Abstract
Introduction
Seizures are common after intracerebral haemorrhage. Tranexamic acid increases the risk of seizures in non-intracerebral haemorrhage population but its effect on post-intracerebral haemorrhage seizures is unknown. We explored the risk factors and outcomes of seizures after intracerebral haemorrhage and if tranexamic acid increased the risk of seizures in the Tranexamic acid for IntraCerebral Haemorrhage-2 trial.
Patients and methods
Seizures were reported prospectively up to day 90. Cox regression analyses were used to determine the predictors of seizures within 90 days and early seizures (≤7 days). We explored the effect of early seizures on day 90 outcomes.
Results
Of 2325 patients recruited, 193 (8.3%) had seizures including 163 (84.5%) early seizures and 30 (15.5%) late seizures (>7 days). Younger age (adjusted hazard ratio (aHR) 0.98 per year increase, 95% confidence interval (CI) 0.97–0.99; p = 0.008), lobar haematoma (aHR 5.84, 95%CI 3.58–9.52; p < 0.001), higher National Institute of Health Stroke Scale (aHR 1.03, 95%CI 1.01–1.06; p = 0.014) and previous stroke (aHR 1.66, 95%CI 1.11–2.47; p = 0.013) were associated with early seizures. Tranexamic acid did not increase the risk of seizure within 90 days. Early seizures were associated with worse modified Rankin Scale (adjusted odds ratio (aOR) 1.79, 95%CI 1.12–2.86, p = 0.015) and increased risk of death (aOR 3.26, 95%CI 1.98–5.39; p < 0.001) at day 90.
Discussion and conclusion: Lobar haematoma was the strongest independent predictor of early seizures after intracerebral haemorrhage. Tranexamic acid did not increase the risk of post-intracerebral haemorrhage seizures in the first 90 days. Early seizures resulted in worse functional outcome and increased risk of death.
Keywords: Seizures, intracerebral haemorrhage, tranexamic acid, randomised controlled trial, incidence
Introduction
Seizures occur in approximately 4% to 14% of patients with acute intracerebral haemorrhage (ICH).1–7 The incidence of seizure is approximately 30% when subclinical or non-convulsive seizures are diagnosed by continuous electroencephalogram (EEG).8,9 Younger age, lobar haematoma, stroke severity and haematoma expansion have been identified as risk factors for early seizures (ESs) after ICH.1,5,9 In patients with ESs, larger haematoma and intraventricular haemorrhage volume, lobar location and lower Glasgow Coma Scale (GCS) increase the risk of recurrent seizures beyond seven days.10 Similarly, cortical involvement, younger age and larger haematoma volume increase the risk of late-onset seizures.10,11 In addition, previous lobar ICH, pre-ICH dementia, presence of white matter disease on neuroimaging and apolipoprotein E ε4, which suggest underlying cerebral amyloid angiopathy are known risk factors for late seizures.10,11
In non-ICH patients, seizure is a known complication of tranexamic acid with an estimated incidence of 2.7%.12 It increased the risk of seizure by approximately five times according to one meta-analysis (pooled OR 5.39, 95% CI 3.29–8.85; I2 = 0%; p < 0.001).12 Tranexamic acid acts directly to increase the excitability of the neural network by inhibiting gamma-aminobutyric acid type A and glycine receptors, both major mediators of inhibition in the central nervous system.13,14 However, as most studies reporting seizures in people treated with tranexamic acid involved patients who underwent cardiac surgery, it is unclear whether tranexamic acid increases the risk of seizures in patients with ICH.
The impact of seizures on outcomes in ICH is debatable. Several studies reported an increased in-patient and 30-day mortality rate, two to three fold higher in patients with seizures compared to those with no seizures.8,15,16 Others found no differences in mortality amongst patients with and without seizures.5 Paradoxically, several studies found that seizures independently decreased morbidity and mortality in patients with ICH.17,18 Mehta et al. and Bruning et al. both found patients with ESs tended to have less severe neurological deterioration and less altered consciousness level at presentation, hence the lower mortality rates.17,18 EEG was not routinely performed in both studies and it is possible that patients who had severe ICH had subclinical or non-motor seizures but were not diagnosed to have seizures.17,18
Given the uncertainties with regards to post-ICH seizures highlighted above, we aimed to explore the risk factors for ESs after ICH and whether administration of tranexamic acid increased the risk of seizure. Secondly, we aimed to examine the effect of post-ICH seizure on clinical outcomes.
Methods
Study design and population
We analysed data from the Tranexamic acid for IntraCerebral Haemorrhage-2 (TICH-2), a prospective phase 3 double-blind placebo-controlled randomised controlled trial that explored the efficacy and safety of tranexamic acid for treatment of acute spontaneous ICH involving 124 centres from 12 countries. Patients aged >18 years with acute spontaneous ICH within 8 h of symptoms onset were eligible while secondary ICHs were excluded. Participants received 2 g of intravenous tranexamic acid or a matching placebo. Details of the trial have been previously described.19,20 Seizure was a pre-specified safety outcome up to 90 days from onset. Site investigators were prompted to file a serious adverse event (SAE) report when seizures occurred. The SAE reports were adjudicated by independent assessors blinded to treatment allocation, based on clinical and diagnostic information provided by site investigators.
Definitions
ES was defined as the occurrence of seizure(s) within seven days from the onset of ICH.21 Late seizure was defined as an occurrence of seizure(s) after seven days from the onset of ICH.21 Any seizure refers to occurrence of seizure within the first 90 days and is the summation of early and late seizures.
Statistical analysis
Descriptive analysis was performed to describe the baseline characteristics of patients who had and did not have seizures using Mann–Whitney U, Chi-square and Student’s t test where appropriate. Due to the small number and relatively short follow-up duration for late seizures, we limited the analyses on predictors and effects of seizures to ESs. Multivariable Cox proportional hazards regression was performed to identify predictors of any seizure and ESs in our cohort, including age, sex and treatment allocation a priori and variables that were significant on univariate analyses. Multiple linear regression and multivariable logistic regression analyses were used to explore the effect of ESs on outcomes, including death, dependency, quality of life, disability, cognition and depression. The 95% confidence intervals (CIs) are given and p values of <0.05 were considered statistically significant. All analyses were performed using SPSS version 24 (IBM, Armonk, NY).
Results
Of 2325 patients recruited, 193 (8.3%) had seizures. ESs comprised 84.5% (n = 163) of seizures, including 79 (41.1%) within 24 h and 31 (16.1%) within 6 h of onset. Late seizures comprised 15.5% (n = 30) of all seizures. Median onset-to-seizure time was 31.5 h (IQR 9.25, 91.75). Eighty-five (44.0%) had a single episode of seizure, 79 (40.9%) had multiple episodes of seizures and for 29 (15%) patients’ data on the number of seizure episodes were not available. In the first seven days, 136 (83.4%) patients received antiepileptic therapy, 16 (9.8%) did not and information was missing for 11 (6.7%). Long-term antiepileptic treatment was not routinely recorded.
Patients with seizures within 90 days and ESs were more likely to have worse premorbid modified Rankin Scale, previous ischaemic stroke and ICH as well as more severe impairment (higher National Institute of Health Stroke Scale (NIHSS) and lower GCS; Table 1).
Table 1.
Clinical characteristics of patients with and without seizures.
| Characteristics |
Any seizure |
Early seizure |
||||
|---|---|---|---|---|---|---|
| Yes (n = 193, 8.3%) | No (n = 2132, 91.7%) | p | Yes (n = 163, 7.0%) | No (n = 2162, 93%) | p | |
| Age, years, mean (SD) | 69.3 (13.3) | 68.9 (13.9) | 0.68 | 69.0 (13.3) | 68.9 (13.9) | 0.92 |
| Male sex, n (%) | 96 (49.7) | 1205 (56.5) | 0.069 | 85 (52.1) | 1216 (56.2) | 0.31 |
| Premorbid mRS, median [IQR] | 0 [0, 1] | 0 [0, 1] | 0.001 | 0 [0, 1] | 0 [0, 1] | 0.015 |
| Systolic blood pressure, mmHg, mean (SD) | 172.8 (30.8) | 175.0 (29.7) | 0.34 | 173.7 (31.0) | 174.9 (29.7) | 0.63 |
| Glasgow Coma Scale, median [IQR] | 14 [11, 15] | 15 [12, 15] | 0.001 | 14 [11, 15] | 15 [12, 15] | 0.004 |
| NIHSS, median [IQR] | 15 [8.5, 20] | 12 [6, 18] | 0.001 | 16 [8, 20] | 12 [6, 18] | 0.002 |
| Prior antiplatelet, n (%) | 58 (30.1) | 553 (26.0) | 0.22 | 51 (31.3) | 560 (25.9) | 0.13 |
| Previous stroke/TIA, n (%) | 42 (22.0) | 288 (13.6) | 0.002 | 35 (21.6) | 295(13.8) | 0.006 |
| Previous ICH, n (%) | 19 (9.9) | 107 (5.0) | 0.005 | 15 (9.3) | 111 (5.2) | 0.027 |
| Ischaemic heart disease, n (%) | 22 (10.8) | 181 (8.6) | 0.17 | 18 (11.1) | 185 (8.7) | 0.29 |
| Hypertension, n (%) | 111 (58.1) | 1310 (61.8) | 0.31 | 97 (59.9) | 1324 (61.6) | 0.66 |
| Diabetes mellitus, n (%) | 26 (13.5) | 286 (13.4) | 0.97 | 20 (12.3) | 292 (13.5) | 0.67 |
| Atrial fibrillation, n (%) | 6 (3.1) | 65 (3.1) | 0.95 | 6 (3.7) | 65 (3.0) | 0.62 |
| Onset-CT time, hours, mean (SD) | 2.4 (1.3) | 2.3 (1.3) | 0.15 | 2.4 (1.3) | 2.3 (1.3) | 0.40 |
| Tranexamic acid, n (%) | 91 (47.2) | 1070 (50.2) | 0.42 | 76 (46.6) | 1085 (50.2) | 0.38 |
ICH: intracerebral haemorrhage; IQR: interquartile range; mRS: modified Rankin Scale; NIHSS: National Institute of Health Stroke Scale; SD: standard deviation; TIA: transient ischaemic attack.
Chi-squared, Student’s t and Mann–Whitney U tests are used for comparisons of categorical variables (n, %), mean (SD) and median (IQR) respectively.
Patients with any seizure and ESs were more likely to have larger, lobar haematoma and midline shift (Table 2). Periventricular leukoaraiosis was more common in patients with any seizures compared to those with no seizures. On 24-h CT scans, patients with any seizures and ESs had more haematoma expansion, intraventricular extension and oedema growth (Table 2).
Table 2.
Baseline and 24-h CT findings in patients with and without seizures.
| Characteristics |
Any seizure |
Early seizure |
||||
|---|---|---|---|---|---|---|
| Yes (n = 193, 8.3%) | No (n = 2132, 91.7%) | p | Yes (n = 163, 7.0%) | No (n = 2162, 93%) | p | |
| Baseline CT | ||||||
| Haematoma location | ||||||
| Lobar, n (%) | 106 (55.8) | 582 (27.8) | <0.001 | 90 (55.6) | 598 (28.2) | <0.001 |
| Deep, n (%) | 78 (41.1) | 1373 (65.6) | <0.001 | 68 (42.0) | 1383 (65.2) | <0.001 |
| Infratentorial, n (%) | 6 (3.2) | 139 (6.6) | 0.060 | 4 (2.5) | 141 (6.6) | 0.036 |
| Intraventricular haemorrhage, n (%) | 66 (34.7) | 641 (30.5) | 0.22 | 55 (34.0) | 652 (30.6) | 0.37 |
| Haematoma volume, mL, mean (SD) | 33.2 (31.4) | 23.1 (26.6) | <0.001 | 32.5 (31.7) | 23.4 (26.7) | <0.001 |
| Perihaematomal oedema volume, mL, mean (SD) | 15.8 (15.3) | 12.7 (15.5) | 0.011 | 15.3 (14.5) | 12.8 (15.5) | 0.057 |
| Midline shift, mm, mean (SD) | 5.9 (2.5) | 5.9 (2.8) | 0.78 | 5.8 (2.4) | 5.9 (2.8) | 0.84 |
| Midline shift ≥5 mm, n (%) | 44 (23.5) | 327 (15.7) | 0.005 | 35 (21.9) | 336 (15.9) | 0.049 |
| Old infarct(s), n (%) | 114 (60.0) | 1279 (60.7) | 0.85 | 93 (57.4) | 1300 (60.9) | 0.38 |
| Cerebral atrophy, n (%) | 178 (93.7) | 1921 (91.2) | 0.24 | 151 (93.2) | 1948 (91.2) | 0.39 |
| Periventricular leukoaraiosis, n (%) | 101 (52.3) | 957 (44.9) | 0.040 | 84 (51.9) | 974 (45.6) | 0.13 |
| 24-h CT | ||||||
| Haematoma expansion, n (%) | 55 (34.8) | 513 (26.4) | 0.031 | 47 (35.1) | 523 (26.9) | 0.040 |
| Absolute haematoma growth, mL, mean (SD) | 7.6 (18.2) | 4.7 (14.7) | 0.052 | 8.4 (19.2) | 4.7 (14.6) | 0.035 |
| Absolute oedema growth, mL, mean (SD) | 9.7 (16.6) | 6.4 (11.8) | 0.014 | 10.6 (16.9) | 6.4 (11.8) | 0.005 |
| Intraventricular haemorrhage extensiona, n (%) | 26 (15.1) | 154 (7.8) | 0.003 | 21 (14.5) | 158 (7.9) | 0.005 |
| Change in midline shift, mm, mean (SD) | 0.8 (4.2) | 1.2 (3.7) | 0.48 | 1.0 (4.3) | 1.2 (3.7) | 0.83 |
Chi-squared and Student’s t tests are used for comparisons of categorical variables (n, %) and mean (standard deviation) respectively.
aIntraventricular haemorrhage extension was present if intraventricular haemorrhage was present on 24-h CT but not on baseline CT.
From the Cox regression analysis, which was adjusted for a priori variables of age, sex and treatment allocation and variables that were significant on univariate analyses, the strongest predictor of any seizure was lobar location with an adjusted hazard ratio (aHR) of 4.83 (95%CI 3.07–7.60; p < 0.001). Similarly, lobar location was the strongest predictor of ES with aHR 5.84 (95%CI 3.58–9.52; p < 0.001). Other significant predictors of any seizure and ESs include younger age, higher NIHSS and previous ischaemic stroke (Table 3). Larger haematoma volume was not a significant predictor.
Table 3.
Multivariable Cox regression analyses of predictors of seizures within 7 and 90 days.
| Variables | Any seizure ≤90 d Adjusteda HR (95%CI) | p | Early seizure ≤7 d Adjusteda HR (95%CI) | p |
|---|---|---|---|---|
| Age (years) | 0.98 (0.97–0.99) | 0.004 | 0.98 (0.97–0.99) | 0.008 |
| Sex (male) | 0.96 (0.70–1.30) | 0.77 | 1.01 (0.72–1.40) | 0.98 |
| Premorbid mRS | 1.15 (1.00–1.32) | 0.051 | 1.07 (0.91–1.25) | 0.41 |
| NIHSS | 1.03 (1.01–1.05) | 0.019 | 1.03 (1.01–1.06) | 0.014 |
| Prior stroke/TIA | 1.69 (1.17–2.43) | 0.005 | 1.66 (1.11–2.47) | 0.013 |
| Prior intracerebral haemorrhage | 1.11 (0.65–1.89) | 0.71 | 1.20 (0.68–2.14) | 0.53 |
| Lobar location | 4.83 (3.07–7.60) | <0.001 | 5.84 (3.58–9.52) | <0.001 |
| Baseline haematoma volume (per 10 mL) | 1.07 (0.98–1.17) | 0.13 | 1.07 (0.97–1.17) | 0.16 |
| PHO volume (per 10 mL) | 1.08 (0.87–1.33) | 0.49 | 1.12 (0.90–1.40) | 0.31 |
| Midline shift ≥5 mm | 1.30 (0.85–1.99) | 0.23 | 1.11 (0.69–1.78) | 0.67 |
| Periventricular leukoaraiosis | 1.41 (1.00–1.99) | 0.049 | 1.31 (0.91–1.90) | 0.15 |
| Tranexamic acid | 0.89 (0.66–1.19) | 0.42 | 0.86 (0.62–1.18) | 0.34 |
CI: confidence interval; HR: hazard ratio; mRS: modified Rankin Scale; NIHSS: National Institute of Health Stroke Scale; PHO: perihaematomal oedema; TIA: transient ischaemic attack.
aAdjusted for a priori variables of age, sex and treatment allocation and variables that were significant on univariate analyses.
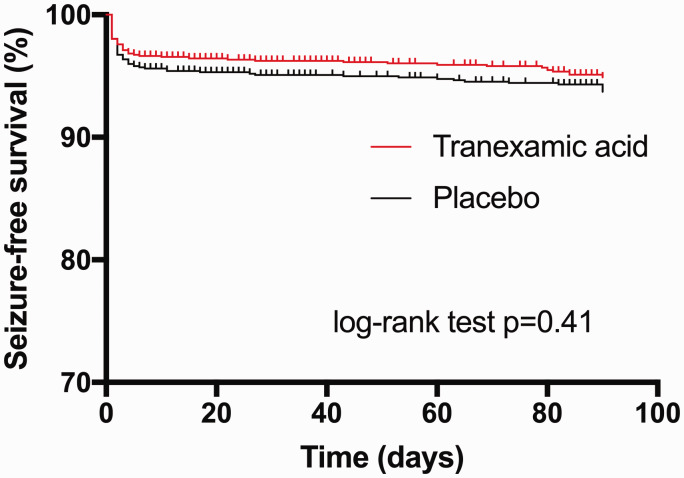
Tranexamic acid did not increase the risk of any seizures (aHR 0.89, 95%CI 0.66–1.19; p = 0.42) or ESs (aHR 0.86, 95%CI 0.62–1.18; p = 0.34; Table 3). Kaplan–Meier curves showed no significant difference in seizure-free survival between tranexamic acid and placebo (Figure 1). Exploratory analysis showed that the incidence of seizures did not differ between the treatment groups whether a full dose (2 g) or less than full dose was given, or by the degree of renal impairment (examined since TXA is renally excreted), although only 38 participants had an eGFR <30 mL/min (Table 4).
Figure 1.
Kaplan–Meier curves show seizure-free survival after intracerebral haemorrhage according to treatment group. There was no significant difference in the seizure event curves between treatment group (log-rank test p = 0.41).
Table 4.
Incidence of seizures by treatment groups according to subgroups of treatment completion and estimated glomerular filtration rate.
|
Any seizures ≤90 days |
||||
|---|---|---|---|---|
| Subgroups | N | Tranexamic acid | Placebo | p |
| Treatment completiona | ||||
| Full dose | 2207 | 80 (7.3) | 89 (8.0) | 0.49 |
| Less than full dose | 100 | 8 (16.7) | 13 (24.5) | 0.33 |
| eGFR (mL/min/1.73 m2)b | ||||
| ≥60 | 1857 | 73 (7.8) | 83 (9.0) | 0.34 |
| 30–59 | 280 | 10 (7.5) | 13 (8.8) | 0.69 |
| <30 | 38 | 3 (13.6) | 1 (6.3) | 0.46 |
Data are number (%) and statistics are Chi-squared test.
aTreatment not given at all in 15 patients; completion status was uncertain in 3 patients.
bEstimated glomerular filtration rate (eGFR) not available in 150 patients.
Within the first seven days, ESs were associated with increased neurological deterioration (n = 102, 63% vs. 618, 29.1% in those with no ES; p < 0.001), neurosurgery (22, 13.5% vs. 95, 4.5%; p = 0.001), invasive ventilation (28, 17.2% vs. 133, 6.2%; p < 0.001) and intensive care unit admission (37, 22.7% vs. 190, 8.9%; p < 0.001). Amongst patients who survived beyond seven days, day 90 death, dependency, disability, mood, cognition and quality of life were all significantly worse in patients with ES (Table 5). Sensitivity analyses showed that patients with seizure <6 h, <24 h and with single or multiple seizures similarly had worse day 90 outcomes (Supplemental Tables 1 and 2). The hazard ratio of death within 90 days was increased in patients with ESs (aHR 2.40, 95%CI 1.67–3.46; p < 0.001; Supplemental Table 3 with Supplemental Figure 1 showing a Kaplan–Meier curve of time to death in patients with ESs and no ESs).
Table 5.
Effects of early seizures on day 90 outcomes.
| Outcomes | Early seizure (n = 139, 85.3%) | No early seizure (n = 1962, 90.7%) | OR/MD (95% confidence interval) | p |
|---|---|---|---|---|
| Deatha | 38 (27.9) | 237 (12.2) | 3.26 (1.98, 5.39) | <0.001 |
| mRS >3a | 88 (64.7) | 947 (48.6) | 1.79 (1.12, 2.86) | 0.015 |
| Barthel Indexb | 42.6 (43.9) | 60.7 (41.1) | −11.8 (−17.1, −6.6) | <0.001 |
| EQ-5D HUSb | 0.26 (0.40) | 0.39 (0.40) | −0.08 (−0.14, −0.03) | 0.005 |
| TICS-mb | 10.6 (12.7) | 17.4 (11.5) | −3.0 (−4.9, −1.1) | 0.002 |
| ZDSb | 73.1 (31.4) | 59.1 (26.9) | 6.6 (1.7, 11.4) | 0.008 |
EQ-5D HUS: EuroQoL-5 Dimensions Health Utility Status; ICU: intensive care unit; mRS: modified Rankin Scale; TICS-m: modified Telephone Interview Cognitive Status; ZDS: Zung Depression Scale.
aBinary logistic regression (OR, odds ratio) and bmultiple linear regression (MD, mean difference) adjusted for age, sex, premorbid modified Rankin Scale, prior antiplatelet therapy, National Institute of Health Stroke Scale, systolic blood pressure, onset to CT <3 h, baseline haematoma volume, intraventricular haemorrhage and lobar location. Analysis limited to 2101 participants who survived beyond seven days.
Discussion
Lobar location of haematoma was the strongest independent predictor of seizure within 90 days and ESs after ICH. This is in agreement with previous studies, which had reported lobar location as a risk factor for ES with an odds ratio of 2 to 16.1,5,7,22 In addition, ICH severity as indicated by worse NIHSS and previous stroke/transient ischaemic attack (TIA) were independent predictors of ES. The pathophysiology of ESs after ICH is unclear but was hypothesised to be related to physical disruption of cortical networks by haematoma or irritation by blood products.5,10 Our findings do not support haematoma or oedema size or mass effect as an independent risk factor for early post-ICH seizures. Previous studies found haematoma size as a risk factor for seizures23 while others did not.1,5,7,22 Indeed, one study found the risk of ESs to be lower with larger haematoma.4 Haematoma expansion was reported as a risk factor in one study.9 Younger age was a predictor for ES in our study in agreement with De Herdt et al.7 and Woo et al.,1 though other studies found no significant association between age and occurrence of ESs.4,5
Tranexamic acid did not increase the risk of seizures in the current large study and this should allay concerns about using tranexamic acid in ICH. The risk of seizures with tranexamic acid was highlighted in a systematic review by Lin and Xiaoyi which reported a pooled odds ratio of 5.4.12 However, the population studied were largely patients who underwent cardiac surgery and mostly used higher doses of tranexamic acid (>50 mg/kg). In the same study, higher doses of tranexamic acid were associated with a higher incidence of seizures.12 Comparatively, the dose used in the TICH-2 trial was relatively low (2 g or approximately 28 mg/kg for an average adult weight of 70 kg).
There were also concerns that adverse events may be increased in patients with renal impairment as tranexamic acid is mainly excreted through the kidneys. A pharmacokinetic study of tranexamic acid in patients who underwent cardiac surgery reported elevated plasma levels of tranexamic acid and high incidence of seizures (8%) in patients with advanced kidney disease.24 The authors proposed that a safe dose of tranexamic acid in patients with eGFR of <60 mL/min/1.73 m2 to be a loading dose of <30 mg/kg and maintenance of <10 m/kg.24 In our cohort, 318 (13.7%) patients had an eGFR of <60 mL/min/1.73 m2 but the risk of seizures was not increased in these patients. This may be because the dose used in TICH-2 was lower than the threshold dose that may increase the risk of seizures in patients with renal impairment.
ESs were associated with neurological deterioration in the first week and led to worse day 90 outcomes in terms of higher death, dependency, disability, cognition, depression and quality of life. This is despite accounting for other known prognostic factors such as age, baseline haematoma volume, IVH and lobar location. This is perhaps indicative that early excitotoxicity after ICH is detrimental and supports the need for preventive or treatment strategies. Current guidelines found little evidence in recommending primary seizure prophylaxis for ICH.25 One small randomised controlled trial found that valproic acid did not reduce the risk of seizures at one year post-ICH although the incidence of ESs was reduced.26
The strength of the current analysis is that it was a prospective multicentre trial with a large sample size, involving patients from 12 countries. Seizure was a pre-specified safety outcome and mandated reporting, which may have increased the capture rate. All SAE reports were adjudicated blinded to treatment allocation by independent expert assessors, further improve the validity of the data. This study was also the first randomised controlled trial to prospectively study the risk of seizures with the use of tranexamic acid in ICH. One limitation of the study is a relatively small number of patients with late seizures. In addition, as the latency of post-ICH epilepsy is much longer than 90 days27,28 we could not reliably determine the predictors or the effect of late seizures. This is perhaps best studied by longer-term registry-based research. Some data were not available, namely seizure semiology in a large proportion of patients, seizure classification, previous history of epilepsy and prior anti-epileptic use. The diagnoses of seizure were mainly made by clinical observation. As EEG was not routinely performed, some subclinical or non-convulsive seizures might have been missed, especially in people with depressed conscious level.8,9 These limitations occurred, as the primary research design was not to study post-ICH seizures but rather to ensure the safety of tranexamic acid with regards to seizures. Nevertheless, this represents a pragmatic real-world scenario where neurology review and EEG are not always available to patients with seizure.
In conclusion, tranexamic acid did not increase the risk of seizures in spontaneous ICH within the first 90 days. Lobar haematoma was the strongest risk factor for seizures after ICH. ES was associated with a worse clinical outcome. Future research on prevention and treatment of ES after ICH should consider focus on patients with lobar ICH.
Supplemental Material
Supplemental material, ESO901391 Supplemental Material for Incidence and predictors of early seizures in intracerebral haemorrhage and the effect of tranexamic acid by Zhe Kang Law, Timothy J England, Amit K Mistri, Lisa J Woodhouse, Lesley Cala, Rob Dineen, Serefnur Ozturk, Maia Beridze, Ronan Collins, Philip M Bath and Nikola Sprigg in European Stroke Journal
Acknowledgements
We would like to acknowledge Alessandro Adami (Al A) and Ana Casado (AC) for assistance in adjudicating neuroimaging data; and Azlina Ali and Kailash Krishnan in measurement of haematoma and oedema volumes. Clinical Trial registration URL: https://www.isrctn.com Unique identifier: ISRCTN93732214.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PMB is Stroke Association Professor of Stroke Medicine. He has received consulting fees from Athersys, Nestle, Phagenesis and ReNeuron; he is an unpaid advisor to Platelet Solutions.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIHR HTA grant (project code 11_129_109) and Swiss Heart Foundation.
Ethical approval
This study was approved by the national and institutional ethics review committee of participating countries/centres.
Informed consent
Written informed consent was obtained from the patient(s) or their representatives for their anonymised information to be published in this article.
Guarantor
NS.
Contributorship
NS and PMB designed the study. TJE and AKM adjudicated SAE reports on seizures. RD and LAC are neuroradiologists who performed adjudications. ZKL measured haematoma and oedema volumes. ZKL wrote the first draft of the manuscript and performed statistical analysis. All authors revised and approved the final draft.
Data sharing
After publication of the planned primary and secondary analyses, the trial data can be shared, upon reasonable request to the corresponding author and trial steering committee.
ORCID iD
Zhe Kang Law https://orcid.org/0000-0002-7900-9037
Supplemental material
Supplemental material is available for this article online.
References
- 1.Woo KM, Yang SY, Cho KT. Seizures after spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc 2012; 52: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Greef BT, Schreuder FH, Vlooswijk MCet al. Early seizures after intracerebral hemorrhage predict drug-resistant epilepsy. J Neurol 2015; 262: 541–546. [DOI] [PubMed] [Google Scholar]
- 3.Sung CY, Chu NS. Epileptic seizures in intracerebral haemorrhage. J Neurol Neurosurg Psychiatry 1989; 52: 1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Passero S, Rocchi R, Rossi Set al. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia 2002; 43: 1175–1180. [DOI] [PubMed] [Google Scholar]
- 5.Bladin CF, Alexandrov AV, Bellavance Aet al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000; 57: 1617–1622. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick CJ, Davis SM, Tress BMet al. Epileptic seizures in acute stroke. Arch Neurol 1990; 47: 157–160. [DOI] [PubMed] [Google Scholar]
- 7.De Herdt V, Dumont F, Henon Het al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology 2011; 77: 1794–1800. [DOI] [PubMed] [Google Scholar]
- 8.Vespa PM, O'Phelan K, Shah Met al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003; 60: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 9.Claassen J, Jette N, Chum Fet al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007; 69: 1356–1365. [DOI] [PubMed] [Google Scholar]
- 10.Biffi A, Rattani A, Anderson CDet al. Delayed seizures after intracerebral haemorrhage. Brain 2016; 139: 2694–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haapaniemi E, Strbian D, Rossi Cet al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014; 45: 1971–1976. [DOI] [PubMed] [Google Scholar]
- 12.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure 2016; 36: 70–73. [DOI] [PubMed] [Google Scholar]
- 13.Lecker I, Wang DS, Romaschin ADet al. Tranexamic acid concentrations associated with human seizures inhibit glycine receptors. J Clin Invest 2012; 122: 4654–4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furtmuller R, Schlag MG, Berger Met al. Tranexamic acid, a widely used antifibrinolytic agent, causes convulsions by a gamma-aminobutyric acid(A) receptor antagonistic effect. J Pharmacol Exp Ther 2002; 301: 168–173. [DOI] [PubMed] [Google Scholar]
- 15.Szaflarski JP, Rackley AY, Kleindorfer DOet al. Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia 2008; 49: 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arboix A, Garcia-Eroles L, Massons JBet al. Predictive factors of early seizures after acute cerebrovascular disease. Stroke 1997; 28: 1590–1594. [DOI] [PubMed] [Google Scholar]
- 17.Mehta A, Zusman BE, Choxi Ret al. Seizures after intracerebral hemorrhage: incidence, risk factors, and impact on mortality and morbidity. World Neurosurg 2018; 112: e385–e392. [DOI] [PubMed] [Google Scholar]
- 18.Bruning T, Awwad S, Al-Khaled M. Do early seizures indicate survival of patients with nontraumatic intracerebral hemorrhage?. Cerebrovasc Dis 2016; 41: 68–73. [DOI] [PubMed] [Google Scholar]
- 19.Sprigg N, Flaherty K, Appleton JPet al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet 2018; 391: 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sprigg N, Robson K, Bath Pet al. Intravenous tranexamic acid for hyperacute primary intracerebral hemorrhage: protocol for a randomized, placebo-controlled trial. Int J Stroke 2016; 11: 683–694. [DOI] [PubMed] [Google Scholar]
- 21.Beghi E, Carpio A, Forsgren Let al. Recommendation for a definition of acute symptomatic seizure. Epilepsia 2010; 51: 671–675. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara S, Sato S, Kodama Tet al. Nonconvulsive status epilepticus in acute intracerebral hemorrhage. Stroke 2018; 49: 1759–1761. [DOI] [PubMed] [Google Scholar]
- 23.Alberti A, Paciaroni M, Caso Vet al. Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vasc Health Risk Manag 2008; 4: 715–720. [PMC free article] [PubMed] [Google Scholar]
- 24.Jerath A, Yang QJ, Pang KSet al. Tranexamic acid dosing for cardiac surgical patients with chronic renal dysfunction: a new dosing regimen. Anesth Analg 2018; 127: 1323–1332. [DOI] [PubMed] [Google Scholar]
- 25.Holtkamp M, Beghi E, Benninger Fet al. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur Stroke J 2017; 2: 103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilad R, Boaz M, Dabby Ret al. Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment?. Epilepsy Res 2011; 95: 227–231. [DOI] [PubMed] [Google Scholar]
- 27.Lahti AM, Saloheimo P, Huhtakangas Jet al. Poststroke epilepsy in long-term survivors of primary intracerebral hemorrhage. Neurology 2017; 88: 2169–2175. [DOI] [PubMed] [Google Scholar]
- 28.Zelano J, Redfors P, Asberg Set al. Association between poststroke epilepsy and death: a nationwide cohort study. Eur Stroke J 2016; 1: 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, ESO901391 Supplemental Material for Incidence and predictors of early seizures in intracerebral haemorrhage and the effect of tranexamic acid by Zhe Kang Law, Timothy J England, Amit K Mistri, Lisa J Woodhouse, Lesley Cala, Rob Dineen, Serefnur Ozturk, Maia Beridze, Ronan Collins, Philip M Bath and Nikola Sprigg in European Stroke Journal