Symptom distress - the degree of discomfort from symptoms experienced by the patient - remains one of the most challenging complications of living with HIV, despite effective HIV antiretroviral therapy. As HIV has become a chronic disease, HIV-associated symptoms have evolved from being related to illness progression and medication side effects to those associated with inflammation, accelerated aging, and age-related co-morbidities (Webel et al., 2018; Willig et al., 2015). While symptom distress in people living with HIV (PLHIV) varies by sex and race (Lee, Dziadkowiec, & Meek, 2014; Webel et al., 2015), it significantly impacts the daily functioning and quality of life in all.
PLHIV often simultaneously experience multiple distressing symptoms, yet symptom management strategies tend only to target one symptom at a time (Schnall et al., 2017). In particular, pharmacological interventions individually treat pain, insomnia, and depression with varying degrees of success, but their use by PLHIV is limited by pill burden and side effects (Gimeno-Gracia, Crusells-Canales, Armesto-Gomez, Compaired-Turlan, & Rabanaque-Hernandez, 2016; Siefried et al., 2018). In contrast, non-pharmacological strategies such as physical activity and diet may provide symptom relief with a lower side effect profile than most prescription drugs.
Indeed, physical activity and a healthy dietary intake can reduce symptoms among individuals with a wide variety of chronic diseases. In individuals without HIV infection, physical activity reduces chronic pain (Geneen et al., 2017), depression (Harvey et al., 2018), fatigue (Salmon, Hewlett, Walsh, Kirwan, & Cramp, 2017) and improves cognitive function (Barha, Davis, Falck, Nagamatsu, & Liu-Ambrose, 2017),while a diet comprised of high fiber, moderate fat, and low in carbohydrates is associated with reduced pain, depression and fatigue (Jacka et al., 2017). Similar responses to physical activity have been observed among PLHIV in smaller studies (Fazeli et al., 2015; O’Brien, Tynan, Nixon, & Glazier, 2016) however, little is known about the relationship between dietary intake and symptoms in PLHIV. Further, PLHIV in the U.S. consume diets low in fiber and high in fat and carbohydrates (Willig, Wright, & Galvin, 2018; Webel et al., 2017) which may exacerbate symptoms. Definitive evidence examining the impact of physical activity and dietary intake on symptoms in PLHIV is needed to provide specific physical activity and dietary recommendations to improve their health and well-being.
In order to address these gaps in the literature, we are currently conducting the “Impact of Physical Activity Routines and Dietary Intake on the Longitudinal Symptom Experience of the people living with HIV (PROSPER-HIV)” study. The purpose of the PROSPER-HIV study is to understand not just if, but which specific aspects of physical activity and dietary intake affect the symptom experience in PLHIV. PROSPER-HIV will be one of the first investigations to longitudinally assess physical activity and dietary intake among PLWH using gold standard assessment techniques. Specifically, we aim to:
Characterize longitudinal, objectively measured physical activity and diet patterns among PLHIV.
Determine which aspects of physical activity patterns (e.g., intensity, frequency, duration) and diet quality are associated with decreased symptom burden and intensity in PLHIV, and if this relationship is moderated by age and sex.
Explore the potential mediating effects of anthropomorphic and physical fitness (waist-hip-ratio, Body Mass Index [BMI], hand-grip strength, Short Performance Physical Battery) variables on the relationships between physical activity, diet patterns, and symptom burden in PLHIV.
Methods
Overview
The PROSPER-HIV study is a four-year, prospective, observational study of 850 participants from the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS). Enrolled participants will complete assessments of physical activity, dietary intake, and physical fitness once a year for three years (Figure 1). As part of their routine clinical care, all participants will also complete a standard CNICS patient-reported outcome assessment (which includes the HIV-Symptom Index) and clinical assessment procedures (Kitahata et al., 2008).
Figure 1.
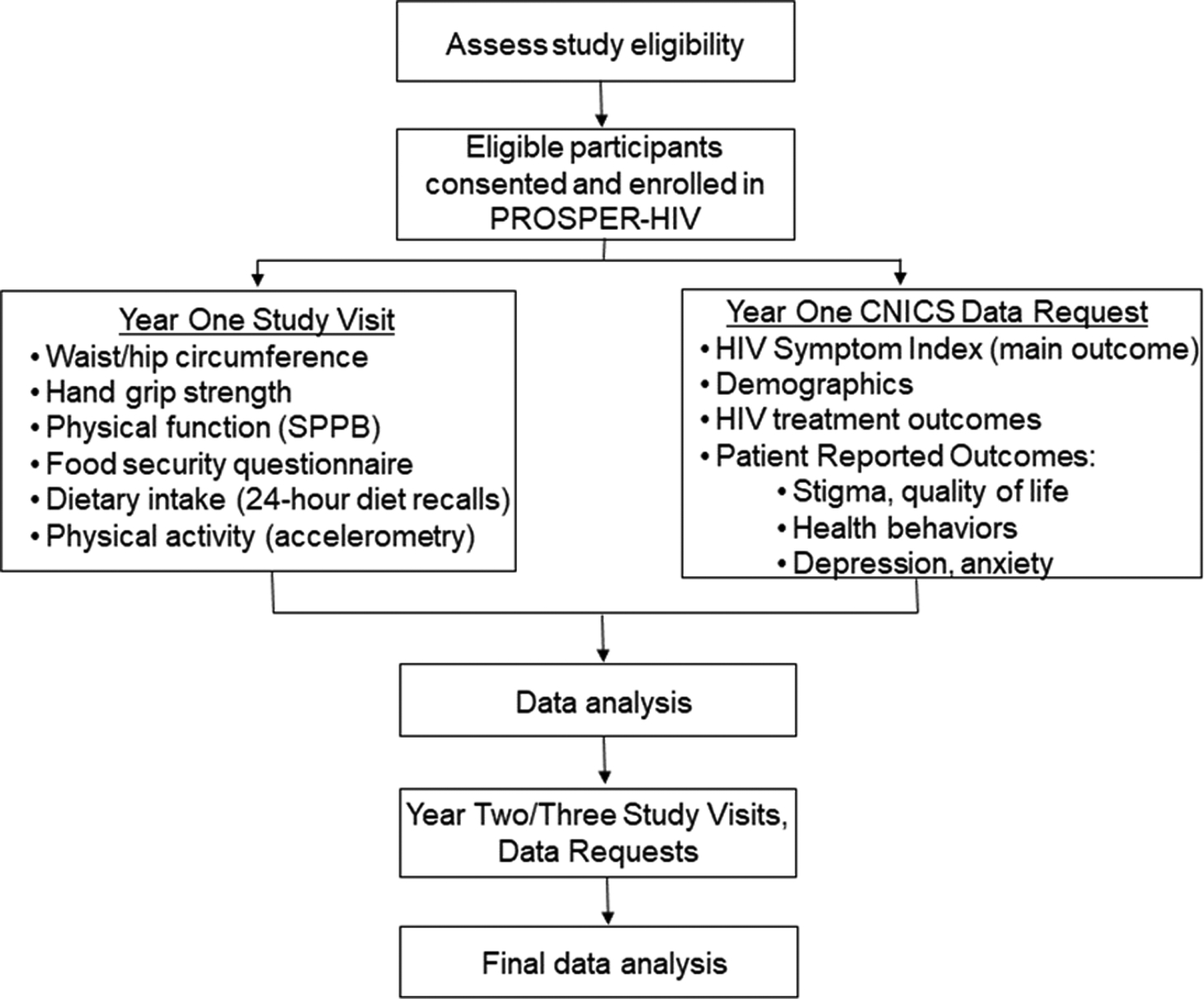
PROSPER-HIVstudy procedure flowchart
Setting
Center for AIDS Research (CFAR) Network of Integrated Clinical Systems.
Any single HIV clinical site typically has insufficient numbers of patients to provide adequate physical activity and dietary intake data to understand the associations with reported symptoms. As such, the PROSPER-HIV study is being conducted as a CNICS (R24AI067039) research protocol. CNICS has incorporated high-quality clinical data from PLHIV in care for the past 25 years. CNICS was established to better define relationships between patient and treatment factors and long-term outcomes among PLHIV (Kitahata et al., 2008). CNICS incorporates new PLHIV as they enter care, ensuring relevance to modern HIV care. It has eight HIV clinical care sites with >32,000 PLHIV enrolled since 1995 with >35 million clinical observations and >200,000 person-years of follow-up. CNICS is diverse in terms of sex, race/ethnicity geography, and age with 18% women, 44% White, 38% Black, 12% Hispanic race/ethnicity, and more than 42% are aged ≥50 years. In addition to working with a large, diverse, generalizable cohort, nesting the PROSPER-HIV study within the CNICS cohort allows us to leverage two other important resources including:
Robust yet brief patient-reported outcomes routinely collected on all participants. These self-administered data are collected at least annually on encrypted touch screen computers and include symptom burden, HIV medication adherence, smoking and substance use, quality of life, interpersonal violence, depression and anxiety and sexual risk behaviors (Fredericksen et al., 2012) CNICS has also recently included measures of housing stability and social support.
Established standards for terminology, format, data verification, and quality assurance for a variety of laboratory, medication, demographic, and health care utilization data that will be used as descriptive variables or co-variates in the PROSPER-HIV study. The use of these resources have resulted in dozens of important manuscripts that have improved HIV clinical care (e.g., Hartzler et al., 2017; Nance et al., 2017); thus CNICS is an ideal platform to incorporate measures of physical activity and dietary intake into the existing data collection framework.
CNICS Approval Process.
Prior to proposing the PROSPER-HIV study to the NIH, a concept sheet had to be approved by the CNICS Research Coordinating Committee. This carefully delineated process is best described on the CNICS website (https://www.uab.edu/cnics) but, in brief, this process involved: (1) Conducting preliminary work demonstrating the significance of the unmitigated symptoms in PLHIV and data supporting the hypothesis that physical activity and a healthy dietary intake are likely to reduce symptom burden. (2) Checking the study feasibility, which included ensuring that PROSPER-HIV was unique from other CNICS projects and contained the data elements needed to complete the study. (3) Working with a CNICS collaborator to develop a concept proposal application. (4) Participating in a review call with the CNICS Research Coordinating Committee to discuss the study purpose, overview, procedures, and impact on the individual participating sites (goals and content of this call are tailored to type of study proposed). (5) Receiving notification of the Research Coordinating Committee’s decision. For the PROSPER-HIV study, steps 2–5 of the review process took approximately 4 months with most of that time devoted to writing the concept proposal.
PROSPER-HIV Sites.
The PROSPER-HIV study is being conducted at four academic medical centers (Case Western Reserve University, University of Alabama at Birmingham, University of Washington at Seattle, and Fenway Health Institute) that provide HIV care for diverse PLHIV who are broadly representative of the U.S. PLHIV population (Table 1). All sites are longstanding members of CNICS and have dedicated space and technological infrastructure for research visits. All PROSPER-HIV procedures occur in this existing CNICS research space.
Table 1.
Demographics of PLHIV engaged in the CNICS PROs at the sites selected for this study1
| Total CNICS participants | Age (SD) | % Female | % Black | % Hispanic | Median Symptom Count (IQR)2 | |
|---|---|---|---|---|---|---|
| Case Western Reserve University | 1020 | 51 (12.8) | 21% | 54% | 4% | 7 (3, 12) |
| University of Alabama at Birmingham | 2649 | 47.5 (12.3) | 25% | 62% | <1% | 6 (3,10) |
| University of Washington at Seattle | 2080 | 45.1 (10.8) | 15% | 21% | 14% | 6 (3,12) |
| Fenway Health Institute | 1147 | 48.7 (10.9) | 2% | 10% | 16% | 7 (3,12) |
Data collected from participants seen in the respective clinics in the past 18 months.
Median symptom count was calculated from the PRO’s completed 1/1/2018–4/15/2018; Symptom count range from 0–20 with higher numbers indicting more symptoms
Participants
The PROSPER-HIV study enrollment goal is 850 PLHIV. This sample size was determined using a power analysis based on our preliminary work describing symptom rates and the standard deviations of self-reported physical activity (Webel, et al., 2019). We determined that with 850 PLHIV we would be able to detect the odds of symptom reduction being 93% higher with an increase of 30-minutes of MVPA with 85% power. To be eligible, participants must be (1) an active CNICS participant (i.e., they must have a have a CNICS consent and have completed the current patient reported outcome assessment); (2) aged ≥18 years; (3) prescribed ART as part of CNICS care; and (4) HIV Viral Load < 200 copies/mL at time of enrollment (>90% of PLHIV in CNICS care are undetectable (Nance, et al., 2018)). Participants will be excluded if he or she (1) did not complete the HIV Symptom Index in a recent assessment, (2) is pregnant, breast-feeding, or planning a pregnancy during the study period, (3) does not have telephone or internet access to complete 24-hour diet recalls, (4) plans to move out of the area in the next 36 months. Additionally, we plan to have a minimum of 50% (n=425) African-American, Hispanic, Asian, and 30% female (n≥255) participants, resulting in representative finding that will allow us to examine sex as a potential moderator on our outcomes.
Procedures.
At the routine patient clinic visit, participants complete the standard CNICS patient reported outcome assessment battery as usual. After participants complete this assessment, the Research Assistant explains the PROSPER-HIV study procedures, potential risks and benefits, and asks the participant if he or she would like to enroll. If they agree, the Research Assistant obtains informed consent and then helps participants complete the PROSPER-HIV assessment which includes (1) waist and hip circumference measures, (2) hand-grip strength using the Jamar Hand Grip Dynamometer, (3) Short Physical Performance Battery, (4) Food Security Questionnaire, and (5) explain and schedule three telephone dietary interviews with the University Hospitals, Cleveland Medical Center’s Clinical Research Unit Bionutrition Core over the next 30 days. The Research Assistant then initializes the participant’s accelerometer, instructs him or her in the proper use of the ActiGraph accelerometer, and provides instructions for returning it ~10 days later (either in person or by mail). In the first months of study recruitment, this assessment is taking ~ 20–25 minutes to complete and is typically done at the end of the participant’s regularly scheduled clinic visit. These procedures are repeated once every 12 months for three years. The study is approved by the University Hospitals, Cleveland Medical Center’s Institutional Review Board (IRB) which is the IRB of record for the PROSPER-HIV study, and the IRB’s at the other three sites have sanctioned the study protocol. The PROSPER-HIV study is registered at ClinicalTrials.gov #NCT03790501.
Measures
Symptom Distress
The primary outcome in the PROSPER-HIV study is symptom distress which is being assessed with the-20 item HIV Symptom Index (Justice et al., 2001). This HIV-specific scale assesses the presence and intensity of 20 common symptoms reported by PLHIV (e.g., pain, anxiety, fatigue) over the past four weeks. All symptoms are measured on a 1–5 ordinal scale where 1 is “I do not have the symptom” and 5 is “I have this symptom and it bothers me a lot”. The reliability of the HIV Symptom Index in the CNICS cohort is 0.92 (Webel et al., 2019). The PROSPER-HIV endpoints are 1) total symptom count (sum all symptoms that are reported as having the symptom and it bothers the participant at least a little); and 2) a total count of symptoms that bother the participant a lot (high symptom distress).
Physical Activity
Physical activity is measured with the ActiGraph accelerometer (ActiGraph, LLC, Fort Walton Beach, FL) (Strath et al., 2013). The intraclass correlation for actigraphy ranges from 0.94–0.99 (Sasaki & Freedson, 2011). Participants wear the monitor for 7–10 consecutive days on their non-dominant hip. A valid wear cycle has data recorded for a minimum of 10 hours per day for at least 4 days (Haskell et al., 2012). Non-wear time is defined as 0 counts per minute for ≥60 minutes. Participants not meeting wear time standards are asked to re-wear the ActiGraph. Data are sampled at 30 Hz, using 60-second epochs, and the low frequency filter (Migueles et al., 2017). Activity ≥ 2690 counts per minute and ≥10 minutes is defined as exercise. The primary exercise endpoints are (1) time spent in moderate-to-vigorous physical activity, and (2) sedentary time. These endpoints are set with the Sasaki, John, and Freedson (2011) adult cutpoints.
Diet Intake
Food security status is determined using the two-item Food Security Questionnaire which classifies respondents as (1) food secure, (2) food secure with hunger (low food security), or (3) food insecure with hunger (very low food security) (Young, Jeganathan, Houtzager, Di Guilmi & Purnomo, 2009; Muhammad, Fernandez, Clay, Saag, Overton & Willig, 2019). Diet intake is measured using a standardized triple-pass 24-hour recall (partial correlation =0.27) obtained by a trained Registered Dietitian (RD). The recalls capture dietary intake for two weekdays and one weekend and within a 30-day window (Moshfegh, Rhodes, Baer, Murayi, Clemens, et al, 2008; Satija, Willett, & Hu, 2015). The RD calls each participant to conduct a nutrition interview. During this interview, the participant recalls what and how much was consumed in the previous 24 hours. Responses are simultaneously entered into the Nutrition Data System for Research Nutritional Analysis Software (University of Minnesota). The Healthy Eating Index-2015 (HEI-2015), a composite measure of diet is the PROSPER-HIV endpoint (Freedman, Guenther, Krebs-Smith, & Kott, 2008; Guenther, Reedy, Krebs-Smith, & Reeve, 2008). We will also assess the intake of dietary fiber, protein, and simple carbohydrates.
Physical Fitness
Physical Fitness is assessed with two measures, handgrip strength and the Short Performance Physical Battery. Handgrip strength is commonly used and correlates to overall muscle strength. It is measured with the Jamar hand-held dynamometer. Each completes two trials with their self-reported dominant hand (Webel et al., 2019). The maximum strength of dominant hand (all attempts) is recorded as the endpoint.
The Short Physical Performance Battery (SPPB) is a brief measure physical performance or functional status that includes a timed walk, repeated chair stands, and several balance tests. The SPPB is a well-regarded, valid (test-rest reliability =0.87), objective assessment of physical function, particularly lower extremity function, and associated with short-term mortality, disability, hospitalizations, and nursing home admission (Guralnik et al., 1994). Each measure on the SPPB is assigned a score from 0–4, with 0 indicating inability to complete the test, yielding a summary score ranging from 0 (frail) to 12 (not frail) (Guralnik et al., 1994). This summery score will be analyzed as our endpoint (Willig, Wright, & Galvin, 2018; Crane, Miller, Pierce, Willig, Case, et al., 2019).
Analysis
The outcomes for the PROSPER-HIV study are to: (1) characterize longitudinal physical activity and dietary intake patterns in PLHIV; (2) determine which aspects of physical activity patterns and diet quality are associated with symptom burden; and (3) explore mediating effects of other factors that may impact the relationships of activity and diet with symptom burden. To characterize physical activity (MVPA) and dietary intake (HEI-2015), linear models maximized using the method of generalized estimating equations (GEE) will be used. Additionally, individuals will be grouped together based on similar trajectories for activity and nutrition using a semi-parametric group-based trajectory estimation (Jones, Magin, and Roeder, 2001). These trajectory groups will be investigated as “exposure” groups in these analyses.
To determine which physical activity and diet patterns are associated with symptom burden, we will implement multiple analyses to determine the relationship between activity and nutrition and HIV symptoms. We will utilize GEE, as described above, with auto-regressive working correlation and robust standard errors. HIV symptoms in two forms, symptom count and serious symptom count, will be examined with separate Poisson regressions within GEE resulting in estimated risk ratios to estimate associations of physical activity, nutrition, time and other covariates with HIV symptoms.
Finally, to assess mediating factors on the relationship between physical activity, dietary intake and symptoms, a counterfactual approach to mediation analysis will be employed, allowing for the estimation of direct and indirect effects (VanderWeele, 2009; Vanderweele, 2015). Given the assumption of normality for each of the potential mediators (waist-hip-ratio, BMI, hand-grip strength, and Short Performance Physical Battery score), we anticipate using the SAS macro published by Valeri and Vanderweele or the newly created PROC CAUSALMED in SAS, and examine potential mediator-mediator interactions.
As in any longitudinal study, it is anticipated that some participants may die, withdraw consent, or be lost to follow-up prior to the end of the study and will have missing outcomes. To deal with missing data, an investigation of the mechanisms for missing data will be conducted. Apart from changing analysis from GEE to mixed models, sensitivity analysis will include multiple imputation as described by Schafer (Schafer, 1997). Once missing values have been imputed, each multiply-imputed data set can be analyzed. Final parameter estimates and their standard errors will be calculated using Rubin’s formula for combining results from multiply imputed data sets (Rubin, 2004). We will report final study results with and without employing the multiple imputation strategy and examine and describe any discrepancies found.
Timeline
The PROSPER-HIV study is prospectively examining symptom burden over time and has four years of research procedures. Data collection started in January 2019 at two sites, and was scaled up to all four sites in April 2019. Enrollment and baseline procedures are expected to continue through March 2020 and all study procedures should be complete by March 2023.
Expected Outcomes
At the conclusion of the PROSPER-HIV study, we will have characterized longitudinal physical activity and dietary patterns in PLHIV, determined which aspects of physical activity and diet quality are associated with decreased symptom burden, and described the effect of potential mediating factors on the relationships between physical activity, dietary patterns, and symptom burden in PLHIV. Further, while we anticipate limited number of participants who identify as transgender, we plan to disaggregate data by sex and gender to better understand these relationships and the potential influence of gender affirming hormones. This new evidence will fill important gaps in the literature, enabling future research on the biological underpinnings of symptoms in PLHIV. These insights will set the stage for feasible, targeted, non-pharmacological interventions for use by HIV clinicians and patients beyond CNICS to reduce symptom burden and improve quality of life in PLHIV.
Conclusion
Every day nurses care for patients living with HIV who have a high symptom burden and have few tools to help reduce their symptoms. The PROSPER-HIV data have tremendous potential to impact care for these patients. This study will provide data on symptom patterns that can be used to help HIV clinicians to develop a clinic-based physical activity and diet intervention to improve symptom management, physical function, and quality of life among PLHIV.
Acknowledgments
This study was funded by the National Institutes of Nursing Research #R01 NR018391 (PIs: Webel and Willig) and by the National Institute of Allergy and Infectious Diseases #R24 AI067039 (PI: Saag).
Footnotes
Registration number: NCT03790501
Disclosures
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest
Contributor Information
Allison R. Webel, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA.
Dustin Long, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Benigno Rodriguez, School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA..
Christine Horvat Davey, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA..
Thomas W. Buford, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Heidi M. Crane, School of Medicine, University of Washington, Seattle Washington, USA..
Kenneth Mayer, The Fenway Institute, Boston, Massachusetts, USA..
References
- Barha CK, Davis JC, Falck RS, Nagamatsu LS, & Liu-Ambrose T (2017). Sex differences in exercise efficacy to improve cognition: A systematic review and meta-analysis of randomized controlled trials in older humans. Frontiers in Neuroendocrinology, 46, 71–85. [DOI] [PubMed] [Google Scholar]
- Crane HM, Miller ME, Pierce J, Willig AL, Case ML, Wilkin AM, Brown S, Asirot MG, Fredericksen RJ, Saag MS, Landay AL & High KP (2019) Physical Functioning Among Patients Aging With Human Immunodeficiency Virus (HIV) Versus HIV Uninfected: Feasibility of Using the Short Physical Performance Battery in Clinical Care of People Living With HIV Aged 50 or Older, Open Forum Infectious Diseases, 6 (3), 10.1093/ofid/ofz038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli PL, Marquine MJ, Dufour C, Henry BL, Montoya J, Gouaux B, … HNRP Group. (2015). Physical activity is associated with better neurocognitive and everyday functioning among older adults with HIV disease. AIDS and Behavior, 19, 1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen RJ, Crane PK, Tufano J, Ralston J, Schmidt S, Brown T, … Crane HM (2012). Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. Journal of AIDS and HIV Research, 4(2), 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LS, Guenther PM, Krebs-Smith SM, & Kott PS (2008). A population’s mean Healthy Eating Index-2005 scores are best estimated by the score of the population ratio when one 24-hour recall is available. Journal of Nutrition, 138, 1725–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, & Smith BH (2017). Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database of Systematic Reveiws. [Google Scholar]
- Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gomez FJ, Compaired-Turlan V, Rabanaque-Hernandez MJ (2016). Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clinical Interventions in Aging, 11, 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther PM, Reedy J, Krebs-Smith SM, & Reeve BB (2008). Evaluation of the Healthy Eating Index-2005. Journal of the American Dietetic Association, 108, 1854–1864. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, … Wallace RB (1994). A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of Gerontolgy, 49, M85–94. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH Mathews CW, … Donovan DM (2017). Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS and Behavior, 21, 1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SB, Overland S, Hatch SL, Wessely S, Mykletun A, & Hotopf M (2018). Exercise and the prevention of depression: Results of the HUNT cohort study. The American Journal of Psychiatry, 175, 28–36. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Troiano RP, Hammond JA, Phillips MJ, Strader LC, Marquez DX, … Ramos E (2012). Physical activity and physical fitness: Standardizing assessment with the PhenX Toolkit. American Journal of Prevtative Medicine, 42, 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacka FN, O’Neil A, Opie R, Itsiopoulos C, Cotton S Mohebbi M, … Berk M (2017). A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Medicine, 15, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, Roeder K (2001). A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research, 29:374–393. [Google Scholar]
- Justice AC, Holmes W,. Gifford AL, Rabeneck L, Zackin R, Sinclair G, … Adult AIDS Clinical Trials Unit Outcomes Committee. (2001). Development and validation of a self-completed HIV symptom index. Journal of Clinical Epidemiology, 54,S77–90. [DOI] [PubMed] [Google Scholar]
- Kitahata MM, Rodriguez B, Haubrich R, Boswell S Matthews WC, Lederman MM, … Saag M (2008). Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International Journal of Epidemiology, 37, 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA, Dziadkowiec O, & Meek P (2014). A systems science approach to fatigue management in research and health care. Nursing Outlook, 62, 313–321. [DOI] [PubMed] [Google Scholar]
- Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nystrom C, Mora-Gonzalez J, Lof M, … Ortega FB (2017). Accelerometer data collection and processing criteria to assess physical activity and other outcomes: A systematic review and practical considerations. Sports Medicine, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LA, Staples RC, Cleveland LE. (2008) The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. The American Journal of Clinical Nutrition. 88(2):324–32. [DOI] [PubMed] [Google Scholar]
- Muhammad JN, Fernandez JR, Clay OJ, Saag MS, Overton ET, Willig AL. (2019)Associations of food insecurity and psychosocial measures with diet quality in adults aging with HIV. AIDS Care. 31(5):554–62. doi: 10.1080/09540121.2018.1554239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance RM, Delaney JC, Golin CE, Wechsberg WM, Cunningham C, Altice F, … Crane HM (2017). Co-calibration of two self-reported measures of adherence to antiretroviral therapy. AIDS Care, 29, 464–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KK, Tynan AM, Nixon SA, & Glazier RH (2016). Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infectious Diseases, 16, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB (2004) Multiple imputation for nonresponse in surveys. Vol 81: John Wiley & Sons; [Google Scholar]
- Salmon VE, Hewlett S, Walsh NE, Kirwan JR, & Cramp F (2017). Physical activity interventions for fatigue in rheumatoid arthritis: A systematic review. Physical Therapy Reviews, 22, 12–22. [Google Scholar]
- Sasaki JE, John D, & Freedson PS (2011). Validation and comparison of ActiGraph activity monitors. Journal of Science and Medicine in Sport, 14, 411–416. [DOI] [PubMed] [Google Scholar]
- Satija A, Yu E, Willett WC, Hu FB. (2015) Understanding nutritional epidemiology and its role in policy. Advances in Nutrition, 6(1):5–18. doi: 10.3945/an.114.007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL (1997) Analysis of incomplete multivariate data London: Chapman & Hall. [Google Scholar]
- Schnall R, Liu J, Cho H, Hirshfield S, Siegel K, Olender S (2017). A health-related quality-of-life measure for use in patients with HIV: A validation study. AIDS Patient Care and STDS, 31, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefried KJ, Mao L, Cysique LA, Rule J, Giles ML Smith DE, …PAART study investigators. (2018). Concomitant medication polypharmacy, interactions and imperfect adherence are common in Australian adults on suppressive antiretroviral therapy. AIDS, 32, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, … Swartz AM (2013). Guide to the assessment of physical activity: Clinical and research applications: a scientific statement from the American Heart Association. Circulation, 128, 2259–2279. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ (2009) Conceptual issues concerning mediation, interventions and composition. Statistics and Its Interface, 2(4), 457–468. [Google Scholar]
- VanderWeele TJ (2015) Explanation in causal inference: Methods for mediation and interaction. Oxford University Press. [Google Scholar]
- Webel A, Jenkins T Longenecker C, Vest M, Horvat Davey C, Currie J, …Josephson R (2018). Relationship of HIV Status and Fatigue, Cardiorespiratory Fitness, Myokines, and Phyiscal Activity. Journal of the Association of Nurses in AIDS Care, doi: 10.1097/JNC.0000000000000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel AR, Sattar A, Funderburg NT, Kinley B, Longenecker CT, Labbato D, … McComsey GA (2017). Alcohol and dietary factors associate with gut integrity and inflammation in HIV-infected adults. HIV Med, 18, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel A, Wantland D, Dawson Rose C, Kemppainen J, Holzemer WL, Chen WT,…Portillo C (2015). A Cross-Sectional Relationship Between Social Capital, Self-Compassion and Perceived HIV Symptoms. Journal of Pain and Symptom Management, 50(1), 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webel A, Willig A, Liu W, Sattar A Boswell S, Crane HM, … Rodriguez B (2018). Physical Activity intensity is associated with symptom distress in the CNICS cohort. AIDS and Behavior, 23(3), 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig A, Wright L, & Galvin TA (2018). Practice Paper of the Academy of Nutrition and Dietetics: Nutrition intervention and human immunodeficiency virus infection. Journal of the Academy of Nutrition and Dietetics, 118(3), 486–498. [DOI] [PubMed] [Google Scholar]
- Young J, Jeganathan S, Houtzager L, Di Guilmi A, Purnomo J. (2009) A valid two-item food security questionnaire for screening HIV-1 infected patients in a clinical setting. Public Health Nutrition.12 (11):2129–32. Epub 2009/05/30. doi: 10.1017/S1368980009005795. [DOI] [PubMed] [Google Scholar]


