Abstract
Background:
Fatigue, inactivity, and falls are major health issues for people with multiple sclerosis (MS). We examined the extent to which fatigue and low walking activity are associated with quality of life and increased fall risk in people with MS.
Methods:
People with MS (N = 210, aged 21–74 years) were categorized as having either high or low reported fatigue and walking activity levels and were then followed up for falls using monthly fall diaries for 6 months.
Results:
A high level of fatigue was significantly associated with higher MS Disease Steps scores, worse balance, high composite physiological (Physiological Profile Assessment) fall risk scores, greater fear of falling, lower World Health Organization Disability Assessment Schedule (WHODAS) quality of life scores, and more prospectively recorded falls. Low walking activity was significantly associated with higher MS Disease Steps scores, reduced proprioception, worse standing and leaning balance, slow stepping, slow gait speed, worse fine motor control, high Physiological Profile Assessment fall risk scores, more fear of falling, and lower WHODAS quality of life scores.
Conclusions:
Increased fatigue and low walking activity levels were significantly associated with increased fall risk and lower quality of life in people with MS. Interventions aimed at addressing fatigue and inactivity may have multiple benefits for this group.
Fatigue is one of the most common and disabling symptoms experienced by people with multiple sclerosis (MS).1 Fatigue affects approximately 75% of people with MS and heavily influences mental health, general health status, employment levels, and quality of life.2 People with MS are also less physically active than their peers without MS. This is evident from a recent meta-analysis that indicated a nearly 1 SD lower level of physical activity in people with MS compared with controls.3 However, evidence is indicating that physical activity improves or maintains walking mobility, muscle strength, fatigue, depression, cognition, and health-related quality of life.4
Falls represent a major mobility-related health issue for people with MS, as demonstrated by an epidemiologic study showing that approximately 60% of people with MS fall one or more times over 3 months.5 Recent studies have identified a range of neuropsychological, physical, health, and lifestyle risk factors for falls in people with MS.6,7 Although fatigue and physical activity levels have been extensively investigated in people with MS, few studies have examined relationships between fatigue and falls in this group. Some studies have documented that people with MS are likely to attribute their falls to fatigue.8,9 However, risk factor studies have produced inconsistent findings. Coote et al.7 found that Fatigue Severity Scale (FSS) scores were significantly increased in past fallers compared with past nonfallers. In contrast, Nilsagård et al.10 found no significant difference in FSS scores between those who did and did not fall in a 3-month follow-up period. Research in this area has also been limited by relatively small sample sizes, heterogeneity of patient populations, and a restricted range of important associated cognitive, psychological, physical functioning, and quality of life measures.
To address these issues, we conducted a large prospective cohort study using a comprehensive battery of validated measures to examine interrelationships among these important health and lifestyle factors. The main aims were 1) to document levels of fatigue and physical activity in a large community-living sample of people with MS and 2) to determine associations between fatigue, planned exercise, and walking activity and MS disease severity, cognitive functioning, functional ability, health-related quality of life, fear of falling, and falls in this group.
Methods
Participants
Participants (N = 210), recruited from among outpatients of an MS clinic in Sydney, Australia, were included if they were 18 years or older, had received a definite diagnosis of MS (any type) by a neurologist, and were able to stand unsupported for 30 seconds and walk 10 m with or without a mobility aid. The sole exclusion criterion was an inability to understand instructions relating to the study questionnaires and fall diaries due to impaired cognitive function or insufficient English. The study was approved by the Human Research Ethics Committee, University of New South Wales, Sydney, Australia. Participation was voluntary, and informed consent was obtained from all the participants before assessment.
Assessment Measures
The questionnaire and assessment measures used in this study encompassed health, sensorimotor, balance, and neuropsychological factors required for safe mobility. They were administered at one time point by trained therapists and took approximately 1.5 hours to complete.
Demographic, Health, and Disability Measures
Participants completed a structured questionnaire to provide information about age, sex, previous falls and fall injuries in the past year, number of years diagnosed as having MS, type of MS, and walking aids used. Participants' level of disability was assessed using the validated Disease Steps scale.11
Fatigue
Fatigue was assessed using the FSS,12 a commonly used self-report questionnaire for measuring fatigue in people with MS. Participants were asked to rate their level of agreement on nine fatigue-related statements, ranging from 1 (strongly disagree) to 7 (strongly agree). Participants were classified into two groups with respect to their fatigue levels: low fatigue (mean FSS item scores <5) and high fatigue (mean FSS item scores ≥5). This criterion was used because Valko et al.12 reported mean (SD) FSS item scores of 4.66 (1.64) in patients with MS, and Lerdal et al.13 indicated that FSS item scores greater than 4 are indicative of fatigue.
Planned Exercise and Walking Activity
Physical activity was assessed using items from the Incidental and Planned Exercise Questionnaire (IPEQ).14 This self-report questionnaire covers the frequency and duration of planned and incidental physical activities and has high reported test-retest repeatability (intraclass correlation coefficient = 0.84). The IPEQ consists of ten questions about physical activity pertaining to an average week in the past 3 months. Participants were classified into two groups with respect to their planned activity levels: taking part in no planned activities per week or taking part in 1 or more planned activities per week. Planned exercises were defined as physical exercises aimed at improving and maintaining balance, strength, flexibility, and coordination. Excluded activities were walking (included in a separate question), routine activities such as gardening and house cleaning, and seated activities such as bingo and piano playing. Participants were also classified with respect to walking activity: low walking activity (≤1 h/wk) or high walking activity (>1 h/wk).
Sensorimotor Function
Visual contrast sensitivity was assessed using the Melbourne Edge Test.15 Proprioception was measured with participants sitting using a lower-limb–matching task.15 Maximal isometric quadriceps strength was included as an outcome measure because it is a major lower-limb muscle group important for sit-to-stand, transfers, gait, and stair climbing. It was measured in both legs while participants were seated on a high chair with the hips and knees flexed to 90°. A strain gauge was fixed horizontally with straps on the lower shin, after which the participant was given a total of three attempts for each leg to push against the strap as forcefully as possible. The timed Nine-Hole Peg Test (NHPT)16 was administered to gain a measure of the extent of disease affecting complex upper-extremity function. Performance on the NHPT was examined by asking seated participants to pick up nine pegs from a shallow container one at a time as quickly as possible, place them in nine holes, and then remove them again as quickly as possible, one at a time, replacing them in the container. Simple Reaction Time was measured with a light as a stimulus and a finger press as the response.15
Balance
Postural sway was assessed using a sway meter with demonstrated high external validity and reliability.17 Testing was performed with participants standing on the floor and on a medium-density foam rubber mat (65 × 65 × 15 cm thick) with eyes open and closed. Controlled leaning balance was measured using two tests with demonstrated validity and reliability: the maximal balance range and coordinated stability tests.18 In these tests, the sway meter was attached anteriorly to the participant. In the maximal balance range test, participants were required to lean as far forward and as far back as possible without moving the feet or bending at the hips. The coordinated stability test required participants to adjust balance by leaning or rotating the body without moving the feet so that the pen followed and remained within the borders of a 1.5-cm-wide convoluted track marked on an A4 size paper sheet.
Stepping and Mobility
Stepping was assessed with a test of choice stepping reaction time.19 Participants stood on a nonslip black mat (0.8 × 1.2 cm) marked with four rectangular panels (32 × 13 cm), one in front of each foot and one to the side of each foot. Participants were instructed to step onto specific rectangle panels in sequence as quickly as possible. Mobility was assessed by walking speed over 10 m with and without a secondary cognitive task (counting backward by threes starting at 100). To allow for acceleration and deceleration, 2 m was provided at either end of a 10-m marked course. The secondary cognitive task was included because it has been established that such tasks significantly affect balance and gait in people with MS.20
Neuropsychological Assessment
Cognitive processing was assessed using the Trail Making Test (TMT),21 including Part A (TMT-A) testing simple attention and Part B (TMT-B) testing complex attention. In the TMT-A, participants were asked to draw lines connecting numbered circles in numerical order. The TMT-B included a similar task but the circles contained numbers and letters (eg, 1-A-2-B). Total time to complete each test was recorded. The difference between parts A and B (TMT-B-A) was calculated to remove the motor speed element from the test evaluation, leaving an estimate of executive function. This test was chosen because it has consistently been found to discriminate significantly between fallers and nonfallers in samples of older people and people with MS.6,22
Physiological Profile Assessment Fall Risk Score
The Physiological Profile Assessment (PPA) provides a fall risk index score composed of weighted values from five of the previously mentioned sensorimotor and balance measures: visual contrast, lower-limb proprioception, quadriceps strength, reaction time, and sway on the foam mat with eyes open. In studies of older people, PPA fall risk index scores discriminate between multiple and nonmultiple fallers with accuracies up to 75%, with scores less than 0 indicating a low risk of falling; 0 to 1, a moderate risk of falling; and 2 or greater, a high risk of falling.15
Quality of Life
Quality of life was measured using the cross-culturally validated 12-item version of the World Health Organization Disability Assessment Schedule (WHODAS) 2.0 questionnaire. The questionnaire captures an individual's level of functioning in six major life domains: cognition, mobility, self-care, getting along, life activities, and participation in society.23
Falls and Fear of Falling
A fall was defined as “unintentionally coming to the ground or some lower level and other than as a consequence of sustaining a violent blow, loss of consciousness, sudden onset of paralysis as in stroke, or an epileptic seizure.”24 Falls were monitored by using monthly postal fall diaries prospectively over 6 months, with monthly telephone follow-up if required. Fear of falling was examined with the Falls Efficacy Scale-International.25
Statistical Analysis
Data were analyzed using a statistical software program (IBM SPSS Statistics for Windows, version 22.0; IBM Corp, Armonk, NY). For continuously scaled variables with right-skewed distributions, log-transformations were used before analysis. Associations between the ordinally scaled Disease Steps measure and continuously scored variables were calculated using Spearman correlation coefficients. Independent t tests and Mann-Whitney U tests were used as appropriate to compare the neuropsychological, sensorimotor, balance, gait, quality of life, Disease Steps, and fall measures with dichotomized (high and low levels) measures of fatigue, planned exercise, and walking activity as the dependent variables. Complementary χ2 tests for contingency tables were also used to assess associations among the dichotomized measures of fatigue, planned exercise, and walking activity between these variables and faller group status. Additional variables, such as disease duration, disability levels, and treatments, were not included in the analyses as possible confounders because the underlying premise is to identify functional/physiological predictors,15 and the inclusion of medical conditions may result in overadjusting and dilution of important explanatory findings.26
Results
Participants
The mean (SD) age of participants was 50.8 (11.1) years, and 152 were women (72.4%). Table 1 shows the demographic and MS-specific disease characteristics of the sample.
Table 1.
Demographic and disease-specific characteristics of the 210 study participants
| Characteristic | Value |
|---|---|
| Age, mean (SD) [range], y | 50.8 (11.1) [21–74] |
| Sex, F/M, No. (%) | 152 (72.4)/58 (27.6) |
| Disease duration, mean (SD) [range], y | 9.9 (7.0) [0.2–31.1] |
| Disease type, No. (%) | |
| Primary progressive | 26 (12.4) |
| Relapsing-remitting | 121 (57.6) |
| Secondary progressive | 27 (12.9) |
| Unknowna | 36 (17.1) |
| Disease Steps score, No. (%) | |
| 0 | 28 (13.3) |
| 1 | 57 (27.1) |
| 2 | 25 (11.9) |
| 3 | 37 (17.6) |
| 4 | 37 (17.6) |
| 5 | 26 (12.4) |
| Mobility aids, No. (%) | |
| No aids | 108 (51.4) |
| Walking stick | 58 (27.6) |
| Frame or rollator | 18 (8.6) |
| Crutches | 7 (3.3) |
| Ankle brace | 5 (2.4) |
| Others (wheelchair, scooter, etc.) | 14 (6.7) |
| Disease-modifying therapy, No. (%) | |
| None | 73 (34.8) |
| Interferon beta-1a | 48 (22.9) |
| Interferon beta-1b | 21 (10.0) |
| Natalizumab | 21 (10.0) |
| Glatiramer acetate | 38 (18.1) |
| Not sure | 9 (4.3) |
| Antispasticity medication, No. (%) | |
| None | 165 (78.6) |
| Baclofen | 30 (14.3) |
| Valium | 6 (2.9) |
| Botulin toxin | 2 (1.0) |
| Not sure | 7 (3.3) |
| Falls and fall-related injuries in the past 12 mo | |
| Falls, mean (SD) [range], No. | 2.5 (2.1) [0–6] |
| ≥1 fall injuries, No. (%) | 71 (33.8) |
| ≥1 fall-related fractures, No. (%) | 34 (16.2) |
aParticipants did not report what type of multiple sclerosis they had.
Fatigue
The FFS was completed by 203 (96.7%) participants. Mean FSS scores were weakly but significantly associated with MS Disease Steps scores (Spearman rho = 0.164, P = .019). Overall, 126 participants (60.0%) reported a high level of fatigue (mean FSS item scores ≥5). Participants categorized as having a high level of fatigue ranged from 40.7% in those with a Disease Steps score of 0 to 73.1% in those with a Disease Steps score of 5 (Figure 1). Participants with a high level of fatigue were also significantly more likely to have a low level of walking activity (χ2 = 5.210, df = 1, P = .030) but not more likely to do no planned exercise (χ2 = 2.423, df = 1, P = .132).
Figure 1.
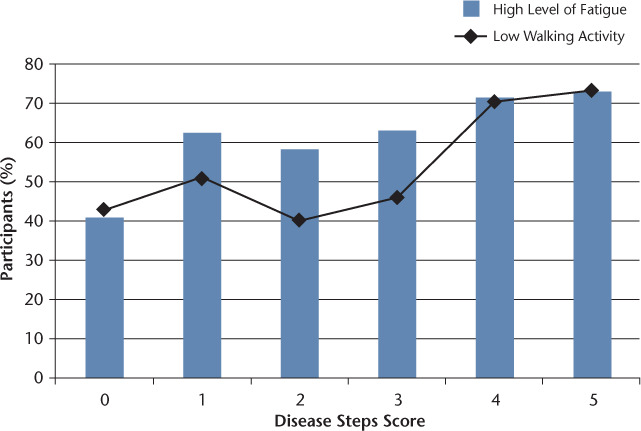
Combined bar and line graph of high levels of fatigue and low walking activity according to Disease Steps score
Table 2 shows the mean (SD) scores for the continuously scored test measures for people with mean FSS item scores above and below the cut-point score of ≥5. Participants with high levels of reported fatigue had significantly increased sway with eyes closed while standing on the floor, high PPA fall risk scores, greater concern about falling, and worse scores on the WHODAS 2.0. The Spearman correlation coefficient for the association between mean FSS item scores and the summed WHODAS 2.0 score was 0.57 (P < .001), and greater fatigue levels were significantly associated with worse function in all of the individual WHODAS 2.0 items (Spearman rho = 0.22–0.58, P < .01); relationships are presented in Supplementary Figure 1 (151.7KB, pdf) , which is published in the online version of this article at ijmsc.org.
Table 2.
Test scores for sensorimotor, balance, gait, neuropsychological function, and quality of life measures for participants categorized as having low or high fatigue, 0 or 1 or more planned exercise per week, and less than 1 or 1 or more hours of walking activity per week
| Measure | Fatigue | Planned exercise | Walking activity | |||
|---|---|---|---|---|---|---|
| FSS mean score <5 (n = 84) | FSS mean score ≥5 (n = 126) | 0/wk (n = 72) | ≥1/wk (n = 138) | <1 h/wk (n = 113) | ≥1 h/wk (n = 97) | |
| MS Disease Steps scorea | 2.0 (1.0–3.0) | 3.0 (1.0–4.0)b | 2.0 (1.0–3.8) | 2.0 (1.0–4.0) | 3.0 (1.0–4.0) | 2.0 (1.0–3.0)c |
| Visual contrast sensitivity, dB | 21.5 (1.7) | 21.9 (2.0) | 22.0 (1.9) | 21.7 (1.9) | 21.7 (2.0) | 21.7 (1.6) |
| Proprioception, degree error | 4.1 (2.9) | 3.9 (2.6) | 4.0 (2.4) | 4.0 (2.8) | 4.4 (3.1) | 3.5 (1.9)b |
| Quadriceps strength, kg force Sway, mm | 29.2 (11.8) | 29.0 (11.1) | 29.6 (12.9) | 29.0 (10.6) | 29.0 (11.6) | 29.6 (12.3) |
| Eyes open on floor | 136 (100) | 180 (144) | 173 (147) | 157 (121) | 168 (116) | 160 (150) |
| Eyes closed on floor | 241 (204) | 329 (249)c | 343 (271) | 270 (211) | 316 (228) | 277 (244)b |
| Eyes open on foam | 302 (214) | 359 (245) | 350 (252) | 329 (226) | 380 (239) | 295 (228)c |
| Eyes closed on foam | 663 (390) | 686 (378) | 649 (412) | 690 (367) | 760 (385) | 588 (360)c |
| Coordinated stability, error score | 21.7 (20.8) | 21.7 (18.0) | 20.6 (17.3) | 22.1 (20.0) | 25.1 (18.7) | 18.3 (19.2)b |
| Choice stepping reaction time, s | 36.3 (19.0) | 41.0 (19.7) | 38.1 (20.0) | 39.7 (19.3) | 44.3 (20.8) | 33.8 (16.6)c |
| 10 m gait time, s | 12.6 (11.8) | 13.2 (9.0) | 12.7 (10.6) | 13.0 (9.9) | 15.0 (11.3) | 10.5 (7.7)c |
| 10 m dual-task gait time, s | 17.2 (17.5) | 18.6 (13.8) | 17.9 (14.9) | 18.1 (15.5) | 20.9 (16.1) | 14.6 (13.3)c |
| NHPT, s | 28.4 (11.2) | 28.5 (9.6) | 28.6 (11.8) | 28.4 (9.3) | 30.5 (12.7) | 26.5 (6.9)c |
| TMT-B-A, s | 54.2 (32.5) | 66.8 (50.8) | 69.3 (52.5) | 58.0 (39.9) | 70.4 (53.7) | 53.5 (32.7) |
| PPA fall risk (z) score | 1.84 (1.38) | 2.27 (1.55)b | 2.10 (1.61) | 2.10 (1.45) | 2.39 (1.51) | 1.78 (1.45)c |
| FES-I score | 29.5 (9.0) | 38.3 (11.4)c | 35.8 (11.6) | 34.5 (11.3) | 37.5 (11.8) | 32.2 (10.4)c |
| WHODAS 2.0 score | 22.6 (6.6) | 31.8 (8.3)c | 30.0 (10.1) | 27.3 (8.1)b | 30.4 (8.8) | 25.9 (8.4)c |
Abbreviations: FES-I, Falls Efficacy Scale-International; MS, multiple sclerosis; NHPT, Nine-Hole Peg Test; PPA, Physiological Profile Assessment; TMT, Trail Making Test; WHODAS, World Health Organization Disability Assessment Schedule.
Note: Data are given as mean (SD) except where indicated otherwise.
aMedian (interquartile range) with comparisons assessed by Mann-Whitney U tests. All other comparisons assessed with group t tests.
bSignificant at P < .05.
cSignificant at P < .01.
Complete fall follow-up was achieved with the system of diaries and follow-up telephone calls. One hundred twenty-six participants (60%) reported at least one fall in the 6-month follow-up period. Of those who fell, 32 (25%) fell one time, 25 (20%) fell twice, and 69 (55%) fell three times or more. The median fatigue score for fallers was 5.8 (interquartile range = 4.3–6.8), which was significantly higher than that for nonfallers, that is, 5.1 (interquartile range = 4.0–6.1) (Mann Whitney U = 4114.50, z = 2.06, P = .039). There were also significant associations between individual FSS items and faller status when dichotomized into yes-no variables. Forty of the 82 nonfallers with FFS data (48.8%) reported that exercise brings on fatigue compared with 83 of the 121 fallers with FFS data (68.6%) (χ2 = 8.04, df = 1, P = .005). Similarly, 48 nonfallers (58.5%) reported that fatigue prevents sustained physical functioning compared with 91 fallers (75.2%) (χ2 = 6.29, df = 1, P = .014).
Planned Exercise and Walking Activity
Walking activity levels were significantly associated with MS Disease Steps levels (Spearman rho = .225, P = .008). Seventy-two participants (34.3%) did not participate in any planned exercise, and 113 (53.8%) had low walking activity (≤1 h/wk). The percentage of participants with walking activity of 1 h/wk or less ranged from 42.9% in participants with a Disease Steps score of 0 to 73.1% in participants with a Disease Steps score of 5 (Figure 1).
Table 2 shows the mean (SD) scores for the continuously scored test measures for people who did nil or one or more planned exercise activities and who had low or high walking activity. Planned exercise as a dichotomized variable was significantly associated with only one measure: summed WHODAS 2.0 scores. In contrast, low walking activity as a dichotomized variable was significantly associated with several measures: worse proprioception, worse balance as indicated by three of the four sway tests and the coordinated stability test, slow choice stepping reaction times, slow walking speed (with and without the conduct of a secondary cognitive task), worse fine motor control, high PPA fall risk scores, worse scores on the WHODAS 2.0, and greater concern about falling.
No significant differences between the activity measures and falls were evident: 46 of the 122 fallers with planned exercise data (37.7%) and 26 of the 82 nonfallers with planned exercise data (31.7%) reported no planned exercise (χ2 = 0.77, df = 1, P = .46), and 63 of the 126 fallers with walking activity data (50.0%) and 50 of 83 nonfallers with walking activity data (60.2%) reported low levels of walking activity (χ2 = 0.15, df = 1, P = .16).
Discussion
The study findings revealed that people with MS with high fatigue levels performed significantly worse on tests of balance and had high composite physiological (PPA) fall risk scores, greater fear of falling, lower WHODAS 2.0 quality of life scores, and more falls in the 6-month period after assessment than people with MS with low fatigue levels. These findings demonstrate the significant and broad range of health and lifestyle factors associated with fatigue, including MS Disease Steps and reduced walking activity, and build on previous studies that have found that people with MS identify fatigue as a contributing factor to their falls8,9 and reduced quality of life.27,28
People with MS rate walking as one of the most important bodily functions and state that mobility is the most valued activity of daily living.29 We examined two measures of physical activity from the IPEQ: planned exercise and total walking activity; of these, walking activity was more strongly associated with physical performance, functional activity, and fall efficacy. Low walking activity (<1 h/wk) was significantly associated with reduced proprioception, worse standing and leaning balance, slow stepping, slow gait speed, worse fine motor control, high PPA fall risk scores, high levels of fear of falling, and worse WHODAS 2.0 quality of life scores, whereas no planned exercise was associated with only reduced total WHODAS 2.0 quality of life scores. Self-reported walking activity over a 1-week period seems to be a simple and appropriate marker of disability, Disease Steps score, and disease progression.30 In contrast, it seems that the construct of planned exercise as used in the present questionnaire was too broad and ill-defined, leading to study participants interpreting it in different ways and rendering it less useful as a screening tool. This construct may be more accurately measured with questionnaires or interview items that more tightly specify physical activities.
Not unexpectedly, participants with higher fatigue levels were also more likely to have low activity levels. This finding is consistent with a report of a survey of 417 individuals with MS that identified fatigue, along with impairment and lack of time, as among the top three barriers to exercise.31 Several nonsignificant findings warrant discussion. Higher levels of fatigue were not significantly associated with reduced quadriceps strength, slow stepping, slower gait speed (with or without a secondary cognitive task), poor NHPT coordination, and reduced executive function assessed using the TMT. Previous studies have found that fatigue is associated with impaired cognition and less functional cerebral activation in several regions involved in motor planning and execution.32,33 It may be that the present participants performed the tests in an unfatigued state, minimizing any variance on this measure, and that significant associations may become more readily apparent after participants undertake a fatiguing activity. Previous studies have also documented that reduced muscle strength is significantly associated with low levels of physical activity in people with MS,4 but such an association was not apparent herein. It is possible that the isometric measure of strength did not sufficiently represent functional strength or that Disease Steps and the wide age range (21–73 years) may have confounded the association. The inclusion of a measure of knee flexion strength may also have been instructive because knee extension strength often remains greater than flexion strength, even at an advanced stage of walking disability, in part due to spasticity.
We also acknowledge other limitations. We used questionnaires to measure fatigue and physical activity because this is the most practical method for gaining this information in studies requiring large sample sizes.34 These self-report assessments have advantages in that they have demonstrated validity12,13 and reflect participants' symptom descriptions12 and activity types, but self-report assessments also have disadvantages regarding subjectivity and recall bias. Future studies could use simple clinical fatigue routines, such as balance assessment before and after a 6-minute walk35 and remote activity monitoring using accelerometers, to assess physical activity over extended periods.36 Second, cognitive status was based on the clinical judgment of the assessing physiotherapist, and no formal cognitive screen was administered. However, because no participants had difficulties completing the TMTs or following other test instructions, it is evident that the sample was without significant cognitive impairment. Third, we did not include variables such as disease duration, disability levels, or disease type in the analyses owing to the functional/physiological approach used. Further studies could include these variables as covariates or in mediational analyses to further elucidate fall risk in people with MS. Finally, the inclusion of other signs and symptoms, such as joint contractures and spasticity, depression, education, and social status, may have revealed additional information regarding physical activity and fall risk in people with MS.
In conclusion, the study findings indicate that increased fatigue and low walking activity levels are significantly associated with increased fall risk and lower quality of life in people with MS. High fatigue levels were additionally associated with prospectively measured falls. Interventions aimed at addressing fatigue and inactivity may have multiple benefits for this group.
PracticePoints
Fatigue, reduced physical activity, and falls are major health problems for people with MS.
Increased fatigue and low walking activity levels are significantly associated with increased fall risk and lower quality of life in people with MS.
Interventions addressing fatigue and inactivity may have multiple benefits for this group.
Supplementary Material
Acknowledgments
We thank Multiple Sclerosis Limited and its physiotherapists for help with recruitment of participants and Ms. Connie Severino for assistance with establishing and populating the database for this study.
Footnotes
Financial Disclosures: The PPA (FallScreen) is commercially available through Neuroscience Research Australia (NeuRA), Sydney, Australia.
Funding/Support: This study was supported by Multiple Sclerosis Research Australia and the National Health and Medical Research Council (of Australia). Ms. Vister and Ms. Tijsma received scholarships from the Foundation of MS Research Nederland–Stichting MS Research (grant S15-2a/b).
References
- 1.Bakshi R. Fatigue associated with multiple sclerosis: diagnosis, impact and management. Mult Scler. 2003;9:219–227. doi: 10.1191/1352458503ms904oa. [DOI] [PubMed] [Google Scholar]
- 2.Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler. 2001;7:340–344. doi: 10.1177/135245850100700511. [DOI] [PubMed] [Google Scholar]
- 3.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11:459–463. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 4.Motl RW, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nat Rev Neurol. 2012;8:487–497. doi: 10.1038/nrneurol.2012.136. [DOI] [PubMed] [Google Scholar]
- 5.Nilsagard Y, Gunn H, Freeman J et al. Falls in people with MS: an individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult Scler. 2015;21:92–100. doi: 10.1177/1352458514538884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoang PD, Cameron MH, Gandevia SC, Lord SR. Neuropsychological, balance, and mobility risk factors for falls in people with multiple sclerosis: a prospective cohort study. Arch Phys Med Rehabil. 2014;95:480–486. doi: 10.1016/j.apmr.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Coote S, Hogan N, Franklin S. Falls in people with multiple sclerosis who use a walking aid: prevalence, factors, and effect of strength and balance interventions. Arch Phys Med Rehabil. 2013;94:616–621. doi: 10.1016/j.apmr.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Nilsagard Y, Lundholm C, Denison C, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis: a longitudinal study. Clin Rehabil. 2009;23:259–269. doi: 10.1177/0269215508095087. [DOI] [PubMed] [Google Scholar]
- 9.Gunn H, Creanor S, Haas B, Marsden J, Freeman J. Frequency, characteristics, and consequences of falls in multiple sclerosis: findings from a cohort study. Arch Phys Med Rehabil. 2014;95:538–545. doi: 10.1016/j.apmr.2013.08.244. [DOI] [PubMed] [Google Scholar]
- 10.Nilsagård Y, Denison E, Gunnarsson LG, Boström K. Factors perceived as being related to accidental falls by persons with multiple sclerosis. Disabil Rehabil. 2009;31:1301–1310. doi: 10.1080/09638280802532639. [DOI] [PubMed] [Google Scholar]
- 11.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology. 1995;45:251–255. doi: 10.1212/wnl.45.2.251. [DOI] [PubMed] [Google Scholar]
- 12.Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31:1601–1607. doi: 10.1093/sleep/31.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lerdal A, Wahl A, Rustoen T, Hanestad BR, Moum T. Fatigue in the general population: a translation and test of the psychometric properties of the Norwegian version of the fatigue severity scale. Scand J Public Health. 2005;33:123–130. doi: 10.1080/14034940410028406. [DOI] [PubMed] [Google Scholar]
- 14.Delbaere K, Hauer K, Lord SR. Evaluation of the Incidental and Planned Activity Questionnaire (IPEQ) for older people. Br J Sports Med. 2010;44:1029–1034. doi: 10.1136/bjsm.2009.060350. [DOI] [PubMed] [Google Scholar]
- 15.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83:237–252. [PubMed] [Google Scholar]
- 16.Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am J Occup Ther. 2003;57:570–573. doi: 10.5014/ajot.57.5.570. [DOI] [PubMed] [Google Scholar]
- 17.Sturnieks DL, Arnold R, Lord SR. Validity and reliability of the Swaymeter device for measuring postural sway. BMC Geriatr. 2011;11:63. doi: 10.1186/1471-2318-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lord SR, Ward JA, Williams P. Exercise effect on dynamic stability in older women: a randomized controlled trial. Arch Phys Med Rehabil. 1996;77:232–236. doi: 10.1016/s0003-9993(96)90103-3. [DOI] [PubMed] [Google Scholar]
- 19.Delbaere K, Gschwind YJ, Sherrington C, Barraclough E, Garrués-Irisarri MA, Lord SR. Clin Rehabil. Validity and reliability of a simple “low-tech” test for measuring choice stepping reaction time in older people [published online October 27, 2015] doi:10.1177/0269215515613422. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton F, Rochester L, Paul L, Rafferty D, O'Leary CP, Evans JJ. Walking and talking: an investigation of cognitive-motor dual tasking in multiple sclerosis. Mult Scler J. 2009;15:1215–1227. doi: 10.1177/1352458509106712. [DOI] [PubMed] [Google Scholar]
- 21.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 22.Delbaere K, Kochan NA, Close JC et al. Mild cognitive impairment as a predictor of falls in community-dwelling older people. Am J Geriatr Psychiatry. 2012;20:845–853. doi: 10.1097/JGP.0b013e31824afbc4. [DOI] [PubMed] [Google Scholar]
- 23.Ustun TB, Chatterji S, Kostanjsek N et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ. 2010;88:815–823. doi: 10.2471/BLT.09.067231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gisbon MJ, Andres RO, Isaacs B et al. The prevention of falls in later life: a report of the Kellogg International Work Group on the Prevention of Falls by the Elderly. Dan Med Bull. 1987;34(suppl 4):1–24. [PubMed] [Google Scholar]
- 25.Van Vliet R, Hoang P, Lord S, Gandevia S, Delbaere K. Falls efficacy scale-international: a cross-sectional validation in people with multiple sclerosis. Arch Phys Med Rehabil. 2013;94:883–889. doi: 10.1016/j.apmr.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Breslow N. Design and analysis of case-control studies. Ann Rev Public Health. 1982;3:29–54. doi: 10.1146/annurev.pu.03.050182.000333. [DOI] [PubMed] [Google Scholar]
- 27.Flensner G, Landtblom AM, Soderhamn O, Ek AC. Work capacity and health-related quality of life among individuals with multiple sclerosis reduced by fatigue: a cross-sectional study. BMC Public Health. 2013;13:224. doi: 10.1186/1471-2458-13-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smedal T, Beiske AG, Glad SB et al. Fatigue in multiple sclerosis: associations with health-related quality of life and physical performance. Eur J Neurol. 2011;18:114–120. doi: 10.1111/j.1468-1331.2010.03090.x. [DOI] [PubMed] [Google Scholar]
- 29.Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14:988–991. doi: 10.1177/1352458508088916. [DOI] [PubMed] [Google Scholar]
- 30.Pearson OR, Busse ME, van Deursen RW, Wiles CM. Quantification of walking mobility in neurological disorders. QJM. 2004;97:463–475. doi: 10.1093/qjmed/hch084. [DOI] [PubMed] [Google Scholar]
- 31.Asano M, Duquette P, Andersen R, Lapierre Y, Mayo NE. Exercise barriers and preferences among women and men with multiple sclerosis. Disabil Rehabil. 2013;35:353–361. doi: 10.3109/09638288.2012.742574. [DOI] [PubMed] [Google Scholar]
- 32.Krupp LB, Elkins LE. Fatigue and declines in cognitive functioning in multiple sclerosis. Neurology. 2000;55:934–939. doi: 10.1212/wnl.55.7.934. [DOI] [PubMed] [Google Scholar]
- 33.Andreasen AK, Jakobsen J, Petersen T, Andersen H. Fatigued patients with multiple sclerosis have impaired central muscle activation. Mult Scler. 2009;15:818–827. doi: 10.1177/1352458509105383. [DOI] [PubMed] [Google Scholar]
- 34.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLoughlin JV, Barr CJ, Crotty M, Sturnieks DL, Lord SR. Six minutes of walking leads to reduced lower limb strength and increased postural sway in people with multiple sclerosis. NeuroRehabil. 2014;35:503–508. doi: 10.3233/NRE-141143. [DOI] [PubMed] [Google Scholar]
- 36.Brodie MA, Lord SR, Coppens MJ, Annegarn J, Delbaere K. Eight-week remote monitoring using a freely worn device reveals unstable gait patterns in older fallers. IEEE Trans Biomed Eng. 2015;62:2588–2594. doi: 10.1109/TBME.2015.2433935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


