as higher concentrations of KYNA inhibit the obligatory glycineB site of the NMDAR in a competitive manner (Birch et al. 1988; Kessler et al. 1989). Indeed, DCS readily prevented the WM dysfunction induced by 4-Cl-KYN, the bioprecursor of the selective glycineB receptor antagonist 7-Cl-KYNA, in the present study.
Admittedly, the systemic route of administration of the precursors KYN and 4-Cl-KYN in the present study makes it challenging to define the precise target region(s) which accounted for the observed DNMTP deficits. However, we assume a central role of the PFC since the integrity of prefrontal cholinergic and glutamatergic transmission is critical for several components of cognitive control, including attention (Benn and Robinson 2014; Parikh and Sarter 2008; St Peters et al. 2011) and WM (Aultman and Moghaddam 2001; Furey 2000). Interestingly, acute administration of KYN not only raises extracellular KYNA levels but also causes a secondary, GAL-sensitive decrease in extracellular glutamate in the PFC (Alexander et al. 2012; Wu et al. 2010). In line with earlier studies demonstrating the presence of functional α7nAChRs on glutamatergic nerve terminals in the PFC (Dickinson et al. 2008; Marchi etal. 2002; Udakis etal. 2016), these datathere- fore suggest an attractive mechanism, linking elevated prefrontal KYNA levels indirectly to glutamate hypofunction and WM deficits. Taken together, the results described here provide significant support for the hypothesis that KYNA malfunction in the brain is causally involved in the pathophysiology of major psychiatric diseases (cf. Introduction).
Finally, while the accuracy data were calculated on the basis of completed trials, the patterns depicted in Figs. 5 and 6 suggest that the impairments seen in KYN- and 4-Cl-KYN- treated animals did not simply reflect drug-induced reductions in motivation or sensorimotor capacity. Moreover, the procognitive effects of GAL and DCS were clearly dissociable from their inability to affect numbers of omissions. This dissociation of the omission effects of KYN from performance is consistent with other findings showing increases in errors of radial arm maze accuracy in the absence of changes in locomotor behavior or latency to consume reward (Chess et al. 2007).
Taken together, the data described here add substantive knowledge regarding the impact of KYNA, a well-established, astrocyte-derived neuromodulator (Schwarcz and Stone 2017), on cholinergic and glutamatergic processes involved in cognitive functions. Using informative pharmacological tools, our experiments provide new conceptual insights through proof-of-principle studies. From a therapeutic perspective, the results provide further justification for accelerated development of cognition-enhancing agents based on the positive modulation of both α7nACh and NMDA receptors (Koola 2018).
Acknowledgements
This work was supported by USPHS grants RO1 MH083729 (JB & RS) and P50 MH103222 (RS; Conte Center for Translational Research).
Footnotes
Compliance with ethical standards
Animal care and experimentation was performed in accordance with protocols approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee and was consistent with the NIH Guide for the Care and Use of Laboratory Animals.
Publisher′s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdi H (2007) The Bonferonni and Šidák corrections for multiple comparisons. In: Encyclopedia of measurement and statistics. Sage Publications, Inc, Thousand Oaks, pp 103–107. 10.4135/9781412952644 [DOI] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y et al. (2002) Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci 22(9):3708–3719. 10.1523/JNEUROSCI.22-09-03708.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Schwarcz R (2013) Kynurenic acid as an antagonist of α7 nicotinic acetylcholine receptors in the brain: facts and challenges. Biochem Pharmacol 85(8):1027–1032. 10.1016/j.bcp.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Wu HQ, Schwarcz R, Bruno JP (2012) Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology 220(3):627–637. 10.1007/s00213-011-2539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Pocivavsek A, Wu H-Q, Pershing ML, Schwarcz R, Bruno JP (2013) Early developmental elevations of brain kynurenic acid impair cognitive flexibility in adults: reversal with galantamine. Neuroscience 238:19–28. 10.1016/j.neuroscience.2013.01.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araud T, Graw S, Berger R, Lee M, Neveu E, Bertrand D, Leonard S (2011) The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7*nAChR function. Biochem Pharmacol 82(8):904–914. 10.1016/j.bcp.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias HR, Gu R-X, Feuerbach D, Guo B-B, Ye Y, Wei D-Q (2011) Novel positive allosteric modulators of the human α7 nicotinic acetylcholine receptor. Biochemistry 50(23):5263–5278. 10.1021/bi102001m [DOI] [PubMed] [Google Scholar]
- Arias HR, Ravazzini F, Targowska-Duda KM, Kaczor AA, Feuerbach D, Boffi JC, Draczkowski P, Montag D, Brown BM, Elgoyhen AB, Jozwiak K, Puia G (2016) Positive allosteric modulators of α7 nicotinic acetylcholine receptors affect neither the function of other ligand- and voltage-gated ion channels and acetylcholinesterase, nor p-amyloid content. Int J Biochem Cell Biol 76:19–30. 10.1016/j.biocel.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B (2001) Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology 153(3):353–364. 10.1007/s002130000590 [DOI] [PubMed] [Google Scholar]
- Beggiato S, Antonelli T, Tomasini MC, Tanganelli S, Fuxe K, Schwarcz R, Ferraro L (2013) Kynurenic acid, by targeting α7 nicotinic acetylcholine receptors, modulates extracellular GABA levels in the rat striatum in vivo. Eur J Neurosci 37(9):1470–1477. 10.1111/ejn.12160 [DOI] [PubMed] [Google Scholar]
- Beggiato S, Tanganelli S, Fuxe K, Antonelli T, Schwarcz R, Ferraro L (2014) Endogenous kynurenic acid regulates extracellular GABA levels in the rat prefrontal cortex. Neuropharmacology 82(2014): 11–18. 10.1016/j.neuropharm.2014.02.019 [DOI] [PubMed] [Google Scholar]
- Benn A, Robinson ESJ (2014) Investigating glutamatergic mechanism in attention and impulse control using rats in a modified 5-choice serial reaction time task. PLoS One 9(12):e115374 10.1371/journal.pone.0115374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JA, Talmage DA, Role LW (2007) Cholinergic circuits and signaling in the pathophysiology of schizophrenia. Int Rev Neurobiol 78(06):193–223. 10.1016/S0074-7742(06)78007-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S, Javitt DC (2009) Neurophysiological and neurochemical animal models of schizophrenia: focus on glutamate. Behav Brain Res 204(2):352–362. 10.1016/j.bbr.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel U, Thomsen T, Weber W, Fischer JP, Bachus R, Nitz M, Kewitz H (1991) Pharmacokinetics of galanthamine in humans and corresponding cholinesterase inhibition. Clin Pharmacol Ther 50(4): 420–428. 10.1038/clpt.1991.159 [DOI] [PubMed] [Google Scholar]
- Birch PJ, Grossman CJ, Hayes AG (1988) Kynurenic acid antagonises responses to NMDA via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol 154(1):85–87. 10.1016/0014-2999(88)90367-6 [DOI] [PubMed] [Google Scholar]
- Bortz DM, Wu H-Q, Schwarcz R, Bruno JP (2017) Oral administration of a specific kynurenic acid synthesis (KAT II) inhibitor attenuates evoked glutamate release in rat prefrontal cortex. Neuropharmacology 121(2017):69–78. 10.1016/j.neuropharm.2017.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosia M, Buonocore M, Bechi M, Spangaro M, Pigoni A, Croci M et al. (2017) Cognitive remediation and functional improvement in schizophrenia: is it a matter of size? Eur Psychiatry 40(2017):26–32. 10.1016/j.eurpsy.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Castel AD, Balota DA, McCabe DP (2009) Memory efficiency and the strategic control of attention at encoding: impairments of value-directed remembering in Alzheimer’s disease. Neuropsychology 23(3):297–306. 10.1037/a0014888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess AC, Simoni MK, Alling TE, Bucci DJ (2007) Elevations of endogenous kynurenic acid produce spatial working memory deficits. Schizophr Bull 33(3):797–804. 10.1093/schbul/sbl033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess AC, Landers AM, Bucci DJ (2009) L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cuespecific fear conditioning. Behav Brain Res 201(2):325–331. 10.1016/j.bbr.2009.03.013 [DOI] [PubMed] [Google Scholar]
- Choueiry J, Blais CM, Shah D, Smith D, Fisher D, Illivitsky V, Knott V (2019) Combining CDP-choline and galantamine: effects of a selective α7 nicotinic acetylcholine receptor agonist strategy on P50 sensory gating of speech sounds in healthy volunteers. J Psychopharmacol 33(6):688–699. 10.1177/0269881119836217 [DOI] [PubMed] [Google Scholar]
- Dickinson JA, Kew JNC, Wonnacott S (2008) Presynaptic 7- and 2- containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol 74(2):348–359. 10.1124/mol.108.046623 [DOI] [PubMed] [Google Scholar]
- Didriksen M, Skarsfeldt T, Arnt J (2007) Reversal ofPCP-induced learning and memory deficits in the Morris’ water maze by sertindole and other antipsychotics. Psychopharmacology 193(2):225–233. 10.1007/s00213-007-0774-3 [DOI] [PubMed] [Google Scholar]
- Dudchenko PA (2004) An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev 28(7):699–709. 10.1016/j.neubiorev.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Dunnett SB (1985) Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology 87(3):357–363. 10.1007/BF00432721 [DOI] [PubMed] [Google Scholar]
- Elvevåg B, Goldberg TE (2000) Cognitive impairment in schizophrenia is the core of the disorder. Crit RevNeurobiol 14(1):1–21. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11253953 [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G (2001) Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313(1–2):96–98. 10.1016/S0304-3940(01)02242-X [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Emanuelsson C, Geyer M (2004) Endogenous kynurenic acid disrupts prepulse inhibition. Biol Psychiatry 56(4): 255–260. 10.1016/j.biopsych.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Everett J, Lavoie K, Gagnon JF, Gosselin N (2001) Performance of patients with schizophrenia on the Wisconsin Card Sorting Test (WCST). J Psychiatry Neurosci 26(2):123–130 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11291529 [PMC free article] [PubMed] [Google Scholar]
- Flores-Barrera E, Thomases DR, Cass DK, Bhandari A, Schwarcz R, Bruno JP, Tseng KY (2017) Preferential disruption of prefrontal GABAergic function by nanomolar concentrations of the α7nACh negative modulator kynurenic acid. J Neurosci 37(33):7921–7929. 10.1523/JNEUROSCI.0932-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest CM, McNair K, Pisar M, Khalil OS, Darlington LG, Stone TW (2015) Altered hippocampal plasticity by prenatal kynurenine administration, kynurenine-3-monoxygenase (KMO) deletion or galantamine. Neuroscience 310:91–105. 10.1016/j.neuroscience.2015.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML (2000) Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science 290(5500): 2315–2319. 10.1126/science.290.5500.2315 [DOI] [PubMed] [Google Scholar]
- Gabriele A, Packard MG (2007) D-Cycloserine enhances memory consolidation of hippocampus-dependent latent extinction. Learn Mem 14(7):468–471. 10.1101/lm.528007 [DOI] [PubMed] [Google Scholar]
- Galizio M, Deal M, Hawkey A, April B (2012) Working memory in the odor span task: effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology 225(2):397–406. 10.1007/s00213-012-2825-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DC (2012) D-cycloserine: an evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull 38(5):936–941. 10.1093/schbul/sbs012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1994) Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 6(4):348–357. 10.1176/jnp.6.4.348 [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1999) The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry 46(5):650–661. 10.1016/S0006-3223(99)00130-4 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA (2012) NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 38(5):950–957. 10.1093/schbul/sbs010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF (1996) What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 153(3): 321–330. 10.1176/ajp.153.3.321 [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK (2004) Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 72(1):41–51. 10.1016/j.schres.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Kerr SJ, Smythe GA, Smith DG, Kapoor V, Armati PJ et al. (2001) Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J Neurochem 78(4):842–853. 10.1046/j.1471-4159.2001.00498.x [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX (2001) The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci 21(19):7463–7473 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11567036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood WF, Compton RP, Monahan JB (1989) D-cycloserine: a ligand for the coupled glycine receptor has partial agonist characteristics. Neurosci Lett 98(1):91–95. 10.1016/0304-3940(89)90379-0 [DOI] [PubMed] [Google Scholar]
- Howes O, McCutcheon R, Stone J (2015) Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 29(2):97–115. 10.1177/0269881114563634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC (2012) Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull 38(5):911–913. 10.1093/schbul/sbs100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JA, Foster AC, Leeson PD, Priestley T, Tridgett R, Iversen LL, Woodruff GN (1988) 7-Chlorokynurenic acid is a selective antagonist at the glycine modulatory site of the N-methyl-D-aspartate receptor complex. Proc Natl Acad Sci U S A 85(17):6547–6550. 10.1073/pnas.85.17.6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Terramani T, Lynch G, Baudry M (1989) A glycine site associated with N-methyl-d-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem 52(4): 1319–1328. 10.1111/j.1471-4159.1989.tb01881.x [DOI] [PubMed] [Google Scholar]
- Kita Y, Ago Y, Takano E, Fukada A, Takuma K, Matsuda T (2013) Galantamine increases hippocampal insulin-like growth factor 2 expression via α7 nicotinic acetylcholine receptors in mice. Psychopharmacology 225(3):543–551. 10.100/s00213-012-2841-7 [DOI] [PubMed] [Google Scholar]
- Konradsson-Geuken Å, Wu HQ, Gash CR, Alexander KS, Campbell A, Sozeri Y, Pellicciari R, Schwarcz R, Bruno JP (2010) Cortical kynurenic acid bi-directionally modulates prefrontal glutamate levels as assessed by microdialysis and rapid electrochemistry. Neuroscience 169(4):1848–1859. 10.1016/j.neuroscience.2010.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koola MM (2018) Potential role of antipsychotic-galantamine-memantine combination in the treatment of positive, cognitive, and negative symptoms of schizophrenia. Mol Neuropsychiatry 4(3):134–148. 10.1159/000494495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroker KS, Moreth J, Kussmaul L, Rast G, Rosenbrock H (2013) Restoring long-term potentiation impaired by amyloid-beta oligomers: comparison of an acetylcholinesterase inhibitior and selective neuronal nicotinic receptor agonists. Brain Res Bull 96:28–38. 10.1016/j.brainresbull.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Leslie JC, Norwood K, Kennedy PJ, Begley M, Shaw D (2012) Facilitation of extinction of operant behaviour in C57Bl/6 mice by chlordiazepoxide and D-cycloserine. Psychopharmacology 223(2): 223–235. 10.1007/s00213-012-2710-4 [DOI] [PubMed] [Google Scholar]
- Li S, Nai Q, Lipina TV, Roder JC, Liu F (2013) α7nAchR/NMDAR coupling affects NMDAR function and object recognition. Mol Brain 6(1):58 10.1186/1756-6606-6-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Hsu FC, Baumann BH, Coulter DA, Lynch DR (2014) Cortical synaptic NMDA receptor deficits in α7 nicotinic acetylcholine receptor gene deletion models: implications for neuropsychiatric diseases. Neurobiol Dis 63(2014):129–140. 10.1016/j.nbd.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm K, Powell S, Olsson E, Holtze M, Snodgrass R, Erhardt S (2010) Role of the NMDA-receptor in prepulse inhibition in the rat. Int J Tryptophan Res 3(1):1–12 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22084584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone PD, Dickinson JA, Srinivasan J, Kew JNC, Wonnacott S (2010) Glutamate-dopamine crosstalk in the rat prefrontal cortex is modulated by Alpha7 nicotinic receptors and potentiated by PNU-120596. J Mol Neurosci 40(1–2):172–176. 10.1007/s12031-009-9232-5 [DOI] [PubMed] [Google Scholar]
- Lopes C, Pereira EFR, Wu H-Q, Purushottamachar P, Njar V, Schwarcz R, Albuquerque EX (2007) Competitive antagonism between the nicotinic allosteric potentiating ligand galantamine and kynurenic acid at 7* nicotinic receptors. J Pharmacol Exp Ther 322(1):48–58. 10.1124/jpet.107.123109 [DOI] [PubMed] [Google Scholar]
- Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK (2012) Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J Neurosci 32(22): 7651–7661. 10.1523/JNEUROSCI.6246-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Gold JM (2008) The construct of attention in schizophrenia. Biol Psychiatry 64(1):34–39. 10.1016/j.biopsych.2008.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Hoffle-Maas A, Samochocki M, Luttmann E, Albuquerque EX, Fels G, Maelicke A (2010) Localization by site-directed mutagenesis of a galantamine binding site on α7 nicotinic acetylcholine receptor extracellular domain. J Recept Signal Transduction 30(6): 469–483. 10.3109/10799893.2010.505239 [DOI] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA (2003) Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol 551(2):539–550. 10.1113/jphysiol.2003.045492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M (2002) Direct evidence that release-stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. J Neurochem 80(6):1071–1078. https://doi.org/10.1046Zj.0022-3042.2002.00805.x [DOI] [PubMed] [Google Scholar]
- Mathew SV, Law AJ, Lipska BK, Dávila-Garcia MI, Zamora ED, Mitkus SN, Hyde TM (2007) Alpha7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum Mol Genet 16(23):2921–2932. 10.1093/hmg/ddm253 [DOI] [PubMed] [Google Scholar]
- Merritt K, McGuire P, Egerton A (2013) Relationship between glutamate dysfunction and symptoms and cognitive function in psychosis. Front Psychiatry 4(NOV):151 10.3389/fpsyt.2013.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Llenos IC, Dulay JR, Weis S (2006) Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res 1073–1074(1):25–37. 10.1016/j.brainres.2005.12.056 [DOI] [PubMed] [Google Scholar]
- Mohn C, Rund BR (2016) Neurocognitive profile in major depressive disorders: relationship to symptom level and subjective memory complaints. BMC Psychiatry 16(1):1–6. 10.1186/s12888-016-0815-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL et al. (2010) Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther 128(3):419–432. 10.1016/j.pharmthera.2010.07.004 [DOI] [PubMed] [Google Scholar]
- Nielsen RE, Levander S, Kjaersdam Telléus G, Jensen SOW, Østergaard Christensen T, Leucht S (2015) Second-generation antipsychotic effect on cognition in patients with schizophrenia—a meta-analysis of randomized clinical trials. Acta Psychiatr Scand 131(3):185–196. 10.1111/acps.12374 [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M (2009) CNTRICS final task selection: control of attention. Schizophr Bull 35(1): 182–196. 10.1093/schbul/sbn158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M (2008) Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci 1129:225–235. 10.1196/annals.1417.021 [DOI] [PubMed] [Google Scholar]
- Park S, Holzman P (1992) Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 49(12):975–982. 10.1001/archpsyc.1992.01820120063009 [DOI] [PubMed] [Google Scholar]
- Paz RD, Tardito S, Atzori M, Tseng KY (2008) Glutamatergic dysfunction in schizophrenia: from basic neuroscience to clinical psychopharmacology. Eur Neuropsychopharmacol 18(11):773–786. 10.1016/j.euroneuro.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD (2001) Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatr 158(7): 1105–1113. 10.1176/appi.ajp.158.7.1105 [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Wu H-Q, Potter MC, Elmer GI, Pellicciari R, Schwarcz R (2011) Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 36(11):2357–2367. 10.1038/npp.2011.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R (2012) Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci 35(10):1605–1612. 10.1111/j.1460-9568.2012.08064.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poels EMP, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA et al. (2014) Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Mol Psychiatry 19(1):20–29. 10.1038/mp.2013.136 [DOI] [PubMed] [Google Scholar]
- Potasiewicz A, Kos T, Ravazzini F, Puia G, Arias HR, Popik P, Nikiforuk A (2015) Pro-cognitive activity in rats of 3-furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the α7 nicotinic acetylcholine receptor. Br J Pharmacol 172(21):5123–5135. 10.1111/bph.13277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potasiewicz A, Holuj M, Kos T, Popik P, Arias HR, Nikiforuk A (2017) 3-Furan-2-yl-N-p-tolyl-acrylamide, a positive allosteric modulator of the α7 nicotinic receptor, reverses schizophrenia-like cognitive and social deficits in rats. Neuropharmacology 113:188–197. 10.1016/j.neuropharm.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu H-Q, Schwarcz R (2010) Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology 35(8):1734–1742. 10.1038/npp.2010.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Astur RS, Jung RE, Bustillo JR, Lauriello J, Yeo RA (2005) Selective cognitive impairments associated with NMDA receptor blockade in humans. Neuropsychopharmacology 30(3):633–639. 10.1038/sj.npp.1300642 [DOI] [PubMed] [Google Scholar]
- Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, Schwarcz R (2011) Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull 37(6):1147–1156. 10.1093/schbul/sbq112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilström B, Ivanov VB, Wiker C, Svensson TH (2007) Galantamine enhances dopaminergic neurotransmission in vivo via allosteric potentiation of nicotinic acetylcholine receptors. Neuropsychopharmacology 32(1):43–53. 10.1038/sj.npp.1301087 [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Stone TW (2017) The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology 112: 237–247. 10.1016/j.neuropharm.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu H-Q, Medoff D, Tamminga CA, Roberts RC (2001) Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50(7):521–530. 10.1016/S0006-3223(01)01078-2 [DOI] [PubMed] [Google Scholar]
- Shaw D, Norwood K, Sharp K, Quigley L, McGovern SFJ, Leslie JC (2009) Facilitation of extinction of operant behaviour in mice by D-cycloserine. Psychopharmacology 202(1–3):397–402. 10.1007/s00213-008-1312-7 [DOI] [PubMed] [Google Scholar]
- Snitz BE, MacDonald AW, Carter CS (2006) Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull 32(1): 179–194. 10.1093/schbul/sbi048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M (2011) Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci 31(26):9760–9771. 10.1523/JNEUROSCI.1902-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilâinen OPH, Ingason A, Steinberg S et al. (2008) Large recurrent microdeletions associated with schizophrenia. Nature 455(7210):232–236. 10.1038/nature07229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW (2019) Does kynurenic acid act on nicotinic receptors? An assessment of the evidence. J Neurochem,.14907 10.1111/jnc.14907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, Darlington LG (2013) The kynurenine pathway as atherapeu- tic target in cognitive and neurodegenerative disorders. Br J Pharmacol 169(6): 1211–1227. 10.1111/bph.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targowska-Duda KM, Wnorowski A, Budzynska B, Jozwiak K, Biala G, Arias HR (2016) The positive allosteric modulator of α7 nicotinic acetylcholine receptors, 3-furan-2-yl-N-p-tolyl-acrylamide, enhances memory processes and stimulates ERK½ phosphorylation in mice. Behav Brain Res 302:142–151. 10.1016/j.bbr.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Thoma P, Daum I (2013) Comorbid substance use disorder in schizophrenia: a selective overview of neurobiological and cognitive underpinnings. Psychiatry Clin Neurosci 67(6):367–383. 10.1111/pcn.12072 [DOI] [PubMed] [Google Scholar]
- Thoma P, Wiebel B, Daum I (2007) Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophr Res 92(1–3):168–180. 10.1016/j.schres.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Udakis M, Wright VL, Wonnacott S, Bailey CP (2016) Integration of inhibitory and excitatory effects of α7 nicotinic acetylcholine receptor activation in the prelimbic cortex regulates network activity and plasticity. Neuropharmacology 105:618–629. 10.1016/j.neuropharm.2016.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vurbic D, Gold B, Bouton ME (2011) Effects of D-cycloserine on the extinction of appetitive operant learning. Behav Neurosci 125(4): 551–559. 10.1037/a0024403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg M-LG, Manetti D, Romanelli MN, Arias HR (2017) Significance of the nicotinic alpha7 receptor in cognition and antipsychotic-like behavior in the rat. Behav Brain Res 333(June): 129–134. 10.1016/j.bbr.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Wu H-Q, Salituro FG, Schwarcz R (1997) Enzyme-catalyzed production of the neuroprotective NMDA receptor antagonist 7-chlorokynurenic acid in the rat brain in vivo. Eur J Pharmacol 319(1):13–20. 10.1016/S0014-2999(96)00829-1 [DOI] [PubMed] [Google Scholar]
- Wu H-Q, Lee S-C, Schwarcz R (2000) Systemic administration of 4-chlorokynurenine prevents quinolinate neurotoxicity in the rat hippocampus. Eur J Pharmacol 390(3):267–274. 10.1016/S0014-2999(00)00024-8 [DOI] [PubMed] [Google Scholar]
- Wu H-Q, Pereira EFR, Bruno JP, Pellicciari R, Albuquerque EX, Schwarcz R (2010) The astrocyte-derived α7 nicotinic receptor antagonist kynurenic acid controls extracellular glutamate levels in the prefrontal cortex. J Mol Neurosci 40(1–2):204–210. 10.1007/s12031-009-9235-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit S, Spurlock G, Williams H, Norton N, Williams N, O’Donovan MC, Owen MJ (2007) Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use. Br J Psychiatry 191(5):402–407. 10.1192/bjp.bp.107.036129 [DOI] [PubMed] [Google Scholar]
- Zmarowski A, Wu H-Q, Brooks JM, Potter MC, Pellicciari R, Schwarcz R, Bruno JP (2009) Astrocyte-derived kynurenic acid modulates basal and evoked cortical acetylcholine release. Eur J Neurosci 29(3):529–538. 10.1111/j.1460-9568.2008.06594.x [DOI] [PubMed] [Google Scholar]