Abstract
Background:
Delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF) is indicated for the treatment of relapsing multiple sclerosis. Flushing and gastrointestinal (GI) adverse events (AEs) are common within the first few months of starting DMF therapy. Although most symptoms are mild or moderate in severity, transient, and infrequently result in treatment discontinuation, they nevertheless present a challenge for patients to adhere to therapy and achieve an optimal treatment response.
Methods:
This review discusses management strategies for the prophylaxis and treatment of common DMF-associated AEs based on clinical trial evidence and real-world experience in clinical practice settings.
Results:
Before starting DMF therapy, patients should receive counseling on the importance of treatment adherence and the likely occurrence and severity of flushing and GI AEs (nausea, vomiting, diarrhea, and abdominal pain). Management strategies, such as administering DMF with food, using a slower-dose titration schedule, applying temporary dose reductions, and using symptomatic therapies, provide clinicians with several approaches to address DMF tolerability. In particular, DMF coadministration with certain foods (eg, sausage, peanut butter) may prevent or reduce the severity of GI AEs. Taking aspirin 325 mg/day 30 minutes before administering DMF in the first month of therapy can reduce the incidence and severity of flushing without negatively affecting GI-related events.
Conclusions:
Through continual patient education and support and management of treatment-related flushing and GI AEs, clinicians can help patients adhere to and persist with DMF therapy, thus maximizing treatment benefit.
The natural history of multiple sclerosis (MS) is characterized by a variable clinical course punctuated by clinical relapses and remissions and chronic progression, resulting in accumulation of disability and reduction in quality of life.1 Moreover, patients with MS also are burdened by a high level of comorbidity.2 Treatment-emergent adverse events (AEs) have led to nonadherence with some injectable disease-modifying agents in this population and, ultimately, suboptimal treatment outcomes.3,4 Therefore, it is important that new treatment options for managing MS are not limited in their effectiveness by AEs.
Delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF) is an orally available disease-modifying agent approved in the United States for the treatment of relapsing forms of MS and in the European Union as a first-line treatment for relapsing-remitting MS (RRMS).5,6 These regulatory approvals were based on placebo-controlled data from the pivotal phase 3 DEFINE (A Randomized, Multicenter Double-Blind, Placebo-Controlled, Dose-Comparison Study to Determine the Efficacy and Safety of BG000012 in Subjects with Relapsing-Remitting Multiple Sclerosis) and CONFIRM (A Randomized, Multicenter, Placebo-Controlled and Active Referenced [Glatiramer Acetate] Comparison Study to Evaluate the Efficacy and Safety of BG00012 in Subjects with Relapsing-Remitting Multiple Sclerosis) clinical studies in patients with RRMS that demonstrated the efficacy of DMF in preventing relapses in addition to a favorable AE profile.7,8 During treatment with DMF in the clinical trial and real-world settings, patients commonly experienced transient mild-to-moderate flushing and gastrointestinal (GI) AEs, particularly nausea, vomiting, abdominal pain, and diarrhea, especially in the first month of therapy.7–10 These AEs associated with DMF can be particularly bothersome for some patients, with the potential to compromise patient acceptance of and adherence to treatment.
The discussion undertaken in this review will help clinicians take advantage of therapy with DMF by providing generalized and tailored strategies that minimize these common AEs.
To review studies reporting on DMF clinical use, we conducted a search of the US National Library of Medicine National Institutes of Health MEDLINE database for full articles using the following Boolean search term strategy: dimethyl fumarate AND multiple sclerosis limited by words in the title and abstract. Additional references were identified from the bibliographies of published articles and posters presented at preeminent neurology congresses.
DMF for MS
Efficacy
Prespecified integrated analyses of the randomized double-blind DEFINE8 and CONFIRM7 studies revealed that at 2 years, DMF 240 mg twice a day significantly reduced the annualized relapse rate and risk of relapse and improved a range of secondary clinical, magnetic resonance imaging, and patient-reported outcomes versus placebo (Table 1).11,12 Importantly, these benefits of DMF therapy were observed consistently across patient subgroups, including treatment-naive patients and switchers from interferon beta therapy (Table 1).11,13,14 The clinical and magnetic resonance imaging efficacy of DMF was maintained over 5 years in the ENDORSE (A Dose-Blind, Multicenter, Extension Study to Determine the Long-Term Safety and Efficacy of Two Doses of BG00012 Monotherapy in Subjects with Relapsing-Remitting Multiple Sclerosis) study, an ongoing, 8-year extension study of DEFINE and CONFIRM.15
Table 1.
Clinical efficacy of DMF 240 mg twice a day in prespecified integrated analyses of DEFINE and CONFIRM11 and post hoc subgroup analyses13,14
| Parameter | Entire population | Treatment-naive patients | Previous interferon beta treatment | |||
|---|---|---|---|---|---|---|
| Placebo | DMF | Placebo | DMF | Placebo | DMF | |
| Clinical efficacy at 2 y | ||||||
| Total population, No. | 771 | 769 | 223 | 221 | 140 | 148 |
| Annualized relapse rate | 0.37 | 0.19 | 0.38 | 0.17 | 0.45 | 0.26 |
| Relative reduction vs. placebo, % | — | 48 (P < .0001) | — | 56 (P< .0001) | — | 43 (P = .0021) |
| Risk of relapse, % | 44 | 28 | 42 | 21 | — | — |
| Relative reduction vs. placebo, % | — | 43 (P < .0001) | — | 54 (P < .0001) | — | — |
| Risk of 12-wk confirmed disability progression, % | 22 | 15 | 23 | 7 | — | — |
| Relative reduction vs. placebo, % | — | 32 (P = .0034) | — | 71 (P <.0001) | — | — |
| Neuroradiologic efficacy at 2 y | ||||||
| Total population, No. | 309 | 299 | 100 | 99 | 62 | 63 |
| New or enlarging T2 hyperintense lesions, No. | 16.8 | 3.7 | 20.0 | 4.0 | 18.2 | 3.12 |
| Mean lesion ratio | — | 0.22 (P < .0001) | — | 80% (P < .0001) | — | 83% (P < .0001) |
| Gd+ lesions, No. | 1.9 | 0.4 | 1.9 | 0.3 | 2.4 | 0.2 |
| Odds ratio | — | 0.17 (P <.0001) | — | 92% (P < .0001) | — | 85% (P =.0005) |
| New T1 hypointense lesions, No. | 6.3 | 2.2 | 6.6 | 2.1 | 6.56 | 1.72 |
| Mean lesion ratio | — | 0.35 (P < .0001) | — | 68% (P < .0001) | — | 74% (P <.0001) |
Abbreviations: DMF, delayed-release dimethyl fumarate (also known as gastroresistant DMF); Gd+, gadolinium-enhancing.
Head-to-head comparative studies of disease-modifying therapies in RRMS are limited. Glatiramer acetate was included as a reference comparator in CONFIRM. Although not powered to directly compare the efficacy of DMF versus glatiramer acetate, a post hoc analysis of the CONFIRM data indicated that DMF 240 mg twice a day showed a statistically significantly reduced risk of inflammatory disease activity versus glatiramer acetate.7,16
General Safety
DEFINE and CONFIRM demonstrated that DMF 240 mg twice a day has a favorable risk/benefit profile in RRMS, which was confirmed by the long-term ENDORSE extension study; data from an interim analysis of this study have been published, with a minimum of 5-year follow-up data in patients initially randomized to receive DMF (treatment duration ≤7 years in some patients).7,8,15 The most frequently occurring AEs in DEFINE and CONFIRM (incidence ≥2% higher than with placebo) were flushing, abdominal pain, diarrhea, and nausea.7,8
In a recently published integrated analysis of phase 2b/3/long-term extension studies of DMF in MS (N = 2470), mean absolute lymphocyte counts (ALCs) decreased by approximately 30% during the first year of treatment, then plateaued, remaining higher than the lower limit of normal (0.91 × 109/L) thereafter.17 Of the 2099 patients treated for at least 6 months, 47 (2.2%) had ALCs less than 0.5 × 109/L persisting for at least 6 months.17 Mean ALCs remained greater than or equal to the lower limit of normal in 84% and 76% of patients during the first 6 and 12 months, respectively; of these patients, 0.1% and 0% later developed ALCs less than 0.5 × 109/L persisting for at least 6 months.17
In the ENDORSE ongoing safety extension study, one case has been reported to date of progressive multifocal leukoencephalopathy (PML), an opportunistic infection, in a patient treated with DMF 240 mg three times daily in the setting of prolonged (≥6 months) severe lymphopenia (approximately <0.5 × 109/L of 3.5 years' duration).15 In addition, rare cases of PML also occurred in the postmarketing setting in the presence of prolonged moderate-to-severe lymphopenia. Aside from PML in the setting of prolonged (≥6 months) moderate-to-severe lymphopenia, there is no overall increased risk of serious infections, including other opportunistic infections.15,17
The incidence of alanine aminotransferase elevations of at least 3× the upper limit of normal (ULN) was higher in the DMF versus placebo groups in DEFINE (6% vs. 3%, respectively) but was not observed in CONFIRM, in which incidences of alanine and aspartate aminotransferase levels of at least 3× ULN were comparable between the placebo and DMF treatment arms.7,8 Consistent findings have been observed in ENDORSE; hepatic AEs occurred in 3% or less of patients in any treatment group, and 3% or less of patients treated with DMF for a minimum of 5 years had alanine aminotransferase or aspartate aminotransferase levels of at least 3× ULN; no case fulfilled Hy's law criteria for drug-induced liver injury.15
Limited pregnancy outcomes from clinical trials and postmarketing data suggest no increased risk of adverse pregnancy outcomes during the first trimester. However, DMF should be used during pregnancy only if the benefits outweigh the potential risks.18
GI Tolerability Profile
Delayed-release dimethyl fumarate has a well-characterized GI AE profile in the treatment of RRMS based on its use in DEFINE8 and CONFIRM7 as well as in MANAGE (Multicenter, Open-Label, Single-Arm Study of Gastrointestinal Tolerability in Patients with Relapsing Forms of Multiple Sclerosis Receiving Tecfidera™ [Dimethyl Fumarate] Delayed-release Capsules), a multicenter, open-label, single-arm, phase 4 study designed to explore the tolerability of DMF by evaluating GI-related events in patients with RRMS in clinical practice in the United States.9 In both settings, DMF treatment-emergent GI AEs were generally mild or moderate in severity, occurred most frequently in the first month of treatment before decreasing thereafter, had a variable duration, and were manageable with standard medical interventions.9,10
In DEFINE and CONFIRM, the mean duration of exposure to study treatment was 77 weeks in both treatment groups, and a comparable proportion of patients in the DMF (70%) and placebo (65%) groups completed 2 years of the study.10 Except during the first month of treatment, the incidence of GI AEs was low for the duration of the study period.10 Abdominal pain, upper abdominal pain, nausea, vomiting, and diarrhea all occurred at a higher incidence in the DMF group than in the placebo group during the 2-year follow-up study periods and during the first 3 months of the study (Supplementary Table 1 (179.6KB, pdf) , which is published in the online version of this article at ijmsc.org). Most GI AEs (>90%) were of mild or moderate severity and resolved during the study. The median duration of resolved events was less than 10 days for all common GI AEs, although ascertainment of time to resolution was not reported rigorously. A higher proportion of patients in the DMF group than in the placebo group received symptomatic therapy for GI symptoms during months 0 to 3 (11% vs. 4%, respectively). Importantly, in the DMF group, most resolved events of abdominal pain or upper abdominal pain, nausea or vomiting, and diarrhea did not require use of a symptomatic treatment or any change to the DMF dosage regimen (Figure 1). Common GI AEs were temporally associated with the use of diverse symptomatic therapies, most of which fell into the following therapeutic categories: proton pump inhibitors (for abdominal pain or upper abdominal pain), gastroprokinetics (for nausea or vomiting), and antidiarrheal agents.
Figure 1.
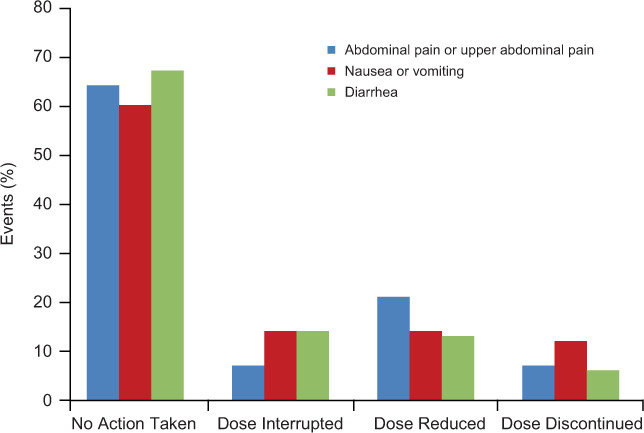
Action taken by prescribers on delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF) treatment in the resolution of specific gastrointestinal adverse events10
MANAGE followed 233 patients with RRMS who took DMF for 12 weeks or less (202 patients completed the study) and specifically recorded information regarding GI AEs using an eDiary and numerical rating scales to give a more detailed account of DMF tolerability than in the pivotal studies.9 Overall, the results were similar to those seen in the DEFINE and CONFIRM integrated analysis of safety in that the incidence of GI AEs was highest in the first month of treatment before declining thereafter, and they were transient in nature and mild to moderate in severity. Most patients (88%) experienced a GI AE, with a higher incidence of acute lower versus upper GI AEs. Four patients (1.7%) experienced at least one GI-related serious AE, including diarrhea, abdominal pain upper, abdominal pain lower, nausea, salivary gland calculus, and vomiting. Seventeen patients (7.3%) discontinued treatment due to GI-related AEs. The incidence of GI-related events was comparable in patients with or without a history of GI abnormalities, who did or did not use alcohol, and who did or did not use tobacco. More than half (54%) of the patients who reported GI AEs used symptomatic therapy, and these patients tended to have a higher incidence of and more severe GI AEs than nonusers of symptomatic therapy.9 Patients in MANAGE tended to use the same symptomatic therapies as those used in DEFINE and CONFIRM.9,10 Except for constipation (duration, 16 hours), all GI AEs had a duration of less than 10 hours.9 The duration of GI AEs was similar among users and nonusers of symptomatic therapy.
Flushing Events
Similar to the GI AE profile of DMF, integrated DEFINE and CONFIRM data indicated that the incidence of flushing events was highest during the first 3 months of treatment (and particularly so in the first month), most were mild or moderate in severity, and they infrequently led to discontinuation of study treatment (Supplementary Table 1 (179.6KB, pdf) ).10 Very few patients in either treatment arm used symptomatic therapies, such as diphenhydramine hydrochloride, loratadine, and acetylsalicylic acid, for flushing events.
Practice Management Strategies for Common AEs
A survey of an international panel of clinicians was completed by trialists from DEFINE and CONFIRM to provide guidance on the management of flushing and GI AEs.19 For GI AEs, this process formed the basis of the first of two Delphi consultations, the second Delphi consultation being directed toward experienced clinicians who prescribe DMF regularly in a real-world setting.20 The Delphi technique uses a questionnaire to obtain data and test hypotheses relating to a particular issue or issues. Subsequent rounds of questions can be used to refine the results, and it is a widely accepted method for gaining consensus from an expert panel. Using the Delphi technique enabled consensus on the most effective strategies to manage the common GI AEs of nausea, vomiting, abdominal pain, and diarrhea, and so reduce discontinuation rates of DMF.20 The results corroborated the safety findings associated with DMF in DEFINE, CONFIRM, and MANAGE,20 and, importantly, the merits of many of the strategies identified by the Delphi consensus-building method have been supported by findings from retrospective and prospective clinical studies. Taken together, these insights provide clinicians with the best known approaches to managing DMF tolerability in patients with MS and, therefore, set appropriate expectations for patients.
Education and Expectation Setting
Patient expectations can be managed more effectively by providing information on MS signs and symptoms, health-related quality of life benefits, and the potential occurrence and likely effect of flushing and GI AEs.19,20 Facilitating an open dialogue with patients is consistent with 2009 guidelines emanating from a systematic review of all disease-modifying agents for MS that highlighted the importance of concordance to improve patient-centered care.21
Individualized patient counseling was shown to improve adherence by efficient management of avoidable AEs in patients with MS who received DMF over 12 months. The incidence of therapy dropout among patients who undertook the counseling program was 11% for coached patients, which compares favorably with the 23% dropout incidence in the uncoached cohort.22 Discontinuation of DMF therapy occurred most often because of GI AEs in the first 3 months after treatment initiation,22 underscoring the importance of coaching calls (eg, suggesting a change in meal composition in favor of dairy products) that have the capacity to resolve most such events.23
It may be helpful to inform patients that the results of a prospective observational study that assessed patient-reported endpoints via clinician-administered assessments indicated that DMF was at least as well tolerated as previous therapies for MS in 81% of patients at week 1 and 67% of patients at week 4.24 Adherence to DMF treatment was high in this study: 89% and 76% of patients reported no missed doses by weeks 1 and 4, respectively.24 At weeks 1 and 4, on the Treatment Satisfaction Questionnaire for Medication, satisfaction with DMF adverse effects was 98% and 100%, respectively, and global satisfaction with DMF treatment was 74% and 69%, respectively, where a score of 0% equates to being not at all satisfied and 100% signifies the highest level of treatment satisfaction.24 Overall, these findings are consistent with the health-related quality of life benefits provided by DMF therapy in DEFINE and CONFIRM.12
Coadministration with Food
Before treatment initiation, clinicians may want to suggest to their patients that coadministering DMF with food is a useful prophylactic measure to mitigate GI AEs.9,19,20 In MANAGE, the incidence of mild GI AEs was higher, and of severe or extreme GI AEs lower, in patients who always took DMF with food than in patients who did not (Figure 2).9 This observation is supported by data from an expert consensus panel showing that administering DMF with food resulted in a reduction in the incidence and severity of nausea, vomiting, and abdominal pain among their patients (Figure 2).20 Because DMF is taken twice a day, it may be useful to align drug administration times with breakfast and dinner. Furthermore, it seems that the type of food eaten has an effect on GI AEs because DMF coadministration with high-fat, high-protein, and low-starch foods (eg, sausage, peanut butter, almond butter, yogurt, bacon, applesauce, cheese, and eggs) can either circumvent GI AEs or reduce their severity (Figure 3).20 Encasing the pill in peanut butter or cream cheese, or eating a tablespoon of peanut butter 5 minutes before taking DMF, are strategies mentioned anecdotally by some experts. Finally with regard to food, the patient's general health and any comorbidities should be considered if suggesting a high-fat diet, and reevaluation of diet after a few months of therapy would be prudent, especially if GI AEs have subsided over time.
Figure 2.
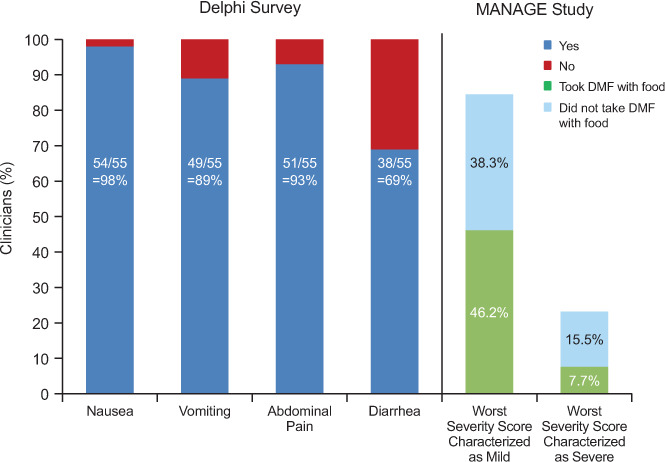
Percentage of clinicians who agree with the use of food as a management strategy to reduce the incidence and severity of delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF)–associated nausea, vomiting, abdominal pain, and diarrhea (Delphi survey; yes/no, consensus ≥70%)20
In addition, gastrointestinal adverse events were less severe in patients who regularly took DMF with a meal in MANAGE (Multicenter, Open-Label, Single-Arm Study of Gastrointestinal Tolerability in Patients with Relapsing Forms of Multiple Sclerosis Receiving Tecfidera™ [Dimethyl Fumarate] Delayed-Release Capsules).9
Figure 3.
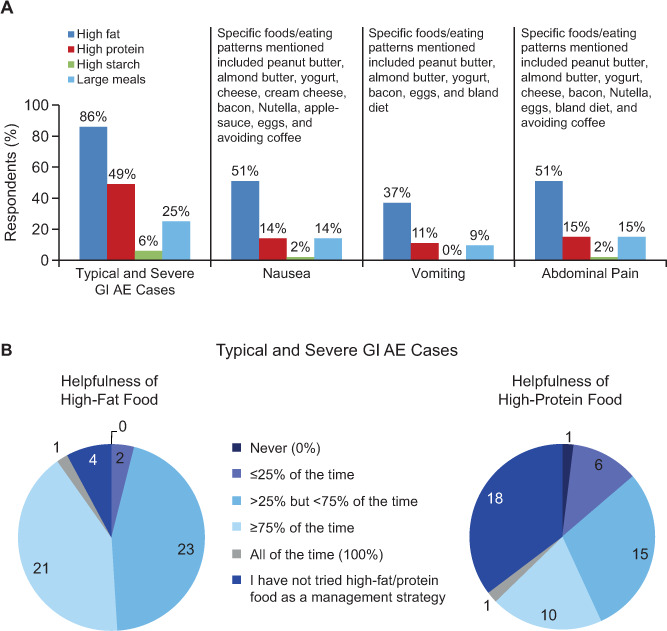
Food types most frequently recommended as a management strategy or observed as having an effect in managing individual adverse events (AEs) (A) or reported to be helpful for preventing individual AEs (B)20
All the responses were provided by the expert physicians participating in the Delphi survey. GI, gastrointestinal.
Titrating the Dose
The DMF prescribing information recommends a starting dose of 120 mg twice a day for 7 days followed by a maintenance dose of 240 mg twice a day,6 as used in DEFINE and CONFIRM.7,8 Consensus expert opinion is that in some cases a slower DMF dose titration may be needed to avoid GI AEs but still reach the approved maintenance dose of 240 mg twice a day.20 Specifically, it is advised that the 7-day period in which DMF is up-titrated from 120 mg twice a day to 240 mg twice a day is extended to 4 weeks or less because this strategy is associated with reducing the incidence and severity of nausea, vomiting, abdominal pain, and diarrhea.20
Starting patients on a DMF dosage regimen of 120 mg once daily for 1 week before up-titrating to 240 mg twice a day over the next 3 weeks is an option. Although this approach did not result in a reduction in the incidence of GI AEs when used in an 8-week study of healthy volunteers, it is possible that some patients will respond favorably.25 For instance, retrospective data from a structured nursing protocol initiative for commencing DMF therapy underscored the importance of a modified titration schedule combined with several other strategies, including educational measures and premedication recommendations to help patients manage DMF GI AEs, instruction on specific dietary requirements, and regular follow-up visits.26 The research group compared the DMF treatment discontinuation rate at 6 weeks in 124 patients who started taking DMF according to the manufacturer's instructions and in 205 patients who received DMF 120 mg once daily for 14 days, then 240 mg once daily for 14 days, then 240 mg twice a day thereafter. At 6 weeks, a lower proportion of patients who started taking DMF more gradually than the protocol recommended in the prescribing information had discontinued because of GI AEs (8% vs. 2%; P = .0215).26
An alternative strategy, based on a summation of expert comments,20,25,26 for slow-dose DMF titration might be to begin with a dosage of 120 mg once daily for 7 days, increasing the dose frequency to 120 mg twice a day for 7 days, then to 120 mg in the morning and 240 mg at night for 7 days, followed by 240 mg twice a day thereafter.
Dose Modification
A dose reduction of 50% (ie, from 240 mg twice a day to 120 mg twice a day) for 4 weeks or less was permitted in DEFINE and CONFIRM in patients unable to tolerate blinded study treatment owing to flushing or GI disturbances.7,8 Although this DMF dose (120 mg twice a day) was not effective, temporarily reducing the DMF dose level for at least 1 week may reduce the incidence or severity of nausea, vomiting, abdominal pain, and diarrhea and may increase the likelihood that patients can remain on therapy.20 Longer dose-reduction periods of 4 weeks or less may be required depending on the severity of the AE.20 In patients with dose reduction due to GI AEs, it is important that DMF is re-titrated slowly as discussed previously herein.20
Discontinuation of DMF use should be considered for patients unable to tolerate a return to the maintenance dose of 240 mg twice a day.6
Pharmaceutical Symptom Management
Table 2 summarizes the range of medicines considered highly useful for managing GI AEs in a consensus-building survey20 and in MANAGE.9 In MANAGE, immediate and pronounced reductions in the severity of GI AEs were evident with the use of bismuth subsalicylate, acid-secretion blockers, and antidiarrheal agents.9 Ondansetron, promethazine, and bismuth subsalicylate have shown utility for managing both nausea and vomiting, as has the use of antacids for nausea.9,20 Abdominal pain can be managed with bismuth subsalicylate, antacids, and antisecretory drug treatment, and diarrhea can be managed with loperamide and diphenoxylate/atropine.9,20
Table 2.
Usefulness of symptomatic therapies in the management of GI adverse events associated with DMF use in the real-world setting
| Symptom | Therapy | Clinicians agreeing therapy was useful, No. (%) (n = 56)20 | Beneficial effect of therapy observed in MANAGE for alleviating the severity of the specified GI-related event9 |
|---|---|---|---|
| Nausea | Ondansetron | 52 (93) | ✓ |
| Antacids | 41 (73) | ✓ | |
| Bismuth subsalicylate | 40 (71) | ✓ | |
| Promethazine | 40 (71) | ✓ | |
| Vomiting | Ondansetron | 52 (93) | ✓ |
| Bismuth subsalicylate | 40 (71) | ||
| Promethazine | 40 (71) | ✓ | |
| Abdominal pain | Proton pump inhibitors | 45 (80) | ✓ |
| Bismuth subsalicylate | 43 (77) | ✓ | |
| Antacids | 42 (75) | ||
| H2 blockers | 41 (73) | ✓ | |
| Diarrhea | Loperamide | 53 (95) | ✓a |
| Diphenoxylate/atropine | 51 (91) | ✓ a |
Abbreviations: DMF, delayed-release dimethyl fumarate (also known as gastroresistant DMF); GI, gastrointestinal; MANAGE, Multicenter, Open-Label, Single-Arm Study of Gastrointestinal Tolerability in Patients with Relapsing Forms of Multiple Sclerosis Receiving Tecfidera™ [Dimethyl Fumarate] Delayed-Release Capsules.
aAlso alleviated vomiting in MANAGE.
Findings from an 8-week, double-blind, placebo-controlled, phase 1 study of 175 healthy volunteers (PREVENT, A Multicenter, Double Blind, Placebo-Controlled Study of Pepto-Bismol® [Bismuth Subsalicylate] on Gastrointestinal Tolerability in Healthy Volunteers Receiving Oral TECFIDERA™ [Dimethyl Fumarate] Delayed-Release Capsules Twice Daily) highlighted the utility of bismuth subsalicylate,27 which was associated with reducing the incidence of severity scores of “severe” and “extreme” for flatulence, diarrhea, upper abdominal pain, indigestion, and vomiting. Use of the selective orally active leukotriene receptor antagonist montelukast also was effective for attenuating DMF-related GI symptoms in a small open-label study; this approach is being formally assessed in an ongoing multicenter, randomized, double-blind, placebo-controlled study (MITIGATE, A Multicenter, Double-Blind, Placebo-Controlled Study of Montelukast on Gastrointestinal Tolerability in Patients With Relapsing Forms of Multiple Sclerosis Receiving Tecfidera® [Dimethyl Fumarate] Delayed Release Capsules).28
Pretreatment with aspirin reduced the incidence and severity of flushing in a randomized single-center study of 173 healthy volunteers who received placebo, DMF alone, aspirin 325 mg/day taken 30 minutes before DMF for 4 weeks, and slow-dose titrated DMF.25 Aspirin pretreatment was associated with reducing the incidence, severity, and number of flushing events and had no adverse effect on GI-related events.25 Over time, the incidence of flushing events remained constant in the DMF alone and slow-dose titration DMF groups and declined slightly in the placebo group, whereas in the DMF plus aspirin group, the incidence of flushing decreased from 63% during week 1 to 52%, 28%, and 36% during weeks 2, 3, and 4, respectively, before the incidence rose when aspirin pretreatment was withdrawn.25 The DMF-associated flushing, and its mitigation by aspirin pretreatment, was correlated with plasma levels of prostaglandin D metabolites.25 It is plausible that the mechanism underlying DMF-induced flushing involves release of vasodilatory prostanoids since pretreatment with aspirin controlled the transient rise in prostaglandin D2 metabolites in plasma and urine observed in some individuals in the first few days after DMF administration.29
Discussion
Early intervention with disease-modifying therapy has the benefit of reducing disability progression in patients in the relapsing phase of MS, but this therapeutic window of opportunity is usually lost once the progressive, inflammation-independent, degenerative phase has begun.30 Therefore, it is imperative that disease-modifying therapy is used in an optimal way before the patient's treatment options become limited.
Delayed-release dimethyl fumarate is an efficacious treatment for RRMS,11 and its oral route of administration represents an attractive feature for patients currently receiving other disease-modifying therapy. Although DMF has demonstrated an acceptable safety profile in clinical studies, flushing and GI AEs can be bothersome in some patients. Therefore, proactively implementing management strategies that maintain patients on therapy is warranted, particularly during the first few months, when flushing and GI AE incidence are at their peak (Figure 4). All patients should receive early counseling regarding the importance of treatment adherence, the likely occurrence and impact of flushing and GI AEs, and potential management strategies. In particular, patients should receive information on how food and diet can be used to alleviate symptoms.
Figure 4.
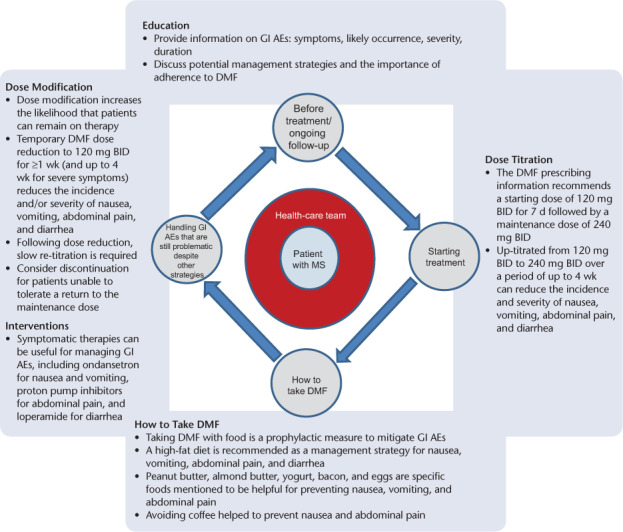
Management structure for the ongoing management of delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF) therapy
AE, adverse event; BID, twice a day; GI, gastrointestinal.
Through continual patient education and support and the management of treatment-related flushing and GI AEs, clinicians can help patients adhere to and persist with DMF therapy, thus maximizing treatment benefit.
PracticePoints
To help prepare patients for treatment of MS with delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF), counseling should be given on the long-term benefits of DMF as well as the adverse events (AEs) that could be experienced during the first few months of therapy, when the incidences of flushing and gastrointestinal AEs are at their highest. Helping patients adhere to and persist with DMF therapy helps to maximize treatment benefit.
Administering DMF with food, using a slower-dose titration schedule, applying temporary dose reductions, and using symptomatic therapies may avoid or relieve flushing and gastrointestinal AEs.
Supplementary Material
Acknowledgments
Malcolm J. M. Darkes (Excel Scientific Solutions, Horsham, UK) wrote the first draft of the manuscript based on input from the authors; Becky Gardner (Excel Scientific Solutions, Horsham, UK) also provided writing support, and Kristen DeYoung (Excel Scientific Solutions, Southport, CT, USA) copyedited and styled the manuscript per journal requirements. This medical writing support was funded by Biogen, which reviewed and provided feedback on the manuscript. The authors had full editorial control of the manuscript and provided their final approval of all content.
Footnotes
Financial Disclosures: Dr. Phillips has received consulting fees from Acorda, Biogen, Genentech, Genzyme, Merck Serono, Sanofi-Aventis, and Xenoport. Ms. Agrella has received consulting fees from Acorda, Biogen, Genzyme, Pfizer, Serono, and Teva. Dr. Fox has received consulting fees from and been a member of advisory committees for Actelion, Biogen, MedDay, Novartis, Questcor, Teva, and Xenoport and has received research support from Novartis.
Funding/Support: This review was supported by Biogen (Cambridge, MA, USA).
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Marrie RA, Cohen J, Stuve O et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21:263–281. doi: 10.1177/1352458514564491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devonshire V, Lapierre Y, Macdonell R et alThe Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol. 2011;18:69–77. doi: 10.1111/j.1468-1331.2010.03110.x. .; GAP Study Group. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg SC, Faris RJ, Chang CF, Chan A, Tankersley MA. Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig. 2010;30:89–100. doi: 10.2165/11533330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.European Medicines Agency Summary of product characteristics: Tecfidera 120 mg gastro-resistant hard capsules. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002601/WC500162069.pdf Accessed November 10, 2015.
- 6.Tecfidera (dimethyl fumarate) delayed-release capsules [prescribing information] Cambridge, MA: Biogen: 2015. [Google Scholar]
- 7.Fox RJ, Miller DH, Phillips JT et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. CONFIRM Study Investigators. [DOI] [PubMed] [Google Scholar]
- 8.Gold R, Kappos L, Arnold DL et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. DEFINE Study Investigators. [DOI] [PubMed] [Google Scholar]
- 9.Fox EJ, Vasquez A, Grainger W et al. Gastrointestinal tolerability of delayed-release dimethyl fumarate in a multicenter, open-label study of patients with relapsing forms of multiple sclerosis (MANAGE) Int J MS Care. 2016;18:9–18. doi: 10.7224/1537-2073.2014-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips JT, Selmaj K, Gold R et al. Clinical significance of gastrointestinal and flushing events in MS patients treated with delayed-release dimethyl fumarate. Int J MS Care. 2015;17:236–243. doi: 10.7224/1537-2073.2014-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viglietta V, Miller D, Bar-Or A et al. Efficacy of delayed-release dimethyl fumarate in relapsing-remitting multiple sclerosis: integrated analysis of the phase 3 trials. Ann Clin Transl Neurol. 2015;2:103–118. doi: 10.1002/acn3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kita M, Fox RJ, Gold R et al. Effects of delayed-release dimethyl fumarate (DMF) on health-related quality of life in patients with relapsing-remitting multiple sclerosis: an integrated analysis of the phase 3 DEFINE and CONFIRM studies. Clin Ther. 2014;36:1958–1971. doi: 10.1016/j.clinthera.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Gold R, Giovannoni G, Phillips JT et al. Efficacy and safety of delayed-release dimethyl fumarate in patients newly diagnosed with relapsing-remitting multiple sclerosis (RRMS) Mult Scler. 2015;21:57–66. doi: 10.1177/1352458514537013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez O, Giovannoni G, Fox R et al. Efficacy of delayed-release dimethyl fumarate for RRMS in prior interferon users in the DEFINE and CONFIRM studies. Neurology. 2015;84(suppl 14) P7.231. [Google Scholar]
- 15.Gold R, Arnold DL, Bar-Or A Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: interim analysis of ENDORSE, a randomized extension study. Mult Scler. [published online ahead of print May 19, 2016] [DOI] [PMC free article] [PubMed]
- 16.Kremenchutzky M, Fox RJ, Phillips JT Efficacy of delayed-release dimethyl fumarate vs glatiramer acetate on a novel composite outcome measure of inflammatory disease activity: post-hoc analysis of the CONFIRM study. Poster presented at: 31st Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 7–10, 2015. Barcelona, Spain. [Google Scholar]
- 17.Fox RJ, Gold R, Phillips JT et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate–treated MS patients: patient management considerations. Neurol Clin Pract. 2016;6:220–229. doi: 10.1212/CPJ.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold R, Phillips JT, Havrdova E et al. Delayed-release dimethyl fumarate and pregnancy: preclinical studies and pregnancy outcomes from clinical trials and postmarketing experience. Neurol Ther. 2015;4:93–104. doi: 10.1007/s40120-015-0033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips JT, Hutchinson M, Fox R, Gold R, Havrdova E. Managing flushing and gastrointestinal events associated with delayed-release dimethyl fumarate: experiences of an international panel. Mult Scler Relat Disord. 2014;3:513–519. doi: 10.1016/j.msard.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Phillips JT, Erwin AA, Agrella S et al. Consensus management of gastrointestinal events associated with delayed-release dimethyl fumarate: a Delphi study. Neurol Ther. 2015;4:137–146. doi: 10.1007/s40120-015-0037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandes DW, Callender T, Lathi E, O'Leary S. A review of disease-modifying therapies for MS: maximizing adherence and minimizing adverse events. Curr Med Res Opin. 2009;25:77–92. doi: 10.1185/03007990802569455. [DOI] [PubMed] [Google Scholar]
- 22.Mäurer M, Voltz R, Begus-Nahrmann Y, Niemczyk G, Schmid B, Kieseier BC. Individualized patient counselling can improve adherence by efficient management of avoidable adverse events. Poster presented at: 1st Congress of the European Academy of Neurology; June 20–23, 2015; Berlin, Germany. [Google Scholar]
- 23.Mäurer M, Voltz R, Begus-Nahrmann Y, Niemczyk G, Schmid B, Kieseier BC. Adherence project with German MS-patients: can an approach of individualized patient counseling improve adherence. Poster presented at: 2014 Joint ACTRIMS-ECTRIMS Meeting; September 10–13, 2014; Boston, MA. [Google Scholar]
- 24.Holliday S, Robinson A. Dimethyl fumarate tolerability and treatment adherence amongst patients with multiple sclerosis enrolled in specialty pharmacy services. Poster presented at: Academy of Managed Care Pharmacy Annual Meeting & Expo; April 1–4, 2014; Tampa, FL. [Google Scholar]
- 25.O'Gorman J, Russell HK, Li J, Phillips G, Kurukulasuriya NC, Viglietta V. Effect of aspirin pretreatment or slow dose titration on flushing and gastrointestinal events in healthy volunteers receiving delayed-release dimethyl fumarate. Clin Ther. 2015;37:1402–1419.e5. doi: 10.1016/j.clinthera.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Sammarco C, Laing L, Minetti J, Desanctis C, Herbert J. Strategies to reduce adverse events related to oral dimethyl fumarate. Poster presented at: 2014 Joint ACTRIMS-ECTRIMS Meeting; September 10–13, 2014; Boston, MA. [Google Scholar]
- 27.Tornatore C, Li J, Ma TS, von Hehn C, Walsh J, Zambrano J. Effect of bismuth subsalicylate on gastrointestinal events associated with delayed-release dimethyl fumarate: a double-blind, placebo-controlled study. Poster presented at: 2014 Joint ACTRIMS-ECTRIMS Meeting; September 10–13, 2014; Boston, MA. [Google Scholar]
- 28.Tornatore C, Walsh J, Mann M, Li J, Hehn C. Effect of montelukast on gastrointestinal tolerability in patients with relapsing forms of multiple sclerosis receiving delayed-release dimethyl fumarate: a multicenter, randomized, double-blind, placebo-controlled study (MITIGATE). Poster presented at: 67th Annual Academy of Neurology Annual Meeting; April 18–25, 2015; Washington, DC. [Google Scholar]
- 29.Sheikh SI, Nestorov I, Russell H et al. Tolerability and pharmacokinetics of delayed-release dimethyl fumarate administered with and without aspirin in healthy volunteers. Clin Ther. 2013;35:1582–1594.e9. doi: 10.1016/j.clinthera.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Ziemssen T, De Stefano N, Pia Sormani M, Van Wijmeersch B, Wiendl H, Kieseier BC. Optimizing therapy early in multiple sclerosis: an evidence-based view. Mult Scler Relat Disord. 2015;4:460–469. doi: 10.1016/j.msard.2015.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


