Abstract
Background:
Assessments of balance and walking are often performed in rehabilitation of people with multiple sclerosis (MS). The Functional Gait Assessment (FGA) is a test of walking balance including challenging items such as walking with a narrow base of support, with eyes closed, and backward. The aim was to investigate the validity (concurrent, discriminant, and known-groups) and sensitivity to change of the modified FGA in ambulatory individuals with MS.
Methods:
A convenience sample of 87 individuals with MS was included (mean age, 54 years; 79% women). Concurrent and discriminant validity was investigated using tests of dynamic balance and the Multiple Sclerosis Walking Scale and Multiple Sclerosis Impact Scale (MSIS). Known-groups validity was investigated with self-reported number of falls and use of walking devices. Sensitivity to change was investigated with data from a group balance training study.
Results:
The median FGA score was 15 (range, 1–26). Concurrent validity with tests of dynamic balance was moderate to strong, with the Timed Up and Go test having the highest correlation coefficient (rho = −0.74). Discriminant validity was shown with a low correlation coefficient with the MSIS psychological subscale (rho = 0.14). The FGA scores differed significantly for users of walking devices versus nonusers but not for reported falls. Sensitivity to change was moderate to low.
Conclusions:
The FGA is a valid measure of balance during walking in people with MS, but further investigation is required for the ability to detect people at risk for falls.
Individuals with multiple sclerosis (MS) often display a variety of symptoms, including significantly disabling symptoms such as difficulty walking and decreased balance.1,2 Gait and balance impairment can be present in the early stages of the disease.3 Impaired walking ability and decreased balance are strongly associated with a greater risk of accidental falls, and more than 50% of individuals with MS are considered fallers.4–6 In clinical practice, it is important to have appropriate measures that identify balance and walking impairment and risk of falls. One clinical measure that can be used for evaluating ambulatory and balance deficits is the Functional Gait Assessment (FGA). The FGA was developed by Wrisley et al.7 by using items in the Dynamic Gait Index (DGI). The DGI consists of eight items with walking tasks such as changing speed, walking and turning the head, walking around or over obstacles, and walking with pivot turn.8 Good validity and reliability of the DGI has been established in several diagnoses, as well as in MS.9–12 The FGA includes seven of the items in the DGI (one item, walking around obstacles, was regarded as being too easy), and three more challenging items were added: walking with a narrow base of support (heel-to-toe with arms crossed), walking with eyes closed, and walking backwards. The FGA covers several aspects: quality of movement, deviation from the intended path, the need for a walking device, and time required to perform the task.
In a recent study, we examined the reliability and validity of a slightly modified version (using centimeters and meters instead of inches) of the FGA in people with peripheral vestibular disorders.13 The interrater (0.73) and intrarater (0.94) reliability were high, and concurrent validity with the Timed Up and Go (TUG) test was strong (rho = −0.69). The TUG test is a test of functional mobility and includes walking and turning, tasks that are also found in the FGA. Strong concurrent validity of the FGA with the Berg Balance Scale (BBS) and the TUG test have also been shown in studies including individuals with Parkinson disease (rho = 0.57–0.85).14,15 In stroke, concurrent validity was strong with the BBS (rho = 0.93)16 and 10-m walking (rho = 0.81).16,17 Furthermore, in community-dwelling older people concurrent validity was strong with the BBS (rho = 0.84) and the TUG test (rho = 0.84).18 High test-retest and interrater and intrarater reliability of the FGA have been established in several studies.7,15–17 The FGA seems to be a suitable measure of balance during walking activities, but its psychometric properties have not been investigated for MS.
The aim of the present study was to investigate the following properties of the modified FGA in a sample of ambulatory individuals with MS: 1) concurrent validity with the BBS,19 the Four Square Step Test,20 the TUG test,21 a timed sit-to-stand test,22 and two patient-rated diagnosis-specific measures: the Multiple Sclerosis Walking Scale (MSWS)23 and the physical subscale of the Multiple Sclerosis Impact Scale (MSIS)24; 2) discriminant validity with use of the MSIS psychological subscale; 3) known-groups validity determined by the use of walking devices and self-reported history of falls; and 4) sensitivity to change. The hypothesis was that correlation coefficients between FGA scores and the tests of balance and walking are moderate to strong, with a positive direction for the BBS and negative directions for the other tests, as well as the MSWS and the MSIS. A low correlation coefficient was expected between the FGA and the psychological subscale of the MSIS.
Methods
This cross-sectional study used data from the baseline assessment in a randomized, controlled, multicenter trial25 investigating the effect of group balance training. A secondary analysis was performed on the baseline data. Data were collected between August 1, 2012, and June 30, 2013. The randomized controlled trial is registered in the ClinicalTrials.gov database (NCT01582126).
Participants
The inclusion criteria were a diagnosis of MS26 by a neurologist based on the McDonald criteria, able to walk 100 m with or without an assistive device, able to get up from the floor with minor support (hence, being able to participate in the intervention), and unable to maintain tandem stance heel-to-toe for 30 seconds with arms alongside the body (study-specific test of balance impairment). The exclusion criteria were cognitive or linguistic difficulties that would prohibit filling in the self-rating scales and having difficulties following commands. Possible participants (age >18 years) were identified in the records at seven centers located in five county councils in central Sweden or were known by the research physical therapist at each center. Eligible individuals with MS (n = 208) were given written and verbal information about the randomized controlled trial; 14 did not meet the inclusion criteria (mainly owing to difficulties getting up from the floor), and 107 declined to participate. The final study group for the randomized controlled trial and, hence, this study comprised 87 individuals. Participation in the study required written consent, and the Uppsala-Örebro Ethical Review Board approved the study.
Procedure
Data for the validation were collected at the baseline assessment. The self-rated scales (the MSWS and the MSIS) were completed by the participants during the testing session. Data collection was performed by specially trained research physical therapists.
The intervention with follow-up data collection that was used for the sensitivity to change analysis consisted of a 60-minute exercise program targeting core stability, dual tasking, and sensory strategies.25 The program was conducted twice weekly for 7 weeks, supervised by a physical therapist. Exercises were performed in the supine, four-point kneeling, sitting, and standing positions. Examples of exercises are supine position with knees bent, slowly sliding one heel forward to straighten leg; walking while juggling a balloon; and standing and walking on uneven surfaces. The BBS was the primary outcome measure. Follow-up assessments were performed at weeks 8, 16, and 24. Data from the follow-up at week 8 were used in the analysis of sensitivity to change in the present study. All data collection was performed by independent assessors who were blinded to group allocation.
Measures
Demographic characteristics, type of MS, use of assistive walking devices indoors and outdoors, and the number of self-reported falls during the previous 2 months were gathered at the baseline assessment. A fall was defined as an unexpected event in which the participant came to rest on the ground, the floor, or a lower level.27
The FGA consists of ten items: walking on an even surface, changing speed, walking with horizontal and vertical head turns, turning, stepping over obstacles, walking with a narrow base of support, walking with eyes closed, walking backward, and stair climbing. Each item is graded from 0 (severe impairment) to 3 (normal performance), giving a maximum score of 30.7 The slightly modified version of the FGA with centimeters and meters was used.13 A 6-m-long, 30-cm-wide walkway and lines marking 15-, 25-, and 38-cm deviations were taped on the floor. Two boxes (25 cm wide and either 11 or 23 cm high) were used as obstacles.
The BBS consists of 14 items covering dynamic and static balance in sitting and standing; each of these is graded between 0 and 4, giving a maximum score of 56.19,28 A higher score indicates better balance performance.
The Four Square Step Test consists of stepping over 2.5-cm-high sticks placed in a cross formation.20 The participant is required to step forward, sideways, backward, sideways, and then the same way back. Time was recorded, and the better of two trials was registered.
The TUG test is a measure of basic mobility. It records the time taken to rise from a chair, walk 3 m, turn, walk back, and sit down.10,21 The test was performed once at forced speed, with an assistive walking device used if necessary. The participants were asked to walk as fast, but safe, as possible.
A timed sit-to-stand test was used to assess the functional strength in the lower extremities.22 Time was measured as the participants rose ten times from a chair with hand rails.
The MSWS is a frequently used self-rated scale measuring walking limitations caused by MS during the past 2 weeks.23 The scale includes 12 items that are rated from 1 (not at all limited) to 5 (extremely limited). Scores are summed, and the maximum score of 60 represents severe limitation. A validated Swedish translation of the MSWS was used.29
The MSIS is a self-rated measure of the impact of MS during the past 2 weeks.24 It includes 29 items, with the first 20 summed into a physical subscale and the last 9 into a psychological subscale. Each item is rated from 1 (not at all limited) to 5 (extremely limited/bothered), and so a higher score represents a more severe impact. The total score on the physical subscale ranges from 20 to 100, and the psychological subscale ranges from 9 to 45. A Swedish translation of the MSIS was used.30
Statistical Analysis
The distributions of the variables are given as mean, SD, median, minimum, and maximum for continuous variables and ordinal variables and as number and percentage for categorical variables. Spearman correlation coefficients were calculated between scores on the FGA and the other measures for analysis of concurrent and discriminant validity, with correlation coefficients less than 0.30 interpreted as weak, 0.30 to 0.59 as moderate, and 0.60 or greater as strong.31 The Mann-Whitney U test was used to analyze differences in scores on the FGA when investigating known-groups validity (fallers vs. nonfallers, users of assistive walking devices vs. nonusers). All significance tests were two-sided and were conducted at the 5% significance level. IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY) and SAS software version 9 (SAS Institute Inc, Cary, NC) were used for data analysis.
Sensitivity to change was analyzed using both homogeneous and heterogeneous approaches. In the homogeneous approach, sensitivity to change was analyzed using standardized response mean, Cohen's effect size, and Guyatt's responsiveness statistics with bootstrapped 95% confidence intervals.32 Stable participants in Guyatt's responsiveness statistics were defined with the BBS as the anchor: 1) an improvement of 1.5 points or less11 and 2) an improvement of 6 points or less after 7 weeks of group balance training.33 Sensitivity to change was assessed as high (≥0.8), moderate (0.5–0.79), or low (0.2–0.49).32 In the heterogeneous approach, Spearman correlation coefficients were calculated between change on the BBS and change on the FGA. An analysis of covariance was also performed with FGA data from the test occasion after the balance intervention as the dependent variable, the dichotomized anchor (BBS) as the independent variable, and baseline FGA values as covariates. If the dichotomized anchor was significant, then the FGA could differentiate between participants who had improved and those who had not.
Results
Characteristics of the 87 participants are presented in Table 1. The mean participant age was 54 years (range, 28–75 years). The participants had lived with their MS diagnosis for 1 to 46 years. Fifteen participants (17%) reported having other diseases/problems, although their main balance and walking disabilities were due to MS: musculoskeletal problems such as backache (n = 9), heart problems (n = 2), chronic obstructive lung disease (n = 1), stroke (n = 1), and Ehlers-Danlos syndrome (n = 2).
Table 1.
Characteristics of the 87 study participants
| Characteristic | Value |
|---|---|
| Age, mean (SD), y | 53.8 (10.9) |
| Time since diagnosis, mean (SD), y | 15.0 (9.8) |
| Female sex, No. (%) | 69 (79) |
| Type of MS, No. (%) | |
| Relapsing-remitting | 40 (46.0) |
| Secondary progressive | 37 (42.5) |
| Primary progressive | 10 (11.5) |
| Use of an assistive device indoors, No. (%) | |
| None | 75 (86) |
| Unilateral crutch | 4 (5) |
| Bilateral crutch | 1 (1) |
| Rolling walker | 7 (8) |
| Use of an assistive device outdoors, No. (%) | |
| None | 35 (40) |
| Unilateral crutch | 15 (17) |
| Bilateral crutch | 12 (14) |
| Rolling walker | 14 (16) |
| Wheelchair | 11 (13) |
Abbreviation: MS, multiple sclerosis.
Median scores on the items in the FGA are presented in Table 2. The most difficult items to perform were walking with a narrow base of support and walking with eyes closed. A total of 71 participants (82%) had severe impairment in walking with a narrow base of support (score 0). None of the participants could walk with their eyes closed without deviating from the walkway or walking very slowly. Floor and ceiling effects on the FGA were not apparent, with scores ranging from 1 to 26. The distribution of scores is presented in Figure 1.
Table 2.
The Functional Gait Assessment (FGA) score data
| Item | Score, median (range) | Participants with a maximum score of 3, No. (%) (N = 87) |
|---|---|---|
| Gait on level surface | 2 (0–3) | 17 (19.5) |
| Change in gait speed | 2 (0–3) | 24 (27.6) |
| Gait with horizontal head turns | 2 (0–3) | 5 (5.7) |
| Gait with vertical head turns | 2 (0–3) | 9 (10.3) |
| Gait and pivot turn | 2 (0–3) | 33 (37.9) |
| Stepping over obstacles | 1 (0–3) | 16 (18.4) |
| Gait with a narrow base of support | 0 (0–3) | 6 (6.9) |
| Gait with closed eyes | 1 (0–2) | 0 |
| Ambulating backward | 2 (0–3) | 11 (12.6) |
| Stair climbing | 2 (1–3) | 20 (23.0) |
| Total FGA score, mean (SD)/median (range) | 14.9 (5.4)/15 (1–26) | — |
Figure 1.
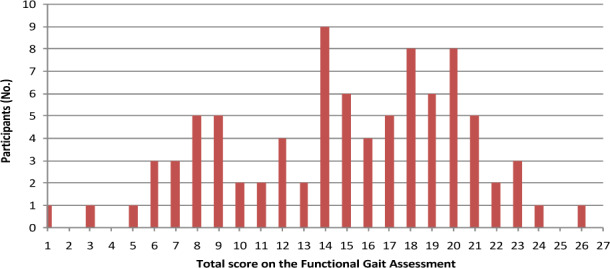
Distribution of total scores on the Functional Gait Assessment
Concurrent validity was strong for the BBS, the Four Square Step Test, and the TUG test; moderate for the timed sit-to-stand test and the MSWS; and weak for the MSIS physical subscale (Table 3). The correlation coefficient was weak (rho = 0.14) and nonsignificant for the MSIS psychological subscale, hence establishing discriminant validity.
Table 3.
Spearman correlation coefficients between the Functional Gait Assessment total score and the other tests and descriptive statistics (N = 87 unless otherwise stated)
| Measure (score range) | Score | Correlation coefficient | P value | |
|---|---|---|---|---|
| Mean (SD) | Median (range) | |||
| Berg Balance Scale (0–56) | 47 (7.8) | 50 (19–56) | 0.72 | <.001 |
| Four Square Step Test, s (n = 80) | 26.3 (22.5) | 16.4 (8.1–106.4) | −0.72 | <.001 |
| Timed Up and Go test, s | 16.1 (8.6) | 12.7 (6.3–49.4) | −0.74 | <.001 |
| Timed sit-to-stand test, s | 38.4 (14.3) | 34.9 (19.8–88.0) | −0.49 | <.001 |
| MSWS (12–60) | 41.4 (10.1) | 42 (17–60) | −0.46 | <.001 |
| MSIS physical subscale (20–100) | 56.0 (15.8) | 56 (26–91) | −0.21 | .047 |
| MSIS psychological subscale (9–45) | 22.2 (8.8) | 22 (9–45) | 0.14 | .197 |
Known-groups validity was established for use of assistive walking devices but not for history of falls. Forty-nine participants (56%) reported no falls during the 2 months before the baseline assessment, 16 (18%) reported one fall, and 22 (25%) reported two or more falls. Characteristics of the groups are presented in Table 4. There were no significant differences in FGA total scores between participants reporting one or more falls (median, 14.50; interquartile range [IQR], 8.8–18.0) and those reporting no falls (median, 17.0; IQR, 11.5–20.0). Participants using any kind of assistive walking device indoors had significantly (P < .001) lower total scores on the FGA (median, 8; IQR, 7–12) than nonusers (median, 17; IQR, 14–20). Similarly, significantly (P < .001) lower scores on the FGA were found for users of assistive walking devices outdoors (median, 14; IQR, 8.2–16) compared with nonusers (median, 19; IQR, 17–21).
Table 4.
Characteristics of groups in the known-groups validity analyses
| Characteristic | Falls in the previous 2 mo | Indoor walking device use | Outdoor walking device use | |||
|---|---|---|---|---|---|---|
| Yes (n = 38) | No (n = 49) | Yes (n = 15) | No (n = 72) | Yes (n = 52) | No (n = 35) | |
| Age, mean (SD), y | 51.7 (10.0) | 55.4 (11.4) | 55.2 (13.0) | 53.5 (10.5) | 55.9 (10.7) | 50.6 (10.6) |
| Time since diagnosis, mean (SD), y | 16.0 (10.0) | 14.2 (9.7) | 17.6 (12.0) | 14.4 (9.3) | 16.4 (10.4) | 12.9 (8.6) |
| Female sex, No. (%) | 32 (84) | 37 (76) | 12 (80) | 57 (79) | 45 (87) | 24 (69) |
| Type of MS, No. (%) | ||||||
| Relapsing-remitting | 14 (37) | 26 (53) | 4 (27) | 36 (50) | 18 (35) | 22 (63) |
| Secondary progressive | 21 (55) | 16 (33) | 9 (60) | 28 (39) | 26 (50) | 11 (31) |
| Primary progressive | 3 (8) | 7 (14) | 2 (13) | 8 (11) | 8 (15) | 2 (6) |
| Use of a walking device, No. (%) | ||||||
| Indoors | 9 (24) | 6 (12) | – | – | 15 (29) | 0 |
| Outdoors | 28 (74) | 24 (49) | 15 (100) | 37 (51) | – | – |
Abbreviation: MS, multiple sclerosis.
In the homogeneous approach, the FGA showed moderate-to-low sensitivity to change; the standardized response mean was 0.65 (95% confidence interval [CI], 0.40–0.95), and Cohen's effect size was 0.43 (95% CI, 0.27–0.61). Guyatt's responsiveness statistics were 0.60 (95% CI, 0.37–0.92) for the anchor of 1.5 points or less on the BBS and 0.66 (95% CI, 0.40–0.99) for the anchor of 6 points or less. In the heterogeneous approach, the Spearman correlation coefficient between change on the BBS and change on the FGA was 0.31. The analysis of covariance showed significant dichotomized anchor (BBS) values for both conditions: 1.5 points or less (P = .003) and 6 points or less (P = .007).
Discussion
This study provides evidence that the FGA is a valid measure of balance during walking for people with MS who have balance deficits. Concurrent validity was considered good, with a moderate-to-strong relationship between the FGA and the BBS, as well as the timed measures. Discriminant validity was also established through the low correlation coefficient with the MSIS psychological subscale. Moderate-to-strong correlation coefficients with different established measures, such as the BBS, the TUG test, and the Four Square Step Test, have also been found in Parkinson disease,14 in community-dwelling older adults,18 in stroke,16 and in people with vestibular disorders.13 The correlation coefficients with the MSWS and the MSIS physical subscale were lower than hypothesized. In an earlier study investigating the validity of the DGI, we found correlations of 0.72 and 0.58, respectively, for these measures.9 However, the MSWS includes aspects of functioning beyond walking ability, such as running and walking distance, that are not directly related to the items in the FGA. A previous study found correlations of 0.32 to 0.37 between the MSWS and measures of walking and balance, the BBS, the Four Square Step Test, and the TUG test.29 Correlation coefficients between the FGA and the subscales of the MSIS were low. The FGA seems weakly related to the perceived impact of MS.
The total score ranged from 1 to 26, indicating no apparent floor and ceiling effects in this sample. However, on four items, 10% or less of the participants received a score of 3: gait with closed eyes, gait with narrow base of support, and gait with horizontal or vertical head turns (Table 2). Balance deficits may be due to visual, somatosensory, and vestibular impairments, and often all three sensory systems are affected. In this sample, it was evident that receiving visual information is a crucial component. The items of the FGA provide the possibility to assess all three parts of the sensory system. Performing horizontal and vertical head turns was also a challenge, and several of the participants reported dizziness when performing these items. Similar findings were reported in studies evaluating the DGI.9,10 The items of the FGA reflect, to a large extent, activities that are performed when walking around in the community. Stepping over obstacles, changing speed, and turning one's head may pose challenges to individuals with MS.
The FGA has been suggested as a potential screening tool for fall risk in community-dwelling older adults.34 In Parkinson disease, the FGA has been found to be moderately sensitive for identifying fallers, with suggested cutoff points of 15 points or less15 and 18 points or less.14 Unexpectedly, we found no significant difference between participants reporting a history of falls and those with no such history. Most of the sample reported falls, which supports the fact that the risk of falling is a reality for people with MS. Measures that assess balance and walking must be able to distinguish individuals with a risk of falls. The predictive validity of the FGA as a measure to identify fall risk in people with MS should be further investigated. The ecological validity of the FGA was supported in the present study in that significant differences were found between participants who did and did not use an assistive walking device, when considering use both indoors and outdoors.
The ability of the FGA to detect change after the balance intervention was moderate to low. The BBS was considered a relevant anchor because the measure was the primary outcome in the randomized controlled trial, although there are no walking items. The limit of a 1.5-point improvement was based on a study by Cattaneo et al.11 that presented a standard error of measurement of 1.5 in a sample of people with MS. The mean score on the BBS was 47 (range, 31–56), which is similar to that in the present study (Table 3). The other limit of 6 points was based on a systematic review article by Downs33 that presents that with a change of 2.8 to 6.6 points, one can be 95% confident that there has been a real change. The analysis of covariance showed that the FGA could significantly distinguish participants who had improved after the balance intervention. Establishing minimal clinically important differences is essential for a measure that will be used in clinical practice, and in MS further studies are needed. In other diagnoses, minimal clinically important differences of 4 to 6 points on the FGA have been suggested.35,36
The FGA was feasible to perform in clinical settings. The test took approximately 10 to 15 minutes to complete. All items apart from climbing stairs can be performed in a hallway. Taping the walkway and deviations requires approximately 30 minutes of preparation. A disadvantage of the FGA is that the rating process is comprehensive, with a multitude of parameters to consider: time, movement quality, deviation from the walkway, and use of assistive device. The rating process can be confusing for a new user of the FGA, and some training to feel confident with the items and the rating is recommended before applying the test in clinical practice.
All the participants were unable to stand in tandem for more than 30 seconds, as this was an inclusion criterion in the randomized controlled trial. We then could conclude that all the participants had objective balance impairment. Because of the inclusion criteria, many of the participants subsequently were unable to walk heel-to-toe in the item challenging gait with narrow base of support. It could be a limitation that the participants were predisposed to have a problem with this particular item. However, the range of scores indicates that participants with a wide range of balance ability were included. In further studies of psychometric properties of the FGA, a sample of people with mild-to-severe MS should be included, hence representing slight to major balance disability.
That as many as 107 individuals declined participation in the randomized controlled trial could be seen as a limitation. Participants in this validation study consist of people who were interested in participating in a training intervention and could be regarded as a subsample of individuals with MS. However, the functioning of the participants varied, with FGA scores ranging from 1 to 26 points. The retrospective reporting of falls is another limitation, and in further studies, prospective reporting is recommended.
Conclusion
The FGA is a valid measure of balance during walking in people with MS, but further studies are needed to investigate its sensitivity to change and ability to detect people at risk for falls.
PracticePoints
The Functional Gait Assessment is a valid measure of balance during walking in ambulatory people with MS.
The test includes walking activities that are challenging for many individuals with MS.
The test is easy to perform in clinical settings but requires preparation such as taping of the walkway.
The administrator of the test needs training because the rating process is comprehensive, with a multitude of parameters to consider.
Acknowledgments
We thank the physiotherapists who assisted with data collection: Ingrid Lundström and Ingmarie Westlund at the Rehabilitation Unit, Västmanland Hospital, Västerås, Sweden; Lena Sanner and Malin Nilsson at the Rehabilitation Unit, Karlstad Central Hospital, Karlstad, Sweden; Lisbeth Franzén and Oskar Davidsson at the Physiotherapy Clinic, Nyköping Hospital, Nyköping, Sweden; and Lena von Koch at the Karolinska Institutet, Solna, Sweden.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This study was supported by the Uppsala-Örebro Regional Research Council (RFR-306241), the Norrbacka-Eugenia Foundation (grant 814/12), and the Research Committee of Region Örebro County (grants OLL-216421 and OLL-317511).
References
- 1.Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4:189–201. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 3.Martin CL, Phillips BA, Kilpatrick TJ et al. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler. 2006;12:620–628. doi: 10.1177/1352458506070658. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda PN, Shumway-Cook A, Ciol MA, Bombardier CH, Kartin DA. Understanding falls in multiple sclerosis: association of mobility status, concerns about falling, and accumulated impairments. Phys Ther. 2012;92:407–415. doi: 10.2522/ptj.20100380. [DOI] [PubMed] [Google Scholar]
- 5.Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis: a longitudinal study. Clin Rehabil. 2009;23:259–269. doi: 10.1177/0269215508095087. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo D, De Nuzzo C, Fascia T, Macalli M, Pisoni I, Cardini R. Risks of falls in subjects with multiple sclerosis. Arch Phys Med Rehabil. 2002;83:864–867. doi: 10.1053/apmr.2002.32825. [DOI] [PubMed] [Google Scholar]
- 7.Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84:906–918. [PubMed] [Google Scholar]
- 8.Shumway-Cook A, Baldwin M, Polissar NL, Gruber W. Predicting the probability for falls in community-dwelling older adults. Phys Ther. 1997;77:812–819. doi: 10.1093/ptj/77.8.812. [DOI] [PubMed] [Google Scholar]
- 9.Forsberg A, Andreasson M, Nilsagard YE. Validity of the dynamic gait index in people with multiple sclerosis. Phys Ther. 2013;93:1369–1376. doi: 10.2522/ptj.20120284. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo D, Regola A, Meotti M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil Rehabil. 2006;28:789–795. doi: 10.1080/09638280500404289. [DOI] [PubMed] [Google Scholar]
- 11.Cattaneo D, Jonsdottir J, Repetti S. Reliability of four scales on balance disorders in persons with multiple sclerosis. Disabil Rehabil. 2007;29:1920–1925. doi: 10.1080/09638280701191859. [DOI] [PubMed] [Google Scholar]
- 12.McConvey J, Bennett SE. Reliability of the Dynamic Gait Index in individuals with multiple sclerosis. Arch Phys Med Rehabil. 2005;86:130–133. doi: 10.1016/j.apmr.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Nilsagard Y, Kollen L, Axelsson H, Bjerlemo B, Forsberg A. Functional Gait Assessment: reliability and validity in people with peripheral vestibular disorders. Int J Ther Rehabil. 2014;21:367–373. [Google Scholar]
- 14.Yang Y, Wang Y, Zhou Y, Chen C, Xing D, Wang C. Validity of the Functional Gait Assessment in patients with Parkinson disease: construct, concurrent, and predictive validity. Phys Ther. 2014;94:392–400. doi: 10.2522/ptj.20130019. [DOI] [PubMed] [Google Scholar]
- 15.Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther. 2011;91:102–113. doi: 10.2522/ptj.20100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thieme H, Ritschel C, Zange C. Reliability and validity of the functional gait assessment (German version) in subacute stroke patients. Arch Phys Med Rehabil. 2009;90:1565–1570. doi: 10.1016/j.apmr.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Lin JH, Hsu MJ, Hsu HW, Wu HC, Hsieh CL. Psychometric comparisons of 3 functional ambulation measures for patients with stroke. Stroke. 2010;41:2021–2025. doi: 10.1161/STROKEAHA.110.589739. [DOI] [PubMed] [Google Scholar]
- 18.Wrisley DM, Kumar NA. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther. 2010;90:761–773. doi: 10.2522/ptj.20090069. [DOI] [PubMed] [Google Scholar]
- 19.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83:S7–S11. [PubMed] [Google Scholar]
- 20.Wagner JM, Norris RA, Van Dillen LR, Thomas FP, Naismith RT. Four Square Step Test in ambulant persons with multiple sclerosis: validity, reliability, and responsiveness. Int J Rehabil Res. 2013;36:253–259. doi: 10.1097/MRR.0b013e32835fd97f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 22.Moller AB, Bibby BM, Skjerbaek AG et al. Validity and variability of the 5-repetition sit-to-stand test in patients with multiple sclerosis. Disabil Rehabil. 2012;34:2251–2258. doi: 10.3109/09638288.2012.683479. [DOI] [PubMed] [Google Scholar]
- 23.Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-Item MS Walking Scale (MSWS-12) Neurology. 2003;60:31–36. doi: 10.1212/wnl.60.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124:962–973. doi: 10.1093/brain/124.5.962. [DOI] [PubMed] [Google Scholar]
- 25.Nilsagard YE, von Koch LK, Nilsson M, Forsberg AS. Balance exercise program reduced falls in people with multiple sclerosis: a single group pretest posttest trial. Arch Phys Med Rehabil. 2014;95:2428–2434. doi: 10.1016/j.apmr.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 28.Learmonth YC, Paul L, McFadyen AK, Mattison P, Miller L. Reliability and clinical significance of mobility and balance assessments in multiple sclerosis. Int J Rehabil Res. 2012;35:69–74. doi: 10.1097/MRR.0b013e328350b65f. [DOI] [PubMed] [Google Scholar]
- 29.Nilsagård Y, Gunnarsson LG, Denison E. Self-perceived limitations of gait in persons with multiple sclerosis. Adv Physiother. 2007;9:136–143. [Google Scholar]
- 30.Hobart J, Cano S, O'Connor R et al. Multiple Sclerosis Impact Scale-29 (MSIS-29): measurement stability across eight European countries. Mult Scler J. 2003;9(suppl 1):S23. [Google Scholar]
- 31.Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil. 2000;81:S15–S20. doi: 10.1053/apmr.2000.20619. [DOI] [PubMed] [Google Scholar]
- 32.Sim J, Jordan K, Lewis M, Hill J, Hay EM, Dziedzic K. Sensitivity to change and internal consistency of the Northwick Park Neck Pain Questionnaire and derivation of a minimal clinically important difference. Clin J Pain. 2006;22:820–826. doi: 10.1097/01.ajp.0000210937.58439.39. [DOI] [PubMed] [Google Scholar]
- 33.Downs S. The Berg Balance Scale. J Physiother. 2015;61:46. doi: 10.1016/j.jphys.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Geller AI, Strasser DC. Analytical review: focus on fall screening assessments. PM R. 2013;5:609–621. doi: 10.1016/j.pmrj.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Marchetti GF, Lin CC, Alghadir A, Whitney SL. Responsiveness and minimal detectable change of the dynamic gait index and functional gait index in persons with balance and vestibular disorders. J Neurol Phys Ther. 2014;38:119–124. doi: 10.1097/NPT.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 36.Beninato M, Fernandes A, Plummer LS. Minimal clinically important difference of the functional gait assessment in older adults. Phys Ther. 2014;94:1594–1603. doi: 10.2522/ptj.20130596. [DOI] [PubMed] [Google Scholar]


