Abstract
Purpose:
Understanding the contribution of tumor genome biology to racial disparities of triple-negative breast cancer (TNBC) is important for narrowing the cancer mortality gap between Black and White women.
Methods:
We evaluated tumor somatic mutations using targeted sequencing of a customized panel of 151 genes and 15 copy number variations (CNVs) within a population of 133 TNBC patients, including 71 Black and 62 White women.
Results:
The overall mutational burden between Black and White women with TNBC was not significantly different, with a median of 5 somatic changes per patient (point mutations and CNVs combined) for the customized panel (range 1–31 for Blacks vs. 1–26 for Whites; p=0.76). Of the 151 genes examined, none were mutated at a significantly higher frequency in Black than in White cases, whereas two genes were mutated at a higher frequency in White cases - PIK3CA and NCOR1. No significant difference in the frequency of CNVs was observed between Black and White women with TNBC in our study population.
Conclusions:
Of gene mutations and CNVs in TNBC tumors from Black and White women, only PIK3CA and NCOR1 had significantly different, although slight, frequencies by race. These results indicate that overall differences observed in the mutation spectra between Black and White women with breast cancer are likely due to the differential distributions of breast cancer subtypes by race.
Keywords: triple-negative breast cancer, racial disparities, African ancestry, somatic mutation, copy number variation
Introduction
African American/Black women with breast cancer are more likely to have aggressive tumors [1, 2] and are 42% more likely to die from their disease than European American/White women [3]. This mortality gap remains even after adjusting for socioeconomic factors [4, 5], and there is wide-ranging evidence for racial differences in tumor biology between Black and White women [6–11]. A notable difference is the high prevalence of triple-negative breast cancer (TNBC) in Black women.
TNBC is a heterogeneous clinical subtype characterized by the absence of detectable expression of receptors for estrogen (ER) and progesterone (PR) and the lack of overexpression of tyrosine kinase-type cell surface receptor HER2/Neu (HER2). TNBC occurs at a disproportionately high rate in young women, and has poor prognosis compared to other subtypes of breast cancer, in part due to fewer options for therapy compared to hormone receptor positive cancers [12]. Population-based incidence rates of TNBC are roughly two-fold higher in Black than White women, which contributes to the poorer survival in the former group [12–14].
However, the question of whether there are differences in survival outcomes or tumor biology within the triple-negative subtype between Black and White women is less clear [15]. Previous studies include conflicting reports, with some studies showing clear racial differences within the TNBC subtype, and other studies reporting that the racial differences were attenuated after accounting for subtype [9–11, 16–18]. Because most breast cancers arise from somatic mutations, it is thus of great interest to compare tumor mutational profiles between Black and White patients with TNBC, to have a better understanding of the roles of tumor biology in cancer disparities. Most studies to date have relied on TCGA data as the sole data source to evaluate tumor somatic mutations in Black and White women with TNBC. Since this cohort is dominated by White cases, studies based on independent non-TCGA data with similar numbers of Black and White cases are needed. In the present study, we sought to determine racial differences in tumor somatic mutations using targeted sequencing within a population of TNBC patients with a comparable number of Black and White cases.
Methods
Study Populations
The Women’s Circle of Health Study (WCHS) is a multi-site, case-control study designed to evaluate risk factors for aggressive breast cancer in Black and White women. Details on study design have been described elsewhere [19]. Briefly, participants were English-speaking women who were 20–75 years old, self-identified as Black or White, had primary, histologically-confirmed invasive breast cancer, were diagnosed between 2001–2017, and had no previous history of cancer other than non-melanoma skin cancer. Cases were first identified from several hospitals in New York City and then from several counties in New Jersey using rapid case ascertainment by the New Jersey State Cancer Registry. As part of the informed consent process, patients were asked to sign a release permitting the use of their tumor tissue for research, and then tumor blocks and pathology reports were requested from treating hospitals. Blood samples were first collected and then saliva samples as a source of genomic DNA. Clinicopathologic variables were extracted from pathology reports. For this study, only patients who were diagnosed with TNBC and had tumor tissues and matched genomic DNA available for sequencing were included. TNBC cases were recorded as negative for ER, PR, and HER2 using immunohistochemistry (IHC) for ER and PR. HER2 status was determined with IHC and fluorescence in situ hybridization (FISH).
To increase the sample size of TNBC cases from White women, archived tumor samples from patients diagnosed between 1998–2011 were obtained from Roswell Park Comprehensive Cancer Center in Buffalo New York. The Pathology Network Shared Resource and Data Bank and BioRepository procure tumor samples and matched genomic DNA extracted from whole blood for research. Black and White TNBC cases were matched on age and cancer stage. This study was approved by the Institutional Review Boards of Roswell Park Comprehensive Cancer Center and Rutgers Cancer Institute of New Jersey.
Genomic Data Acquisition
Tumor DNA was extracted from cores taken from tumor-rich regions chosen by our study pathologist (T.K.) using Covaris truXTRAC FFPE Kits. A customized gene panel was designed for sequencing, which included 151 genes selected from significantly mutated genes identified in previous breast cancer genomic studies [20–23], and in our preliminary analyses of TNBC and Black breast cancer data subsets from TCGA. Sequencing libraries prepared from tumor DNA using Agilent Haloplex Target Enrichment kit were barcoded and multiplexed at 32 samples per lane and matched genomic DNA at 96 samples per lane. Samples were sequenced using an Illumina HiSeq 2500 sequencer in Roswell Park Genomics Shared Resource (GSR), randomized on race (Black vs. White) and study population (WCHS vs. Roswell Park) across sequencing lanes to reduce potential batch effects.
The average sequencing depths were 1,279× for tumor samples and 494× for matched normal samples. The average mapping rates were 94% for tumor samples and 97% for normal samples, with an average of 91% and 95% of targeted regions covering at least 20× for tumor and normal, respectively. After the initial QC based on sequencing data indices, three additional QC steps were taken to filter out low-quality samples and variant calls. First, to remove samples with tumor-normal mismatch, the identified somatic mutations were compared to the public human germline databases including dbSNP, 1000 Genomes Project, and the National Heart, Lung, and Blood Institute’s Exome Sequencing Project to further exclude remaining germline polymorphisms. Samples with higher than expected percent of germline SNPs which were not present in the matched normal sample were excluded from further analysis. Second, for tumor samples whose mapping rates were below 90%, a minimum variant allele fraction of 15% was required for somatic mutations to avoid potential false positives due to artifacts. Third, to exclude likely false positive calls due to last base quality issue, we excluded putative calls where over 95% of the supporting reads had the mutation present at the last base of the read. As a result, the final dataset contained 144 genes, including 20 without any mutations detected, from 133 patients with TNBC (71 Black and 62 White). For each race, we determined the most frequently mutated genes by ranking genes by mutations across the largest number of tumors. Somatic mutation number per tumor across all markers in our panels was calculated.
We also assessed a panel of 15 focal CNVs in regions known to be aberrant in breast tumors, selecting from TCGA data. These regions contain known cancer genes including CCNE1, CDKN2A, CREBBP, EGFR, ERBB2, ETV6, FGFR1, INPP4B, MDM2, MLL3, MYC, PIK3CA, PTEN, RB1, and TP53. For each region, three probes were designed and after probe-sample hybridization, digital counting was conducted using the NanoString nCounter CNV assay performed by Roswell Park GSR according to the manufacturer’s instructions. Along with tumor samples, a panel of normal DNA samples known to have no copy number changes was included for data normalization purposes. Amplification or deletion status of each probe was determined by a Z-test of its count in comparison to the empirical distribution of the same probe from the normal samples. A stringent Holm-Bonferroni method was used to control for family-wise error rate.
Statistical Analysis
The Wilcoxon Rank Sum Test was used to compare the mutation burden between Black and White patients, and Fisher’s exact test was used to test for racial differences in mutation frequency of single genes. Two-tailed P values < 0.05 were considered significant.
Results
After sequencing QC measures were implemented, 124 of 151 genes had data for point mutations from 71 Black and 62 White TNBC patients (Supplemental Table 1). Of these, 65 Black and 57 White patients also had data for 14 informative CNVs (Supplemental Table 2). One CNV did not show variation in either racial group. Descriptive characteristics of Black and White patients in the final analysis are shown in Table 1. There were no significant differences between Blacks and Whites for age at diagnosis, cancer stage, or tumor grade. As shown in Figure 1, there were no significant differences in the number of point mutations between Black and White women with TNBC, with a median of 2 per patient for Blacks (range 1–29) and 2 for Whites (range 1–23, p=0.92). There was no difference in the number of CNVs (Blacks: median = 2, range 0–8; Whites: median = 2, range 0–8, p=0.76) or the total number of point mutations and CNVs combined (Blacks: median = 5, range 1–31; Whites: median = 5, range 1–26, p=0.76) (Figure 1). The average mutation rate was 7.00 mutations/Mb (7.26 for Black women vs. 6.72 for White, p=0.67) based on single-nucleotide variant mutations within the targeted region (Figure 2). The mutation rate was notably higher than those from published exome or genome sequencing data, likely due to the targeted sequencing approach used.
Table 1.
Descriptive characteristics of the TNBC study population
| Black (N=71) | White (N=62) | P | |
|---|---|---|---|
| Age at enrollment, yrs | 51.6 ± 11.9 | 53.7 ± 13.3 | 0.34a |
| Stage at Diagnosis | 0.77b | ||
| I | 22 (31.4) | 21 (34.4) | |
| II | 35 (50.0) | 31 (50.8) | |
| III | 11 (15.7) | 9 (14.8) | |
| IV | 2 (2.9) | 0 (0.0) | |
| Grade | 0.07b | ||
| 2 | 5 (7.0) | 11(17.7) | |
| 3 | 66 (93.0) | 51(82.3) |
Student’s T Test
Fisher’s Exact Test
Fig. 1.
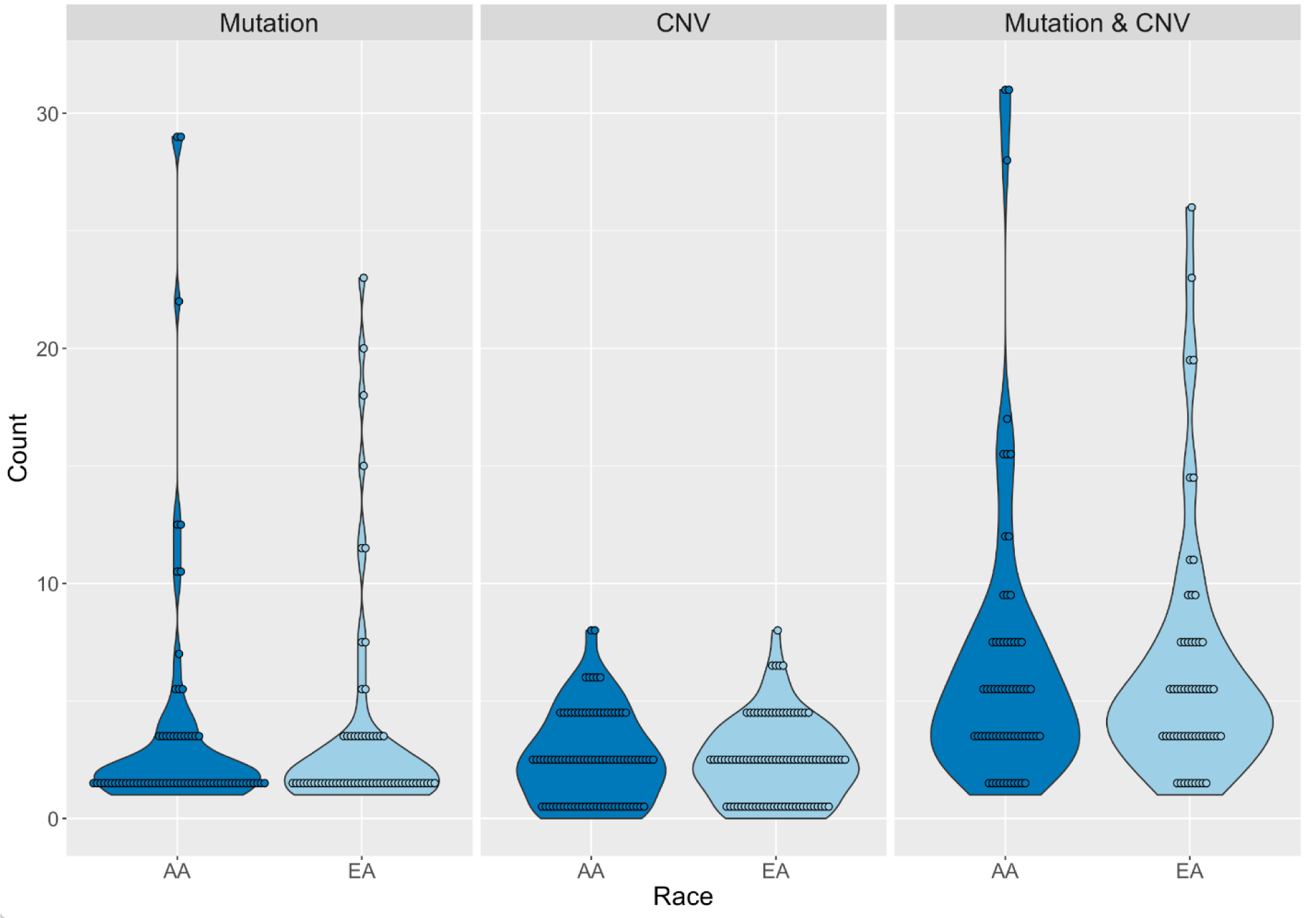
Number of mutations and copy number variations (CNV) carried by African American/Black (Black) and European American/White (White) triple-negative breast cancer patients.
Fig. 2.
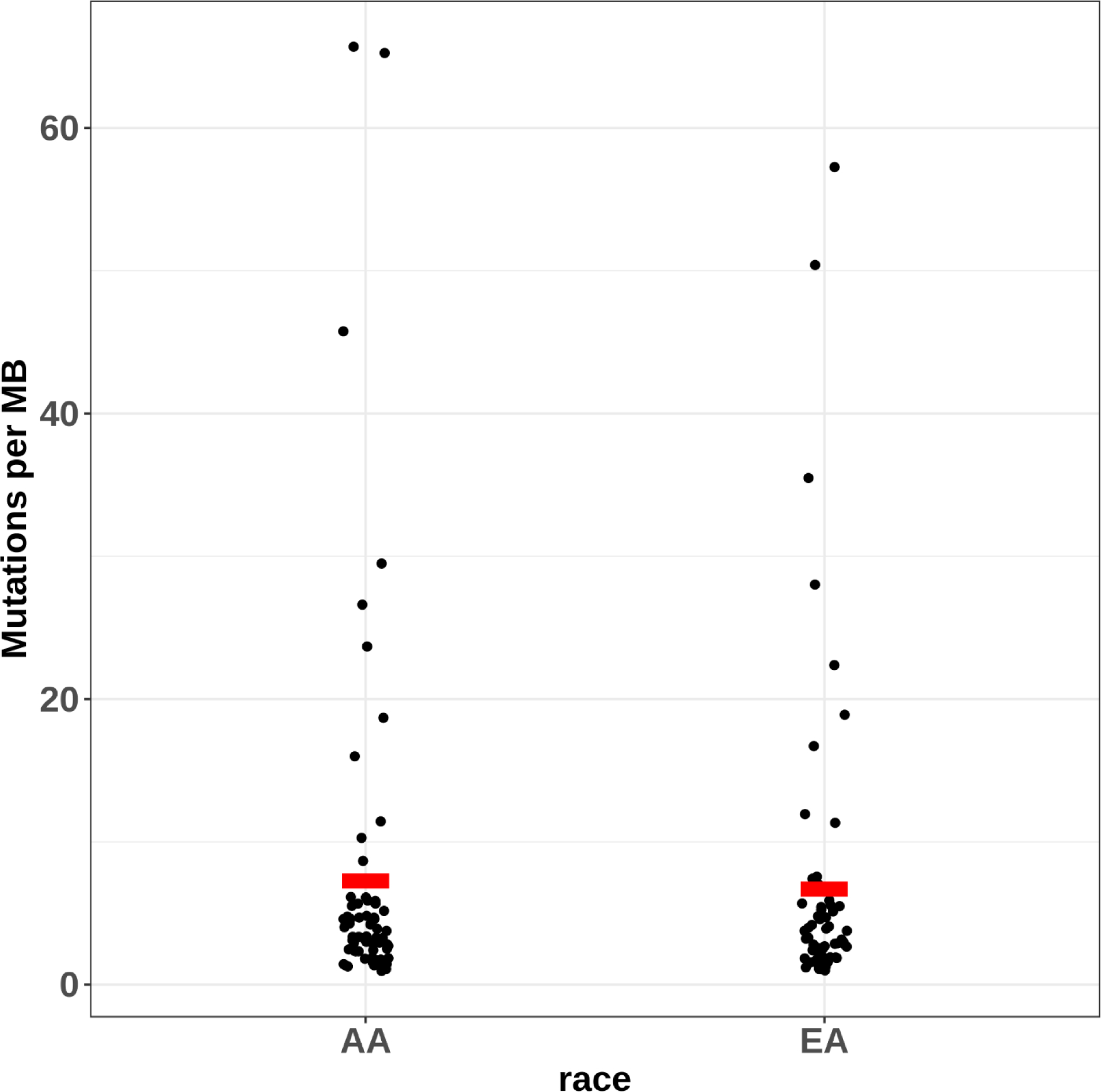
Single-nucleotide variant mutation rate in targeted regions in Black and White women with triple-negative breast cancer. The average mutation rate was 7.26 mutations/Mb for Black women and 6.72 mutations/Mb for White women (denoted with red rectangles).
Table 2 lists the genes harboring point mutations at ≥5% frequency in Black or White TNBC cases. As expected, TP53 was the most frequently mutated gene in both racial groups (63.4% in Blacks vs. 75.8% in Whites, p=0.13). RYR2 was the second most frequently mutated gene in Whites with a frequency of 16.1% compared to 9.9% in Blacks (p=0.31). PIK3CA was the third, mutated at 12.9% in White TNBC cases, significantly higher than in Black cases (2.8%, p=0.045). SYNE1 was the second most frequently mutated gene in Blacks with a frequency of 14.1% vs. 6.5% in Whites (p=0.17). Of all the genes sequenced, no gene was mutated at a significantly higher frequency in Black than in White cases; whereas two genes were mutated at a significantly higher frequency in Whites. In addition to PIK3CA mentioned above, 9.7% of White TNBC cases carried mutations in NCOR1, compared to only 1.4% in Black cases (p=0.050). The differences became non-significant after controlling for multiple testing. BRCA mutations did not differ significantly between the two racial groups (Supplemental Table 1).
Table 2.
Genes harboring point mutations in greater than 5% of Black or White TNBC cases.
| % of TNBC cases | |||
|---|---|---|---|
| Gene symbol | Black N=71 | White N=62 | Pa |
| ADGRG4 | 5.6 | 1.6 | 0.37 |
| AFF2 | 5.6 | 3.2 | 0.68 |
| ARID1A | 5.6 | 3.2 | 0.68 |
| ARID2 | 5.6 | 1.6 | 0.37 |
| ATR | 4.2 | 6.5 | 0.70 |
| BRCA1 | 2.8 | 6.5 | 0.42 |
| COL6A3 | 7.0 | 8.1 | 1.00 |
| HMCN1 | 7.0 | 6.5 | 1.00 |
| KIF4A | 5.6 | 1.6 | 0.37 |
| KMT2A | 5.6 | 8.1 | 0.73 |
| KMT2C | 8.5 | 4.8 | 0.50 |
| KMT2D | 8.5 | 6.5 | 0.75 |
| MDN1 | 9.9 | 4.8 | 0.34 |
| NCOR1 | 1.4 | 9.7 | 0.05 |
| PIK3CA | 2.8 | 12.9 | 0.04 |
| RYR2 | 9.9 | 16.1 | 0.31 |
| SYNE1 | 14.1 | 6.5 | 0.17 |
| SYNE2 | 4.2 | 8.1 | 0.47 |
| TP53 | 63.4 | 75.8 | 0.13 |
| UBR5 | 7.0 | 4.8 | 0.72 |
| USH2A | 5.6 | 3.2 | 0.68 |
| USP9X | 5.6 | 1.6 | 0.37 |
Fisher’s Exact Test
Table 3 lists the genes with CNVs occurring at ≥5% in Black or White TNBC cases, with MYC amplification being the dominant CNV in both groups (70.8% in Blacks vs. 68.4% in Whites). In addition, amplification of CREBBP and PIK3CA regions, and loss of the CDKN2A region were also commonly observed at >20% frequency in both racial groups. Comparisons of the CNVs showed no significant difference in the frequency of any CNVs between Black and White patients with TNBC.
Table 3.
Genes with CNVs in greater than 5% of Black or White TNBC cases
| % of TNBC cases | |||
|---|---|---|---|
| CNV | Black | White | P |
| CCNE1 | 4.6 | 5.3 | 1.00a |
| CDKN2A | 23.1 | 22.8 | 0.97 |
| CREBBP | 49.2 | 35.1 | 0.12 |
| EGFR | 16.9 | 19.3 | 0.73 |
| ERBB2 | 9.2 | 7.0 | 0.75a |
| ETV6 | 4.6 | 8.8 | 0.47a |
| FGFR1 | 12.3 | 14.0 | 0.78 |
| INPP4B | 4.6 | 7.0 | 0.70a |
| MYC | 70.8 | 68.4 | 0.78 |
| PIK3CA | 35.4 | 38.6 | 0.71 |
| PTEN | 18.5 | 10.5 | 0.22 |
| RBI | 7.7 | 7.0 | 1.00a |
| TP53 | 13.9 | 14.0 | 0.98 |
Fisher’s Exact Test
Discussion
Our study specifically examined racial differences in tumor somatic mutation profiles within the TNBC subtype using cases from the WCHS, a large epidemiological study, and tissue banked at Roswell Park Comprehensive Cancer Center. We evaluated differences in tumor mutations in a targeted panel of genes, and only two (PIK3CA and NCOR1) showed significantly different point mutation frequencies between Black and White cases, and only marginally so. These results suggest that if mutation spectra are different between Black and White women with breast cancer, it is likely due to differential distributions of breast cancer subtypes by race. Our data represent one of the few tumor-genomic comparisons to date between Black and White women with TNBC that do not rely on TCGA data.
Keenen et al. used TCGA data for a population of 105 Black women and 663 White women with all subtypes of breast cancer and reported that median somatic mutation counts per tumor were significantly greater in Black women, but after accounting for the TNBC subtype, racial differences were no longer significant [10]. However, mutant allele tumor heterogeneity (MATH), a measure of intratumoral genetic heterogeneity, was significantly higher in Black women, even within the TNBC subtype. In a slightly larger cohort of TCGA patients (N=930, 154 Black, 776 White), Huo et al. investigated a variety of breast tumor molecular features including gene expression, protein expression, somatic mutations, somatic DNA copy number alterations, and DNA methylation patterns and reported a similar pattern of Black-White differences in tumor molecular features being attenuated after accounting for the triple-negative subtype. In this study, expression in 142 genes, 1 protein, and 16 DNA methylation probes remained significant after adjustment for subtype, but no mutations and just four DNA copy number alterations were statistically significant, having higher frequencies in Black women [9]. In an examination of 178 TNBC patients with TCGA data, Ademuyiwa et al. found no compelling differences in the median somatic mutation number per tumor, the frequency of high prevalence genes, expression profiles, or clinical outcomes between Black and White women with TNBC [16].
In a large pan-cancer study of TCGA data, TP53 showed significantly higher mutation frequency in Black patients compared with White patients, and genes of the phosphatidylinositol 3-kinase (PI3K) pathways were less frequently mutated in Black patients, and this trend was also specific to breast cancer (all subtypes) [6]. Other studies of breast cancer have also reported a trend of Black women having significantly more TP53 mutations and fewer PIK3CA mutations, but have also shown that these racial differences were diminished after adjustment for the TNBC subtype [9, 10, 16]. Our analysis of TNBC patients in the WCHS and Roswell Park patient populations is largely consistent with these earlier reports of TCGA data.
The TNBC phenotype is roughly twice as prevalent in Black women than White women, with estimates of approximately 30% in Black women [12]. Studies of genomic profiles in TNBC tumors have generally not shown appreciable differences between Black and White women, consistent with our data. While many studies have shown genomic differences between Black and White women such as genomic instability, genome doubling, intratumoral heterogeneity, CNVs, and mutation counts and frequencies [6, 9, 10, 16], these differences dissipate when the focus narrows to the TNBC subtype. As analyses of the TNBC subtype consist of small sample sizes, it is possible that the lack of significant findings is due to the lack of statistical power, which is also a limitation of the current study. Moreover, we examined a targeted panel of genes that may not be representative of the overall mutational differences between Black and White women with breast cancer.
Other limitations include that TNBC is a heterogeneous group that comprises several subtypes [24, 25], and we did not have access to gene expression data to investigate the TNBC subgroups. We also used a targeted approach and our genes were chosen based on TCGA results, which are predominantly based on tumors from White women. While a targeted approach is less expensive than whole-exome or whole-genome sequencing, there is the drawback that an a priori assumption is made about what cancer genes are important and that they are the same in each racial group. Strengths of our study include the new collection of DNA sequences comparing TNBC breast tumors from a similar number of Black and White women outside of TCGA-related studies. Additional studies of women with TNBC from diverse ancestral backgrounds are needed to fully understand the mutational processes in this specific subtype that may ultimately inform on how to lessen the burden of TNBC in women of African descent.
Supplementary Material
Acknowledgements and Funding Information
This work was supported by the Breast Cancer Research Foundation and the National Cancer Institute (R01 CA100598, R01 CA133264, P01 CA151135, and used Roswell Park Comprehensive Cancer Center’s Pathology Network Shared Resource, Genomics Shared Resource, and Data Bank and BioRepository (P30 CA016056).
Table of Abbreviations
- CNV
Copy number variation
- ER
Estrogen receptor
- GSR
Genomics Shared Resource
- HER2
Receptor tyrosine-protein kinase erbB-2
- PR
Progesterone receptor
- TCGA
The Cancer Genome Atlas
- TNBC
Triple-negative breast cancer
- WCHS
Women’s Circle of Health Study
Footnotes
Ethics approval
The Institutional Review Boards at Rutgers University and Roswell Park Comprehensive Cancer Center provided approval for the use of patient samples in this study. All participants gave informed consent and this study was performed in accordance with the ethical standards outlined in the 1964 Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Data availability statement
All data generated or analyzed during this study are included in this published article in the supplementary information files or available from the author upon request.
References
- 1.Elledge RM, Clark GM, Chamness GC, Osborne CK: Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst 1994, 86(9):705–712. [DOI] [PubMed] [Google Scholar]
- 2.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP et al. : Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. J Clin Oncol 2015, 33(20):2254–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A: Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017, 67(6):439–448. [DOI] [PubMed] [Google Scholar]
- 4.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA: Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol 2006, 24(9):1342–1349. [DOI] [PubMed] [Google Scholar]
- 5.Albain KS, Unger JM, Crowley JJ, Coltman CA Jr., Hershman DL: Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst 2009, 101(14):984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan J, Hu Z, Mahal BA, Zhao SD, Kensler KH, Pi J, Hu X, Zhang Y, Wang Y, Jiang J et al. : Integrated Analysis of Genetic Ancestry and Genomic Alterations across Cancers. Cancer Cell 2018, 34(4):549–560 e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA: Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA 2015, 313(2):165–173. [DOI] [PubMed] [Google Scholar]
- 8.Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, Tsai YC, Williams EH, Lee DH, Stephens RM et al. : Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One 2009, 4(2):e4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huo D, Hu H, Rhie SK, Gamazon ER, Cherniack AD, Liu J, Yoshimatsu TF, Pitt JJ, Hoadley KA, Troester M et al. : Comparison of Breast Cancer Molecular Features and Survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol 2017, 3(12):1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan T, Moy B, Mroz EA, Ross K, Niemierko A, Rocco JW, Isakoff S, Ellisen LW, Bardia A: Comparison of the Genomic Landscape Between Primary Breast Cancer in African American Versus White Women and the Association of Racial Differences With Tumor Recurrence. J Clin Oncol 2015, 33(31):3621–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telonis AG, Rigoutsos I: Race Disparities in the Contribution of miRNA Isoforms and tRNA-Derived Fragments to Triple-Negative Breast Cancer. Cancer Res 2018, 78(5):1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman LA, Kaljee LM: Health Disparities and Triple-Negative Breast Cancer in African American Women: A Review. JAMA Surg 2017, 152(5):485–493. [DOI] [PubMed] [Google Scholar]
- 13.Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, Boscoe FP, Cronin KA, Lake A, Noone AM et al. : Annual Report to the Nation on the Status of Cancer, 1975–2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst 2015, 107(6):djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott LC, Mobley LR, Kuo TM, Il’yasova D: Update on triple-negative breast cancer disparities for the United States: A population-based study from the United States Cancer Statistics database, 2010 through 2014. Cancer 2019, 125(19):3412–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietze EC, Sistrunk C, Miranda-Carboni G, O’Regan R, Seewaldt VL: Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer 2015, 15(4):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ademuyiwa FO, Tao Y, Luo J, Weilbaecher K, Ma CX: Differences in the mutational landscape of triple-negative breast cancer in African Americans and Caucasians. Breast Cancer Res Treat 2017, 161(3):491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindner R, Sullivan C, Offor O, Lezon-Geyda K, Halligan K, Fischbach N, Shah M, Bossuyt V, Schulz V, Tuck DP et al. : Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One 2013, 8(11):e71915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart PA, Luks J, Roycik MD, Sang QX, Zhang J: Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS One 2013, 8(12):e82460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ambrosone CB, Ciupak GL, Bandera EV, Jandorf L, Bovbjerg DH, Zirpoli G, Pawlish K, Godbold J, Furberg H, Fatone A et al. : Conducting Molecular Epidemiological Research in the Age of HIPAA: A Multi-Institutional Case-Control Study of Breast Cancer in African-American and European-American Women. J Oncol 2009, 2009:871250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Genome Atlas N: Comprehensive molecular portraits of human breast tumours. Nature 2012, 490(7418):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G et al. : The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 2012, 486(7403):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, Nik-Zainal S, Martin S, Varela I, Bignell GR et al. : The landscape of cancer genes and mutational processes in breast cancer. Nature 2012, 486(7403):400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerji S, Cibulskis K, Rangel-Escareno C, Brown KK, Carter SL, Frederick AM, Lawrence MS, Sivachenko AY, Sougnez C, Zou L et al. : Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012, 486(7403):405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK, Hilsenbeck SG, Chang JC et al. : Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res 2015, 21(7):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA: Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011, 121(7):2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


