Abstract
Background
The incidence of osteoporotic vertebral fractures (OVCFs) has increased significantly in recent years. In order to assess osteoporotic fracture healing process, it is necessary to study the characteristics after this type of vertebral fracture. However, there are few researches on fracture healing process in severe OVCFs. We aim to investigate the histological healing process and the kinetics of bone turnover markers following severe OVCFs.
Material/Methods
There were 149 patients with severe OVCFs included in this study. Fasting blood samples were obtained to detect bone turnover markers levels. A transpedicular bone biopsy was performed to collect bone biopsy specimens during vertebroplasty surgery. Stratification of healing process was performed: stage I (1–3 days), stage II (4–10 days), stage III (11–20 days), stage IV (21–30 days), stage V (1–3 months), stage VI (3–6 months).
Results
Quantitative analysis of bone histomorphometry showed that a large amount of necrotic bone tissue was observed in stage VI (12.92±3.66%). Bone turnover markers showed the concentration of β-isomerized C-terminal telopeptide (β-CTX) which reflects activity in osteoclast continued to increase in stage VI (0.9±0.33 ng/mL). These results differed from previous reports of other type vertebral fractures.
Conclusions
Bone histomorphometric analysis and bone turnover markers showed that severe osteoporotic vertebral compression fractures often associated with delayed union and nonunion during the healing process.
MeSH Keywords: Bone Density, Osteoporotic Fractures, Spinal Fractures
Background
With the advent of aging population, the incidence of osteoporosis has increased rapidly in recent decades. The prevention and treatment of osteoporotic vertebral fractures (OVCFs) has become the focus of society [1]. Numerous studies have reported on the healing process after long bone fracture [2,3], however, considering differences between vertebral fractures and long bone fractures [4,5], the morphological and biochemical changes in vertebral fractures are still ongoing intense discussion [6–8].
Delayed union and nonunion are common complications in vertebral compression fracture patients with severe osteoporosis. Granchi et al. characterized long bone fractures by measuring bone turnover markers concentrations in serum [9]. But, data on the healing process of severe OVCFs is scarce. We reviewed all the patients with severe OVCFs in the past decade at our institute to investigate the histomorphometric characteristics and dynamic changes of bone turnover during the healing process following severe OVCFs.
Material and Methods
Patients
Between January 2011 and September 2018, 149 patients with severe OVCFs were included in this retrospective study. According to the definition criteria of World Health Organization (WHO), severe osteoporotic vertebral compression fractures were defined as T-values ≤−2.5 along with fragility fractures [10]. Written informed consent was obtained from patients for this study and permission was obtained from the Medical Ethics Committee.
Inclusion criteria were as follows: 1) back pain and aggravation of symptoms after activity; 2) T-score of spine ≤−2.5 was assessed by dual-energy x-ray absorptiometry (DXA); 3) patients were demonstrated magnetic resonance imaging (MRI) criteria of vertebral fracture [11].
Exclusion criteria were as follows: 1) pathological fracture; 2) vertebral fracture caused by spinal infection; 3) non-severe osteoporotic fracture (T-score >−2.5); 4) combined with metabolic bone disease; 5) renal osteodystrophy (serum creatinine greater than 0.20 mmol/L); 6) treatment with drugs that affect bone metabolism; 7) specimen failed to be obtain from fracture area confirmed under intraoperative fluoroscopy.
Preparation of bone biopsies
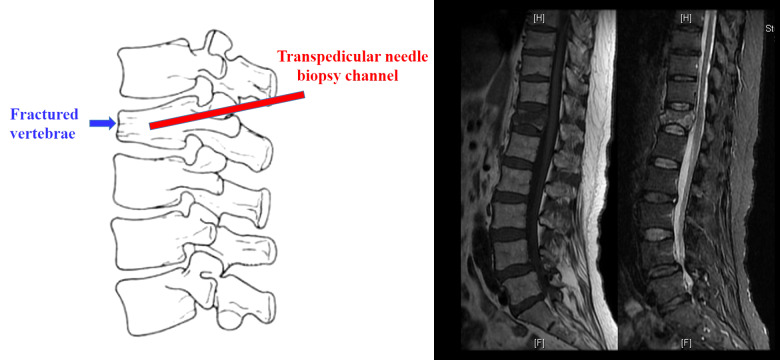
During a vertebroplasty procedure, a needle is placed into the vertebral body to extract tissue by using a transpedicular needle biopsy [12]. Biopsy localization was standardized for all patients and the positioning of biopsy was located to fracture area confirmed by MRI (as shown in Figure 1). Cancellous bone cores were harvested and fixed in 70% ethanol. After that, all specimens were embedded in methyl methacrylate, and sectioned with a heavy microtome [13].
Figure 1.
Diagram of location and operation channel for obtaining bone tissue sample.
Bone tissue quantitative analysis
All specimens were observed using light microscopy at 40× magnification and 100× magnification. Light microscopic analysis of biopsies was performed by a senior pathologist and repeated by another physician of the same specialty. The images were recorded by the microscope and quantitative analysis was performed by Image Pro Plus measurement software (Image Pro Plus, Media Cybernetics, Inc.). The histomorphometric parameters after quantitative analysis were expressed using the American Society for Bone and Mineral Research nomenclature [2,3]: cancellous bone volume/tissue volume (BV/TV, %), fibrous tissue volume/tissue volume (FV/TV, %), endochondral bone volume/tissue volume (EBV/TV, %), necrotic bone volume/tissue volume (NBV/TV, %), woven bone volume/tissue volume (WBV/TV, %), and lamellar bone volume/tissue volume (LBV/TV, %).
Determination of bone turnover markers
Blood samples were collected during pre-operation. Electrochemiluminescence immunoassays (Roche Diagnostics, Germany) were used to detect N-MID osteocalcin (OST), N-terminal propeptide of type I collagen (PINP), and β-isomerized C-terminal telopeptide (β-CTX) [14]. Alkaline phosphatase (ALP) was measured using a kinetic chromogenic method (Beckman Coulter, CA, USA) [15].
Staging
It is well-known that the fracture healing process is divided into 3 stages: inflammatory stage, callus formation stage, and callus remodeling stage [16]. In order to describe changes in bone turnover markers levels and bone tissue content precisely, we adopted a previously published article [17] staging method that subdivided each period into 6 time periods during fracture healing process: stage I (1–3 days), stage II (4–10 days), stage III (11–20 days), stage IV (21–30 days), stage V (1–3 months), stage VI (3–6 months).
Statistical methods
SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA) was used for data analysis. Comparisons between groups were performed by one-way analysis of variance (ANOVA). The bone histomorphometric parameters at different fracture stages were compared, as well as the serum concentrations of bone turnover markers in fracture healing process. The differences in fracture healing process between different ages and genders were also analyzed. P<0.05 was considered statistically significant.
Results
Location and time of specimen acquisition
There were 116 patients with 149 vertebral compression fractures (T-score ≤−2.5) included in the experimental study. Bone biopsy specimens were obtained from 25 male and 91 female patients (mean age 78.9 years) with 55 lumbar fractured vertebrae (L1=25; L3=5; L2=12; L4=6; L5=7) and 61 thoracic fractured vertebrae (T5=1; T6=3; T7=6; T8=6; T9=7; T10=8; T11=8; T12=22). All patients were diagnosed with severe OVCF (T-score ≤−2.5) assessed by DXA.
All cases were divided into 6 phases: Stage I (1–3 days), Stage II (4–10 days), Stage III (11–20 days), Stage IV (21–30 days), Stage V (1–3 months), Stage VI (3–6 months). There were 20 patients (17%) in stage I, 23 patients (20%) in stage II, 23 patients (20%) in stage III, 16 patients (14%) in stage IV, 18 patients (15%) in stage V, and 16 patients (14%) in stage VI.
Morphological characteristics and bone histomorphometric analysis of severe OVCF
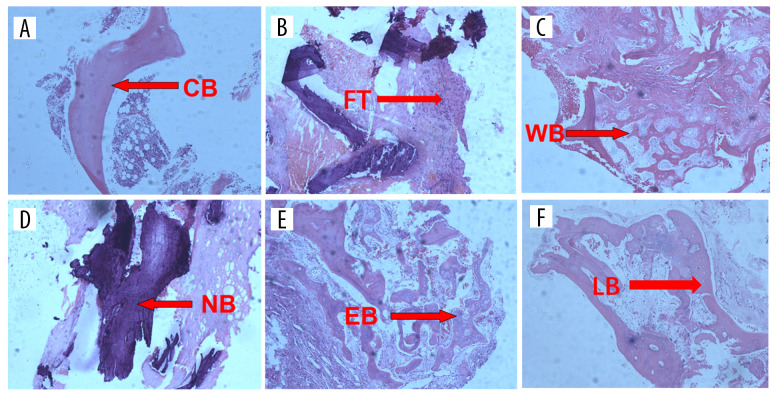
The tissues observed under the light microscopy included the following observations. 1) Cancellous bone was the main component in the vertebral body, and there was no fracture of trabeculae at the early stage of fracture (as shown in Figure 2A). 2) Inflammatory reactions appeared at the fracture area due to trauma at the initial stage of the fracture, and the formation of fibrous tissue marks the beginning of the bone repair process (as shown in Figure 2B). 3) Immature woven bone marked the initial formation of the callus, which plays a supportive and protective role during the transitional phase of vertebral fracture (as shown in Figure 2C). 4) A large amount of necrotic bone tissue was observed during the healing process of severe OVCFs, suggesting that this type of fractures was frequently associated with newly fractures (as shown in Figure 2D). 5) Endochondral bone was produced by mineralization of fibrous tissue osteoblasts (as shown in Figure 2E). 6) Lamellar bone was hard bone tissue formed at the late stage of fracture healing, and lamellar bone plays a critical role in the support of the vertebral body after fracture (as shown in Figure 2F).
Figure 2.
(A–F) Pictures of tissues observed under the light microscopy during severe OVCFs healing process: (A) cancellous bone (BV); (B) fibrous tissue (FV); (C) woven bone (WBV); (D) necrotic bone (NBV); (E) endochondral bone (EBV); (F) lamellar bone structure (LBV).
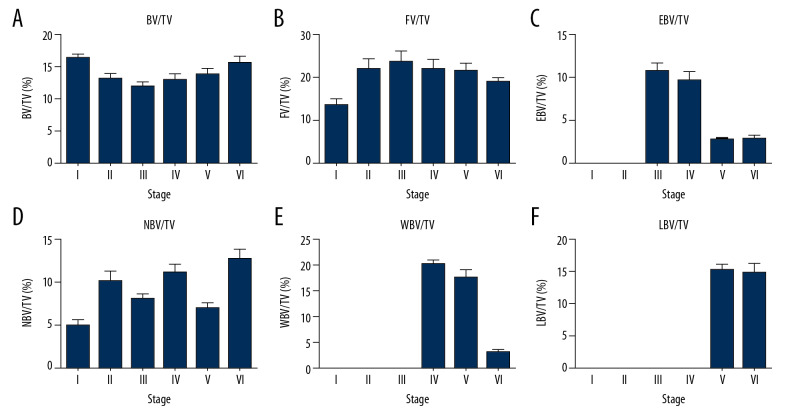
In order to reflect the characteristics of histological changes after severe OVCF fracture, we performed histomorphometric analysis. The results of quantitative analysis showed that the volume of BV/TV decreased gradually in the early stage of fracture and was gradually replaced by necrotic bone tissue (as shown in Figure 3). The volume of BV/TV decreased to the lowest value of 11.96±3.09% at stage III. Necrotic bone tissue appeared (5.13±2.61%) in stage I (as shown in Table 1) and gradually rose to the first peak (10.21±5.22%) in stage II. And then rose again to the second peak (12.92±3.66%) at stage VI. The onset of endochondral bone formation was at stage III with a single peak (10.71±3.97%) during this period, after which the endochondral bone was replaced by woven bone. Woven bone began to appear in stage III and rose to reach a peak of 11.3±3.05% in stage IV. Lamellar bone, as the mineralized bone tissue, appeared in stage V and reached a peak of 15.45±3.43% in this stage. Overlap of different stages were observed apparently in stage IV (9 of 16 specimens, 37.5%), stage V (9 of 18 specimens, 50%), and stage VI (12 of 16 specimens, 75%).
Figure 3.
(A–F) Histomorphometric analysis of fracture healing at different stages after severe OVCFs. A large amount of necrotic bone and fibrous tissue still appeared after more than 1 month of fractures. Cancellous bone volume/tissue volume (BV/TV%), fibrous tissue volume/tissue volume (FV/TV%), endochondral bone volume/tissue volume (EBV/TV%), necrotic bone volume/tissue volume (NBV/TV%), woven bone volume/tissue volume (WBV/TV%), and lamellar bone volume/tissue volume (LBV/TV%) are indicated as mean±standard error of mean (SEM).
Table 1.
Fracture callus morphometry in patients with severe OVCFs classified according to fracture staging.
| Variable | Stage I | Stage II | Stage III | Stage IV | Stage V | Stage VI | P |
|---|---|---|---|---|---|---|---|
| BV/TV (%) | 16.33±2.69 | 13.01±3.98 | 11.96±3.09 | 12.93±3.05 | 13.89±3.63 | 15.52±3.87 | 0.001 |
| FV/TV (%) | 15.06±7.01 | 21.92±11.05 | 23.6±10.22 | 21.89±7.36 | 21.5±7.01 | 7.63±3.86 | <0.001 |
| EBV/TV (%) | 0 | 0 | 10.71±3.97 | 9.51±3.36 | 2.61±0.97 | 2.78±1.39 | <0.001 |
| NBV/TV (%) | 5.13±2.61 | 10.21±5.22 | 8.21±2.33 | 11.3±3.05 | 7.32±2.72 | 12.92±3.66 | <0.001 |
| WBV/TV (%) | 0 | 0 | 0 | 20.39±1.98 | 17.71±6.49 | 3.22±1.68 | <0.001 |
| LBV/TV (%) | 0 | 0 | 0 | 0 | 15.45±3.43 | 14.96±5.07 | <0.001 |
Cancellous bone volume/tissue volume (BV/TV%), fibrous tissue volume/tissue volume (FV/TV%), endochondral bone volume/tissue volume (EBV/TV%), necrotic bone volume/tissue volume (NBV/TV%), woven bone volume/tissue volume (WBV/TV%), and lamellar bone volume/tissue volume (LBV/TV%). All values are expressed as mean±standard deviation (SD).
Kinetic of bone turnover markers after severe OVCF
Bone turnover markers are essential to assess the risk of delayed fracture healing and the progress of fracture healing [18]. We measured bone turnover markers to reflect the activity of osteoblasts and osteoclasts.
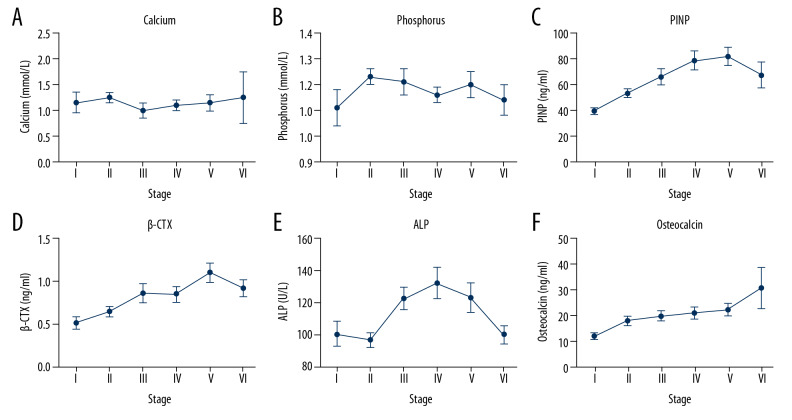
The levels of bone turnover markers showed that OST continued to rise after fracture and reached a peak value of 30.51±25.5 ng/mL in stage VI (as shown in Figure 4). The level of β-CTX rose to a plateau in stage III after fracture, and then continued to rise to its highest value of 1.1±0.4 ng/mL in stage V. PINP continued to rise after fracture and reached a peak of 81.5±26.77 ng/mL in stage V. ALP started to decrease at the beginning of fracture, then increased rapidly to the highest value of 132.17±40.5 U/L in stage IV (as shown in Table 2).
Figure 4.
(A–F) Concentrations of bone turnover markers at different stages after severe OVCFs. Levels of bone resorption markers and bone formation markers were still significantly higher than normal levels at the late stage of fracture. Concentrations of N-terminal propeptide of type I collagen (PINP), β-isomerized C-terminal telopeptide (β-CTX), alkaline phosphatase (ALP), calcium, phosphorus, and osteocalcin are expressed by mean±standard error of mean (SEM).
Table 2.
Bone turnover markers in patients with severe OVCFs classified according to fracture staging.
| Variable | Stage I | Stage II | Stage III | Stage IV | Stage V | Stage VI | P |
|---|---|---|---|---|---|---|---|
| Calcium (mmol/L) | 2.23±0.15 | 2.25±0.09 | 2.21±0.13 | 2.22±0.11 | 2.2±0.15 | 2.25±0.3 | 0.971 |
| Phosphorus (mmol/L) | 1.11±0.23 | 1.23±0.18 | 1.21±0.21 | 1.16±0.14 | 1.2±0.18 | 1.13±0.19 | 0.415 |
| PINP (ng/mL) | 39.22±8.6 | 53.08±15.45 | 65.61±25.09 | 78.3±31.6 | 81.5±26.77 | 67.02±31.87 | <0.001 |
| β-CTX (ng/mL) | 0.53±0.23 | 0.68±0.25 | 0.85±0.46 | 0.8±0.4 | 1.1±0.4 | 0.9±0.33 | 0.002 |
| ALP (U/L) | 100.73±25.37 | 97±19.96 | 122.53±28.07 | 132.17±40.5 | 123.2±36.05 | 100.4±18.39 | 0.002 |
| Osteocalcin (ng/mL) | 11.82±4.5 | 18.72±7.91 | 19.71±8.03 | 20.75±10.01 | 22.21±9.26 | 30.51±25.5 | 0.015 |
Concentrations of N-terminal propeptide of type I collagen (PINP), β-isomerized C-terminal telopeptide (β-CTX), Alkaline phosphatase (ALP), calcium, phosphorus and osteocalcin are indicated as mean±standard deviation (SD).
There were no significant differences in bone histomorphometry and bone turnover markers between different age groups (P>0.05). At the same time, there were also no significant statistical differences in the above indicators between genders (P>0.05). These results indicated that the samples were evenly distributed, avoiding the effects of age and gender (as shown in Table 3).
Table 3.
Clinical demographics in patients with severe vertebral fractures classified according to fracture staging.
| Demographics | Stage I | Stage II | Stage III | Stage IV | Stage V | Stage VI |
|---|---|---|---|---|---|---|
| Number | 20 | 23 | 23 | 16 | 15 | 16 |
| Women | 4 | 6 | 6 | 4 | 4 | 1 |
| Men | 16 | 17 | 17 | 12 | 11 | 15 |
| Sex ratio (Male/Female) | 1:4 | 6:17 | 6:17 | 1:3 | 4:11 | 1:15 |
| Age (years) | 79.1±8.6 | 81.3±9.6 | 83.2±9.1 | 77.5±6.9 | 77.5±6.1 | 79.6±7.6 |
| Weight (kg) | 61.1±5.1 | 50.5±9.7 | 50.1±8.9 | 61.5±1.5 | 59.5±4.5 | 49.6±5 |
| Height (cm) | 159.1±6.7 | 151.1±9.6 | 150.5±9.1 | 155.9±5 | 160.1±4.5 | 150.5±6.7 |
Age, weight, and height of patients are indicated as mean±standard deviation (SD).
Discussion
Fracture healing is a complex process, which is usually divided into 3 stages: 1) hematoma and inflammatory stage; 2) primary callus formation stage; 3) primary callus remodeling stage [19–21]. It begins with a formation of hematoma and inflammatory reaction caused by tissue reactions directly after injury of vascular and soft tissue, and then inflammatory cells participate in the formation of inflammatory granulation tissue [19,20]. The fibrocartilage matrix formed after the inflammatory phase, which is mainly composed of fibroblasts and chondrocytes, providing the initial mechanical stability of the fracture site [20]. After the formation of the primary callus, newly formed endochondral bone and immature woven bone are continuously mineralized to form hard lamellar bone. Mineralized lamellar bone formation marks the completion of the mineralization process of the primary callus [19–21].
In the present study, fracture hematoma and fibrous tissue were observed in biopsy specimens collected in stage I (1–3 days after fracture), with necrotic bone tissue appeared in stage II (3–10 days after fracture). Endochondral bone appeared in stage III (11–20 days after fracture), and woven bone appeared in stage IV (21–30 days after fracture). Lamellar bone formation from in the last stage (3–6 months after fracture).
However, the healing process of vertebral fractures is different from the characteristics of long bones healing process [3]. In our study, significant tissue overlapping was observed during healing in vertebral fractures with a large amount of cancellous bone. This is the reason that effective reduction and fixation can provide a stable growth environment for fracture healing in long bone fractures. It is worth noting that fracture stabilization is not possible in vertebral fractures, especially in patients with severe osteoporosis [8].
The first innovation of this experiment is that we describe the delayed union and nonunion process in severe OVCF by histopathological analysis. In contrast to previous studies, we found that necrotic bone and fibrous tissue were still present in fracture sections over 1 month after vertebral fracture. Particular in the chronic stage of fracture, there were still a large amount of tissues involved in the transition to hard callus, which should have occurred in the subacute stage, such as endochondral bone and immature woven bone. The aforementioned results illustrated that delayed union and newly fractures occurred in patients with severe osteoporotic vertebral compression fractures. Some patients with severe OVCF may even exhibit features of nonunion. Several studies have reported that osteoporosis increases the risk of newly fractures after vertebral surgery [22,23]. Lu et al. [24] showed that bone mineral density (BMD) was the only factor associated with newly fractures after 2 years of follow-up. In this study, we found the highest content of necrotic bone tissue at 3–6 months after fracture. Due to significantly decreased strength of bone structure caused by severe osteoporosis, the endochondral bone and woven bone cannot provide sufficiently stable environment for fracture healing. Furthermore, newly vertebral fractures are often associated with necrotic bone and a large amount of fibrous tissue, which might explain the reason that severe osteoporotic vertebral compression fractures often result in delayed union and nonunion of fractures.
Another innovation of this experiment is that we describe the healing process in severe OVCF by detecting the levels of bone turnover markers. It is well-known that the role of bone turnover markers in assessing the risk of delayed union or nonunion is remarkable [18]. Bone turnover markers are commonly classified into 3 categories: 1) bone resorption markers, 2) osteoclast regulatory proteins, and 3) bone formation markers [14,25,26]. During the normal homeostasis of bone resorption and formation are balanced, but the balance is disrupted when osteoporosis is present [14]. C-telopeptide of type I collagen (CTX) is a collagenous bone resorption marker. Unlike previous studies of non-severe osteoporotic vertebral fractures [17], CTX serum concentrations did not decrease significantly from 3 to 20 days after fracture. This may be related to the increased activity of osteoclast to absorb necrotic bone tissue caused by newly fracture during the healing process. The CTX concentrations were lower than other types of osteoporotic fractures during the same stages. The aforementioned results indicated that the capacity of bone resorption in severe OVCF was more lasting but lower than in non-severe OVCF. PINP is derived from degraded fragments of type I procollagen, which is primarily used to reflect osteoblastic activity [27]. PINP serum concentrations were found to increase gradually after fracture. This is the reason that osteoblasts are involved in the formation of new woven bone and mineralization of immature woven bone. This result is consistent with the findings of Stoffel et al. [28].
In this study, we describe the healing process of severe OVCF by using histomorphometry and determination of bone turnover markers. Necrotic bone tissue and fibrous tissue appeared at the chronic stage after fracture. This suggests that severe OVCF are usually associated with newly fracture and delayed union or nonunion during the healing process. Therefore, systematic anti-osteoporosis treatment and providing a stable state for fracture healing are particularly important in the treatment of severe osteoporotic vertebral compression fractures. However, there were shortcomings to this study. Firstly, we included a small sample size, and secondly, we lacked data on patients with a fracture time over 6 months. We did not assess characteristics of delayed union or microfracture in MRI and computed tomography. Therefore, in future studies we will focus on analyzing more characteristics of radiology in severe osteoporotic vertebral compression fractures to obtain more accurate results.
Conclusions
Although the variations of tissue and bone turnover markers was consistent with the fracture healing process, a large amount of necrotic bone tissue appeared in the chronic stage of fracture. At the same time, there was a large amount of endochondral bone and immature woven bone in the chronic stage, which showed that severe OVCFs were often associated with delayed union and nonunion during the healing process.
Acknowledgement
This work was supported by the Department of Pathology of Shandong University and Pathology Laboratory of Shandong Provincial Hospital for providing specimen preparation and measurement method. We thank the Shandong Medical Imaging Research Institute and Central Laboratory of Shandong Provincial Hospital for providing the data of bone mineral density and bone turnover markers.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998;(355 Supl.):S7–21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 3.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 4.Movrin I, Vengust R, Komadina R. Adjacent vertebral fractures after percutaneous vertebral augmentation of osteoporotic vertebral compression fracture: A comparison of balloon kyphoplasty and vertebroplasty. Arch Orthop Trauma Surg. 2010;130(9):1157–66. doi: 10.1007/s00402-010-1106-3. [DOI] [PubMed] [Google Scholar]
- 5.Ning L, Wan S, Liu C, et al. New levels of vertebral compression fractures after percutaneous kyphoplasty: Retrospective analysis of styles and risk factors. Pain Physician. 2015;18(6):565–72. [PubMed] [Google Scholar]
- 6.Antonacci MD, Mody DR, Rutz K, et al. A histologic study of fractured human vertebral bodies. J Spinal Disord Tech. 2002;15(2):118–26. doi: 10.1097/00024720-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Togawa D, Lieberman IH, Bauer TW, et al. Histological evaluation of biopsies obtained from vertebral compression fractures: Unsuspected myeloma and osteomalacia. Spine. 2005;30(7):781–86. doi: 10.1097/01.brs.0000157478.03349.57. [DOI] [PubMed] [Google Scholar]
- 8.Diamond TH, Clark WA, Kumar SV. Histomorphometric analysis of fracture healing cascade in acute osteoporotic vertebral body fractures. Bone. 2007;40(3):775–80. doi: 10.1016/j.bone.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Granchi D, Gómez-Barrena E, Rojewski M, et al. Changes of bone turnover markers in long bone nonunions treated with a regenerative approach. Stem Cells Int. 2017;2017 doi: 10.1155/2017/3674045. 3674045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glaser L, Kaplan D, Frederick S. Osteoporosis: Definition and clinical presentation. Spine. 1997;22(24 Suppl):12S. doi: 10.1097/00007632-199712151-00003. [DOI] [PubMed] [Google Scholar]
- 11.Voormolen MH, van Rooij WJ, van der Graaf Y, et al. Bone marrow edema in osteoporotic vertebral compression fractures after percutaneous vertebroplasty and relation with clinical outcome. Am J Neuroradiol. 2006;27(5):983–88. [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond TH, Champion B, Clark WA. Management of acute osteoporotic vertebral fractures: A nonrandomized trial comparing percutaneous vertebroplasty with conservative therapy. Ame J Med. 2003;114(4):257–65. doi: 10.1016/s0002-9343(02)01524-3. [DOI] [PubMed] [Google Scholar]
- 13.Diamond TH, Stiel D, Lunzer M, et al. Hepatic osteodystrophy. Static and dynamic bone histomorphometry and serum bone Gla-protein in 80 patients with chronic liver disease. Gastroenterology. 1989;96(1):213–21. [PubMed] [Google Scholar]
- 14.Leeming DJ, Alexandersen P, Karsdal MA, et al. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol. 2006;62(10):781–92. doi: 10.1007/s00228-006-0174-3. [DOI] [PubMed] [Google Scholar]
- 15.Veitch SW, Findlay SC, Hamer AJ, et al. Changes in bone mass and bone turnover markers following tibial shaft fracture. Osteoporos Int. 2006;17(3):364–72. doi: 10.1007/s00198-005-2025-y. [DOI] [PubMed] [Google Scholar]
- 16.Phillips AM. Overview of the fracture healing cascade. Injury. 2005;36(Suppl):S5–7. doi: 10.1016/j.injury.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Pan C, Liu X, Li T, et al. Kinetic of bone turnover markers after osteoporotic vertebral compression fractures in postmenopausal female. J Orthop Surg Res. 2018;13(1):314. doi: 10.1186/s13018-018-1025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cox G, Einhorn TA, Tzioupis C, Giannoudis PV. Bone-turnover markers in fracture healing. J Bone Joint Surg Br. 2010;92-B(3):329–34. doi: 10.1302/0301-620X.92B3.22787. [DOI] [PubMed] [Google Scholar]
- 19.Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: The cellular picture. Semin Cell Dev Biol. 2008;19(5):459–66. doi: 10.1016/j.semcdb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Gerstenfeld LC, Cullinane DM, Barnes GL, et al. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88(5):873–84. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 21.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 2012;8(3):133–43. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 22.Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15(4):721–39. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285(3):320–23. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 24.Lu K, Liang CL, Hsieh CH, et al. Risk factors of subsequent vertebral compression fractures after vertebroplasty. Pain Med. 2012;13(3):376–82. doi: 10.1111/j.1526-4637.2011.01297.x. [DOI] [PubMed] [Google Scholar]
- 25.Civitelli R, Armamento-Villareal R, Napoli N. Bone turnover markers: Understanding their value in clinical trials and clinical practice. Osteoporos Int. 2009;20(6):843–51. doi: 10.1007/s00198-009-0838-9. [DOI] [PubMed] [Google Scholar]
- 26.Looker AC, Bauer DC, Chesnut CH, et al. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporosis International. 2000;11(6):467–80. doi: 10.1007/s001980070088. [DOI] [PubMed] [Google Scholar]
- 27.Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing. A current review. Clinical Orthopaedics and Related Research. 1995;(318):265–78. [PubMed] [Google Scholar]
- 28.Stoffel K, Engler H, Kuster M, Riesen W. Changes in biochemical markers after lower limb fractures. Clinical Chemistry. 2007;53(1):131–4. doi: 10.1373/clinchem.2006.076976. [DOI] [PubMed] [Google Scholar]