Abstract
Background
Lung adenocarcinoma currently accounts for the highest cancer-related mortality rate worldwide. MiR-21-5p has a vital role in various types of cancers. We have analyzed the miR-21-5p expression level, prognosis, and associated molecular pathways in lung adenocarcinoma with multiple bioinformatics databases.
Material/Methods
The Cancer Genome Atlas (TCGA) database was employed to fetch the miR-21-5p expression profile in multiple tumors. We used the UALCAN platform to assess the differential regulation of the miR-21-5p in healthy tissue and lung adenocarcinoma. Also, the survival prognosis of the miR-21-5p in each stage of lung adenocarcinoma was done by the Kaplan-Meier database. The STARBASE and UALCAN databases were employed to predict the miR-21-5p target genes, and the levels of target genes and their prognostic value were analyzed.
Results
MiR-21-5p was overexpressed in the majority of human cancers. MiR-21-5p demonstrated escalated expression in the lung adenocarcinoma tissue in contrast to the normal tissue (P<0.05). Poor prognosis was witnessed in the miR-21-5p high expression group as compared to the low expression group (hazard ratio [HR]= 1.59, P<0.05). PDZD2 was predicted as a miR-21-5p potential target. We found a negative correlation between PDZD2 and miR-21-5p (r=−0.255, P<0.05). PDZD2 was downregulated in lung adenocarcinoma (P<0.05). Overexpression of PDZD2 was associated with a better prognosis of survival in lung adenocarcinoma patients (HR=0.45, P<0.05).
Conclusions
MiR-21-5p exhibits the potential to act as a biomarker for the survival prognosis of lung adenocarcinoma. It might be responsible for the onset and progression of lung adenocarcinoma through PDZD2 regulation.
MeSH Keywords: Carcinoma, Non-Small-Cell Lung; MicroRNAs; PDZ Domains; Adenocarcinoma
Background
Lung cancer is a very commonly occurring cancer type with the highest incidence and mortality rate worldwide [1]. Non-small cell lung cancer (NSCLC) makes a significant contribution (85%) to the total cases of lung cancer. It is further classified as adenocarcinoma, squamous cell, and large cell carcinomas. Among various subtypes of NSCLC, lung adenocarcinoma is most commonly reported, and around 30% to 35% of NSCLC cases fall under this category [2,3]. Previous studies have reported a considerably higher 5-year survival rate for early postoperative lung cancer as compared to advanced lung cancer [4–6]. The occult clinical symptoms associated with the NSCLC stage I and stage II make its successful diagnosis exceptionally challenging, and hence the diagnosis of most cases of NSCLC occurs only in much later disease stages [7–9]. Lung screening has witnessed significant advances as far as the NSCLC diagnosis is concerned, but the traditional diagnostic technologies are still struggling with accurate diagnosis of NSCLC. Numerous studies have reported that microRNAs (miRNAs) have a vital role in NSCLC. For instance, miR-504 was found to restrict the invasion and proliferation of NSCLC cells via lysyl oxidase-like 2 (LOXL2) [10]. Besides, miR-142-5p was found to inhibit NSCLC tumorigenesis by targeting PIK3CA expression [11]. Recent studies have reported miR-21-5p upregulation in NSCLC cases [12]. However, the miR-21-5p expression profile in lung adenocarcinoma, survival prognosis, and mechanism of action remain uninvestigated, which demands verification from a large cohort of the sample.
In the current study, we focused mainly on the clinical prognosis and expression analysis of miR-21-5p in lung adenocarcinoma by bioinformatics methods. We also predicted its gene target, i.e., PDZD2, and analyzed its expression level and prognostic significance in lung adenocarcinoma and normal tissue. It might, thus, potentially act as a target in the clinical therapy of lung adenocarcinoma.
Material and Methods
MiR-21-5p profile in various human cancers
We retrieved the expression profile of miR-21-5p in several human cancer and adjacent normal tissues from The Cancer Genome Atlas (TCGA) database (http://bioinfo.life.hust.edu.cn/miR_path/index.html) [13].
Expression of miR-21-5p in normal lung tissue and lung adenocarcinoma
UALCAN is a user-oriented interactive web resource that can comprehensively analyze cancer OMICS data (http://ualcan.path.uab.edu/index.html) [14]. We determined the level of miR-21-5p expression in normal lung tissue and lung adenocarcinoma by employing the miRNA panel, and the RNA sequencing data from the TCGA platform. We also categorized lung adenocarcinoma patients as per clinicopathological parameters, i.e., gender, ethnicity, age, smoking status, cancer stages, nodal metastasis status, and tumor histology. Subsequently, we analyzed the differences and correlations between the clinicopathological parameters aforementioned and miR-21-5p expression levels.
Differences in miR-21-5p expression and patients’ prognosis with lung adenocarcinoma
We employed the Kaplan-Meier data analysis plotter (http://www.kmplot.com/analysis/) interlinked with the PAN-CANCER software to plot the miR-21-5p survival curve in lung adenocarcinoma [15]. MiR-21-5p was divided into high and low expression groups by median. We staged lung adenocarcinoma patients according to “The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer” [16] and analyzed the survival of each stage.
Predicting the gene targets of miR-21-5p in lung adenocarcinoma
The miR-21-5p gene target prediction in lung adenocarcinoma was made by employing the miRNA-mRNA module of the STARBASE database (http://starbase.sysu.edu.cn/panMirDiffExp.php) [17]. To get a more accurate prediction and narrow down the target gene range, we selected the miRanda and miRmap modules in the PREDICTED PROGRAM, which resulted in the identification of a total 1901 target genes. Besides, a total of 250 downregulated genes in adenocarcinoma of the lung were screened by the TCGA module on the UALCAN portal. Based on the comprehensive analysis of the 2 platforms, the target genes (RTKN2, GCOM1, AKAP2, PALM2-AKAP2, and PDZD2) with low miRNA-21-5p expression in lung adenocarcinoma were screened. Furthermore, the scatter plot demonstrated the association among the expressions of miR-21-5p and target gene, with the best correlation selected for analyzing its expression level and prognosis in lung adenocarcinoma.
Expression of PDZD2 in lung adenocarcinoma and normal tissues
We also analyzed the expression level of PDZD2 in normal and lung adenocarcinoma tissue by using the TCGA module on the UALCAN database platform. The differences and correlations between clinicopathological parameters and PDZD2 expression levels were analyzed.
Effect of differential expression of target gene PDZD2 on lung adenocarcinoma patient prognosis
We used the Kaplan-Meier database platform to analyze the PDZD2 expression in the 720 cases of lung adenocarcinoma. PDZD2 was upregulated in 384 cases and downregulated in 336 cases. The correlation between PDZD2 expression and survival prognosis in lung adenocarcinoma was evaluated by plotting the survival curve. PDZD2 was divided into high and low expression groups by median. Additionally, patients with adenocarcinoma were grouped according to TNM stage, and the prognosis of each group was analyzed.
Results
MiR-21-5p profile in various human cancers
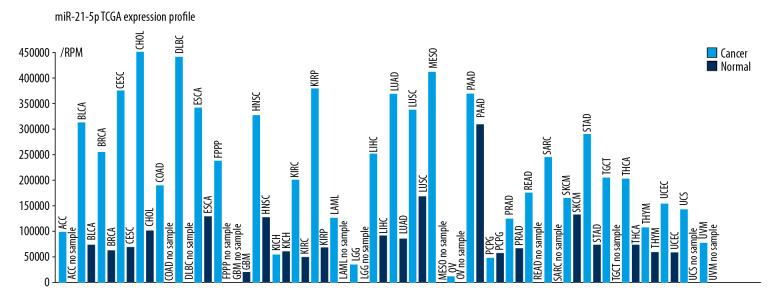
As shown in Figure 1, the miRNA-21-5p expression profile demonstrated that it was overexpressed in the majority of malignancies, such as cholangiocarcinoma, pancreatic cancer, and lung adenocarcinoma. Interestingly, in all these cancers, miRNA-21-5p has been identified as an oncogene, which can lead to cancer development [18–20].
Figure 1.
The miRNA-21-5p expression profile from the TCGA database. MiRNA-21-5p was highly expressed in lung adenocarcinoma tissue as compared to the healthy, normal tissue.
Expression of miR-21-5p in normal lung tissue and lung adenocarcinoma
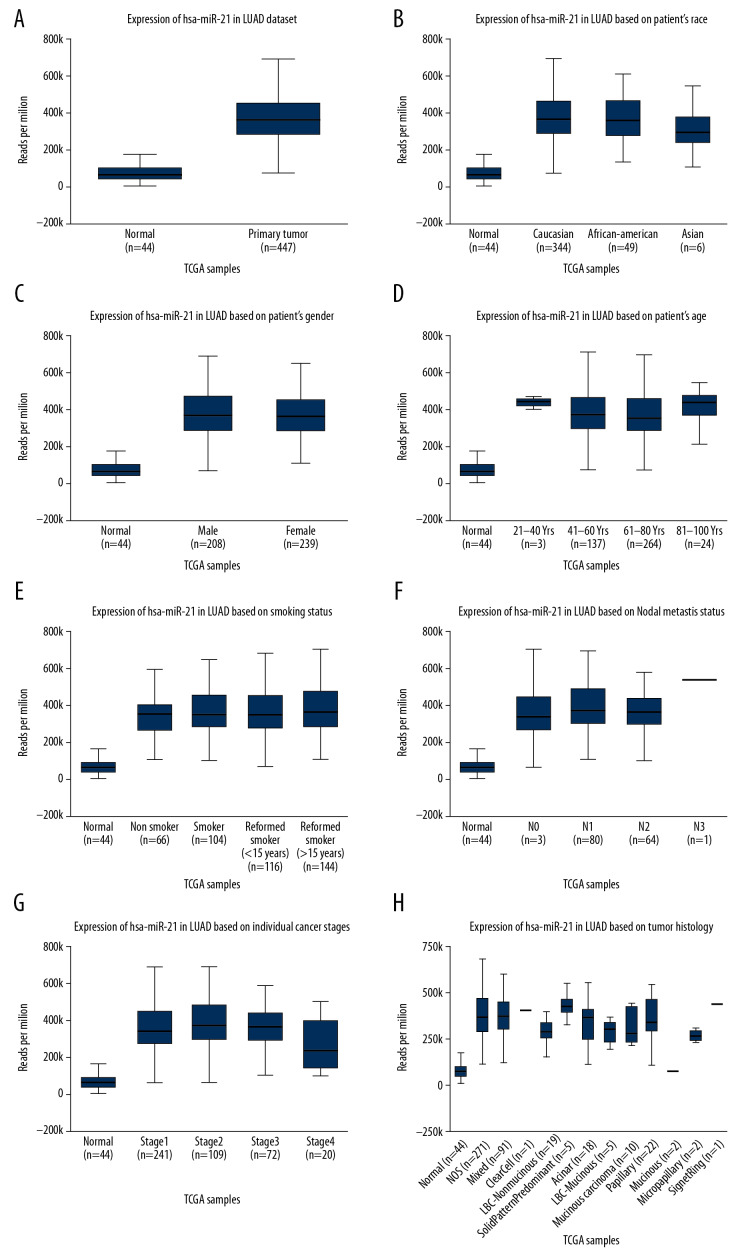
The UALCAN database analyzed a total of 447 lung adenocarcinoma and 44 normal tissue. A significant high expression of miR-21-5p in lung adenocarcinoma was observed in contrast to the normal tissue (P<0.05) (Figure 2A). There was no correlation between the miR-21-5p expression level and race, gender, age, smoking habits, lymph node metastasis in lung adenocarcinoma patients(P>0.05, Figure 2B–2F). However, the miR-21-5p expression level was correlated with TNM stage and tissue types (P<0.05, Figure 2G, 2H).
Figure 2.
Differential expression of miR-21-5p in normal tissue and lung adenocarcinoma. (A) High level of miR-21-5p in lung adenocarcinoma tissue. (B–F) Lung adenocarcinoma patient grouping by race, gender, age, smoking habits, lymph node metastasis, and miR-21-5p expression level analysis. There was no significant difference. (G–H) The miR-21-5p expression level was correlated with TNM stage and tissue types (P<0.05).
Differences in miR-21-5p expression and patient prognosis with lung adenocarcinoma
We plotted the survival curve by using the Kaplan-Meier plotter for 503 patients with lung adenocarcinoma. Out of the 503 cases, miR-21-5p was repressed in 419 cases and upregulated in 84 cases. We categorized these cases by TNM stage classification as stage I (272 cases), stage II (119 cases), stage III (81 cases), stage IV (24 cases), and uncategorized (7 cases). Poor prognosis was associated with a high miR-21-5p expression group in comparison to the low expression group (hazard ratio [HR]=1.91, P<0.05, Figure 3A). The survival curve demonstrated a significant variation in the stage I group (HR=1.98, P<0.05, Figure 3B); however, the variation reported between the stages II, III, and IV groups was not statistically significant (P>0.05, Figure 3C–3E).
Figure 3.
The patient’s survival curves with lung adenocarcinoma in high and low miR-21-5p expression groups. (A) Total survival curve of miR-21-5p in high and low expression groups; (B) survival curve at stage I; (C) survival curve at stage II; (D) survival curve at stage III; (E) survival curve at stage IV. If P<0.05, the difference was statistically significant.
Predicting miR-21-5p target genes in lung adenocarcinoma
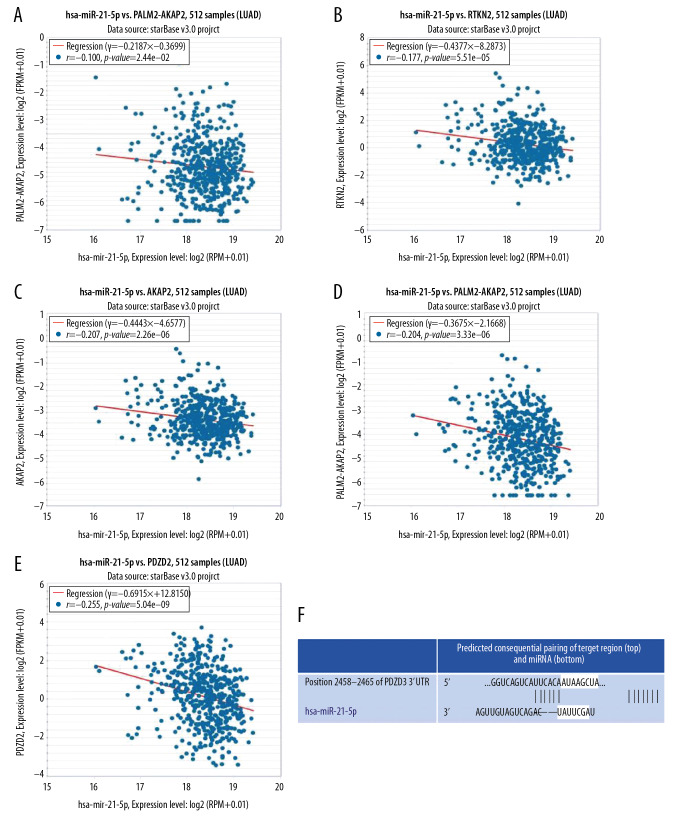
A total of 1901 target genes were predicted in the PANCANCER category with the help of the miRNA-mRNA module of the STARBASE database. Besides, the UALCAN portal was used to predict the first 250 downregulated genes in lung adenocarcinoma. We carried out a comprehensive analysis of the data retrieved from these 2 databases and screened 5 target genes (RTKN2, GCOM1, AKAP2, PALM2-AKAP2, and PDZD2), which could be suppressed by miRNA-21-5p in lung adenocarcinoma. Furthermore, we analyzed their correlation with the miR-21-5p with the help of the scatter plot (Figure 4A–4E). We found that all these 5 target genes were negatively correlated with the miR-21-5p, and the correlation coefficient of PDZD2 was r=−0.255, P<0.05, and it stood out as the most relevant target. Moreover, miR-21-5p binds to PDZD2 mRNA 3′UTR (Figure 4F).
Figure 4.
Correlation analysis and binding sites on miR-21-5p and target genes. (A–E) A negative correlation was found between the RTKN2, GCOM1, AKAP2, PALM2-AKAP2, PDZD2, and miR-21-5p; (F) miR-21-5p and PDZD2 binding sites.
The target gene PDZD2 expression in lung adenocarcinoma and normal lung tissues
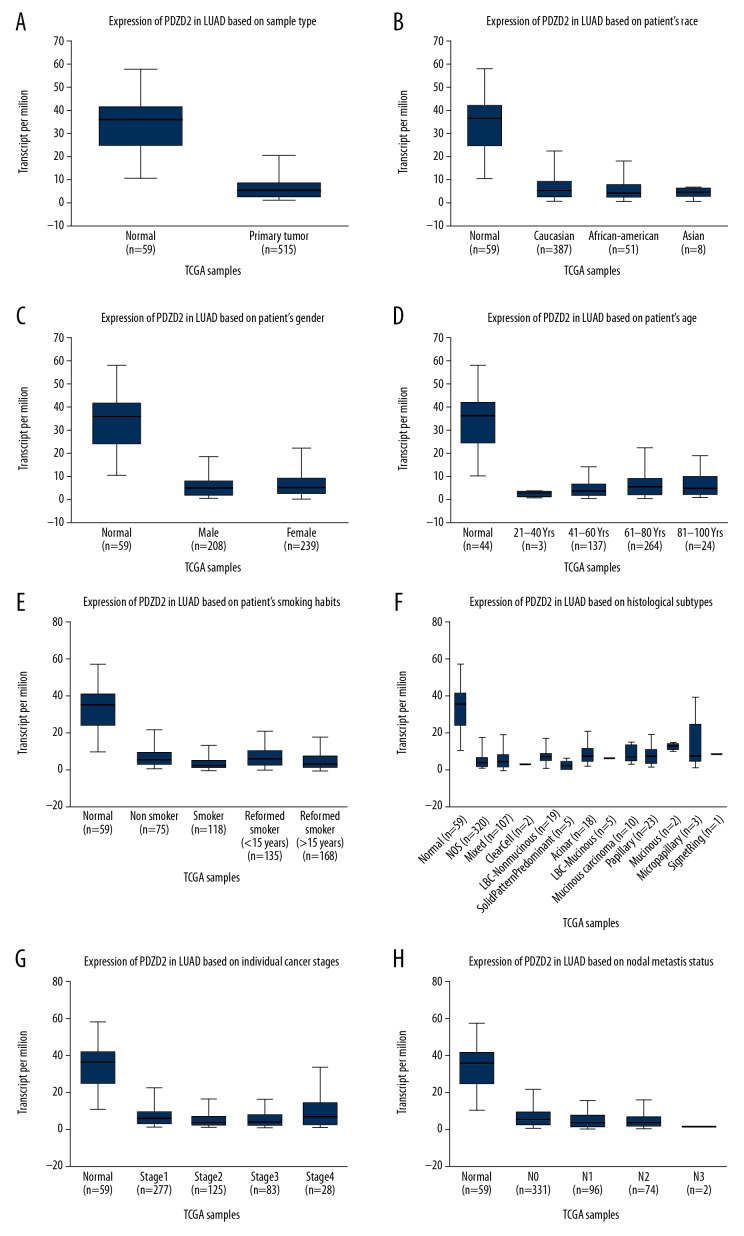
The TCGA data was used to analyze 515 tissues of lung adenocarcinoma and 59 healthy, normal tissues. The level of PDZD2 was low in the lung adenocarcinoma tissue as compared to the normal tissue with a statistically significant difference (Figure 5A). There was no correlation between the PDZD2 expression level and gender, race, age, smoking habits, TNM stage, tissue typing, or lymph node metastasis in lung adenocarcinoma patients (P<0.05, Figures 5B–5H).
Figure 5.
Differential expression of PDZD2 target gene in normal tissue and lung adenocarcinoma tissue. (A) Low level of PDZD2 in lung adenocarcinoma tissue. (B–H) Lung adenocarcinoma patient grouping by gender, race, age, smoking habits, TNM staging, tissue typing, lymph node metastasis, and PDZD2 expression level analysis. There was no significant difference.
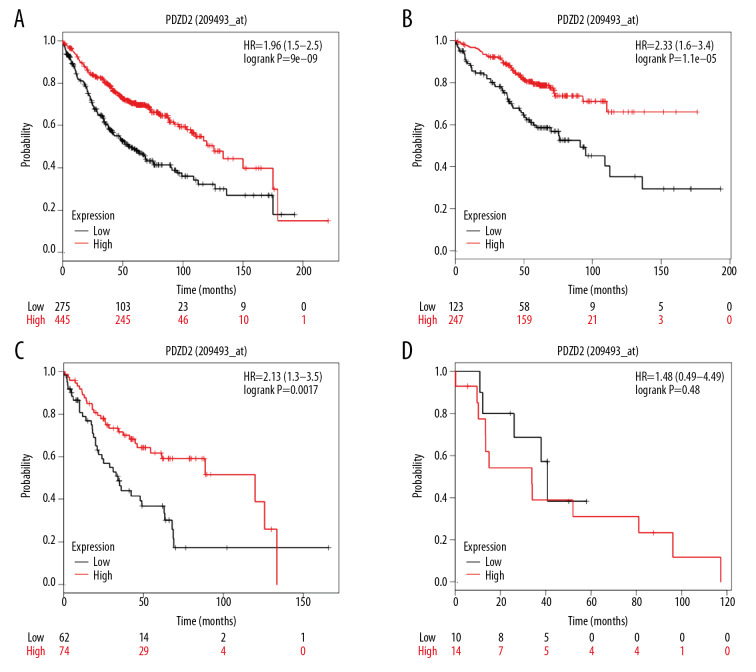
Effect of differential expression of target gene PDZD2 on lung adenocarcinoma patient prognosis
The survival curve of 720 lung adenocarcinoma patients obtained from the Kaplan-Meier plotter revealed a significantly better survival prognosis for the PDZD2 high expression group in contrast to the low expression group, which might substantially contribute to the survival time of patients (HR=1.96, P<0.05, Figure 6A). Also, we categorized the lung adenocarcinoma patients by TNM stages: stage I (370 cases), stage II (136 cases), stage III (24 cases), stage IV (4 cases), and uncategorized (186 cases). The survival curves of stage I and II suggested an increased survival prognosis for the PDZD2 high expression group (P<0.05, Figures 6B, 6C); however, no significant difference was observed in stage III (P<0.05, Figure 6D). We could not draw any statistically significant inference in stage IV due to the small sample size.
Figure 6.
The effect of PDZD2 expression differences in prognosis of lung adenocarcinoma patients. (A) PDZD2 high expression group had a better survival prognosis; (B) stage I survival curve; (C) stage II survival curve; (D) stage III survival curve. If P<0.05, the difference was statistically significant.
Discussion
MiRNA-21-5p is one of the most important members of the miRNA family and is located on human chromosome 17q23.1. Jiang et al. reported that the miRNA-21-5p functions as an oncogene in gastric cancer via the target gene SMAD7, which promotes the proliferation of gastric cancer [21]. Liu et al. showed that miRNA-21-5p inhibited tongue squamous cell carcinoma cell line apoptosis via the target gene-PDCD4, and the anti-apoptotic effect was through the regulation of the PI3K/Akt/FOXO1 signaling pathway [22]. MiRNA-21-5p is overexpressed in NSCLC tissue and leads to the onset and progression of NSCLC by target gene regulation [23,24]. So far, none of the studies have been able to report on the molecular function of the miRNA-21-5p in NSCLC due to small sample sizes of NSCLC and normal tissues. In this study, we used the UALCAN database to analyze a total of 447 lung adenocarcinoma and 44 normal tissues to exhaustively investigate the difference in miRNA-21-5p expression level in lung adenocarcinoma and normal tissue. Additionally, we plotted a survival curve with the help of the Kaplan-Meier plotter to analyze the miRNA-21-5p expression effect on lung adenocarcinoma patients’ survival prognosis. Our analysis was found to be in line with the study by Liang et al. [18], which reported the significant overexpression of the miRNA-21-5p in a group having lung adenocarcinoma compared with a healthy, normal group. This suggests that miRNA-21-5p expression in lung adenocarcinoma tissue could be a potential clinical prognostic indicator. In our study, the high expression group was associated with poor prognosis as compared to the low expression group, which significantly reduced the survival time of patients and suggested that miRNA-21-5p might function as an oncogene in lung adenocarcinoma.
To unravel the molecular mode of action of miR-21-5p in lung adenocarcinoma, we performed a molecular investigation of a total of 1901 target genes with the help of the STARBASE and UALCAN platforms. We identified 5 target genes (RTKN2, GCOM1, AKAP2, PALM2-AKAP2, and PDZD2) of miRNA-21-5p that could be downregulated by miRNA-21-5p in lung adenocarcinoma. The correlation analysis between target genes and miR-21-5p led to the identification of PDZD2 as the miR-21-5p gene target in lung adenocarcinoma. Previous studies have suggested PDZD2 as an oncogene, which is overexpressed in human primary prostate tumors and cell lines of prostate tumor. Recently it was shown that human secreted PDZD2 (sPDZD2) is anti-tumorigenic; besides, sPDZD2 induces prostate cancer cells senescence through mutation or transcriptional activation of wild type p53, and via genotoxic stress makes cancer cells more sensitive to apoptosis [25]. Moreover, Tam et al. have demonstrated that sPDZD2 exerts anti-proliferative effects in human cancer cells by influencing S-phase cell cycle arrest [26].
PDZD2 (PDZ domain-containing 2) is a protein with multi-PDZ domains, which is expressed in various tissues such as the brain, spleen, lungs, and kidneys. Previous studies have reported its role in insulinoma proliferation and its overexpression in prostate cancer [27–29]. However, no attempt has been made to explore the function of PDZD2 in lung adenocarcinoma.
In this study, we have analyzed a large cohort of tissue sample data and found that PDZD2 was under expressed in lung adenocarcinoma tissue, which suggested that PDZD2 might act as a tumor suppressor in lung adenocarcinoma. As per our survival curve analysis, the high PDZD2 expression group showed a better prognosis than the low expression group, which could effectively prolong patient survival.
Conclusions
We established the significance of miR-21-5p in patient prognostic with lung adenocarcinoma by analyzing bioinformatics databases, namely TCGA, STARBASE, and UALCAN. Also, miR-21-5p might regulate the onset and progression of lung adenocarcinoma via its target gene PDZD2, but this analysis had some limitations. Since we did not perform any laboratory experiments, we were not able to determine the underlying molecular mechanism. In future studies, we need to perform laboratory experiments to examine the molecular mechanism of PDZD2- mediated action of miR-21-5p in lung adenocarcinoma development.
Footnotes
Conflicts of interest
None.
Source of support: Natural Science Foundation of Guangdong Province, Grant/Award Number: 2019A1515012079
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ma L, Xie X-W, Wang H-Y, et al. Clinical evaluation of tumor markers for diagnosis in patients with non-small cell lung cancer in China. Asian Pac J Cancer Prev. 2015;16:4891–94. doi: 10.7314/apjcp.2015.16.12.4891. [DOI] [PubMed] [Google Scholar]
- 3.Li A, Yu J, Kim H, et al. MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res. 2013;19:3600–10. doi: 10.1158/1078-0432.CCR-12-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominioni L, Imperatori A, Rovera F, et al. Stage I non-small cell lung carcinoma: Analysis of survival and implications for screening. Cancer. 2000;89:2334–44. doi: 10.1002/1097-0142(20001201)89:11+<2334::aid-cncr4>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Ganti AK. Lung cancer screening. Oncologist. 2006;11:481–87. doi: 10.1634/theoncologist.11-5-481. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, et al. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–56. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 7.Brambilla C, Fievet F, Jeanmart M, et al. Early detection of lung cancer: Role of biomarkers. Eur Respir J. 2003;21:36s–44s. doi: 10.1183/09031936.02.00062002. [DOI] [PubMed] [Google Scholar]
- 8.Patz EF, Goodman PC, Bepler G. Screening for lung cancer. N Engl J Med. 2000;343:1627–33. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- 9.Rossi A, Maione P, Colantuoni G, et al. Screening for lung cancer: New horizons? Crit Rev Oncol Hematol. 2005;56:311–20. doi: 10.1016/j.critrevonc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Ye M-f, Zhang J-g, Guo T-x, et al. MiR-504 inhibits cell proliferation and invasion by targeting LOXL2 in non-small cell lung cancer. Biomed Pharmacother. 2018;97:1289–95. doi: 10.1016/j.biopha.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Wang Z, Liu Z, Fang X, et al. MiR-142-5p suppresses tumorigenesis by targeting PIK3CA in non-small cell lung cancer. Cell Physiol Biochem. 2017;43:2505–15. doi: 10.1159/000484459. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y, Sheng B, Xia Q, et al. Association of long non-coding RNA H19 and microRNA-21 expression with the biological features and prognosis of non-small cell lung cancer. Cancer Gene Ther. 2017;24:317–24. doi: 10.1038/cgt.2017.20. [DOI] [PubMed] [Google Scholar]
- 13.Ma Z, Liu T, Huang W, et al. MicroRNA regulatory pathway analysis identifies miR-142-5p as a negative regulator of TGF-β pathway via targeting SMAD3. Oncotarget. 2016;44:71504–13. doi: 10.18632/oncotarget.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandrashekar DS, Bashel B, Balasubramanya SAH, et al. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–58. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyorffy B, Surowiak P, Budczies J, et al. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8(12):e82241. doi: 10.1371/journal.pone.0082241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Li JH, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puik JR, Meijer LL, Le Large TYS, et al. miRNA profiling for diagnosis, prognosis and stratification of cancer treatment in cholangiocarcinoma. Pharmacogenomics. 2017;18:1343–58. doi: 10.2217/pgs-2017-0010. [DOI] [PubMed] [Google Scholar]
- 19.Takikawa T, Masamune A, Yoshida N, et al. Exosomes derived from pancreatic stellate cells. Pancreas. 2017;46:19–27. doi: 10.1097/MPA.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 20.Zhong J, Ren X, Chen Z, et al. miR-21-5p promotes lung adenocarcinoma progression partially through targeting SET/TAF-Iα. Life Sci. 2019;231:116539. doi: 10.1016/j.lfs.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, Zhang M, Guo T, et al. MicroRNA-21-5p promotes proliferation of gastric cancer cells through targeting SMAD7. Onco Targets Ther. 2018;11:4901–11. doi: 10.2147/OTT.S163771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Tong Z, Tan J, et al. MicroRNA-21-5p targeting PDCD4 suppresses apoptosis via regulating the PI3K/AKT/FOXO1 signaling pathway in tongue squamous cell carcinoma. Exp Ther Med. 2019;18:3543–51. doi: 10.3892/etm.2019.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan L, Ma J, Wang Y, et al. miR-21-5p induces cell proliferation by targeting TGFBI in non-small cell lung cancer cells. Exp Ther Med. 2018;16:4655–63. doi: 10.3892/etm.2018.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Wu X. miR-21-5p promotes the progression of non-small-cell lung cancer by regulating the expression of SMAD7. Onco Targets Ther. 2018;11:8445–54. doi: 10.2147/OTT.S172393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam CW, Cheng AS, Ma RYM, et al. Inhibition of prostate cancer cell growth by human secreted PDZ domain-containing protein 2, a potential autocrine prostate tumor suppressor. Endocrinology. 2006;147:5023–33. doi: 10.1210/en.2006-0207. [DOI] [PubMed] [Google Scholar]
- 26.Tam CW, Liu VWS, Leung WY, et al. The autocrine human secreted PDZ domain-containing protein 2 (sPDZD2) induces senescence or quiescence of prostate, breast and liver cancer cells via transcriptional activation of p53. Cancer Lett. 2008;271:64–80. doi: 10.1016/j.canlet.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 27.Ma RYM, Tam TSM, Suen APM, et al. Secreted PDZD2 exerts concentration-dependent effects on the proliferation of INS-1E cells. Int J Biochem Cell Biol. 2006;38:1015–22. doi: 10.1016/j.biocel.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Chaib H, Rubin MA, Mucci NR, et al. Activated in prostate cancer: A PDZ domain-containing protein highly expressed in human primary prostate tumors. Cancer Res. 2001;61:2390–94. [PubMed] [Google Scholar]
- 29.Yeung M-L, Tam TSM, Tsang ACC, et al. Proteolytic cleavage of PDZD2 generates a secreted peptide containing two PDZ domains. EMBO Rep. 2003;4:412–18. doi: 10.1038/sj.embor.embor804. [DOI] [PMC free article] [PubMed] [Google Scholar]