Abstract
Background:
Exposure to fine particulate matter () during wildfire seasons has been associated with adverse health outcomes. Previous studies have focused on daily exposure, but levels in smoke events can vary considerably within 1 d.
Objectives:
We aimed to assess the immediate and lagged relationship between sub-daily exposure to and acute health outcomes during wildfire seasons in British Columbia.
Methods:
We used a time-stratified case-crossover study design to evaluate the association between modeled hourly and ambulance dispatches during wildfire seasons from 2010 to 2015. Distributed lag nonlinear models were used to estimate the lag-specific and cumulative odds ratios (ORs) at lags from 1 to 48 h. We examined the relationship for all dispatches and dispatches related to respiratory, circulatory, and diabetic conditions, identified by codes for ambulance dispatch (AD), paramedic assessment (PA) or hospital diagnosis (HD).
Results:
Increased respiratory health outcomes were observed within 1 h of exposure to a increase in . The 48-h cumulative OR [95% confidence interval (CI)] was 1.038 (1.009, 1.067) for the AD code Breathing Problems and 1.098 (1.013, 1.189) for PA code Asthma/COPD. The point estimates were elevated within 1 h for the PA code for Myocardial Infarction and HD codes for Ischemic Heart Disease, which had 24-h cumulative ORs of 1.104 (0.915, 1.331) and 1.069 (0.983, 1.162), respectively. The odds of Diabetic AD and PA codes increased over time to a cumulative 24-h OR of 1.075 (1.001, 1.153) and 1.104 (1.015, 1.202) respectively.
Conclusions:
We found increased during wildfire seasons was associated with some respiratory and cardiovascular outcomes within 1 h following exposure, and its association with diabetic outcomes increased over time. Cumulative effects were consistent with those reported elsewhere in the literature. These results warrant further investigation and may have implications for the appropriate time scale of public health actions. https://doi.org/10.1289/EHP5792
Introduction
Approximately 3% of the global land surface is burned by landscape fires every year, an area equivalent to nearly 20% of North America (Giglio et al. 2013). Over the past few decades, many areas of the world have reported longer wildfire seasons and more severe wildfire activity in terms of fire frequency, size, and intensity (Dennison et al. 2014; Jain et al. 2017; Jolly et al. 2015; Lucas et al. 2007). These trends are partially attributed to the increasing temperatures and more drought as the global climate changes, and projections suggest a continuation of these trends into the future (Aldersley et al. 2011; Barbero et al. 2015; Westerling et al. 2006; Wotton et al. 2017).
Smoke emitted from wildfires can affect large populations, even those distant from the fire, by degrading air quality at the local, regional, and global scales (Dempsey 2013; Dirksen et al. 2009; Jeong et al. 2008; Miller et al. 2011). Although wildfire smoke is a complex mixture of gases and particles, ambient concentrations of fine particulate matter () are the most widely used as a proxy for the mixture. The use of is based on a) concentrations being consistently elevated during wildfire smoke events, at locations both near and far from the fire (Naeher et al. 2007); b) well-established dose–response relationships with a wide range of health outcomes; and c) the availability of continuous measurements in many locations for the purposes of air quality regulation. The most recent estimates suggest that approximately 3.4 million deaths could be attributed to ambient air pollution in 2017 (Stanaway et al. 2018), and fire emissions accounted for up to 8% of these deaths (Lelieveld et al. 2015).
Exposure to wildfire smoke has also been associated with a wide range of acute cardiopulmonary morbidity (DeFlorio-Barker et al. 2019; Dennekamp et al. 2015; Haikerwal et al. 2015; Liu et al. 2015; Reid et al. 2016; Tinling et al. 2016), and evidence is emerging for other health conditions, such as adverse early life outcomes (Black et al. 2017; Holstius et al. 2012) and reduced diabetic control (Johnston et al. 2018). Although diabetes has not been comprehensively studied in response to short-term changes in , these recent findings for wildfire smoke are consistent with other findings associating with increased risk of diabetic hospitalization (Zanobetti and Schwartz 2002; Zanobetti et al. 2014). In addition, evidence from studies on long-term exposure to exposure suggests an association with the development of type 2 diabetes (Bowe et al. 2018; Pearson et al. 2010; Rao et al. 2015; Thiering and Heinrich 2015). Finally, people with diabetes were more vulnerable to cardiovascular health effects associated with (Forastiere et al. 2008; Pinault et al. 2018; Zanobetti and Schwartz 2002). These findings warrant further investigation into the impact of wildfire smoke exposure on diabetic conditions.
So far, most epidemiological studies on wildfire smoke exposure have focused on 24-h average concentrations, associating same-day or previous-day exposures with acute health outcomes. Very few studies have examined the relationship between these acute health outcomes and sub-daily exposures, measured in 1-h rather than 24-h periods (Liu et al. 2015; Reid et al. 2016). Given that concentrations can vary considerably within 1 d due to changes in the weather and the intermittent nature of wildfire emissions (Saide et al. 2015; Strand et al. 2011), there is uncertainty about the true nature of the exposure–response relationship during smoke episodes. First, it is not clear whether smoke exposure can trigger an acute outcome immediately, or whether there is a time lag between the exposure and the effect. Second, it is not clear whether the effects of smoke are driven by peak exposures within the day or by cumulative exposures throughout the day. Although from wildfire smoke is the focus of this work, the same uncertainties apply to from other sources. These questions are important to address for the development of effective public health response plans with the appropriate time sensitivity.
There are two key challenges in studying the health effects of sub-daily smoke exposures. The first, addressed in our previous study (Yao et al. 2018), is to generate spatially resolved estimates at the 1-h time scale. The second is to identify population-based health outcomes recorded on a similar time scale. Because wildfire smoke events are usually sporadic, large populations and long time-series of data are needed to provide enough statistical power to detect their effects. This research is most feasible using routinely collected administrative health data, such as hospital admissions or medical billings. However, many of these databases do not have precise information on the exact time and location of the health events, and this information is necessary for studying sub-daily exposures.
Ambulance dispatches are a promising alternative to other types of administrative health data for assessing the sub-daily impacts of air pollution, including wildfire smoke. These databases typically have records of the exact location (in latitude and longitude) and time (in minutes and seconds) of the dispatch call. Although the spatial and temporal resolutions of ambulance dispatch data are ideal for studying sub-daily exposures, dispatch codes have uncertain diagnostic value in the absence of contextual information from trained medical personnel. As such, recent studies in Australia have found a general association between ambulance dispatch and wildfire smoke exposure (Dennekamp et al. 2015; Johnston et al. 2018; Salimi et al. 2017) but have not been able to examine a wide range of cause-specific dispatches at the sub-daily temporal scale.
We extend this prior work to North America and examine the association between the sub-daily exposure to wildfire smoke and acute health outcomes by combining a previously developed exposure model with ambulance dispatch data that have been uniquely linked to subsequent paramedic reports and hospital admissions. This method allows more complete examination of the relationship between wildfire smoke and all ambulances dispatches, as well as those subsets most likely to be due to cardiovascular, respiratory, and diabetic conditions.
Methods
Study Area and Study Period
British Columbia (BC) is the westernmost province in Canada, with a population of approximately 5 million people in 2018, over half of which reside in the greater Vancouver area located in the southwestern corner. With more than 70% of land covered by forests (Austin et al. 2008), the province is prone to seasonal wildfires, and smoke from these fires is the dominant source of elevated during the summer months (McKendry 2006). The health outcomes and exposure data for this study cover the wildfire seasons (1 April to 30 September) from 2010 to 2015, including three severe seasons in 2010, 2014, and 2015 with over 300,000 hectares burned in each of those years.
Health Outcome Data
We obtained data for all emergency ambulance dispatches during the study period from BC Emergency Health Services, which is the sole provider of ambulance and emergency health services across the province. The data included the date and time of the call, geographic coordinates of the event, and the reason for the call recorded as one of 33 codes (Table S1) assigned by the dispatcher using the Medical Priority Dispatch System (MPDS) (Clawson et al. 2015). The MPDS is a standardized set of protocols produced by the International Academies of Emergency Dispatch. Calls without a dispatch location or calls from callers who made more than four calls during the study period (5% of all unique callers) were excluded. The latter was done to minimize the occurrence of multiple calls within a short period of time, which may violate the assumptions of the case-crossover design. If there were multiple calls from the same caller within a 24-h period, only the first call was included in the analysis.
Each call in the dispatch database was provided with a linked patient care report as completed by the attending paramedics. Key information retrieved from these reports included the Personal Health Number (PHN, a lifetime unique identifier for health care in the province), the age and sex of the patient, and any assessment of medical conditions by the paramedics, assigned as one of the 180 Paramedic Impression (PI) codes (Table S2) (BC Emergency Health Services 2019). Although each patient had a care report, not all patients had an impression code because it is not a mandatory field for the paramedics to complete.
We also obtained hospital discharge data from the BC Ministry of Health (Canadian Institute for Health Information 2017), which included the date of hospital admission and the primary diagnosis, coded according to the International Classification of Diseases, 10th Revision (ICD-10). The primary diagnosis reflects the primary reason for the total length of the hospital stay and so may or may not reflect the initial reason for admission. Hospital diagnoses were linked to a dispatch call by PHN and included for the study whether a) the admission occurred within a 7-d period of the ambulance dispatch, and b) it was the admission closest to the date of the dispatch for cases where multiple admissions were found within the 7-d period.
Given this chain of data linkage, we could have had up to three measures of health outcome for each dispatch call: a) the MPDS code assigned by the dispatcher at the call center [Ambulance Dispatch (AD)]; b) the PI code assigned by the paramedics at the dispatched location [Paramedic Assessment (PA)]; and c) the primary ICD-10 code associated with the hospital admission record [Hospital Diagnosis (HD)]. These three measures have different advantages and disadvantages for the purposes of our study. The AD code was assigned to every single dispatch (no missing data), and it was assessed at the time closest to the onset of the emergency event. However, it was generally based on information self-reported by a lay caller and recorded as broad categories of health problems. The PA code was assigned by professionals with medical training after a physical examination the patient, but the assessment can be constrained by time, equipment, and demand and is not available on every record. The HD code provided the most robust medical assessment among the three, but it was available only for the most severe cases (i.e., those admitted), and it could have been made hours or even days after the initial call. Considering these different features, we decided to first provide a summary of the relationship among them to assess consistency and then to examine the dispatches related to cardiovascular, respiratory, and diabetic conditions, as identified by each of these three measures (Table 1).
Table 1.
Definitions and number of cases for each health outcome measure.
| Case groups | Definition | Number of cases |
|---|---|---|
| All | 676,401 | |
| Cause-specific cases identified by Ambulance Dispatch (AD) codes based on the Medical Priority Dispatch System (MPDS)a | ||
| Breathing problems | 46,277 | |
| Chest pain | 51,996 | |
| Arrest | 3,527 | |
| Stroke | 21,173 | |
| Heart problems | 12,039 | |
| Diabetic problems | 5,987 | |
| Other Codes | 535,402 | |
| Cause-specific cases identified by Paramedic Assessment (PA) codes based on Paramedic Impressions (PI)b | ||
| Circulatory | PI starts with 08 or 1830 | 44,122 |
| Stroke | 17,495 | |
| Myocardial infarction | 1,724 | |
| Other circulatory | 24,903 | |
| Respiratory | PI starts with 09 | 23,392 |
| Asthma/COPD | 5,824 | |
| Other respiratory | 17,568 | |
| Diabetic | or 0315 | 4,722 |
| Hyperglycemia | 1,535 | |
| Hypoglycemia | 3,187 | |
| Other codes | 346,910 | |
| Cause-specific cases identified by Hospital Diagnosis (HD) codes based on the International Classification of Diseases, 10th Revision (ICD-10) | ||
| Circulatory | to I99 | 37,078 |
| Stroke | to I69 | 10,373 |
| Ischemic heart diseases | to I25 | 10,653 |
| Other circulatory | 16,052 | |
| Respiratory | to J99 | 22,038 |
| Asthma/COPD | to J45 | 9,084 |
| Lower respiratory infection | to J22 | 7,708 |
| Other respiratory | 5,246 | |
| Diabetic | to E14 | 2,921 |
| Other codes | 114,246 | |
More about Medical Priority Dispatch System codes can be found in Clawson et al. 2015.
More about PI codes can be found at BC Emergency Health Services 2019.
Exposure Assessment and Assignment
Hourly exposures to during the study period for all subjects were estimated with the 1-h Optimized Statistical Smoke Exposure Model (OSSEM-1h) previously developed and described elsewhere (Yao et al. 2018). Briefly, OSSEM-1h generates hourly estimates across all populated areas of BC at a spatial resolution using a random forests model, with input data including measurements from monitoring stations, fire activity observed by satellites, meteorology assimilated from observations and modeling, and geographic information. Compared with observations, model predictions had a correlation of 0.93 and a root mean squared error of . The intent was to model fire-related by using only data in fire seasons and incorporating fire-related predictors. Model training, however, necessarily relied on total measured at air-quality monitors; thus, its predictions do not strictly reflect from wildfire smoke alone. Exposure for each dispatch call was assigned based on the date and hour of the call, as well as the dispatch location (latitude and longitude) that was matched to the OSSEM-1h grid.
Statistical Analysis
A time-stratified case-crossover study design (Maclure 1991) was used to assess the association between dispatches and estimated exposure during wildfire seasons. Exposure during the case window was compared with exposures during a series of control windows. The case window was defined as the hour immediately before the ambulance was dispatched, and the control windows were defined as the same hour on the same day of the week in the same calendar month of the dispatch to control for day-of-week effects and seasonal trends. Control window exposures were assigned at the same location as the case window exposure. Using conditional logistic regression, individual factors that do not vary over a short time period (e.g., age, smoking status) can be controlled because the exposures during the case and control windows are compared in the same individual.
To examine the lag structure of the association between exposure and outcome, a distributed lag nonlinear model (DLNM) (Gasparrini et al. 2010) was used. This type of model can simultaneously describe complex exposure–response and lag–response relationships by combining the functions for both relationships in the same model. This approach has been applied in studies on the acute health effects of air pollution and ambient temperature (Buteau et al. 2018; Gasparrini et al. 2015; Guo et al. 2011; Guo 2017). We allowed for delayed relationship up to 48 h (lag 1–48 h) because most of previous studies using 24-h average exposures to wildfire smoke found the strongest association or best model fit at lags of 0–2 d (Borchers-Arriagada et al. 2019; Delfino et al. 2009; Elliott et al. 2013; Henderson et al. 2011; Johnston et al. 2007; Morgan et al. 2010; Youssouf et al. 2014). A natural cubic B-spline with 2 or 3 degrees of freedom (df), depending on the health outcome, was used for the lag–response relationship based on exploratory analyses to minimize the Akaike Information Criterion (AIC). Other functions including polynomials and penalized splines, with varying degrees of freedom, were also tested to describe the lag structure in the exploratory analyses, which produced similar results and less desirable model fit compared with cubic splines based on AIC. Both the lag-specific and cumulative odds ratios (ORs) were calculated to evaluate the time course and the overall association, respectively.
A linear exposure–response relationship was assumed in the analyses after preliminary evaluation of linear and nonlinear options found the linear models provided the best fit for most health outcomes (Figures S1–S3). This assumption also simplified the presentation of the results and allowed us to focus on the lag–response relationship.
Models were adjusted for the same-day and previous-day maximum apparent temperatures from the nearest weather station maintained by Environment and Climate Change Canada, using a natural cubic B-spline with 3 df. All data preparation and statistical analyses were conducted using R software (version 3.5.1; R Development Core Team). The dlnm package was used to fit DLNM (Gasparrini 2011). Cox regression with Breslow ties was used to fit conditional logistic regression models, adopting the example code provided in a previous publication (Guo 2017). The study was approved by the Behavioural Research Ethics Board at the University of British Columbia (H15-02269).
Results
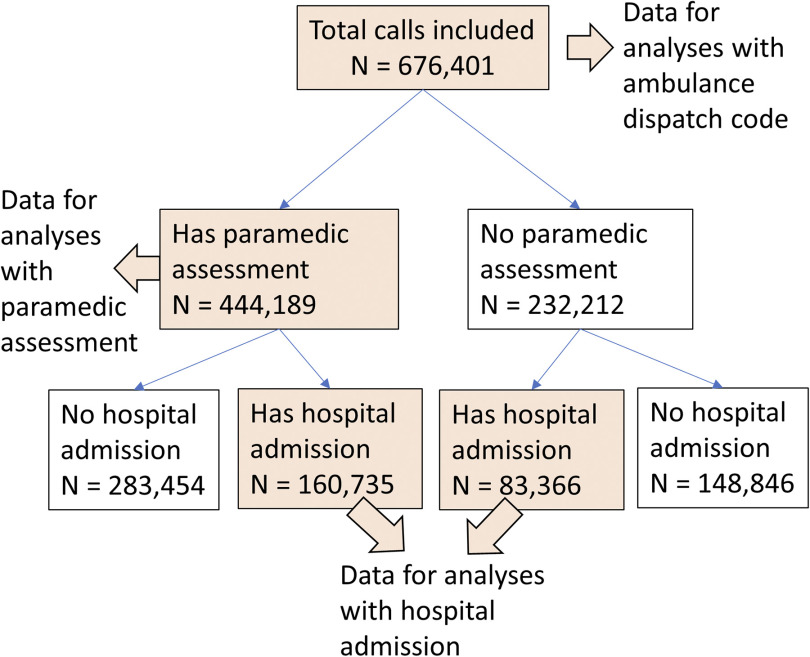
A total of 676,401 dispatch calls from 500,302 unique individuals were included in the study, among which all calls had an AD code; 444,189 (65.7%) had a PA code; and 244,101 (36.1%) were linked to HD codes (Figure 1). Paramedics arrived at the dispatched location within 1 h of the call in 99% of the cases, regardless of PA code group. Hospital admissions occurred within the same calendar day of the dispatch calls for 73% to 81% of cases, depending on the HD code group.
Figure 1.
Flowchart of analytic data selection.
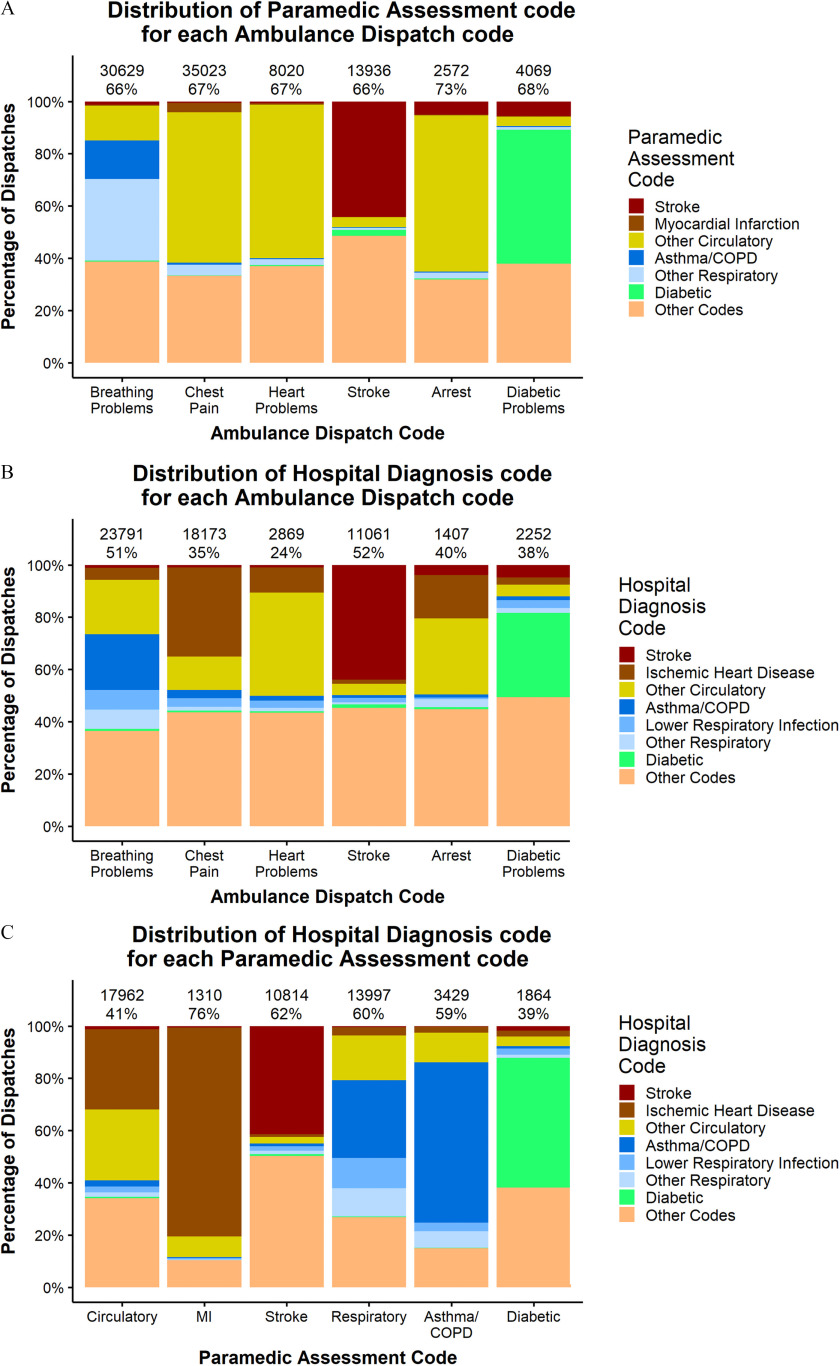
For each of the six AD code groups, 66%–73% of the calls also had a PA code (Figure 2A). For calls with the AD code Breathing Problems, the most prevalent (45.8%) PA code was related to respiratory conditions (Asthma/COPD and Other Respiratory in Figure 2A). Most of the calls with an AD code for Chest Pain, Heart Problems, and Arrest (61.0%, 59.2%, and 60.0%, respectively) had PA codes related to circulatory conditions (Myocardial Infarction and Other Circulatory in Figure 2A). On the other hand, only 44.2% of calls with the AD code for Stroke had a PA code for Stroke, and 51.2% of calls with an AD code for Diabetic Problems had the PA code for Diabetic (Figure 2A).
Figure 2.
Distribution of (A) Paramedic Assessment code for each Ambulance Dispatch code; (B) Hospital Diagnosis code for each Ambulance Dispatch code; (C) Hospital Diagnosis code for each Paramedic Assessment code. Numbers at the top row indicate the number of calls included in the analysis and percentages at the second row indicate the percentage of calls without missing data.
For each of the six AD codes groups assessed, 24%–52% were linked to HD codes (Figure 2B). Compared with the linkage between AD and PA codes, the linkage between AD and HD codes had a larger proportion of cases in the Other Codes category (Figure 2B), indicating a somewhat weaker correspondence. The strongest relationship was observed between PA codes and HD codes (Figure 2C). For example, there were z cases with a PA code for Myocardial Infarction and a subsequent hospital admission, of which 76% also had a HD code for Ischemic Heart Disease, of which Myocardial Infarction was the primary subtype.
The mean (interquartile range) of exposures during the case and control windows in the 1-h window prior to dispatch were 5.5 (3.1, 6.5) and 5.4 (3.0, 6.4) , respectively. The means of the maximum apparent temperature on the case days and control days were 17.6°C (13.5°C, 21.7°C) and 17.6°C (13.4°C, 21.7°C), respectively.
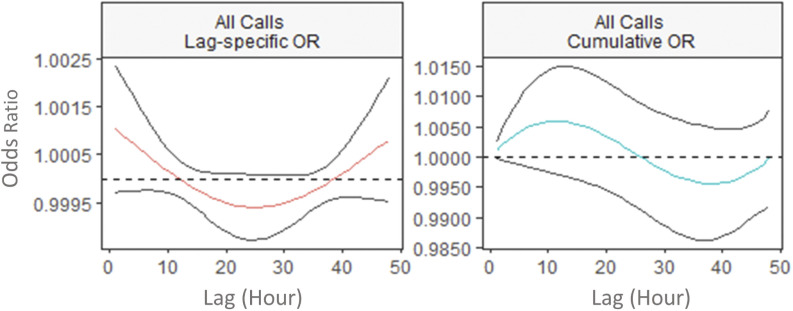
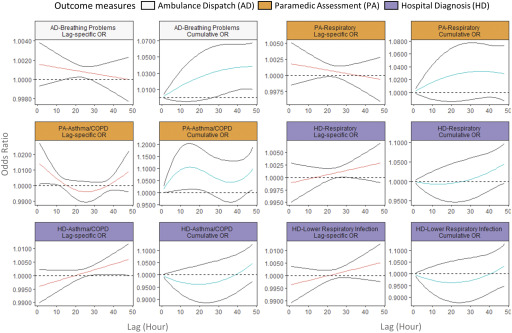
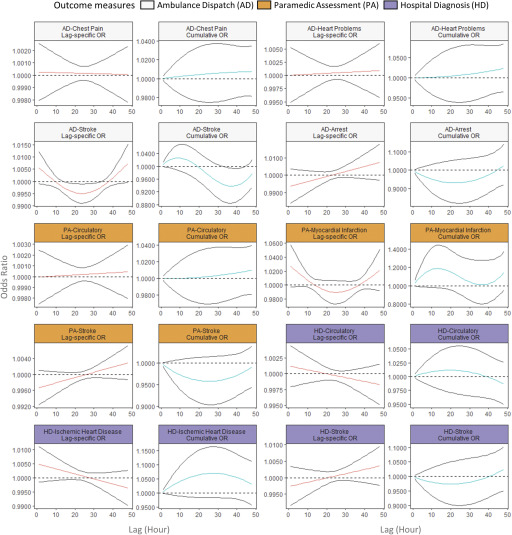
The lag–response relationship between exposures and the AD, PA, and HD codes varied by health outcome. There was a small increase in the odds of any AD [at lag 1 h, OR (0.999, 1.002)] within 1 h following increased exposure (Figure 3). An increase in odds was also observed for respiratory conditions identified by the AD code Breathing Problems [at lag 1 h, OR (0.999, 1.004)] and the PA codes Asthma/COPD [at lag 1 h, OR (1.001, 1.027)] and Respiratory [at lag 1 h, OR (0.999, 1.005)]. In all cases, the increase was largest at the 1-h lag interval. On the other hand, the ORs for respiratory outcomes identified by the HD codes Respiratory, Asthma/COPD, and Lower Respiratory Infection increased over time (Figure 4), and the maximum ORs at lag 48 h were 1.003 (0.999, 1.007), 1.006 (1.000, 1.012), and 1.005 (0.998, 1.013), respectively.
Figure 3.
Lag-specific and cumulative odds ratio (OR) of all ambulance dispatches associated with a increase in fine particulate matter () in the lag period of 1–48 h in 2010–2015 wildfire seasons in British Columbia. The figure shows the lag–response curves estimated from distributed lag nonlinear model (with 95% CI), assuming a linear exposure–response relationship, adjusted for same-day and previous-day maximum apparent temperatures. Lag-specific OR refers to the OR associated with the single hour exposure, whereas cumulative OR refers to OR associated with the cumulative exposure up to the specific hour. The y-axes for each panel are different to clearly show the shape of the lag–response curves.
Figure 4.
Lag-specific and cumulative odds ratio (OR) of respiratory health outcomes associated with a increase in fine particulate matter () in the lag period of 1–48 h in 2010–2015 wildfire seasons in British Columbia. The figure shows the lag–response curves estimated from distributed lag nonlinear model (with 95% CI), assuming a linear exposure–response relationship, adjusted for same-day and previous-day maximum apparent temperatures. Lag-specific OR refers to the OR associated with the single hour exposure, whereas cumulative OR refers to OR associated with the cumulative exposure up to the specific hour. The y-axes for each panel are different to clearly show the shape of the lag–response curves.
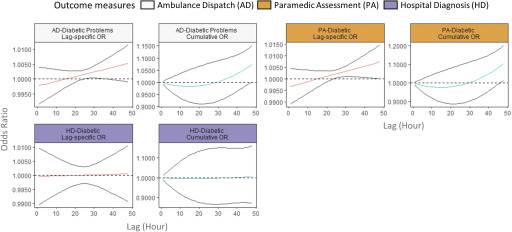
Switching to cardiovascular outcomes, the AD codes for Chest Pain and Heart Problems did not show any increase associated with exposure, but elevated odds were observed within 1 h after exposure in the PA code for Myocardial Infarction [at lag 1 h, OR (0.997, 1.057)] and in the HD codes for Ischemic Heart Disease [at lag 1 h, OR (0.999, 1.011)]. The OR for the Ambulance Dispatch code Arrest increased from negative to positive over time, with the largest OR observed at lag 48 h [1.007 (0.997, 1.013)] (Figure 5). The lag–response relationship for Stroke varied by outcome measure: An immediate increase in odds was observed in AD code, whereas the ORs increased from negative to positive over time for the PA and HD codes (Figure 5). Finally, the odds of Diabetic outcomes for the AD and PA codes both increased over time, and the OR became positive at approximately 24 h after the exposure, but the same was not observed for the HD codes (Figure 6). When we stratified the analysis for the PA codes into Hypoglycemia and Hyperglycemia, the lag–response relationship was different for the two conditions (Figure S4).
Figure 5.
Lag-specific and cumulative odds ratio (OR) of circulatory health outcomes associated with a increase in fine particulate matter () in the lag period of 1–48 h in 2010–2015 wildfire seasons in British Columbia. The figure shows the lag–response curves estimated from distributed lag nonlinear model (with 95% CI), assuming a linear exposure–response relationship, adjusted for same-day and previous-day maximum apparent temperatures. Lag-specific OR refers to the OR associated with the single hour exposure, whereas cumulative OR refers to OR associated with the cumulative exposure up to the specific hour. The y-axes for each panel are different to clearly show the shape of the lag–response curves.
Figure 6.
Lag-specific and cumulative odds ratio (OR) of diabetic outcomes associated with a increase in fine particulate matter () in the lag period of 1–48 h in 2010–2015 wildfire seasons in British Columbia. The figure shows the lag–response curves estimated from distributed lag nonlinear model (with 95% CI), assuming a linear exposure–response relationship, adjusted for same-day and previous-day maximum apparent temperatures. Lag-specific OR refers to the OR associated with the single hour exposure, whereas cumulative OR refers to OR associated with the cumulative exposure up to the specific hour. The y-axes for each panel are different to clearly show the shape of the lag–response curves.
Many outcomes reached their maximum cumulative ORs (95% CI) at a 48-h lag, including: 1.038 (1.009, 1.067) for the AD code Breathing Problems; 1.046 (0.995, 1.098) for the HD code Respiratory; 1.034 (0.948, 1.128) for Lower Respiratory Infection; 1.075 (1.001, 1.153) for the AD code Diabetic Problems; and 1.104 (1.015, 1.202) for the PA code Diabetic. On the other hand, the HD code Ischemic Heart Disease reached a cumulative maximum of 1.070 (0.983, 1.165) at a 28-h lag, and the PA codes for Myocardial Infarction and Asthma/COPD reached maximums of 1.189 (0.979, 1.442) at a 13-h lag and 1.106 (1.015, 1.205) at a 15-h lag, respectively (Figures 3–5).
Discussion
In this study, we found that a) cause-specific AD codes matched to subsequent PA and HD codes agreed reasonably well, providing more confidence in ambulance dispatches as a measure of health; b) exposure to elevated during wildfire seasons was associated with increased odds of dispatches related to respiratory and cardiovascular conditions, and the largest point estimates were observed in the hour immediately after the exposure; and c) exposure to elevated during wildfire seasons was also associated with dispatches related to diabetic conditions, with positive associations observed after a 24-h lag in exposure.
An important novelty of this study was the focus on the lag–response relationship at the hourly time scale, using a modern statistical method. Previous studies have examined such relationships by including the averaged exposure in specific lag hours as multiple independent variables in one regression model (Bhaskaran et al. 2011), or including exposure averaged over different lag windows in separate models (Ensor et al. 2013; Evans et al. 2017; Gardner et al. 2014; Peters et al. 2001; Rosenthal et al. 2008; Sullivan et al. 2005; Wichmann et al. 2013). The biggest limitation of these methods was that they did not account for the correlation between exposures at different lags, whereas the distributed lag nonlinear model (DLNM) framework used in this study accounts for such correlation. As a result, the lag-specific effect estimates from this study may not be directly comparable with estimates from previous studies using conventional methods, and the following discussion will focus on comparison of the lag–response relationship and the cumulative effect estimates.
The associations between respiratory outcomes and estimates were consistent with previous reports. Studies using ambulance data in Australia found an association between daily and breathing problems [, 95% CI: 1.02, 1.05, per increase in ] (Salimi et al. 2017), as well as asthma/COPD calls (; 95% CI: 1.01, 1.11, per increase in ) (Johnston et al. 2018), similar to the 24-h and 48-h cumulative ORs we report. These cumulative ORs were also consistent with those estimated for respiratory medication dispensations (Elliott et al. 2013; Yao et al. 2016), physician visits (Yao et al. 2016), and hospital admissions (Henderson et al. 2011) from studies using daily measurements during wildfire events in the same region. Increased airway inflammation and decreased lung function have been observed in children with asthma and in the elderly immediately following exposure to ambient , with lagged associations lasting from 5 to 12 h (Adamkiewicz et al. 2004; Delfino et al. 2006; Mar et al. 2005; Yamazaki et al. 2011). We also found an immediate increase in ambulance dispatches for respiratory codes following exposure, and the association declined over time.
Odds of myocardial infarction as measured by PA were elevated immediately following exposure, as were odds of ischemic heart disease as measured by HD. Neither result was reported by the previous Australian study (Johnston et al. 2018). However, a few studies using a similar case-crossover design found immediate associations for myocardial infarction following exposure to elevated ambient (Evans et al. 2017; Gardner et al. 2014; Peters et al. 2001; Rosenthal et al. 2008). Controlled exposure studies in humans have also suggested there is an increase in subclinical indications of acute cardiovascular responses within hours of elevated exposure to , including increased cardiac arrhythmia, blood pressure, arterial stiffness, and thrombus formation (He et al. 2011; Lucking et al. 2008; Lundbäck et al. 2009; Soppa et al. 2017; Urch et al. 2005).
Although previous studies have reported significant associations between out-of-hospital cardiac arrests and wildfire smoke exposure (Dennekamp et al. 2015; Salimi et al. 2017), we did not find the same for the AD code Arrest. Results from studies on ambient and out-of-hospital cardiac arrests have also been inconsistent, where some found immediate associations following exposure (Pradeau et al. 2015; Rosenthal et al. 2008, 2013) and others found no association (Ensor et al. 2013; Raza et al. 2014; Wichmann et al. 2013). These inconsistencies could be due to different risk factors for cardiac arrest in different regions. For example, drug overdose was one of the major PA code for cardiac/respiratory arrest calls in this study, but not in the Australian study (Salimi et al. 2017).
Short-term exposure to has been associated with insulin resistance (Dang et al. 2018; Haberzettl et al. 2016) and hospitalization for diabetes (Zanobetti and Schwartz 2002; Zanobetti et al. 2014), and long-term exposure has been associated with increased incidence and prevalence of type 2 diabetes (Eze et al. 2015; Pearson et al. 2010; Yang et al. 2018). On the other hand, the association between and other chronic conditions, such as heart diseases, may be modified by diabetic comorbidity (Pinault et al. 2018; Zanobetti et al. 2014). The mechanism behind these associations remains inconclusive, but some studies suggest that it may be linked to oxidative stress and systematic inflammation induced by the exposure (Haberzettl et al. 2016; Sun et al. 2009). In this study, we found that the association between diabetic problems and wildfire smoke exposure increased over time and became positive at a 24-h lag. This finding adds to the limited evidence that has been available on the lag–response relationship, especially at the hourly time scale. When we modeled PA codes for hypoglycemia and hyperglycemia separately, the lag–response relationship was different (Figure S4). A recent study in Australia found that daily exposure was associated with increased same-day and next-day dispatches for hypoglycemia but not hyperglycemia (Johnston et al. 2018). These findings warrant further studies that examine the potential mechanisms behind the association between wildfire smoke and different diabetic conditions.
This study has some unique strengths. The temporal resolution of the exposure and ambulance dispatch data allowed the examination of the exposure–response lag structure on an hourly scale. The linkage among ambulance dispatches, paramedic assessments, and hospital admissions provided the opportunity to evaluate the quality of the data and the internal consistency of the study results. In addition, having a single provider of ambulance services in British Columbia enabled us to conduct a population-based study over a very large geographic area with variable wildfire smoke impacts.
There were also several limitations. First, exposure misclassification was possible due to a) error in the exposure model, b) uncaptured variability within the prediction grid, c) the assumption that each subject was exposed at the dispatch location during the control windows, and d) the potential lag time between exposure and the dispatch call. Second, some populations may be more likely than others to call ambulance services (Kerr et al. 2006; Rucker et al. 1997). With the case-crossover study design, we were able to control for the confounding effect of this factor, but the results might not be generalizable to the entire population. Third, there is still uncertainty about the temporal relationship between exposure, symptom onset, ambulance call, and subsequent care for any given study subject. Although the ambulance dispatch data have finer temporal resolution than many other administrative data sets, there may still be a lag between the onset of symptoms and the action of calling an ambulance. Further, there was also a lag between some dispatches and hospital admissions, the reasons for which are unclear. In some cases, the subject may have been transported to a hospital and held under observation in the emergency room prior to admission, or admission was delayed due to limited availability of hospital beds. Unfortunately, the emergency room data were disparately collected for each hospital and were not available through an integrated database for the study period. All of these uncertainties may affect the characterization of the lag structure. Finally, with our study design, we are not able to rule out the possibility that some of the increased ambulance calls may be due to the elevated stress level from the perception of fire or smoke, instead of air pollution, during wildfire smoke events.
This study adds to the limited evidence on the acute health effects from sub-daily exposure to , especially during wildfire seasons. We found associations with some respiratory and cardiovascular outcomes within 1 h of exposure, whereas the association with some diabetic increased over time and became positive at approximately 24 h after exposure. The results varied by whether the outcomes were coded for AD, PA, or HD. These results warrant further investigation into the health effects of sub-daily exposures and may have implications for the appropriate time scale of air quality standards and public health actions during air pollution events.
Supplementary Material
Acknowledgments
This work is supported by the Australian Research Council Linkage Program (LP130100146) and the British Columbia Lung Association. The authors would like to thank the editor and reviewers for helping to strengthen this work with their insights. The authors also want to thank British Columbia Emergency Health Services for sharing their data with us, because this work would not be possible otherwise. We are grateful to PopData BC for helping with the data request and linkage and providing the secure research environment. All inferences, opinions, and conclusions drawn in this article are those of the authors and do not reflect the opinions or policies of the data stewards.
References
- Adamkiewicz G, Ebelt S, Syring M, Slater J, Speizer FE, Schwartz J, et al. 2004. Association between air pollution exposure and exhaled nitric oxide in an elderly population. Thorax 59(3):204–209, PMID: 14985553, 10.1136/thorax.2003.006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldersley A, Murray SJ, Cornell SE. 2011. Global and regional analysis of climate and human drivers of wildfire. Sci Total Environ 409(18):3472–3481, PMID: 21689843, 10.1016/j.scitotenv.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Austin M, Buffett D, Nicolson D, Scudder G, Stevens V. 2008. Taking Nature’s Pulse: The Status of Biodiversity in British Columbia. Victoria, British Columbia, Canada: Biodiversity BC. [Google Scholar]
- Barbero R, Abatzoglou J, Larkin N, Kolden C, Stocks B. 2015. Climate change presents increased potential for very large fires in the contiguous United States. Int J Wildland Fire 24(7):892–899, 10.1071/WF15083. [DOI] [Google Scholar]
- BC Emergency Health Services. 2019. Patient Care Report Standard Operating Procedure, version 5.6. https://handbook.bcehs.ca/operations/siren-reference-epcr/pcr-guidelines-and-procedures/pcr-standard-operating-procedures/ [accessed 19 November 2019].
- Bhaskaran K, Wilkinson P, Smeeth L. 2011. Cardiovascular consequences of air pollution: what are the mechanisms? Heart 97(7):519–520, PMID: 21148574, 10.1136/hrt.2010.212183. [DOI] [PubMed] [Google Scholar]
- Black C, Gerriets JE, Fontaine JH, Harper RW, Kenyon NJ, Tablin F, et al. 2017. Early life wildfire smoke exposure is associated with immune dysregulation and lung function decrements in adolescence. Am J Respir Cell Mol Biol 56(5):657–666, PMID: 28208028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers-Arriagada N, Horsley JA, Palmer AJ, Morgan GG, Tham R, Johnston FH. 2019. Association between fire smoke fine particulate matter and asthma-related outcomes: systematic review and meta-analysis. Environ Res 179(part A):108777, PMID: 31593836, 10.1016/j.envres.2019.108777. [DOI] [PubMed] [Google Scholar]
- Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. 2018. The 2016 global and national burden of diabetes mellitus attributable to PM2.5 air pollution. Lancet Planet Health 2(7):e301–e312, PMID: 30074893, 10.1016/S2542-5196(18)30140-2. [DOI] [PubMed] [Google Scholar]
- Buteau S, Goldberg MS, Burnett RT, Gasparrini A, Valois M-F, Brophy JM, et al. 2018. Associations between ambient air pollution and daily mortality in a cohort of congestive heart failure: case-crossover and nested case-control analyses using a distributed lag nonlinear model. Environ Int 113:313–324, PMID: 29361317. [DOI] [PubMed] [Google Scholar]
- Canadian Institute for Health Information. 2017. Discharge Abstract Database (Hospital Separations). Part V2. Population Data BC: Data Extract. MOH (2017), http://www.popdata.bc.ca/data.
- Clawson JJ, Dernocoeur KB, Murray C. 2015. Principles of Emergency Medical Dispatch. 6th ed. Salt Lake City, UT: National Academy of EMD. [Google Scholar]
- Dang J, Yang M, Zhang X, Ruan H, Qin G, Fu J, et al. 2018. Associations of exposure to air pollution with insulin resistance: a systematic review and meta-analysis. IJERPH 15(11):2593, PMID: 30463387, 10.3390/ijerph15112593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFlorio-Barker S, Crooks J, Reyes J, Rappold AG. 2019. Cardiopulmonary effects of fine particulate matter exposure among older adults, during wildfire and non-wildfire periods, in the United States 2008–2010. Environ Health Perspect 127(3):037006, PMID: 30875246, 10.1289/EHP3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Brummel S, Wu J, Stern H, Ostro B, Lipsett M, et al. 2009. The relationship of respiratory and cardiovascular hospital admissions to the Southern California wildfires of 2003. Occup Environ Med 66(3):189–197, PMID: 19017694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Gillen D, Tjoa T, Sioutas C, Fung K, et al. 2006. Personal and ambient air pollution is associated with increased exhaled nitric oxide in children with asthma. Environ Health Perspect 114(11):1736–1743, PMID: 17107861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey F. 2013. Forest fire effects on air quality in Ontario: evaluation of several recent examples. Bull Amer Meteor Soc 94(7):1059–1064, 10.1175/BAMS-D-11-00202.1. [DOI] [Google Scholar]
- Dennekamp M, Straney LD, Erbas B, Abramson MJ, Keywood M, Smith K, et al. 2015. Forest fire smoke exposures and out-of-hospital cardiac arrests in Melbourne, Australia: a case-crossover study. Environ Health Perspect 123(10):959–964, PMID: 25794411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennison PE, Brewer SC, Arnold JD, Moritz MA. 2014. Large wildfire trends in the western United States, 1984–2011. Geophys Res Lett 41(8):2928–2933, 10.1002/2014GL059576. [DOI] [Google Scholar]
- Dirksen RJ, Folkert Boersma K, de Laat J, Stammes P, van der Werf GR, Val Martin M, et al. 2009. An aerosol boomerang: rapid around-the-world transport of smoke from the December 2006 Australian forest fires observed from space. J Geophys Res Atmos 114:D21201, 10.1029/2009JD012360. [DOI] [Google Scholar]
- Elliott CT, Henderson SB, Wan V. 2013. Time series analysis of fine particulate matter and asthma reliever dispensations in populations affected by forest fires. Environ Health 12(1):11, PMID: 23356966, 10.1186/1476-069X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensor KB, Raun LH, Persse D. 2013. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation 127(11):1192–1199, PMID: 23406673, 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- Evans KA, Hopke PK, Utell MJ, Kane C, Thurston SW, Ling FS, et al. 2017. Triggering of ST-elevation myocardial infarction by ambient wood smoke and other particulate and gaseous pollutants. J Expo Sci Environ Epidemiol 27(2):198–206, PMID: 27072425, 10.1038/jes.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, et al. 2015. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect 123(5):381–389, PMID: 25625876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forastiere F, Stafoggia M, Berti G, Bisanti L, Cernigliaro A, Chiusolo M, et al. 2008. Particulate matter and daily mortality: a case-crossover analysis of individual effect modifiers. Epidemiology 19(4):571–580, PMID: 18467959, 10.1097/EDE.0b013e3181761f8a. [DOI] [PubMed] [Google Scholar]
- Gardner B, Ling F, Hopke PK, Frampton MW, Utell MJ, Zareba W, et al. 2014. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: a case-crossover study. Part Fibre Toxicol 11(1):1, PMID: 24382024, 10.1186/1743-8977-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 2011. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Soft 43(8):1, PMID: 22003319, 10.18637/jss.v043.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29(21):2224–2234, PMID: 20812303, 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. 2015. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386(9991):369–375, PMID: 26003380, 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio L, Randerson JT, Werf GR. 2013. Analysis of daily, monthly, and annual burned area using the fourth-generation global fire emissions database (GFED4). J Geophys Res Biogeosci 118(1):317–328, 10.1002/jgrg.20042. [DOI] [Google Scholar]
- Guo Y. 2017. Hourly associations between heat and ambulance calls. Environ Pollut 220:1424–1428, PMID: 27825842, 10.1016/j.envpol.2016.10.091. [DOI] [PubMed] [Google Scholar]
- Guo Y, Barnett AG, Pan X, Yu W, Tong S. 2011. The impact of temperature on mortality in Tianjin, china: a case-crossover design with a distributed lag nonlinear model. Environ Health Perspect 119(12):1719–1725, PMID: 21827978, 10.1289/ehp.1103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P, O’Toole TE, Bhatnagar A, Conklin DJ. 2016. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect 124(12):1830–1839, PMID: 27128347, 10.1289/EHP212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikerwal A, Akram M, Del Monaco A, Smith K, Sim MR, Meyer M. 2015. Impact of fine particulate matter (PM2.5) exposure during wildfires on cardiovascular health outcomes. J Am Heart Assoc 4(7):e001653, PMID: 26178402, 10.1161/JAHA.114.001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Shaffer ML, Rodriguez-Colon S, Yanosky JD, Bixler E, Cascio WE, et al. 2011. Acute effects of fine particulate air pollution on cardiac arrhythmia: the APACR study. Environ Health Perspect 119(7):927–932, PMID: 21398201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SB, Brauer M, Macnab YC, Kennedy SM. 2011. Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population-based cohort. Environ Health Perspect 119(9):1266–1271, PMID: 21659039, 10.1289/ehp.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstius DM, Reid CE, Jesdale BM, Morello-Frosch R. 2012. Birth weight following pregnancy during the 2003 Southern California wildfires. Environ Health Perspect 120(9):1340–1345, PMID: 22645279, 10.1289/ehp.1104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P, Wang X, Flannigan MD. 2017. Trend analysis of fire season length and extreme fire weather in North America between 1979 and 2015. Int J Wildland Fire 26(12):1009–1020, 10.1071/WF17008. [DOI] [Google Scholar]
- Jeong JI, Park RJ, Youn D. 2008. Effects of Siberian forest fires on air quality in East Asia during May 2003 and its climate implication. Atmos Environ 42(39):8910–8922, 10.1016/j.atmosenv.2008.08.037. [DOI] [Google Scholar]
- Johnston FH, Bailie RS, Pilotto LS, Hanigan IC. 2007. Ambient biomass smoke and cardio-respiratory hospital admissions in Darwin, Australia. BMC Public Health 7(1):240, PMID: 17854481, 10.1186/1471-2458-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston FH, Salimi F, Williamson GJ, Henderson SB, Yao J, Dennekamp M, et al. 2018. Ambient particulate matter and paramedic assessments of acute diabetic, cardiovascular, respiratory conditions. Epidemiology 30(1):11–19, PMID: 30334919, 10.1097/EDE.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly WM, Cochrane MA, Freeborn PH, Holden ZA, Brown TJ, Williamson GJ, et al. 2015. Climate-induced variations in global wildfire danger from 1979 to 2013. Nat Commun 6:7537, PMID: 26172867, 10.1038/ncomms8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr D, Holden D, Smith J, Kelly A-M, Bunker S. 2006. Predictors of ambulance use in patients with acute myocardial infarction in Australia. Emerg Med J 23(12):948–952, PMID: 17130609, 10.1136/emj.2006.038414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J, Evans J, Fnais M, Giannadaki D, Pozzer A. 2015. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525(7569):367–371, PMID: 26381985, 10.1038/nature15371. [DOI] [PubMed] [Google Scholar]
- Liu JC, Pereira G, Uhl SA, Bravo MA, Bell ML. 2015. A systematic review of the physical health impacts from non-occupational exposure to wildfire smoke. Environ Res 136:120–132, PMID: 25460628, 10.1016/j.envres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C, Hennessy K, Mills G, Bathols J. 2007. Bushfire Weather in Southeast Australia: Recent Trends and Projected Climate Change Impacts. Melbourne, Australia: Bushfire Cooperative Research Centre. [Google Scholar]
- Lucking A, Lundback M, Mills N, Faratian D, Barath S, Pourazar J, et al. 2008. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J 29(24):3043– 3051, PMID: 18952612. [DOI] [PubMed] [Google Scholar]
- Lundbäck M, Mills NL, Lucking A, Barath S, Donaldson K, Newby DE, et al. 2009. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part Fibre Toxicol 6:7, PMID: 19284640, 10.1186/1743-8977-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclure M. 1991. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 133(2):144–153, PMID: 1985444, 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- Mar TF, Jansen K, Shepherd K, Lumley T, Larson TV, Koenig JQ. 2005. Exhaled nitric oxide in children with asthma and short-term PM2.5 exposure in Seattle. Environ Health Perspect 113(12):1791–1794, PMID: 16330366, 10.1289/ehp.7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendry IG. 2006. Background Concentrations of PM2.5 and Ozone in British Columbia, Canada, https://www2.gov.bc.ca/assets/gov/environment/air-land-water/air/reports-pub/background_pm25_ozone.pdf [accessed 29 May 2020]. [Google Scholar]
- Miller DJ, Sun K, Zondlo MA, Kanter D, Dubovik O, Welton EJ, et al. 2011. Assessing boreal forest fire smoke aerosol impacts on U.S. air quality: a case study using multiple data sets. J Geophys Res Atmos 116:D22209, 10.1029/2011JD016170. [DOI] [Google Scholar]
- Morgan G, Sheppeard V, Khalaj B, Ayyar A, Lincoln D, Jalaludin B, et al. 2010. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 21(1):47–55, PMID: 19907335. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, et al. 2007. Woodsmoke health effects: a review. Inhal Toxicol 19(1):67–106, PMID: 17127644, 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. 2010. Association between fine particulate matter and diabetes prevalence in the US. Diabetes Care 33(10):2196–2201, PMID: 20628090, 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Dockery D, Muller J, Mittleman M. 2001. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 103(23):2810–2815, PMID: 11401937, 10.1161/01.CIR.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Pinault L, Brauer M, Crouse DL, Weichenthal S, Erickson A, van Donkelaar A, et al. 2018. Diabetes status and susceptibility to the effects of PM2.5 exposure on cardiovascular mortality in a national Canadian cohort. Epidemiology 29:784–794, PMID: 30074537. [DOI] [PubMed] [Google Scholar]
- Pradeau C, Rondeau V, Lévèque E, Guernion P-Y, Tentillier E, Thicoipé M, et al. 2015. Air pollution and activation of mobile medical team for out-of-hospital cardiac arrest. Am J Emerg Med 33(3):367–372, PMID: 25577313. [DOI] [PubMed] [Google Scholar]
- Rao X, Montresor-Lopez J, Puett R, Rajagopalan S, Brook RD. 2015. Ambient air pollution: an emerging risk factor for diabetes mellitus. Curr Diab Rep 15(6):603, PMID: 25894943, 10.1007/s11892-015-0603-8. [DOI] [PubMed] [Google Scholar]
- Raza A, Bellander T, Bero-Bedada G, Dahlquist M, Hollenberg J, Jonsson M, et al. 2014. Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur Heart J 35(13):861–868, PMID: 24302272, 10.1093/eurheartj/eht489. [DOI] [PubMed] [Google Scholar]
- Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT. 2016. Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect 124(9):1334–1343, PMID: 27082891, 10.1289/ehp.1409277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal F, Carney J, Olinger M. 2008. Out-of-hospital cardiac arrest and airborne fine particulate matter: a case-crossover analysis of emergency medical services data in Indianapolis, Indiana. Environ Health Perspect 116(5):631–536, PMID: 18470283, 10.1289/ehp.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal FS, Kuisma M, Lanki T, Hussein T, Boyd J, Halonen JI, et al. 2013. Association of ozone and particulate air pollution with out-of-hospital cardiac arrest in Helsinki, Finland: evidence for two different etiologies. J Expo Sci Environ Epidemiol 23(3):281–288, PMID: 23361443, 10.1038/jes.2012.121. [DOI] [PubMed] [Google Scholar]
- Rucker DW, Edwards RA, Burstin HR, O’Neil AC, Brennan TA. 1997. Patient-specific predictors of ambulance use. Ann Emerg Med 29(4):484–491, PMID: 9095009, 10.1016/S0196-0644(97)70221-X. [DOI] [PubMed] [Google Scholar]
- Saide PE, Peterson DA, da Silva A, Anderson B, Ziemba LD, Diskin G, et al. 2015. Revealing important nocturnal and day-to-day variations in fire smoke emissions through a multiplatform inversion. Geophys Res Lett 42(9):3609–3618, 10.1002/2015GL063737. [DOI] [Google Scholar]
- Salimi F, Henderson SB, Morgan GG, Jalaludin B, Johnston FH. 2017. Ambient particulate matter, landscape fire smoke, and emergency ambulance dispatches in Sydney, Australia. Environ Int 99:208–212, PMID: 27887782, 10.1016/j.envint.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Soppa VJ, Schins RP, Hennig F, Nieuwenhuijsen MJ, Hellack B, Quass U, et al. 2017. Arterial blood pressure responses to short-term exposure to fine and ultrafine particles from indoor sources–a randomized sham-controlled exposure study of healthy volunteers. Environ Res 158:225–232, PMID: 28662448. [DOI] [PubMed] [Google Scholar]
- Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. 2018. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet 392(10159):1923–1994, PMID: 30496105, 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand T, Larkin N, Rorig M, Krull C, Moore M. 2011. PM2.5 measurements in wildfire smoke plumes from fire seasons 2005–2008 in the northwestern United States. J Aerosol Sci 42(3):143–155, 10.1016/j.jaerosci.2010.09.001. [DOI] [Google Scholar]
- Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. 2005. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology 16(1):41–48, PMID: 15613944, 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, et al. 2009. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119(4):538–546, PMID: 19153269, 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Heinrich J. 2015. Epidemiology of air pollution and diabetes. Trends Endocrinol Metab 26(7):384–394, PMID: 26068457, 10.1016/j.tem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Tinling MA, West JJ, Cascio WE, Kilaru V, Rappold AG. 2016. Repeating cardiopulmonary health effects in rural North Carolina population during a second large peat wildfire. Environ Health 15(1):12, PMID: 26818940, 10.1186/s12940-016-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urch B, Silverman F, Corey P, Brook J, Lukic K, Rajagopalan S, et al. 2005. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect 113(8):1052–1055, PMID: 16079078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling AL, Hidalgo HG, Cayan DR, Swetnam TW. 2006. Warming and earlier spring increase western U.S. forest wildfire activity. Science 313(5789):940–943, 10.1126/science.1128834. [DOI] [PubMed] [Google Scholar]
- Wichmann J, Folke F, Torp-Pedersen C, Lippert F, Ketzel M, Ellermann T, et al. 2013. Out-of-hospital cardiac arrests and outdoor air pollution exposure in Copenhagen, Denmark. PLoS One 8(1):e53684, PMID: 23341975, 10.1371/journal.pone.0053684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotton B, Flannigan M, Marshall G. 2017. Potential climate change impacts on fire intensity and key wildfire suppression thresholds in Canada. Environ Res Lett 12(9):095003, 10.1088/1748-9326/aa7e6e. [DOI] [Google Scholar]
- Yamazaki S, Shima M, Ando M, Nitta H, Watanabe H, Nishimuta T. 2011. Effect of hourly concentration of particulate matter on peak expiratory flow in hospitalized children: a panel study. Environ Health 10(1):15, PMID: 21392385, 10.1186/1476-069X-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B-Y, Qian ZM, Li S, Chen G, Bloom MS, Elliott M, et al. 2018. Ambient air pollution in relation to diabetes and glucose-homoeostasis markers in china: a cross-sectional study with findings from the 33 Communities Chinese Health Study. Lancet Planet Health 2(2):e64–e73, PMID: 29615239, 10.1016/S2542-5196(18)30001-9. [DOI] [PubMed] [Google Scholar]
- Yao J, Brauer M, Raffuse SM, Henderson S. 2018. Machine learning approach to estimate hourly exposure to fine particulate matter for urban, rural, and remote populations during wildfire seasons. Environ Sci Technol 52(22):13239–13249, PMID: 30354090, 10.1021/acs.est.8b01921. [DOI] [PubMed] [Google Scholar]
- Yao J, Eyamie J, Henderson SB. 2016. Evaluation of a spatially resolved forest fire smoke model for population-based epidemiologic exposure assessment. J Expo Sci Environ Epidemiol 26(3):233–240, PMID: 25294305, 10.1038/jes.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssouf H, Liousse C, Roblou L, Assamoi EM, Salonen RO, Maesano C, et al. 2014. Non-accidental health impacts of wildfire smoke. Int J Environ Res Public Health 11(11):11772–11804, PMID: 25405597, 10.3390/ijerph111111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Dominici F, Wang Y, Schwartz JD. 2014. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ Health 13(1):38, PMID: 24886318, 10.1186/1476-069X-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. 2002. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology 13(5):588–592, 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.