Abstract
Introduction
Alterations in haemoglobin levels are frequent in stroke patients. The prognostic meaning of anaemia and polyglobulia on outcomes in patients treated with intravenous thrombolysis is ambiguous.
Patients and methods
In this prospective multicentre, intravenous thrombolysis register-based study, we compared haemoglobin levels on hospital admission with three-month poor outcome (modified Rankin Scale 3–6), mortality and symptomatic intracranial haemorrhage (European Cooperative Acute Stroke Study II-criteria (ECASS-II-criteria)). Haemoglobin level was used as continuous and categorical variable distinguishing anaemia (female: <12 g/dl; male: <13 g/dl) and polyglobulia (female: >15.5 g/dl; male: >17 g/dl). Anaemia was subdivided into mild and moderate/severe (female/male: <11 g/dl). Normal haemoglobin level (female: 12.0–15.5 g/dl, male: 13.0–17.0 g/dl) served as reference group. Unadjusted and adjusted odds ratios with 95% confidence intervals were calculated with logistic regression models.
Results
Among 6866 intravenous thrombolysis-treated stroke patients, 5448 (79.3%) had normal haemoglobin level, 1232 (17.9%) anaemia – of those 903 (13.2%) had mild and 329 (4.8%) moderate/severe anaemia – and 186 (2.7%) polyglobulia. Anaemia was associated with poor outcome (ORadjusted 1.25 (1.05–1.48)) and mortality (ORadjusted 1.58 (1.27–1.95)). In anaemia subgroups, both mild and moderate/severe anaemia independently predicted poor outcome (ORadjusted 1.29 (1.07–1.55) and 1.48 (1.09–2.02)) and mortality (ORadjusted 1.45 (1.15–1.84) and ORadjusted 2.00 (1.46–2.75)). Each haemoglobin level decrease by 1 g/dl independently increased the risk of poor outcome (ORadjusted 1.07 (1.02–1.11)) and mortality (ORadjusted 1.08 (1.02–1.15)). Anaemia was not associated with occurrence of symptomatic intracranial haemorrhage. Polyglobulia did not change any outcome.
Discussion
The more severe the anaemia, the higher the probability of poor outcome and death. Severe anaemia might be a target for interventions in hyperacute stroke.
Conclusion
Anaemia on admission, but not polyglobulia, is a strong and independent predictor of poor outcome and mortality in intravenous thrombolysis-treated stroke patients.
Keywords: Anaemia, polyglobulia, haemoglobin, intravenous thrombolysis, outcome, stroke
Introduction
Alterations in haemoglobin levels (HLs) on admission are frequently (anaemia up to 25%) observed in acute stroke patients.1,2 In general stroke populations, anaemia was associated with poorer outcomes1,3–5 except for one study.6 Only one smaller study (n = 217) has investigated the effect of anaemia on outcomes in stroke patients treated with intravenous thrombolysis (IVT). In this study, the development of anaemia or worsening of anaemia in the first days after admission was associated with poor functional outcome and mortality but not the presence of anaemia on admission.7 A second study, including both IVT (n = 466) and endovascular treated patients (n = 712), found anaemia on admission being an independent predictor of poor functional outcome and mortality.8 From a pathophysiological point of view, low HL at stroke onset is likely to impair outcomes due to a mismatch between increased metabolic requirements of the penumbral brain tissue and lowered oxygen transport capacity and reduced blood perfusion. Furthermore, HL might also affect outcomes via alterations in cerebrovascular autoregulation, blood coagulation and inflammatory mediators.1,9–12
On the other side of the spectrum, the presence of polyglobulia in the general stroke population was associated with mortality in one1 but not in another study.4 Therefore, the prognostic meaning of anaemia and polyglobulia on outcomes in patients treated with IVT remains unclear.
The aim of this study was to investigate the effect of baseline HL on functional outcome, mortality and bleeding risk in a large cohort of IVT-treated stroke patients.
Methods
For this cohort study, we used prospectively collected data from the ThRombolysis in Ischemic Stroke Patients (TRISP) registry which has been previously described.13 Ten TRISP centres participated in this study (eTable 1). A complete list of all TRISP centres is presented in the online supplement (eAppendix 1). Data collection was done locally in each stroke centre using a standardised form with predefined parameters.14,15 Data of the local registries were pooled and analysed at the stroke centre Basel. Parameters of interest for the present study were age, sex, National Institutes of Health Stroke Scale (NIHSS) score,16 blood pressure prior to IVT treatment, onset-to-treatment time, estimated glomerular filtration rate using the CKD-EPI formular,17 glucose levels and HL in blood serum on admission, vascular risk factors according to predefined criteria13,18 and prior treatment with antithrombotic agents (antiplatelet agents or anticoagulants). Outcome parameters were mortality and the modified Rankin Scale (mRS) score at three months assessed either by outpatient visits or telephone calls with patients and/or relatives. Poor functional outcome was defined as a mRS score of 3–6. As safety outcome we defined the occurrence of symptomatic intracranial haemorrhage (sICH) using the ECASS-II-criteria.19 Intracranial haemorrhage was monitored by follow-up CT or MRI – in most cases performed at 24 h after start of IVT and immediately in case of neurological detorientation – as described in prior research.15,20
Included data were collected up to 30 January 2014 (eTable 1). All patients with missing data on (i) HL, (ii) three-month outcome and (iii) sICH-data were excluded.
Statistical analyses
Statistical analyses were performed with SPSS Statistics version 25 (IBM) and STATA version 14.1 (StataCorp LP).
We investigated associations between HL and outcome measures using HL as a (i) continuous variable and as a (ii) categorical variable distinguishing anaemia (female: <12 g/dl; male: <13 g/dl) and polyglobulia (female: >15.5 g/dl; male: >17 g/dl) defined by the criteria of the World Health Organisation (WHO).21 Normal HL (female: 12–15.5 g/dl; male: 13–17 g/dl) served as reference group. According to the grading system of the WHO, anaemia was further subdivided into mild (female: 11–11.9 g/dl; male: 11–12.9 g/dl) and moderate/severe (female/male: <11 g/dl).22
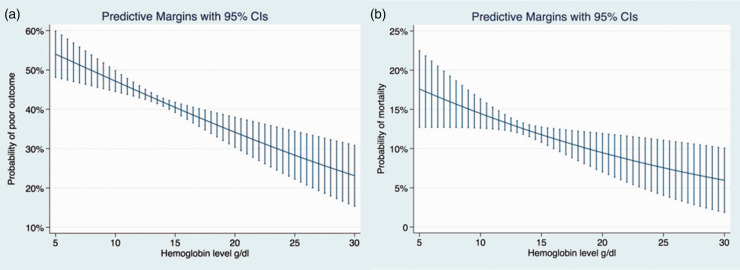
Continuous data were summarised as median and interquartile range. We used Chi2-test and Fisher’s exact test for categorical variables where appropriate and the Mann–Whitney U-test for continuous variables. The association between HL and each outcome was estimated by calculating odds ratios (OR) with 95% confidence intervals (95% CI), using binary logistic regression models. All variables with p < 0.05 were included in the multivariable analyses using stepwise regression with backward elimination. To avoid overfitting, the maximum number of potential confounders in the final model was restricted to one-tenth of the number of outcome events. Predictive margins of the adjusted logistic regression models are displayed in Figure 1.
Figure 1.
Predictive margins with 95% CI of the adjusted logistic regression models showing the association of haemoglobin levels and outcomes in IVT-treated stroke patients: (a) adjusted for age, stroke severity (NIHSS), glucose, eGFR, RF diabetes and (b) adjusted for age, stroke severity (NIHSS), RF diabetes.
Receiver operating characteristic (ROC) and area under the curve (AUC) were calculated to show the accuracy of HL to predict poor outcome and mortality.
The study was approved by the ethics committee in Basel, Switzerland. The requirement for additional local ethical approval differed between participating centres and was obtained if required. Anonymised data will be shared by request from any qualified investigator.
Results
Data were eligible for analysis in 6866 (92.8%) of the 7395 IVT-treated patients. Reasons for excluding patients were missing data on HL (n = 213; 2.9%), three-month outcome (n = 257; 3.5%) or sICH (n = 59; 0.8%).
Among study patients, 5448 (79.4%) had normal HL, 1232 (17.9%) anaemia and 186 (2.7%) polyglobulia.
Anaemia versus normal HL
Baseline characteristics are presented in Table 1. Patients with anaemia were older, had more severe strokes, lower median systolic blood pressure, lower eGFR at stroke onset, more frequently on antithrombotics (antiplatelets and/or anticoagulation) and were more likely to have a history of atrial fibrillation, coronary artery disease, diabetes mellitus, hypertension and prior stroke compared to patients with normal HL. Patients with anaemia more often had poor functional outcome (55.5% versus 39.5%) and died more often (22.4% versus 11.3%) during the three-month follow-up, while the proportion of patients with symptomatic ICH did not differ significantly (4.5% versus 4.3%). Data of recurrent strokes within three months after stroke onset were available in a subgroup of patients only (n = 2518, centres of Amsterdam, Basel, Belgrade, Bern, Brescia and Modena). Frequency of recurrent stroke did not differ significantly between patients with anaemia and normal HL (3.3% versus 3.4%) as well as between polyglobulia and normal HL (3.3% versus 4.9%). (Table 1)
Table 1.
Clinical characteristics and frequency of outcome events of IVT-treated stroke patients divided into groups depending on their haemoglobin level (HL) at stroke onset.
| Normal HL | Anaemiaa | Normal HL versus anaemia | Mild anaemiab | Normal HL versus mild anaemia | Moderate/severe anaemiac | Normal HL versus moderate/severe anaemia | Polyglobuliad | Normal HL versus polyglobulia | |
|---|---|---|---|---|---|---|---|---|---|
| n = 5448 | n = 1232 | P value | n = 903 | P value | n = 329 | P value | n = 186 | P value | |
| Hb on admission, in g/dl, median (IQR) | 14.2 (13.4–14.9) | 11.6 (10.9–12.2) | <0.001 | 11.9 (11.5–12.4) | <0.001 | 10.2 (9.5–10.6) | <0.001 | 16.7 (16.0–17.4) | <0.001 |
| Age, years, median (IQR) | 70 (59.8–78) | 76 (67–82) | <0.001 | 76 (67–82) | <0.001 | 77 (66–83) | <0.001 | 64.9 (57–75) | 0.001 |
| Men, n (%) | 3158 (58) | 697 (56,6) | 0.371 | 569 (63.0) | 0.005 | 128 (38.9) | <0.001 | 78 (41.9) | <0.001 |
| Stroke severity, NIHSS, median (IQR) | 9 (5–15) | 12 (7–17) | <0.001 | 11 (6–17) | <0.001 | 13 (8–18) | <0.001 | 9 (5–15) | 0.870 |
| Systolic blood pressure, mmHg, median (IQR) | 157 (140–173) | 150 (136–169) | <0.001 | 151 (138–170) | <0.001 | 150 (132–164) | <0.001 | 163 (148–180) | <0.001 |
| Onset-to-treatment, min, median (IQR) | 142 (105–180) | 145 (110–180) | 0.215 | 145 (109–180) | 0.316 | 145 (110–185) | 0.400 | 138 (95.5–184) | 0.550 |
| eGFR on admission ml/min, median (IQR) | 80.6 (63.1–93) | 72.2 (51.1–88) | <0.001 | 73.4 (53.5–88.1) | <0.001 | 68.8 (43.7–87.5) | <0.001 | 80.3 (64–92.7) | 0.766 |
| Glucose on admission mmol/l, median (IQR) | 6.6 (5.8–7.9) | 6.5 (5.6–7.9) | 0.019 | 6.6 (5.7–8.0) | 0.348 | 6.3 (5.4–7.8) | 0.001 | 6.95 (6.0–8.88) | 0.015 |
| Prior antithrombotics, n (%) | 1851 (35.1) | 588 (48.9) | <0.001 | 419 (46.4) | <0.001 | 169 (51.4) | <0.001 | 62 (34.5) | 0.696 |
| Antiplatelets, n (%) | 1504 (30.8) | 466 (42.1) | <0.001 | 333 (40.8) | <0.001 | 133 (45.5) | <0.001 | 55 (30.6) | 0.953 |
| Anticoagulation with or without antiplatelets, n (%)e | 195 (4.0) | 70 (6.3) | 0.001 | 54 (6.6) | 0.001 | 16 (5.5) | 0.211 | 7 (3.9) | 0.946 |
| Atrial fibrillation, n (%) | 1388 (25.7) | 409 (33.7) | <0.001 | 293 (32.4) | <0.001 | 116 (35.3) | <0.001 | 55 (29.6) | 0.234 |
| Hypertension, n (%) | 3569 (65.6) | 892 (72.6) | <0.001 | 652 (72.2) | <0.001 | 240 (72.9) | 0.005 | 130 (69.9) | 0.239 |
| Current (or stopped < 2y) smoking, n (%) | 978 (23.1) | 144 (14.6) | <0.001 | 112 (12.4) | <0.001 | 32 (9.7) | <0.001 | 59 (37.8) | <0.001 |
| Hypercholesterolemia, n (%) | 2282 (42.0) | 508 (41.5) | 0.773 | 375 (41.5) | 0.913 | 133 (40.4) | 0.686 | 85 (45.7) | 0.327 |
| Diabetes mellitus, n (%) | 889 (16.4) | 297 (24.2) | <0.001 | 211 (23.4) | <0.001 | 86 (26.1) | <0.001 | 30 (16.1) | 1.000 |
| Coronary artery disease, n (%) | 953 (17.6) | 338 (27.7) | <0.001 | 238 (26.4) | <0.001 | 100 (30.4) | <0.001 | 32 (17.2) | 0.866 |
| Prior stroke, n (%) | 738 (13.6) | 221 (18.1) | <0.001 | 161 (18) | 0.001 | 60 (18.2) | 0.017 | 23 (12.4) | 0.743 |
| Poor outcome, n (%) | 2151 (39.5) | 684 (55.5) | <0.001 | 478 (52.9) | <0.001 | 206 (62.6) | <0.001 | 67 (36.0) | 0.360 |
| Mortality, n (%) | 613 (11.3) | 276 (22.4) | <0.001 | 187 (20.7) | <0.001 | 89 (27.1) | <0.001 | 19 (10.2) | 0.724 |
| Symptomatic ICH(ECASS-II criteria), n (%) | 235 (4.3) | 56 (4.5) | 0.700 | 43 (4.8) | 0.539 | 13 (4) | 0.889 | 6 (3.2) | 0.582 |
| Recurrent strokef | 67 (3.4) | 17 (3.3) | 0.883 | 13 (3.6) | 0.905 | 4 (2.7) | 0.6213 (4.9) | 0.538 |
ECASS-II-criteria: European Cooperative Acute Stroke Study II-criteria; eGFR: estimated glomerular filtration rate; ICH: intracerebral haemorrhage; IQR: interquartile range; IVT: intravenous thrombolysis; NIHSS: National Institutes of Health Stroke Scale.
aAnaemia = haemoglobin female: <12 g/dl; male: <13 g/dl.
bMild anaemia = haemoglobin female: 11–11.9 g/dl; male: 11–12.9 g/dl.
cModerate/severe anaemia = haemoglobin <11 g/dl.
dPolyglobulia = haemoglobin female: >15.5 g/dl; male: >17 g/dl.
eType of antithrombotic treatment not known in 152 patients.
fInformation available in a subgroup of patients only, n = 2518.
Anaemia was associated with poor functional outcome (ORunadjusted 1.91, 95% CI 1.69–2.17 and ORadjusted 1.25, 95% CI 1.05–1.48) and mortality (ORunadjusted 2.28, 95% CI 1.94–2.67 and ORadjusted 1.58, 95% CI 1.27–1.95) but not with sICH (ORunadjusted 1.04, 95% CI 0.82–1.33 and ORadjusted 0.94, 95% CI 0.69–1.30). The lack of association between HL and sICH remained irrespective of type of antithrombotic treatment (antiplatelet and/or anticoagulation) (Tables 2 and 3).
Table 2.
Univariate analysis of clinical characteristics (odds ratio with 95% confidence interval) in IVT patients.
| Putative predicting variables | sICH | Poor outcomea | Mortality |
|---|---|---|---|
| Age (each year) | 1.01 (1.01–1.03) p < 0.001 | 1.05 (1.04–1.05) p < 0.001 | 1.07 (1.06–1.08) p < 0.001 |
| Sex | 1.07 (0.88–1.23) p=0.516 | 1.51 (1.37–1.67) p < 0.001 | 1.34 (1.16–1.54) p < 0.001 |
| NIHSS (each point) | 1.07 (1.06–1.09) p < 0.001 | 1.18 (1.17–1.20) p < 0.001 | 1.16 (1.14–1.17) p < 0.001 |
| Systolic blood pressure (each mmHg) | 1.00 (1.00–1.01) p = 0.032 | 1.00 (1.00–1.01) p = 0.011 | 1.00 (1.00–1.01) p = 0.032 |
| Stroke-to-needle (each minute) | 1.00 (0.99–1.00) p = 0.235 | 1.00 (1.00–1.00) p = 0.136 | 1.00 (0.99–1.00) p = 0.389 |
| Decreasing eGFR (by 10 ml/min) | 1.00 (1.00–1.01) p < 0.001 | 1.02 (1.02–1.02) p < 0.001 | 1.03 (1.02–1.03) p < 0.001 |
| Glucose (each mmol/l) | 1.03 (1.01–1.06) p = 0.004 | 1.09 (1.07–1.11) p < 0.001 | 1.09 (1.06–1.11) p < 0.001 |
| Prior antithrombotics | 1.22 (0.96–1.55) p = 0.113 | 1.47 (1.33–1.63) p < 0.001 | 1.99 (1.73–2.29) p < 0.001 |
| Antiplatelets | 1.20 (0.93–1.55) p = 0.163 | 1.34 (1.20–1.40) p < 0.001 | 1.71 (1.47–1.99) p < 0.001 |
| Anticoagulation with or without antiplatelets | 0.93 (0.50–1.72) p = 0.817 | 1.88 (1.47–2.41) p < 0.001 | 2.12 (1.59–2.83) p < 0.001 |
| Atrial fibrillation | 1.45 (1.19–1.78) p < 0.001 | 1.93 (1.74–2.16) p < 0.001 | 2.01 (1.74–2.33) p < 0.001 |
| Hypertension | 1.19 (0.96–1.46) p = 0.107 | 1.50 (1.35–1.66) p < 0.001 | 1.61 (1.37–1.89) p < 0.001 |
| Smoking | 0.67 (0.51–0.90) p = 0.007 | 0.70 (0.62–0.81) p < 0.001 | 0.50 (0.40–0.63) p < 0.001 |
| Hypercholesterolemia | 1.10 (0.91–1.33) p = 0.321 | 0.86 (0.78–0.95) p = 0.003 | 0.85 (0.74–0.98) p = 0.025 |
| Diabetes mellitus | 1.33 (1.06–1.68) p = 0.014 | 1.67 (1.47–1.87) p < 0.001 | 1.67 (1.41–1.97) p < 0.001 |
| Coronary artery disease | 1.38 (1.10–1.72) p = 0.005 | 1.44 (1.27–1.62) p < 0.001 | 1.95 (1.67–2.28) p < 0.001 |
| Prior stroke | 1.17 (0.90–1.51) p = 0.237 | 1.35 (1.18–1.54) p < 0.001 | 1.48 (1.24–1.78) p < 0.001 |
| Anaemiab versus normal HL | 1.04 (0.82–1.33) p = 0.744 | 1.91 (1.69–2.17) p < 0.001 | 2.28 (1.94–2.67) p < 0.001 |
| Mild anaemiac versus normal HL | 1.11 (0.80–1.55) p = 0.542 | 1.72 (1.50–1.99) p < 0.001 | 2.06 (1.72–2.47) p < 0.001 |
| Moderate/severe anaemiad versus normal HL | 0.91 (0.52–1.61) p = 0.753 | 2.57 (2.04–3.23) p < 0.001 | 2.93 (2.26–3.78) p < 0.001 |
| Polyglobuliae versus normal HL | 0.79 (0.41–1.50) p = 0.469 | 0.86 (0.64–1.17) p = 0.343 | 0.90 (0.55–1.45) p = 0.660 |
| Decreasing HL (by 1 g/dl) | 1.04 (0.98–1.10) p = 0.160 | 1.22 (1.19–1.26) p < 0.001 | 1.24 (1.19–1.29) p < 0.001 |
eGFR: estimated glomerular filtration rate; HL: haemoglobin level; IVT: intravenous thrombolysis; NIHSS: National Institutes of Health Stroke Scale; sICH: symptomatic intracerebral haemorrhage (ECASS II definition).
aPoor outcome: modified Rankin Scale 3–6.
bAnaemia = haemoglobin female: < 12 g/dl; male: < 13 g/dl.
cMild anaemia = haemoglobin female: 11–11.9 g/dl; male: 11–12.9 g/dl.
dModerate/severe anaemia = haemoglobin < 11 g/dl.
ePolyglobulia = haemoglobin female: >15.5 g/dl; male: >17 g/dl.
Table 3.
Multivariate analysis of outcomes (odds adjusted for variables with p < 0.05 in the univariate analysis). Odds ratio (95% confidence interval), p-value.
|
Outcome measures |
|||
|---|---|---|---|
| Putative predicting variables | sICH | Poor outcomea | Mortality |
| Anaemia versus normal HL | 0.94 (0.69–1.30)b p = 0.718 | 1.25 (1.05–1.48)c p = 0.012 | 1.58 (1.27–1.95)d p < 0.001 |
| Mild anaemia versus normal HL | 1.03 (0.73–1.46)e p = 0.865 | 1.29 (1.07–1.55)f p = 0.009 | 1.45 (1.15–1.84)g p = 0.002 |
| Moderate/severe anaemia versus normal Hb | 0.72 (0.40–1.30)h p = 0.277 | 1.48 (1.09–2.02)i p = 0.013 | 2.00 (1.46–2.75)j p<0.001 |
| Decreasing HL (by 1 g/dl) | 1.02 (0.94–1.11)k p = 0.652 | 1.07 (1.02–1.11)l p = 0.004 | 1.08 (1.02–1.15)m p = 0.010 |
HL: haemoglobin level; sICH: symptomatic intracerebral haemorrhage (ECASS II definition).
aPoor outcome: modified Rankin Scale 3–6.
bNIHSS on admission, age, CKD-EPI, pre-antithrombotic any, anaemia.
cNIHSS on admission, age, glucose on admission, CKD-EPI, RF diabetes, RF hypertension, RF coronary artery disease, gender, smoking, pre-antithrombotic any, RF atrial fibrillation, RF hypercholesterolemia, RR systole on admission, RF prior ischaemic stroke, anaemia.
dNIHSS on admission, age, glucose on admission, CKD-EPI, RF diabetes, RF hypertension, RF coronary artery disease, gender, smoking, pre-antithrombotic any, RF atrial fibrillation, RF hypercholesterolemia, RR systole on admission, RF prior ischaemic stroke, anaemia.
eNIHSS on admission, age, CKD-EPI, pre-antithrombotic any, mild anaemia.
fNIHSS on admission, age, stroke-to-needle-time, glucose on admission, CKD-EPI, RF diabetes, RF hypertension, RF coronary artery disease, gender, pre-antithrombotic any, RF atrial fibrillation, RF hypercholesterolemia, RR systole on admission, RF prior ischaemic stroke, mild anaemia.
gNIHSS on admission, age, stroke-to-needle-time, glucose on admission, CKD-EPI, RF diabetes, RF hypertension, RF coronary artery disease, gender, pre-antithrombotic any, RF atrial fibrillation, RF hypercholesterolemia, RR systole on admission, RF prior ischaemic stroke, mild anaemia.
hNIHSS on admission, severe anaemia.
iNIHSS on admission, age, stroke-to-needle-time, glucose on admission, CKD-EPI, RF diabetes, RF hypertension, RF coronary artery disease, gender, pre-antithrombotic any, RF atrial fibrillation, RF hypercholesterolemia, RR systole on admission, RF prior ischaemic stroke, current smoking, severe anaemia.
jNIHSS on admission, age, gender, glucose on admission, CKD-EPI, prior stroke, RR on admission, serve anaemia.
kNIHSS on admission, age, glucose on admission, CKD-EPI, RF diabetes, RF coronary artery disease, smoking, pre-antithrombotic any, RF atrial fibrillation, RR systole on admission, decreasing HL in g/dl.
lNIHSS on admission, age, glucose on admission, CKD-EPI, RF diabetes, RF hypertension, RF coronary artery disease, gender, smoking, pre-antithrombotic any, RF atrial fibrillation, RF hypercholesterolemia, RR systole on admission, RF prior ischaemic stroke, decreasing HL in g/dl.
mNIHSS on admission, age, glucose on admission, CKD-EPI, RF diabetes, RF hypertension, RF coronary artery disease, gender, smoking, pre-antithrombotic any, RF atrial fibrillation, RF hypercholesterolemia, RR systole on admission, RF prior ischaemic stroke, decreasing HL in g/dl.
Mild and moderate to severe anaemia versus normal HL
Of 1232 patients with anaemia, 903 (73.3%) had mild anaemia and 329 (26.7%) moderate/severe anaemia. Baseline characteristics of both anaemia subgroups are presented in Table 1. Patients with mild and moderate/severe anaemia had more frequently poor functional outcome (52.9 and 62.6% versus 39.5% in patients with normal HL) and mortality (20.7 and 27.1% versus 11.3% in normal HL). Frequency of sICH did not differ significantly between patients with mild anaemia (4.8%), moderate/severe anaemia (4.0%) and normal HL (4.3%) (Table 1). Mild anaemia was associated with poor functional outcome (ORunadjusted 1.72, 95% CI 1.50–1.99) and mortality (ORunadjusted 2.06, 95% CI 1.72–2.47). These associations remained significant after adjustment for potential confounders (poor functional outcome: OR 1.29, 95% CI 1.07–1.55 and mortality: OR 1.45, 95% CI 1.15–1.84). Similarly, moderate/severe anaemia was associated with poor functional outcome (ORunadjusted 2.57, 95% CI 2.04–3.23; ORadjusted 1.48, 95% CI 1.09–2.02) and mortality (ORunadjusted 2.93, 95% CI 2.26–3.78; ORadjusted 2.0, 95% CI 1.46–2.75). Neither mild nor moderate/severe anaemia was associated with the occurrence of sICH (Tables 2 and 3).
Polyglobulia versus normal HL
Compared to normal HL, polyglobulia (n = 186; 2.71%) did not significantly change the odds for any outcome (poor functional outcome ORunadjusted 0.86, 95% CI 0.64–1.17; mortality ORunadjusted 0.9, 95% CI 0.55–1.45; sICH ORunadjusted 0.79, 95% CI 0.41–1.50) (Tables 1 to 3).
HL as a continuous variable
The adjusted logistic regression model showed an increasing probability of poor outcome and mortality with decreasing HL. A decrease in HL by 1 g/dl was associated with an OR of 1.07 (95% CI 1.02–1.11) for poor outcome and with an OR of 1.08 (95% CI 1.02–1.15) for mortality. HLs were not significantly associated with sICH (Figure 1 and Table 3).
The ability of HL to predict poor functional outcome or mortality was low (AUC of ROC curve for poor functional outcome: 0.59 (95% CI 0.58–0.61, p < 0.001); for mortality: 0.60 (95% CI 0.58–0.62, p < 0.001) (eFigure 1).
Discussion
This study showed the following key results for the association between HL and outcomes in acute ischaemic stroke patients treated with IVT: (i) 17.9% of IVT-treated stroke patients had anaemia on admission. (ii) Anaemia was an independent predictor for poor functional outcome and mortality in IVT-treated stroke patients, but not for symptomatic ICH. (iii) The more severe the anaemia the higher the probability of poor functional outcome and mortality. (iv) Polyglobulia was not associated with any outcome.
In our study population, 17.9% of patients had anaemia (according to the WHO criteria) on admission and before IVT administration. Presence of anaemia independently increased the probability of poor functional outcome after three months of follow-up by 25% and the probability of mortality by 60%. Previously, only one smaller observational study (n = 217) has investigated the impact of HL on outcomes in IVT-treated stroke patients.7 Although the proportion of patients with anaemia on admission was higher in patients with poor functional outcome (20.4%) compared to patients with favourable functional outcome (10.5%; p = 0.04), HLs on admission were not independently associated with poor functional outcome (p = 0.20) or mortality (p = 0.45). However, the number of patients with anaemia was small (n = 33), though the proportion (i.e. 15%) resembled that of the present study (i.e. 17.9%). Interestingly, after inclusion of patients who had developed anaemia during the first five days of hospitalisation into analysis (n = 86), anaemia was independently associated with poor functional outcome (OR 2.61, 95% CI 1.33–5.11) but still not with mortality (p = 0.13). A recent study, including IVT and endovascular treated patients, found that anaemia at hospital admission and any decrease of haemoglobin were associated with poor functional outcome and higher mortality.8
The large sample size in the present study allowed a subgroup analysis of patients with mild and moderate/severe anaemia. Data on anaemia subgroups are scarce. One retrospective study after mechanical thrombectomy23 (n = 90) reported an association between severe anaemic patients (Hb <10 g/dl) and poor functional outcome but not for patients with mild anaemia. In our study, both mild and moderate/severe anaemic patients were independently associated with poor functional outcome and mortality but the odds were higher in the moderate/severe group. In line, the probability for poor functional outcome and mortality increased with decreasing HL. Every decrease in HL by 1 g/dl increased the probability of poor outcome by 7% and the probability of mortality by 8%.
Several underlying pathophysiological mechanisms could explain the association between anaemia and an increased probability of poor functional outcome and mortality. Arterial oxygen content depends on HL and arterial oxygen saturation. The oxygen content and cerebral blood flow determine oxygen delivery to the brain. Consequently, decreasing HL lowers the arterial oxygen content and possibly leads to changes in blood flow resulting in an impaired tissue oxygen supply. Therefore, hypoxia in penumbral lesions may be increased leading to more extensive ischaemic areas.24,25
Furthermore, anaemia could compromise the cerebral autoregulation which maintains cerebral blood flow and oxygen carrying capacity through collaterals to penumbral lesions.26,27 Healthy patients with anaemia might tolerate hypoxia with an HL of approximately down to 8 g/dl.25 However, in anaemic stroke patients who likely have additional comorbidities, cerebral autoregulation is thought to be already impaired.28 In addition, a mathematical model of regional cerebral oxygen uptake also suggested that oxygen uptake in ischaemic penumbra progressively decreases below an HL of 10 g/dl.29 Other potential pathophysiological mechanisms include the modulation of adhesion molecules by hyperdynamic circulation in anaemic patients30 and the upregulation of inflammatory mediators.1,31 In line, a recent study found a positive correlation between decreasing baseline HL and increasing final infarct volume in acute stroke patients.8
The clear and independent association between anaemia on admission and poor functional outcome and mortality in acute ischaemic stroke patients carries the chance for hyperacute interventions. Such an intervention could be red blood cell transfusion (RBCT). In one retrospective study investigating the optimal management of HL and RBCT in patients with severe ischaemic stroke, RBCT was not associated with any clinical improvement.32 However, the sample size was small (n = 109), and stroke severity was high (median NIHSS 19). Furthermore, RBCT was performed at the discretion of the neurologic ICU physician in charge, based on the general aim to keep HL between 8 and 10 g/dl over the course of the hospitalisation and not necessarily during the hyperacute phase when the penumbra might still be preserved.
Furthermore, the optimal HL threshold for RBCT is unclear.33 In general, RBCT is not considered in patients with HL >10 g/dl. In some ischaemic populations (i.e. acute coronary syndrome) the threshold for RBCT is between 8 and 10 g/dl.34 Data on RBCT in ischaemic patients (i.e. myocardial infarction) with anaemia remain ambiguous: in some studies, RBCT reduced mortality35,36 while in others RBCT increased adverse outcomes after percutaneous coronary intervention37 and in ST-elevation myocardial infarction.38 In anaemic patients with aneurysmal subarachnoid haemorrhage, RBCTs are suggested to be beneficial when there was no considerable anaemia beforehand.39 On the other hand, in the setting of perioperative procedures RBCT was suggested to be associated with stroke.40 In patients undergoing coronary artery bypass surgery, the use of solvent/detergent treated plasma and platelet transfusions seemed to have a larger impact on the development of stroke than RBCT.41
Nevertheless, the strong association of anaemia with poor functional outcome and mortality in acute setting of IVT-treated stroke patients and the high vulnerability of penumbral brain tissue to hypoxia might justify a randomised controlled trial investigating RBCT in the hyperacute phase of ischaemic stroke.
In our study, polyglobulia was not associated with functional outcome, mortality or sICH. In the general stroke population, a U-shaped relationship between HL and short-term mortality (up to one month) in men has been suggested.1 This might indicate that patients with polyglobulia have a special benefit of IVT. However, the proportion of patients with polyglobulia was small (2.7%; n = 186) in our study and results should be interpreted with caution.
Our study has the following strengths: (i) the large sample size reduced the risk of chance findings and allowed adjustment for several confounding variables, (ii) the low number of missing data on HL (2.9%) and clinical outcome at three months (3.5%) reduced the risk of bias and (iii) the prospective and systematic approach of data assessment was not influenced by the current research question.
This study has limitations: (i) the TRISP registry is not monitored and does not provide data from comparison group of patients not treated with IVT. Thus, we were not able to calculate the treatment effect of IVT stratified to HL categories. (ii) We were neither able to classify the type of anaemia nor did we have any information about the underlying cause of anaemia, which may affect functional three-month outcome and mortality, e.g. in the case of cancer-related anaemia, anaemia of chronic disease or malnutrition. Due to insufficient data it was also not possible to fully incorporate potential confounders such as frailty (i.e. by a standard measure of comorbidity) which is associated with anaemia and poor outcome. However, we were able to adjust for parameters that are relevant in anaemia of chronic disease most importantly for renal function (via estimated glomerular filtration rate) and for vascular risk factors. Hence chronic disease as a possible confounder on functional outcome and mortality is partly addressed in the multivariate analyses. In addition, anaemia might be a manifestation of chronic, e.g. gastrointestinal, bleeding. In these cases, IVT treatment is likely to deteriorate outcomes. However, because IVT is contraindicated in haemorrhagic diseases (acute or chronic), the number of these patients is expected to be very small. (iii) Further, we have no information on the course of the HL over time and whether HLs were deliberately altered with RBCT or iron supplements. (iv) The investigated data were collected from high volume stroke centres. These results may not apply to low volume centres.
Conclusions
Anaemia was independently associated with poor clinical outcome and mortality in IVT-treated stroke patients. The more severe the anaemia, the stronger the association with poor clinical outcome and mortality. Therefore, severe anaemia might be a target for interventions in hyperacute stroke. Anaemia was not associated with occurrence of sICH and no significant association between polyglobulia and any outcome was found.
Supplemental Material
Supplemental material, ESO889468 Supplementary material for Effect of haemoglobin levels on outcome in intravenous thrombolysis-treated stroke patients by Valerian L Altersberger, Lars Kellert, Abdulaziz S Al Sultan, Nicolas Martinez-Majander, Christian Hametner, Ashraf Eskandari, Mirjam R Heldner, Sophie A van den Berg, Andrea Zini, Visnja Padjen, Georg Kägi, Alessandro Pezzini, Alexandros Polymeris, Gian M DeMarchis, Marjaana Tiainen, Silja Räty, Stefania Nannoni, Simon Jung, Thomas P Zonneveld, Stefania Maffei, Leo Bonati, Philippe Lyrer, Gerli Sibolt, Peter A Ringleb, Marcel Arnold, Patrik Michel, Sami Curtze, Paul J Nederkoorn, Stefan T Engelter, Henrik Gensicke and for the Thrombolysis in Stroke Patients (TRISP) collaborators in European Stroke Journal
Acknowledgements
All TRISP Centers and Collaborators are listed in the supplemental material.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Valerian L Altersberger, Abdulaziz S Al Sultan, Nicolas Martinez-Majander, Christian Hametner, Ashraf Eskandari, Mirjam R Heldner, Sophie A van den Berg, Visnja Padjen, Alessandro Pezzini, Alexandros Polymeris, Marjaana Tiainen, Silja Räty, Stefania Nannoni, Thomas P Zonneveld, Stefania Maffei, Gerli Sibolt and Sami Curtze report no disclosure. Lars Kellert has received speaker honoraria and/or travel support and/or honoraria for advisory board from Bristol-Myers Squibb, Boehringer-Ingelheim, Bayer-Vital, Daiichi-Sankyo and Pfizer. Andrea Zini has received funding for speaker honoraria and consulting fees from Boehringer-Ingelheim and Medtronic, for scientific advisory board from Boehringer-Ingelheim, Daiichi-Sankyo and Stryker. Georg Kägi has received modest honoraria for travel and advisory board from Bayer, Boehringer-Ingelheim and Zambon and a research grant from the Swiss Heart Foundation, Swiss Parkinson Foundation, Swiss National Science Foundation. Gian Marco DeMarchis has received support from the Swiss National Science Foundation (No. PBBEP3_139388); Spezialprogramm Nachwuchsförderung Klinische Forschung, University of Basel; Science Funds (Wissenschaftspool) of the University Hospital Basel; Swiss Heart Foundation; Bangerter-Rhyner-Stiftung; Swisslife Jubiläumsstiftung for Medical Research; Swiss Neurological Society; Fondazione Dr Ettore Balli; De Quervain research grant; Thermo Fisher GmbH; consultant and travel honoraria by Bayer; speaker honoraria by Medtronic and BMS/Pfizer. Simon Jung has received research grants from the Swiss National Science Foundation, the Swiss Heart Foundation; honoraria from scientific advisory boards from Boehringer-Ingelheim, Bayer, Pfizer, Amgen. Leo Bonati received grants from the Swiss National Science Foundation, the Swiss Heart Foundation and the University of Basel; an unrestricted research grant from AstraZeneca, consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical, and travel grants from Amgen and Bayer. Philippe Lyrer has served on scientific advisory boards for Bayer, Daiichi-Sankyo, Schering Pharma and Boehringer-Ingelheim has received funding for travel or speaker honoraria from Bayer Schering Pharma, Boehringer-Ingelheim and Shire plc; he has received research support from AstraZeneca, Boehringer-Ingelheim, Sanofi-aventis, PhotoThera, the Swiss National Science Foundation and the Swiss Heart Foundation. Peter A Ringleb has received modest honoraria for lectures and advisory board from Boehringer-Ingelheim. The University Hospital Heidelberg is sponsor of the ECASS4-trial, examining the role of rtPA in an extended time-window, which is financed by Boehringer-Ingelheim. Marcel Arnold received Speaker honoraria from Bayer, Boehringer-Ingelheim and Covidien; Scientific advisory board honoraria from Amgen, Bayer, Boehringer-Ingelheim, BMS, Pfizer, Covidien, Daiichi-Sankyo and Nestlé Health Science. Research grants from the Swiss Heart Foundation and the Swiss National Science Foundation. Patrik Michel has received within the last two years research grants from the Swiss National Science Foundation, the Swiss Heart Foundation and BMS; speaker fees from Medtronic and Amgen; consulting fees from Medtronic and Amgen, and honoraria from scientific advisory boards from Pfizer and BMS. All this support goes to his institution and is used for stroke education and research. Paul J Nederkoorn has received funding from the Dutch heart foundation for acute stroke intervention trials in the Collaboration for New Trials in Stroke (CONTRAST) consortium. Stefan T Engelter has received funding for travel or speaker honoraria from Bayer, Boehringer-Ingelheim and Daiichi-Sankyo. He has served on scientific advisory boards for Bayer, Boehringer-Ingelheim, BMS/Pfizer, MindMaze and on the editorial board of Stroke. He has received an educational grant from Pfizer and research support from the Science Funds (Wissenschaftsfonds) of the University Hospital Basel, the University Basel, the Swiss Heart Foundation and the Swiss National Science Foundation. Henrik Gensicke has received research support from the Swiss National Science Foundation.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was partly supported by the Stroke-[Hirnschlag]-Fund Basel and by grants from the Swiss National Foundation (33CM30-124119; 33CM30-140340/1; P300PB_161071) and the University of Basel.
Ethical approval
The study was approved by the ethics committee in Basel, Switzerland.
Informed consent
The requirement for additional local ethical approval/informed consent differed between participating centres and was obtained if required. Anonymised data will be shared by request from any qualified investigator.
Guarantor
HG.
Contributorship
VLA designed/conceptualised the study, collected data, analysed/interpreted the data, drafted the manuscript. LK designed/conceptualised the study, collected data, revised the manuscript. ASA analysed/interpreted data and revised the manuscript. STE and HG designed/conceptualised and initiated the study, supervised the study, collected data, analysed/interpreted the data, revised the manuscript. All other authors collected data and revised the manuscript.
References
- 1.Barlas RS, Honney K, Loke YK, et al. Impact of hemoglobin levels and anemia on mortality in acute stroke: analysis of UK regional registry data, systematic review, and meta-analysis. J Am Heart Assoc 2016; 5: e003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanne D, Molshatzki N, Merzeliak O, et al. Anemia status, hemoglobin concentration and outcome after acute stroke: a cohort study. BMC Neurol 2010; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimberly WT, Lima FO, O’Connor S, et al. Sex differences and hemoglobin levels in relation to stroke outcomes. Neurology 2013; 80: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park YH, Kim BJ, Kim JS, et al. Impact of both ends of the hemoglobin range on clinical outcomes in acute ischemic stroke. Stroke 2013; 44: 3220–3222. [DOI] [PubMed] [Google Scholar]
- 5.Lasek-Bal A, Holecki M, Stęposz A. The impact of anemia on the course and short-term prognosis in patients with first ever ischemic stroke. Neurol Neurochir Pol 2015; 49: 107–112. [DOI] [PubMed] [Google Scholar]
- 6.Sharma K, Johnson DJ, Johnson B, et al. Hemoglobin concentration does not impact 3-month outcome following acute ischemic stroke. BMC Neurol 2018; 18: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellert L, Martin E, Sykora M, et al. Cerebral oxygen transport failure? Decreasing haemoglobin and haematocrit levels after ischaemic stroke predict poor outcome and mortality: STroke: RelevAnt Impact of haemoGlobin, Haematocrit and Transfusion (STRAIGHT) – an observational study. Stroke 2011; 42: 2832–2837. [DOI] [PubMed] [Google Scholar]
- 8.Bellwald S, Balasubramaniam R, Nagler M, et al. Association of anemia and hemoglobin decrease during acute stroke treatment with infarct growth and clinical outcome. PLoS One 2018; 13: e0203535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellert L, Kloss M, Pezzini A, et al. Anemia in young patients with ischaemic stroke. Eur J Neurol 2015; 22: 948–953. [DOI] [PubMed] [Google Scholar]
- 10.Hare GM, Tsui AK, McLaren AT, et al. Anemia and cerebral outcomes: many questions, fewer answers. Anesth Analg 2008; 107: 1356–1370. [DOI] [PubMed] [Google Scholar]
- 11.Tsui AK, Dattani ND, Marsden PA, et al. Reassessing the risk of hemodilutional anemia: some new pieces to an old puzzle. Can J Anesth 2010; 57: 779–791. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Kang SY. Bleeding and subsequent anemia: a precipitant for cerebral infarction. Eur Neurol 2000; 43: 201–208. [DOI] [PubMed] [Google Scholar]
- 13.Scheitz JF, Gensicke H, Zinkstok SM, et al. TRISP collaboration. Cohort profile: Thrombolysis in Ischemic Stroke Patients (TRISP): a multicentre research collaboration. BMJ Open 2018; 8: e023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelter ST, Soinne L, Ringleb P, et al. IV thrombolysis and statins. Neurology 2011; 77: 888–895. [DOI] [PubMed] [Google Scholar]
- 15.Gensicke H, Al Sultan AS, Strbian D, et al. Intravenous thrombolysis and platelet count. Neurology 2018; 90: e690–e697. [DOI] [PubMed] [Google Scholar]
- 16.Lyden P, Brott T, Tilley B, et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke 1994; 25: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fluri F, Hatz F, Voss B, et al. Restenosis after carotid endarterectomy: significance of newly acquired risk factors. Eur J Neurol 2010; 17: 493–498. [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, et al. Randomised doubleblind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 20.Gensicke H, Zinkstok SM, Roos YB, et al. IV thrombolysis and renal function. Neurology 2013; 81: 1780–1788. [DOI] [PubMed] [Google Scholar]
- 21.Blanc B, Finch CA, Hallberg L, et al. Nutritional anaemias. Report of WHO scientific group. World Health Organ Tech Rep Ser 1968; 405: 1–40. [PubMed] [Google Scholar]
- 22.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: WHO, 2011. [Google Scholar]
- 23.Akpinar CK, Gurkas E, Aytac E. Moderate to severe anemia is associated with poor functional outcome in acute stroke patients treated with mechanical thrombectomy. Intervent Neurol 2018; 7: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao KY, Hsiao CT, Lin LJ, et al. Severe anemia associated with transient ischemic attacks involving vertebrobasilar circulation. Am J Emerg Med 2008; 26: e3–e4. [DOI] [PubMed] [Google Scholar]
- 25.Lelubre C, Bouzat P, Crippa IA, et al. Anemia management after acute brain Injury. Crit Care 2016; 20: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang WY, Chen IC, Meng L, et al. The influence of anemia on clinical presentation and outcome of patients with first-ever atherosclerosis-related ischemic stroke. J Clin Neurosci 2009; 16: 645–649. [DOI] [PubMed] [Google Scholar]
- 27.Li Z, Zhou T, Li Y, et al. Anemia increases the mortality risk in patients with stroke: a metaanalysis of cohort studies. Sci Rep 2016; 6: 26636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gottesman RF, Sojkova J, Beason-Held LL, et al. Patterns of regional cerebral blood flow associated with low hemoglobin in the Baltimore Longitudinal Study of Aging. J Gerontol Biol Sci Med Sci 2012; 67: 963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dexter F, Hindman BJ. Effect of haemoglobin concentration on brain oxygenation in focal stroke: a mathematical modelling study. Br J Anaesth 1997; 79: 346–351. [DOI] [PubMed] [Google Scholar]
- 30.Morigi M, Zoja C, Figliuzzi M, et al. Fluid shear stress modulates surface expression of adhesion molecules by endothelial cells. Blood 1995; 85: 1696–1703. [PubMed] [Google Scholar]
- 31.McLaren AT, Marsden PA, Mazer CD, et al. Increased expression of HIF-1alpha, nNOS, and VEGF in the cerebral cortex of anemic rats. Am J Physiol 2007; 292: R403–R414. [DOI] [PubMed] [Google Scholar]
- 32.Kellert L, Schrader F, Ringleb P, et al. The impact of low hemoglobin levels and transfusion on critical care patients with severe ischemic stroke: STroke: RelevAnt impact of HemoGlobin, Hematocrit and Transfusion (STRAIGHT) – an observational study. J Crit Care 2014; 29: 236–240. [DOI] [PubMed] [Google Scholar]
- 33.Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol 2013; 160: 445–464. [DOI] [PubMed] [Google Scholar]
- 34.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016; 316: 2025. [DOI] [PubMed] [Google Scholar]
- 35.Wu WC, Rathore SS, Wang Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med 2001; 345: 1230–1236. [DOI] [PubMed] [Google Scholar]
- 36.Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J 2013; 165: 964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwok CS, Sherwood MW, Watson SM, et al. Blood transfusion after percutaneous coronary intervention and risk of subsequent adverse outcomes: a systematic review and meta-analysis. JACC Cardiovasc Interv 2015; 8: 436–446. [DOI] [PubMed] [Google Scholar]
- 38.Mincu RI, Rassaf T, Totzeck M. Red blood cell transfusion in patients with ST-elevation myocardial infarction-a meta-analysis of more than 21,000 patients. Neth Heart J 2018; 26: 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayling OGS, Ibrahim GM, Alotaibi NM, et al. Anemia after aneurysmal subarachnoid hemorrhage is associated with poor outcome and death. Stroke 2018; 49: 1859–1865. [DOI] [PubMed] [Google Scholar]
- 40.Whitlock EL, Kim H, Auerbach AD. Harms associated with single unit perioperative transfusion: retrospective population based analysis. BMJ 2015; 350: h3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mikkola R, Gunn J, Heikkinen J, et al. Use of blood products and risk of stroke after coronary artery bypass surgery. Blood Transfus 2012; 10: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ESO889468 Supplementary material for Effect of haemoglobin levels on outcome in intravenous thrombolysis-treated stroke patients by Valerian L Altersberger, Lars Kellert, Abdulaziz S Al Sultan, Nicolas Martinez-Majander, Christian Hametner, Ashraf Eskandari, Mirjam R Heldner, Sophie A van den Berg, Andrea Zini, Visnja Padjen, Georg Kägi, Alessandro Pezzini, Alexandros Polymeris, Gian M DeMarchis, Marjaana Tiainen, Silja Räty, Stefania Nannoni, Simon Jung, Thomas P Zonneveld, Stefania Maffei, Leo Bonati, Philippe Lyrer, Gerli Sibolt, Peter A Ringleb, Marcel Arnold, Patrik Michel, Sami Curtze, Paul J Nederkoorn, Stefan T Engelter, Henrik Gensicke and for the Thrombolysis in Stroke Patients (TRISP) collaborators in European Stroke Journal