Abstract
Background
Cortical spreading depression (CSD) underlies the neurobiology of migraine with aura (MWA). Animal studies reveal networks of microvessels linking brain-meninges-bone marrow. CSD activates the trigeminovascular system, evoking a meningeal inflammatory response. Accordingly, this study examines the upregulation of an inflammatory marker in extra-axial tissues in migraine with visual aura.
Methods
We used simultaneously acquired 11C-PBR28 PET/MRI data of 18kDa translocator protein (an inflammatory marker) in MWA patients (n=11) who experienced headaches and visual aura in the preceding month. We measured mean tracer uptake (SUVR) in four regions of interest comprising the meninges plus the adjacent overlying skull bone (parameningeal tissues, PMT). These data were compared to healthy controls and patients with pain (chronic low-back pain, CLBP).
Results
MWA had significantly higher mean SUVR in PMT overlying occipital cortex than both other groups, though not in the PMT overlying three other cortical areas. A positive correlation was also found between the number of visual auras and tracer uptake in occipital PMT.
Interpretation
A strong persistent extra-axial inflammatory signal was found in meninges and calvarial bone overlying the occipital lobe in migraine with visual auras. Our findings are reminiscent of CSD-induced meningeal inflammation and provide the first imaging evidence implicating inflammation in the pathophysiology of migraine meningeal symptoms. We suspect that this inflammatory focus results from a signal that migrates from underlying brain and if so, may implicate newly discovered bridging vessels that crosstalk between brain and skull marrow, a finding of potential relevance to migraine plus other neuroinflammatory brain disorders.
Keywords: migraine, migraine visual aura, cortical spreading depression, inflammation, skull-brain interactions, meninges
Introduction
According to the World Health Organization, migraine is the sixth most prevalent medical disorder on the planet in terms of Global Burden of Disease1. Migraine with aura constitutes approximately 25% of cases, and the aura, which most often anticipates the headache, may be visual (more than 90%), sensory, speech or motor. Migraine patients also experience symptoms such as photophobia suggesting the presence of meningeal inflammation or irritation.
Cortical spreading depression (CSD) underlies migraine with visual aura (MWA) (2; for review see 3). CSD is a slow (2-5 mm/min), self-propagating wave of nearly complete depolarization of neurons and glia that is easily evoked in cortical grey matter3. Furthermore, CSD triggers an inflammatory response in brain and meninges. It sensitizes and activates trigeminal afferents within meninges 4–6, the only nociceptor-containing tissue within the cranium.
Although suspected, meningeal inflammation has never been documented in a population of migraineurs. Most clinical studies in migraineurs measure blood flow, vessel caliber or BOLD responses within brain, and rarely within meninges. Hence the importance and novelty of this study using a 2nd generation PET ligand 11C-PBR28 that detects activated inflammatory cells (reviewed in 7, 8). Its binding site, the 18-kD translocator protein (TSPO), is located on outer mitochondrial membranes. Previously known as the peripheral benzodiazepine receptor, TSPO, expressed at low levels in healthy CNS tissue, is often used as a marker of glial activation within brain parenchyma9,10 reflecting activated microglia and/or astrocytes in multiple animal models of disease as well as humans8, 11, 12.
In addition, TSPO is upregulated in activated macrophages and other peripheral immune cells13, and therefore can also be used as an imaging marker of peripheral inflammation. Our group recently published a clinical report in MWA showing increased 11C-PBR28 uptake in pain processing pathways including the thalamus, the primary and secondary somatosensory and insular cortices and the spinal trigeminal nucleus SpV, as well as in occipital areas shown to be involved in CSD generation14. Notably, in a rat CSD model, elevated TSPO signal sustained for at least 15 days in ipsilateral hemisphere15, not unlike what we recently observed in primary visual cortex in migraineurs14.
Here we examined parameningeal tissues (PMT) by simultaneous PET/MRI using 11C-PBR28 in humans with migraine visual auras. Data from the same pool of participants reported in 14 were further analyzed here to specifically interrogate parameningeal tissues including meninges and bone marrow, and their relationship to the underlying brain. We sought to determine whether there was a robust inflammatory signal in parameningeal tissues overlying occipital lobe as anticipated from numerous preclinical data. We compared results to regions remote from visual cortex and compared results to either chronic pain patients (CLBP) or healthy controls. We also examined whether the elevated uptake in occipital PMT correlated with the number of visual auras, as well as determined the impact of time since last attack on signal intensity.
The data show that parameningeal inflammation overlying visual cortex is a consistent phenotype in migraine with visual aura. This phenotype may explain the symptoms of meningeal irritation in migraineurs and provide further insights into migraine pathophysiology.
Methods
All participants gave written informed consent and the protocol was approved by the Institutional Review Board (IRB) of Partners Healthcare.
Participants
Among 40 subjects initially responding to a questionnaire, 13 patients were entered into the study (10 females; mean age: 31.15, range 18-65). They all fulfilled the International Headache Society (IHS) criteria for MWA16, suffered from episodic migraine (less than 15 headache days per month) and kept a diary where they reported (a) days (date) when they were experiencing a migraine (with or without aura), (b) the time when the attack started, (c) the severity of the attack, (c) the presence or not of nausea/vomiting, as well as (d) medications taken. The diary was kept for at least four weeks prior to PET/MRI imaging. During enrollment, patients were also interviewed about the quality of their migraine attacks, including whether they usually experienced nausea, phonophobia, and photophobia, and the characteristics of the aura they would typically experience. They were also interviewed about their migraine history (number of years, frequency, length of crises, triggers) and their usual medication. Prior to the scanning session, patients discussed in a second interview the content of their diary including information about the presence/absence of auras. For the present study, we only considered migraineurs who had at least one migraine with visual aura during the previous four weeks, resulting in the inclusion of 11 of the 13 patients in the final analyses.
During an initial screening visit, a blood sample was obtained to genotype subjects for the Ala147Thr TSPO polymorphism, which is known to affect binding affinity for 11C-PBR2817. Low-affinity binders (Thr/Thr) were excluded from participation. Only high-affinity binders (HABs, Ala/Ala) and mixed-affinity binders (MABs, Ala/Thr) were included. Exclusion criteria for migraineurs were contraindications for PET or MR scanning (e.g. pregnancy, claustrophobia, ferromagnetic implants, benzodiazepines). The final group of patients consisted of 11 individuals (1 male; mean age = 29.6, range 18-65). Seven of these 11 participants were HABs, and 4 were MABs.
Every patient experienced at least one episode of migraine headache in the previous 4 weeks of observation (median number of attacks: 3, range 1-11). One patient (#113) experienced a migraine headache with no aura starting during the scanning session but did complete the study. The median number of visual auras experienced was 3 per patient (range 1-6), and aura symptoms were either purely visual or a combination of visual, sensory, language and cognitive. Auras lasted for less than an hour. Descriptions of the visual auras included spots, glitters, blurred vision, scotoma, partial field cut and classical visual aura18. During attacks, 11 patients experienced photophobia, 10 noted phonophobia, and about half reported feeling nauseated. Except for patient #113 (as noted above), migraine subjects were pain free during the imaging study. For our patient population, the median number of days between the last attack and the scanning session was 8 days, and the longest interval between an attack and the scanning session was 18 days (patient #112).
None of the migraineurs were taking preventive medication, and they refrained from taking NSAIDS in the two weeks preceding the scanning session. Clinical details of individual migraineurs can be found in Table 1 and in Albrecht et al.14.
Table 1: Patients’ clinical characteristics.
Binding affinity: HAB= high affinity binder; MAB = mixed affinity binder; Age: age at scan; Aura type: Type of aura experienced by the patient before a migraine attack, as reported in their migraine history; Frequency: reported history of migraine frequency per month; Total # of attacks: total number of migraine attacks in the 4 weeks preceding the scan; # of headaches without aura: number of headaches during the 4 weeks preceding the scan that were not accompanied by a visual aura; # of auras: number of auras (with or without headache, in parenthesis) during the 4 weeks preceding the scan; # of days before scan: number of days between the last migraine attack and the scan.
| ID | Binding affinity | Sex | Age | Aura type | Frequency | Total # of attacks | # of headaches without aura | # of auras | # of days before scan |
|---|---|---|---|---|---|---|---|---|---|
| 104 | HAB | F | 27 | Visual + slurred speech | 2-6 | 3 | 0 | 3 (3/0) | 4 |
| 105 | HAB | F | 22 | Visual + vertigo | 10-15 | 3 | 0 | 3 (1/2) | 4 |
| 109 | MAB | F | 30 | Visual + sensory symptoms + aphasia | 2 | 4 | 0 | 4 (4/0) | 14 |
| 112 | HAB | F | 35 | Visual + sensory symptoms+ slurred speech | 1-3 | 3 | 0 | 3 (3/0) | 18 |
| 113 | MAB | M | 37 | Visual | 1-3 | 11 | 8 | 3 (3/0) | 0 |
| 114 | HAB | F | 22 | Visual | 3-4 | 2 | 0 | 2 (2/0) | 7 |
| 116 | MAB | F | 23 | Visual + speech disturbances + confusion | 2-3 | 5 | 2 | 3 (0/3) | 2 |
| 118 | HAB | F | 65 | Visual + confusion, difficulties finding words + sensory symptoms+ | 8 | 5 | 3 | 2 (2/0) | 9 |
| 123 | HAB | F | 23 | Visual + sensory symptoms | 1 | 1 | 0 | 1 (1/0) | 14 |
| 125 | MAB | F | 23 | Visual + confusion, anxiety + sensory symptoms | 1 | 3 | 0 | 3 (3/0) | 9 |
| 126 | HAB | F | 18 | Visual + slurred speech | 3 | 7 | 1 | 6 (6/0) | 8 |
Patients with photophobia (N=11); Patients with phonophobia (N=10 – all but #112)
Protocol:
The timing of the protocol was chosen based on a report in rodents, where a published study found higher TSPO uptake at 15 days after CSD than at earlier time points (later points were not examined) 15. We instructed patients to start a diary for two weeks, and to call us when they had a migraine attack. Based on this call, we could schedule an imaging session more or less two weeks later. Some did not have any more episodes after calling us (e.g. #109, #112, and #123), whereas others had several more attacks. Our primary aim was to determine whether there was a robust PET signal enhancement in this population with multiple auras.
We compared data from 11 migraineurs with aura to data from two other groups with similar age range and matched for binding affinity: 11 healthy controls (CON) (7 HAB; 6 males; mean age: 34.9, range 22-52) and 11 patients suffering from chronic low-back pain (CLBP) (7 HAB; 3 males; mean age: 45.9, range 27-63). All CLBP patients had a diagnosis of back pain (either with or without radicular pain complaints) dating at least 2 years prior to enrollment, no history of major medical disorders, and were not on benzodiazepines, blood thinners. Opioids were allowed only if at low or moderate doses (< 60 mg morphine equivalents). Healthy controls had the same inclusion / exclusion criteria as the patients, except that they were required not to have a history of migraine or chronic low back pain.
The CLBP cohort was included to evaluate whether any changes observed in the MWA patients were specific to migraine, or represent a more general feature common to disorders accompanied by pain, regardless of etiology. There were more females in the migraine cohort, reflecting the higher female prevalence of migraine and the availability of qualified subjects. There were no significant differences in demographics between CLBP and CON, and between CON and MWA although a one-way ANOVA analysis showed a significant difference in age between the 3 groups (F=4.6, p=0.02) and post-hoc Tukey’s multiple comparison showed that the CLBP were older than migraineurs. CLBP had back pain with or without a radicular component and reported on average pain during the scan of 31.5±24.6 (SD) on a 0–100 numerical rating scale. Neither CLBP patients or healthy controls reported a history of migraine during the history and physical examination.
Image acquisition
The PET data were acquired on a prototype MR-compatible brain PET scanner (“BrainPET”) designed to fit inside the Magnetom Tim Trio 3T MRI scanner (Siemens Healthcare, Erlangen, Germany). Structural T1 images were acquired prior to radiotracer injection. Dynamic PET acquisition started concurrently with an injected IV bolus of 11C-PBR28, and data were stored in listmode format. T1 images were used for the generation of attenuation correction maps19 as well as anatomical localization and spatial normalization of the PET data.
Data processing and analysis
Analytic details are described in Albrecht et al.14, except that here skull/meninges were not masked out (as customary in brain imaging studies), but rather used to create regions-of-interest. Briefly, Standardized Uptake Value (SUV) images (60–90 minutes post 11C-PBR28 administration) were generated, co-registered to the MPRAGE, nonlinearly transformed into MNI space and smoothed with a 2mm FWHM Gaussian kernel, using a combination of FSL (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki), Freesurfer (https://surfer.nmr.mgh.harvard.edu/) and SPM tools (https://www.fil.ion.ucl.ac.uk/spm/). Voxel-wise SUV ratio (SUVR) images were obtained by dividing SUV images by average SUV from a pseudo-reference region, as previously described14. Data were first examined in subjects’ native space (i.e. before transformation into MNI space).
Anatomical regions of interest (ROIs) were created manually on the average MNI brain in FSL, in the meninges/skull marrow over the convexity. They were then automatically applied as a mask on the MNI-normalized brain of each individual subject. These ROIs included the skull marrow as well as the inner periosteum of the skull (dura mater). These two tissues were not discernible by PET due to low inherent imaging resolution; for this reason, we use the term PMT. The ROIs were drawn to test the hypothesis that an enhanced binding signal would be detected within tissues overlying the brain, especially within bone marrow. The ROI covered the PMT over the visual cortex, the orbitofrontal cortex, the dorsolateral prefrontal cortex (DLPFC), and the parietal cortex. All ROIs had the same volume (100 voxels). Regions of interest were chosen based on their proximity to occipital lobe (parietal lobe), their relation to sensory processing and modulation (DLPFC), or as a negative or neutral reference (orbitofrontal cortex) (Figure 1).
Figure 1:
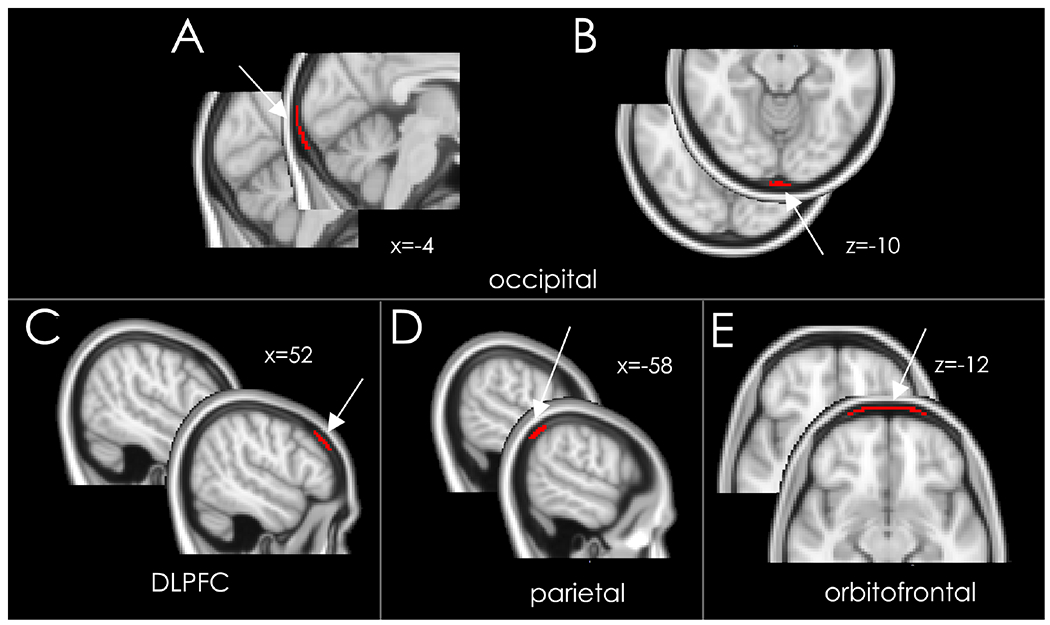
Meninges/skull bone marrow ROI. The ROIs location are indicated by an arrow, and the native image is shown next to each ROI to show the PMT area. Panels A and B shows the occipital meninges/skull ROI, Panel C, D and E the control ROIs, on the MNI 152 brain template. Panel C: DLPFC; Panel D: parietal; Panel E: orbitofrontal. Each ROI contained 100 voxels. x and z refer to the MNI coordinates of the slices shown in each panel.
Using fslmaths, an FSL tool, SUVR were automatically extracted from these ROIs in all three groups. Fslstats (FSL) was used to calculate the mean SUVR in the all ROI and to create a histogram showing the distribution of values, with 10 bins from 0.3 to 3.0. We tested the three following hypotheses: in the meningeal/skull marrow ROI, whether the percentage of voxels showing increased 11C-PBR28 uptake would (i) be higher over the visual cortex in migraineurs than in both control groups; (ii) be similar over the other 3 cortical areas among all three groups; (iii) show a positive correlation with the number of visual aura episodes in the four weeks preceding the scan in the occipital PMT. We also tested whether the signal correlated with the number of migraine attacks (migraine with + without aura) in the past four weeks, and with number of days since the last attack.
Statistical analyses.
ANOVA and post-hoc Tukey-corrected 2-tailed t-tests were used to compare mean SUVR between the three groups. Data were considered significant of P<0.05, and we also report 95% Confidence Intervals. Spearman rho was used for the correlation analyses of the SUVR with (1) the number of visual aura episodes in the previous four weeks (with or without headache), as well as (2) with the total number of migraine attacks in the previous four weeks and (3) with the number of days before the scanning since the last attack. Unpaired t-test was used to compare SUVR between migraineurs scanned shortly after their last migraine attack (0-7 days) vs later (8-18 days). Effect size was computed with Cohen’s d formula.
Data availability.
Anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Consistent with our hypotheses, we found elevated signal in the occipital PMT and not in the other three brain regions in migraineurs compared to other groups. Figure 2 illustrates the spatial distribution of SUVR in occipital cortex and in occipital PMT as represented in the average MNI brain. Compared with CLBP patients and healthy controls, MWA patients exhibit increased uptake in PMT overlying occipital cortex.
Figure 2:
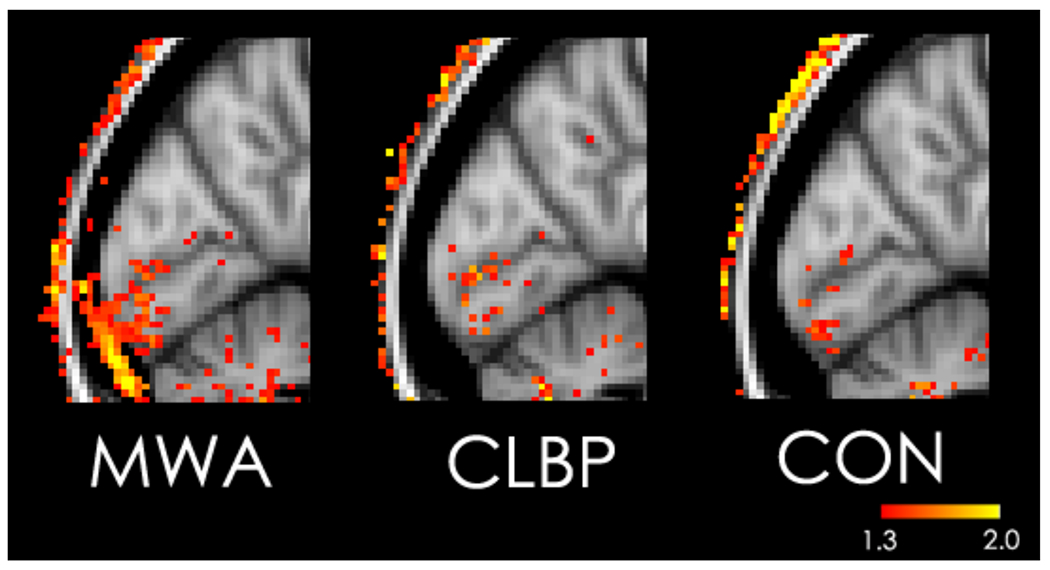
SUVR value in the occipital cortex and PMT in the average data for each group, on the MNI125 brain template. MWA: Migraineurs With Aura; CLBP: Chronic Low Back Pain patients; CON: healthy controls.
We then examined the signal in native space of individual patients. As shown in Figure 3, uptake could be observed overlying the meninges and in the skull area in four representative patients who had experienced migraine and visual auras. Enhanced PET signal was observed in nearly all patients14 in primary visual cortex (black arrows) and in bone marrow (sm, white arrows) and in the expected vicinity of the meninges (m, red arrows).
Figure 3:
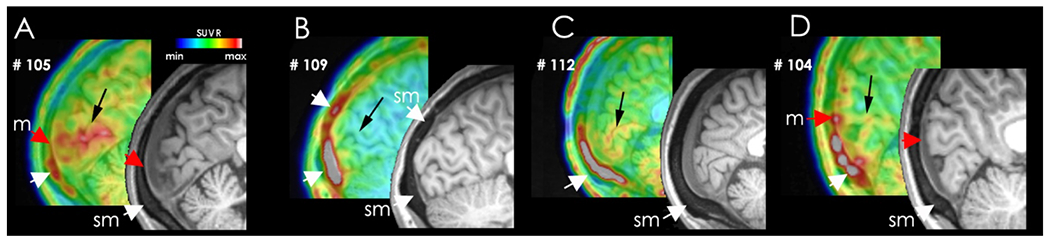
Native images of PET tracer uptake in the skull marrow/meninges in original PET/MRI data in the occipital cortex and adjacent PMT in four representative patients. Black arrows – primary visual cortex; sm – skull marrow; m – location of meninges. PET data are color-coded in a spectrum scale ranging from low (dark blue) to high (grey).
The histogram in Figure 4 shows a rightward skewed distribution of SUVR in MWA compared with CLBP and healthy controls. These data reflect a higher uptake in occipital PMT in migraineurs compared with both other groups.
Figure 4:
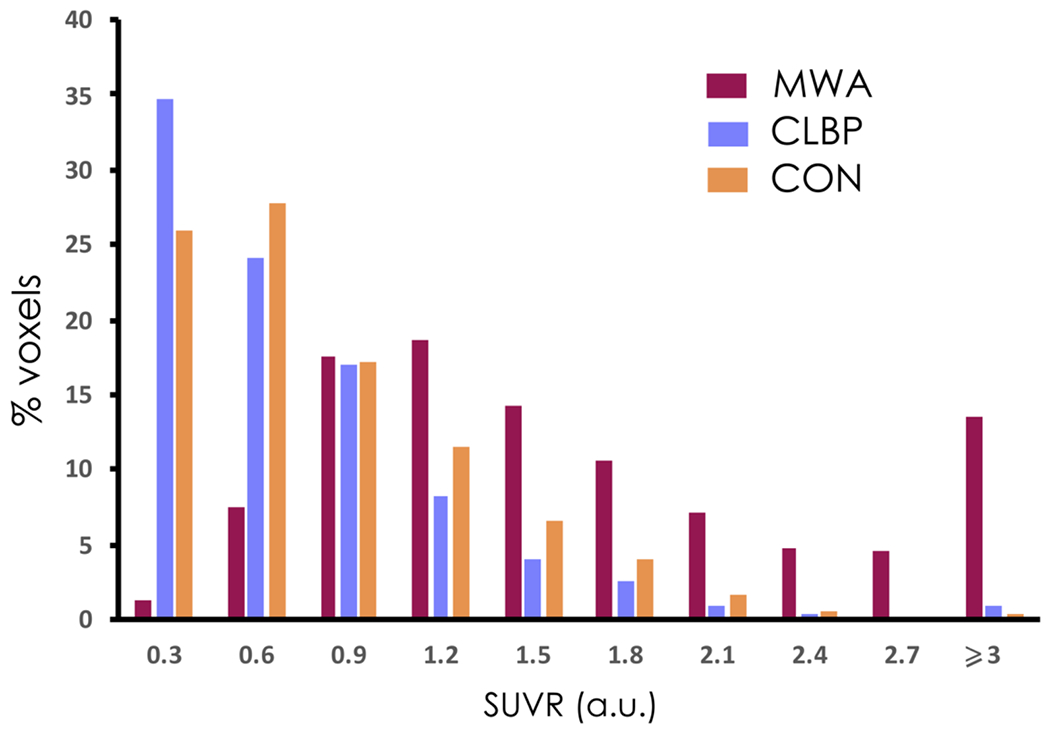
Histogram of distribution of the mean SUVR values (arbitrary units, a.u.) in the occipital PMT for the migraineurs with aura (MWA), Chronic Low Back Pain patients (CLBP) and healthy controls (CON). Note the higher values of SUVRs in MWA compared to the other groups.
Note that within groups, there were no significant PET signal differences in the occipital PMT between the mixed and high affinity binders (MAB and HAB, n=4, 7 in each group, respectively) (migraineurs: t=0.71, p=0.5; CLBP t=1.75, p=0.1; controls: t=0.81, p=0.44).
We then examined whether this increased uptake was specific to the occipital PMT. A 4 [visual, orbitofrontal, dorsolateral prefrontal cortex (DLPFC) and parietal] by 3 (migraineurs, CLBP, controls) ANOVA evaluating SUVR group differences showed significant interaction (F (6,90) =12.16, p<0.0001) (Figure 5).
Figure 5:
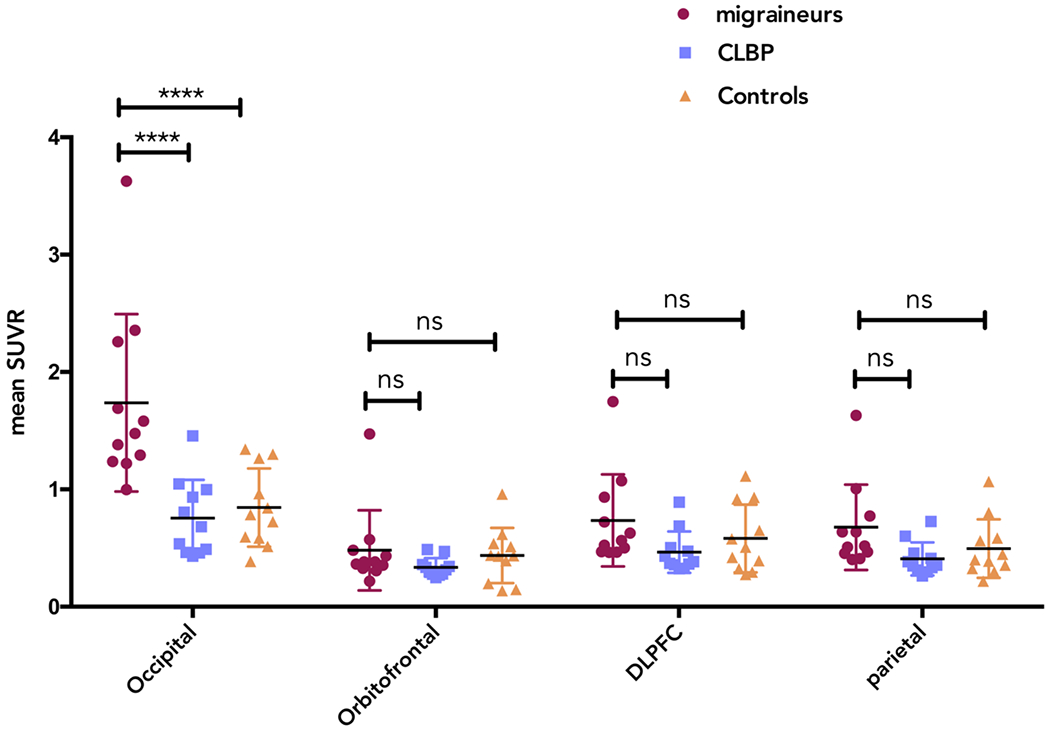
Mean SUVR values in each ROI for each group in PMT overlying the occipital, orbitofrontal, DLPFC and parietal regions. Migraineurs with aura (MWA, circles), Chronic Low Back Pain patients (CLBP, squares) and healthy controls (CON, triangles). **** denotes statistical significance at a probability value of p<0.0001.
For the occipital PMT, Tukey’s multiple comparison test showed significant differences with large effect sizes between MWA and CLBP and between MWA and CON (p<0.0001, 95% CI [0.65-1.32], Cohen’s d = 1.53 and p<0.0001, 95% CI [0.56-1.28], Cohen’s d = 1.69, respectively). No differences were found when CLBP and CON were compared using Tukey multiple comparison test (ns, 95% CI [−0.44-0.26]). For the orbitofrontal PMT, no significant differences were found between groups (MWA vs CLBP 95%CI [−0.21-0.50]; MWA vs. CON 95% CI [−0.31-0.39]; CLBP vs. CON 95% CI [−0.45-0.25]) Similarly, for the DLPFC PMT, there were no significant differences between groups (MWA vs CLBP 95%CI [−0.08-0.62]; MWA vs. CON 95% CI [−0.20-0.51]; CLBP vs. CON 95% CI [−0.47-0.23]) using Tukey multiple comparison test. Likewise, for the parietal PMT, Tukey’s multiple comparison test showed no significant differences between groups (MWA vs CLBP 95%CI [−0.08-0.62]; MWA vs. CON 95% CI [−0.17-0.53]; CLBP vs. CON 95% CI [−0.44-0.26]).
We then tested the hypothesis that elevated tracer uptake in occipital PMT would correlate positively with the total number of visual auras. We found a statistically significant correlation between the mean SUVR and the total number of visual aura episodes (Spearman’s rho=0.74, p=0.01).
There was, however, no significant correlation between PMT uptake and the total number of migraine attacks (with + without aura) in the previous 4 weeks (r=0.14, p=0.7). Moreover, there was no correlation between PMT uptake and the number of days since the last attack (r=0.27, p=0.4) and the enhanced uptake did not appear to diminish with time.
Discussion
This study provides the first evidence of a persistent extra-axial inflammatory signal in a subtype of migraine, in patients with MWA. We found that: (i) Migraine with visual aura is associated with a sustained and enhanced inflammatory signal (i.e. increased 11C-PBR28 binding reflecting increased TSPO expression) in a space occupied by meninges and bone (parameningeal (PMT) signal) overlying activated occipital cortex (also showing increased 11C-PBR28 binding, see 14). This uptake is greater in migraineurs than in both healthy control subjects and those with chronic low back pain, the latter condition also characterized by brain inflammation20 (Figures 2, 4 and 5). (ii) By contrast, this increase in binding was not observed overlying the parietal, DLPFC or orbitofrontal cortex (Figure 5). (iii) The differences between 11C-PBR28 binding in migraineurs and CLBP suggest that the experience of pain may not necessarily be accompanied by PMT inflammation (Figure 5). (iv) The enhanced PET signal in occipital PMT did not diminish with time as there were no differences when comparing scans (SUVR) obtained 0-7 vs 8-18 vs 0-4 days vs 14-18 after the last attack. Taken together, these results are consistent with the conclusion that extra-axial inflammation is significantly augmented and prolonged in tissues overlying cortex generating visual aura. Our study offers evidence that PET imaging of an inflammatory signal provides an “historical fingerprint” of events during migraine with visual aura as well as identifies a phenotype of this migraine subtype.
We considered the merits of a time-dependent study but quickly realized that our study population (subjects with multiple episodes with short attack intervals during a 30-day period) were not ideal for such a study design. We now know this to be true because we detected significant TSPO signal carryover between episodes that remained undiminished for even 18 days since the last attack (patient #112). Therefore, without multiple scanning sessions (isotope injections) before and after attacks in each subject, time-dependent data would have been misleading. Nevertheless, the knowledge gained from studying these 11 patients showing a robust and sustained signal enhancement, better informs us going forward regarding the proper design of a time-dependent study using multiple injections in subjects whose attack intervals are much less frequent (e.g., one per month). Studying migraineurs with multiple episodes did maximize the possibility of detecting an enhanced inflammatory signal. Moreover, it also allowed us to establish a positive statistical relationship between signal enhancement and the number of visual aura episodes.
A site of extra-axial inflammation has not been identified previously in migraine patients. Clinically, aspirin and NSAIDs have long been used to treat migraine; steroids use has also been successful in some patients21. At the preclinical level, neurogenic inflammation has been well studied in migraine models and involves the release of vasoactive neuropeptides such as calcitonin gene-related peptide (CGRP), pituitary adenylyl cyclase activating peptide (PACAP) and substance P from trigeminovascular axons22 that innervate the meninges23 and the bone24. Other implicated inflammatory mediators include protons (acid sensing ion channels), COX-2 generating prostaglandins and other cyclooxygenase products, serine proteases, nitric oxide (iNOS) and IL-6 as well as macrophages, dendritic cells and mast cells21, 25–28. Relevant to migraine aura, CSD generates proinflammatory prostaglandins and nitric oxide29 and promotes mast cell degranulation5, 22, meningeal edema5, 27, dilation of the middle meningeal artery as well as macrophage and dendritic cell activation6.
Inflammation may be implicated even in migraine without aura. In one patient, extensive extravasation of technetium-99m labeled human serum albumin was observed over the convexity and in the epicenter of pain at 3 hours but not after 3 days 30. Moreover, in a model that mimics a human model of migraine without aura31, dural macrophages started to express IL-1,IL-6 and iNOS, as mast cells degranulate hours after administering the triggering agent, nitroglycerin32.
It seems reasonable to accept the notion that meningeal inflammation contributes to the pathophysiology of photophobia and possibly phonophobia. All 11 migraineurs experienced photophobia. One proposed mechanism to explain it involves sensitization of trigeminovascular afferents and their downstream thalamic targets on neurons receiving converging inputs from the retina33. Much less is known about phonophobia, but it may involve similar mechanisms. What is less clear is the relationship of the enhanced PMT signal to pain, headache and its history. Unlike the number of auras, there was no correlation between the number of headaches and SUVR, albeit headache intensity and duration were not considered. Importantly however, TSPO has never been tested as a biomarker for pain, nor as a marker to reflect activity in primary afferent fibers. To more specifically address this complex relationship, scanning cohorts that experience visual auras without headache as well as experience only migraine headache may prove useful.
Inflammation in Skull and Bone Marrow
The extent of the inflammatory signal in calvarial bone was unanticipated. Colocalizing PET images with higher-resolution MRI indicates that this signal also includes the spongy layer of bone between the inner and outer skull table. This tissue contains hematopoietic bone marrow, a blood cell production site that also gives rise to inflammatory leukocytes. In meninges or bone marrow, there are numerous TSPO-expressing cell types that potentially could overexpress and thus provide enhanced binding sites. In the dura mater, these cells include macrophages, dendritic cells and mast cells (www.immgen.org). In the bone marrow, they include macrophages, monocytes, myeloid progenitor cells and neutrophils, even though the latter do not feature in the classic repertoire of migraine-responding cell types. However, macrophages, mast cells and dendritic cells have been implicated. In addition, TSPO is expressed in adipocytes or fat cells and it is well established that adipocytes are a major source of multiple cytokines (e.g., adiponectin34, 35). Although not much is known, the process of age-dependent fat replacement of bone marrow is highly dynamic and the young migraine population under study could well have bone inflammatory signals emanating from adipocytes in addition to bone marrow cells, as by age 31 (the mean age of our migraineurs), it has been shown that in trochanter and femur, 10% and 25 % of the marrow is fat, respectively 36. In normal human skull, this process may be accelerated, although to our knowledge, specimens from patients with neuroinflammatory conditions have not been studied.
Whether the inflammatory signal migrates from underlying brain to meninges to bone marrow is of interest to us. Regarding microvascular channels, the skulls of both mice and also humans contain channels that may provide a bidirectional vascular bridge between meninges and skull bone marrow37 and perhaps cortex37, 38. Existing animal data suggest a centrifugally propagating signal from cortical gray matter to surrounding tissues, possibly via CSF route 39, via microvascular channels or depolarization of traversing trigeminal afferents 40 or a combination of the above. In the mouse, these vascular channels are lined with endothelial cells, are approximately 20 μm wide37, 38, 41 and express alpha-SMA and the basement membrane protein laminin38. In humans, these vessels are estimated to be 100 μm in diameter and may be part of the Haversian Canal system42. After an inflammatory stimulus such as experimental meningitis or stroke, myeloid cells migrate through these channels from bone marrow toward the meninges and brain against the usual direction of blood flow (i.e. away from brain)37, 38. The signal to bone marrow is not known but may come from upregulated cytokines and/or HMGB-1, an alarmin released from neurons during CSD29, stroke43. A second potential mechanism noted above involves signaling from proinflammatory neuropeptides released from sparsely distributed trigeminovascular axons that pass through the calvarium from the meninges24. Furthermore, there are recent examples of brain-bone marrow cross-talk that implicate connecting microvessels and possibly the glymphatic network in this process41, 44, 45. For example, in a model of acute lymphoblastic leukemia, bridging vessels provided the principal route for migrating lymphoblasts invading the subarachnoid space38.
Our data raise questions as to the role of the bone and bone marrow in migraine with aura. We speculate that a sustained signal in the calvarium may serve as a local repository of inflammation and as a potential candidate to trigger subsequent attacks or possibly to promote migraine chronification. One recent case report of chronic migraine-like headache in a patient with petrous apicitis and its response to treatment46 suggests that these relationships are worthy of additional studies.
Shortcomings
One limitation of our study is that the three groups were not perfectly matched in terms of age and sex. To ensure that our results were not driven by demographic differences between groups, we computed statistics including only females, as well as splitting the groups between younger (18-30 years old) and older participants. This did not change our results.
Some of our migraine subjects described disturbances in language, sensation, and speech during attacks (Table 1), but these were too few to make any meaningful analysis. Sex differences were not explored. Furthermore, we did not measure blood or CSF for inflammatory markers. Some success in this direction was reported by sampling internal jugular blood 26. In view of our data, these and other measurements bear expanding upon. For many reasons, migraine is not an easy disorder to study. These shortcomings notwithstanding, we anticipate that future studies will help to determine whether parameningeal inflammation is a distinguishing feature between different migraine subtypes and whether other extra-axial skull regions exhibit the responses we observed overlying visual cortex.
TSPO PET tracers have well-recognized limitations despite reports showing enhanced binding in brain parenchyma in brain diseases - including degenerative brain diseases (e.g. Alzheimer’s Disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis, multiple sclerosis), stroke and migraine – with likely inflammatory components (for review, see 47). It was recently used to image peripheral tissues such as immune cell infiltration in the bone after fracture48, inflamed pannus tissue in osteoarthritis, the activation of macrophage and stroma in the synovial tissue of patients with rheumatoid arthritis49, as well as in the neuroforamen of patients suffering from sciatica50. Our findings in migraine demonstrate that inflammation is consistently present in a pain-sensitive tissue, thereby reinforcing results from preclinical studies5, 51. From our results however, we cannot conclude whether the elevated TSPO signals observed in MWA patients reflects increased protein expression, increased cellular density, or both. On the other hand, the problem of non-specificity of the ligand (i.e., distinguishing among activated cell types) may have been advantageous here by boosting an inflammatory signal coming from multiple cell types.
Regarding the validity of the tracer, we were encouraged by a recent detailed report8 finding a strong correlation between [3H]PBR28 radioligand binding in vitro (the tritiated version of the tracer we used) and the number of TSPO-positive cells across all CNS tissues examined. These include microglia and macrophages and also activated astrocytes (and in smaller measure endothelial cells) in vitro. Another limitation stems from the fact that in the present study we did not perform kinetic modeling radiometabolite-corrected arterial input function, which is considered by some to be the gold standard for quantification of TSPO binding. However, work from our group and others7, 52, 53 suggest that semiquantitative ration metrics may be similarly sensitive to detect pathology-related group differences in 11C-PBR28 signal as classic kinetic modeling techniques. Nevertheless, future studies will need to validate the use of these metrics in migraine datasets.
In summary, this human study provides the first evidence for a robust and persistent inflammatory signal within parameningeal tissues (meninges and bone marrow) overlying cortical areas generating migraine visual aura. These results in humans are compatible with conclusions from numerous animal model studies linking inflammation to CSD and may account for symptoms related to meningeal irritation. Finally, these findings raise important questions about the impact and consequences of an upregulated inflammatory signal in calvarium bone. They suggest that newly explored bridging vessels provide the conduits for possible signal migration from brain with implications for migraine pathophysiology as well as for the pathophysiology of other neuroinflammatory disorders (e.g., head trauma, stroke, Alzheimer’s disease, multiple sclerosis).
Acknowledgements:
This work was supported by 5R21NS082926-02 (NH); 1PO1AT009965 (NH, VN, MLL); 01NS108419 (MAM, MN), NIH R01NS07832201 A1 (CM), 1R01NS095937-01A1 (MLL), 1R01NS095937-01A1 (MLL).
Footnotes
Potential Conflicts of Interests
NH, DSA, CM, EI, NW, CG, NRZ, OA, JMH, GB, JP, VN, MN, MLL and MAM have no conflicts to report.
REFERENCES
- 1.Collaborators GBDH. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018. November;17(11):954–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadjikhani N, Sanchez del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A. 2001;98(8):4687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annual Review of physiology. 2013;75:365–91. [DOI] [PubMed] [Google Scholar]
- 4.Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13(3):1167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002. February;8(2):136–42. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. Journal of Neuroscience. 2010. June 30;30(26):8807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht DS, Granziera C, Hooker JM, Loggia ML. In Vivo Imaging of Human Neuroinflammation. ACS Chem Neurosci. 2016. April 20;7(4):470–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nutma E, Stephenson JA, Gorter RP, et al. A quantitative neuropathological assessment of translocator protein expression in multiple sclerosis. Brain. 2019. November 1;142(11):3440–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagnin A, Kassiou M, Meikle SR, Banati RB. Positron emission tomography imaging of neuroinflammation. Neurotherapeutics. 2007. July;4(3):443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banati RB. Neuropathological imaging: in vivo detection of glial activation as a measure of disease and adaptive change in the brain. Br Med Bull. 2003;65:121–31. [DOI] [PubMed] [Google Scholar]
- 11.Cosenza-Nashat M, Zhao ML, Suh HS, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009. June;35(3):306–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rupprecht R, Papadopoulos V, Rammes G, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature reviews Drug discovery. 2010. December;9(12):971–88. [DOI] [PubMed] [Google Scholar]
- 13.Lacor P, Benavides J, Ferzaz B. Enhanced expression of the peripheral benzodiazepine receptor (PBR) and its endogenous ligand octadecaneuropeptide (ODN) in the regenerating adult rat sciatic nerve. Neuroscience letters. 1996. December 6;220(1):61–5. [DOI] [PubMed] [Google Scholar]
- 14.Albrecht DS, Mainero C, Ichijo E, et al. Imaging of neuroinflammation in migraine with aura: A [(11)C]PBR28 PET/MRI study. Neurology. 2019. April 23;92(17):e2038–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Y, Takashima T, Takashima-Hirano M, et al. 11C-PK11195 PET for the In Vivo Evaluation of Neuroinflammation in the Rat Brain After Cortical Spreading Depression. J Nucl Med. 2009 November 1, 2009;50(11):1904–11. [DOI] [PubMed] [Google Scholar]
- 16.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018. January;38(1):1–211. [DOI] [PubMed] [Google Scholar]
- 17.Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012. January;32(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadjikhani N, Vincent M. Visual Aura In: Press OU, editor. The Migraine Brain, Imaging Structure and Function. Oxford; 2012. [Google Scholar]
- 19.Izquierdo-Garcia D, Hansen AE, Forster S, et al. An SPM8-based approach for attenuation correction combining segmentation and nonrigid template formation: application to simultaneous PET/MR brain imaging. J Nucl Med. 2014. November;55(11):1825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loggia ML, Chonde DB, Akeju O, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015. March;138(Pt 3):604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005. May 24;64(10 Suppl 2):S9–15. [DOI] [PubMed] [Google Scholar]
- 22.Ashina M, Møller Hansen J, Do T, Melo-Carrillo A, Burstein R, Moskowitz MA. The Trigeminovascular System and Migraine – 40 years and counting. The Lancet Neurology. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayberg M, Langer RS, Zervas NT, Moskowitz MA. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981. July 10;213(4504):228–30. [DOI] [PubMed] [Google Scholar]
- 24.Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. The Journal of comparative neurology. 2009. July 20;515(3):331–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conti P, D’Ovidio C, Conti C, et al. Progression in migraine: Role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur J Pharmacol. 2019. February 5;844:87–94. [DOI] [PubMed] [Google Scholar]
- 26.Sarchielli P, Floridi A, Mancini ML, et al. NF-kappaB activity and iNOS expression in monocytes from internal jugular blood of migraine without aura patients during attacks. Cephalalgia. 2006. September;26(9):1071–9. [DOI] [PubMed] [Google Scholar]
- 27.Levy D Endogenous mechanisms underlying the activation and sensitization of meningeal nociceptors: the role of immuno-vascular interactions and cortical spreading depression. Curr Pain Headache Rep. 2012. June;16(3):270–7. [DOI] [PubMed] [Google Scholar]
- 28.Reuter U, Chiarugi A, Bolay H, Moskowitz MA. Nuclear factor-kappaB as a molecular target for migraine therapy. Ann Neurol. 2002. April;51(4):507–16. [DOI] [PubMed] [Google Scholar]
- 29.Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science. 2013. March 1;339(6123):1092–5. [DOI] [PubMed] [Google Scholar]
- 30.Knotkova H, Pappagallo M. Imaging intracranial plasma extravasation in a migraine patient: a case report. Pain Med. 2007. May-Jun;8(4):383–7. [DOI] [PubMed] [Google Scholar]
- 31.Ashina M, Hansen JM, BO AD, Olesen J. Human models of migraine - short-term pain for long-term gain. Nat Rev Neurol. 2017. December;13(12):713–24. [DOI] [PubMed] [Google Scholar]
- 32.Reuter U, Bolay H, Jansen-Olesen I, et al. Delayed inflammation in rat meninges: implications for migraine pathophysiology. Brain. 2001. December;124(Pt 12):2490–502. [DOI] [PubMed] [Google Scholar]
- 33.Noseda R, Copenhagen D, Burstein R. Current understanding of photophobia, visual networks and headaches. Cephalalgia. 2019. November;39(13):1623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimura M, Izumiya Y, Higuchi A, et al. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008. January 15;117(2):216–23. [DOI] [PubMed] [Google Scholar]
- 35.Fantuzzi G Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005. May;115(5):911–9; quiz 20. [DOI] [PubMed] [Google Scholar]
- 36.Tuljapurkar SR, McGuire TR, Brusnahan SK, et al. Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J Anat. 2011. November;219(5):574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herisson F, Frodermann V, Courties G, et al. Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat Neurosci. 2018. September;21(9):1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao H, Price TT, Cantelli G, et al. Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature. 2018. August;560(7716):55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plog BA, Nedergaard M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu Rev Pathol. 2018. January 24;13:379–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996. December 12;384(6609):560–4. [DOI] [PubMed] [Google Scholar]
- 41.Cai R, Pan C, Ghasemigharagoz A, et al. Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull-meninges connections. Nat Neurosci. 2019. February;22(2):317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saper CB, Jarowski CI. Leukemic infiltration of the cerebellum in acute myelomonocytic leukemia. Neurology. 1982. January;32(1):77–80. [DOI] [PubMed] [Google Scholar]
- 43.Roth S, Singh V, Tiedt S, et al. Brain-released alarmins and stress response synergize in accelerating atherosclerosis progression after stroke. Sci Transl Med. 2018. March 14;10(432). [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018. November;17(11):1016–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn JH, Cho H, Kim JH, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019. August;572(7767):62–6. [DOI] [PubMed] [Google Scholar]
- 46.Mancini AJ, Glassman RD, Chang YM, Burstein R, Ashina S. Headache in Petrous Apicitis: A Case Report of Chronic Migraine-like Headache Due to Peripheral Pathology. Headache. 2019. September 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alam MM, Lee J, Lee SY. Recent Progress in the Development of TSPO PET Ligands for Neuroinflammation Imaging in Neurological Diseases. Nucl Med Mol Imaging. 2017. December;51(4):283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cropper HC, Johnson EM, Haight ES, et al. Longitudinal translocator protein-18 kDa-positron emission tomography imaging of peripheral and central myeloid cells in a mouse model of complex regional pain syndrome. Pain. 2019. September;160(9):2136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Narayan N, Owen DR, Mandhair H, et al. Translocator Protein as an Imaging Marker of Macrophage and Stromal Activation in Rheumatoid Arthritis Pannus. J Nucl Med. 2018. July;59(7):1125–32. [DOI] [PubMed] [Google Scholar]
- 50.Albrecht DS, Ahmed SU, Kettner NW, et al. Neuroinflammation of the spinal cord and nerve roots in chronic radicular pain patients. Pain. 2018. May;159(5):968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schain AJ, Melo-Carrillo A, Borsook D, Grutzendler J, Strassman AM, Burstein R. Activation of pial and dural macrophages and dendritic cells by cortical spreading depression. Ann Neurol. 2018. March;83(3):508–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herranz E, Gianni C, Louapre C, et al. Neuroinflammatory component of gray matter pathology in multiple sclerosis. Ann Neurol. 2016. November;80(5):776–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lyoo CH, Ikawa M, Liow JS, et al. Cerebellum Can Serve As a Pseudo-Reference Region in Alzheimer Disease to Detect Neuroinflammation Measured with PET Radioligand Binding to Translocator Protein. J Nucl Med. 2015. May;56(5):701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.


