Abstract
Background
Several European countries recently developed international diagnostic and management guidelines for pemphigus, which have been instrumental in the standardization of pemphigus management.
Objective
We now present results from a subsequent Delphi consensus to broaden the generalizability of the recommendations.
Methods
A preliminary survey, based on the European Dermatology Forum and the European Academy of Dermatology and Venereology guidelines, was sent to a panel of international experts to determine the level of consensus. The results were discussed at the International Bullous Diseases Consensus Group in March 2016 during the annual American Academy of Dermatology conference. Following the meeting, a second survey was sent to more experts to achieve greater international consensus.
Results
The 39 experts participated in the first round of the Delphi survey, and 54 experts from 21 countries completed the second round. The number of statements in the survey was reduced from 175 topics in Delphi I to 24 topics in Delphi II on the basis of Delphi results and meeting discussion.
Limitations
Each recommendation represents the majority opinion and therefore may not reflect all possible treatment options available.
Conclusions
We present here the recommendations resulting from this Delphi process. This international consensus includes intravenous CD20 inhibitors as a first-line therapy option for moderate-to-severe pemphigus.
Keywords: CD20 inhibitor, consensus, guidelines, pemphigus foliaceus, pemphigus vulgaris, treatment
Pemphigus encompasses a spectrum of rare mucocutaneous bullous diseases that are autoimmune in origin. Because of the rarity of these diseases, it can take patients months before their pemphigus is diagnosed, during which time many are treated for other blistering diseases.1,2 Even once the diagnosis has been made, treatment regimens can vary greatly, as there is no defined standard of care owing to the paucity of large-scale clinical trials evaluating their efficacy.1
There have been recent national attempts to standardize the diagnosis and management of pemphigus from individual countries, including in the United Kingdom, France, Japan, and Germany.3–6 However, it was the European Dermatology Forum and the European Academy of Dermatology and Venereology that passed the first international guidelines for the management of pemphigus.7 Although these efforts have been instrumental in the standardization of pemphigus management, the lack of involvement from countries outside of Europe may render these guidelines nongeneralizable to other countries.
In an attempt to garner greater international consensus, the International Bullous Diseases Consensus Group, convened by Dr Dedee Murrell and Dr Victoria Werth, met in March 2016 at the annual American Academy of Dermatology conference in Washington, DC, with the goal of developing international consensus guidelines for the diagnosis and management of pemphigus vulgaris and pemphigus foliaceus. Before the meeting, members of the group, which comprised experts in blistering diseases, completed a Delphi survey based on the European Dermatology Forum and European Academy of Dermatology and Venereology guidelines. Some of the tests and treatments mentioned may not be available or officially registered in all countries and have been assessed on the basis of their scientific usefulness rather than regulation status. The Delphi technique is a consensus-building process in which questionnaires are given to a group of experts in a series of rounds to ultimately achieve opinion convergence.8 The results of the questionnaire were discussed in the meeting and a follow-up survey was sent out to further consensus.
METHODS
The first round of surveys was delivered via email in February 2016 and completed by 39 expert participants. The results of the survey were tallied and delivered to the group. A median score of 70 percent or greater per question was used as the consensus threshold for agreement, and a median score of 30 or lower was established as the consensus threshold for disagreement. Statements that achieved median scores between 30 and 70 were determined as having reached no consensus among participants and discussed during the meeting. Afterward, these statements were revised according to the opinion of the participants and sent out and completed by 54 individuals in the subsequent round. The survey was designed and distributed using RedCAP software.
INITIAL CLINICAL PRESENTATION OF PEMPHIGUS
The initial evaluation of suspected pemphigus should seek to determine the signs or symptoms present that would corroborate the diagnosis of pemphigus, as well as to screen for possible comorbidities.
Major objectives
To verify the diagnosis of pemphigus
To evaluate possible risk factors, severity factors, and comorbidities
To specify the type of initial involvement (skin, mucosa) and its extent
To evaluate the prognosis depending on the age of the patient and general condition (Karnofsky score is optional)
There are 2 clinical scores, the Pemphigus Disease and Area Index (PDAI) and/or Autoimmune Bullous Skin Intensity and Severity Score (ABSIS), which are currently being used as clinical outcome parameters and in clinical trials for the evaluation of the extent and activity of pemphigus Presently, there are no agreed-on cutoff values to define mild, moderate, or severe disease for either the PDAI or the ABSIS; however, there have been 2 studies that have attempted to define these values. In 1 multicenter study based in Japan, researchers evaluated both patients with newly diagnosed and patients with relapsing pemphigus and determined PDAI cutoff values of 0 to 8 for mild, 9 to 24 for moderate, and 25 or higher for severe disease.9 Another multicenter study, conducted internationally, assessed only patients with newly diagnosed pemphigus and determined cutoff values of 15 and 45 for PDAI and 17 and 53 for ABSIS to distinguish between mild, moderate, and severe (significant and extensive) forms of pemphigus.10 Although these studies greatly add to our understanding of disease activity scoring, it is premature to definitively state cutoff values presently.
Specialists involved
The management of patients with pemphigus is the responsibility of dermatologists with experience in treating bullous diseases. If extensive, the initial management of the disease usually requires hospitalization until clinical control of the bullous eruption is achieved. In limited forms of pemphigus, additional diagnostic examinations and clinical monitoring can be done in either an inpatient or outpatient setting.
The overall disease management is coordinated by the dermatologist with the cooperation of the referring dermatologist/family practitioner, the general physician, and other medical specialists and hospital doctors from the center of reference and/or geographic area (if a reference center exists in the particular country).
Rarely, the disease can occur during childhood, and children should be managed by a multidisciplinary team, jointly by a reference center, a pediatric dermatology department, or a pediatrician.
Other health professionals who may serve as supportive adjuncts are as follows:
The referring dermatologist
The patient’s primary care provider to manage comorbidities and monitor for treatment side effects
Other specialists whose expertise is necessary on the basis of comorbidities and/or mucosal locations of pemphigus, such as internists, cardiologists, stomatologists, ophthalmologists, otorhinolaryn-gologists, gastroenterologists, gynecologists, urologists, proctologists, rheumatologists, oncologists, dieticians, physiotherapists, and psychologists
Home health nurses, where available, in selected cases in which home care is required and applicable (eg, elderly or disabled patients with residual mucosal or skin lesions following hospitalization)
A nurse specialist/practitioner to aid in managing stable patients, making phone calls, or changing wound dressings
Diagnosis
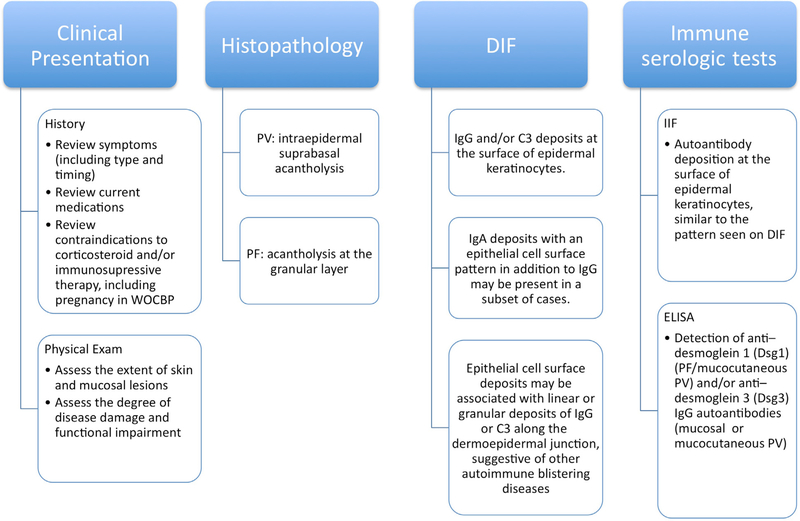
The diagnosis (Fig 1) of pemphigus is based on the following criteria:
Fig 1.
Diagnosis of pemphigus. Diagnosis requires clinical presentation and histopathology that are consistent with pemphigus and either a positive direct immunofluorescence (DIF) microscopy result or serologic detection of autoantibodies against epithelial cell surface antigens. ELISA, Enzyme-linked immunosorbent assay; PF, pemphigus foliaceus; PV, pemphigus vulgaris; WOCBP, women of childbearing potential.
Clinical presentation
Histopathology
Direct immunofluorescence (DIF) microscopy of perilesional skin
Serologic detection of serum autoantibodies against epithelial cell surface by indirect immunofluorescence (IIF) microscopy and/or enzyme-linked immunosorbent assay (ELISA)
Diagnosis requires clinical presentation and histopathology that are consistent with pemphigus and either a positive DIF microscopy or serologic detection of autoantibodies against epithelial cell surface antigens
Clinical evaluation
Medical history
Timing of symptoms
Functional symptoms (ie, pain; pruritus; intensity of dysphagia; ocular and ear, nose, and throat symptoms; dysuria; anogenital problems; and weight loss)
Contraindications of systemic corticosteroid treatment and development of complications of immunosuppressive treatments
Contraception and plans for pregnancy in women of childbearing potential
Medication history, with special attention to causes of drug-induced pemphigus, including D-penicillamine, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and cephalosporins
Psychologic tolerance of possible side effects due to treatment, especially corticosteroid treatment
Impact of disease burden on quality of life
Physical examination
Extent of skin and mucosal lesions and degree of disease damage
- Patient’s overall state of health and comorbidities
- General condition (Karnofsky index)
- Weight
- Vital signs, including blood pressure and temperature
- Comorbidities (neoplastic, cardiovascular, musculoskeletal, etc)
The changes made to previous guidelines are summarized in the Supplemental Table I (available at http://www.jaad.org). The laboratory work-up is delineated in Table I.
Table I.
Laboratory work-up
| Histopathology |
| • A biopsy specimen of a recent (<24 h) small vesicle consisting of the peripheral portion of a blister (1/3 of the sample) and perilesional skin (placed in 4% formalin solution) (2/3 of the sample) should be obtained for routine histopathologic analysis: intraepidermal suprabasal acantholysis in PV or acantholysis at the granular layer in PF |
| DIF microscopy |
| • Skin biopsy of perilesional skin (≤1 cm from a recent lesion), put into a cryotube for transportation in saline (delivery <36 h) in a cylinder of liquid nitrogen or Michel’s fixative for DIF microscopy analysis: |
| ■ DIF microscopy: IgG and/or C3 deposits at the surface of epidermal keratinocytes. The smooth and reticular staining pattern is also referred to as chicken wire, honeycomb, or fishnet-like |
| ■ IgA deposits with an epithelial cell surface pattern in addition to IgG may be present in a subset of cases |
| ■ Epithelial cell surface deposits may be associated with linear or granular deposits of IgG or C3 along the dermoepidermal junction, suggestive of other autoimmune blistering diseases (including paraneoplastic pemphigus or pemphigus erythematosus) or the coexistence of pemphigus and pemphigoid |
| Immune serologic tests |
| Indirect immunofluorescence (IIF) microscopy |
| • IIF microscopy test on monkey esophagus or human skin to search for autoantibodies against surface proteins of epidermal keratinocytes, with a pattern similar to that seen on DIF microscopy |
| • In cases of atypical presentation or the suspicion of another autoimmune bullous disorder, additional immunopathologic tests may be performed, such as IIF microscopy on rat bladder and immunoblot/immunoprecipitation |
| • IIF microscopy on human cells with recombinant expression of desmoglein 1, desmoglein 3, or envoplakin (Euroimmun) is an alternative when desmoglein- or envoplakin-specific ELISA cannot be used |
| ELISA |
| • Detection of anti—desmoglein 1 (PF/mucocutaneous PV) and/or anti—desmoglein 3 IgG autoantibodies (mucosal or mucocutaneous PV) by ELISA (Mannose-Binding Lectin, Euroimmun, Lübeck, Germany) |
| • The detection of IgG autoantibodies by ELISA is positive in more than 90% of cases |
| • In general, the ELISA index correlates with the extent and/or activity of disease (see earlier remark) and prognostic value for relapse, helping to guide treatment. Large prospective cohort studies are, however, missing in this context to provide reliable data about predictive value |
| Work-up before corticosteroid or immunosuppressive therapy |
| • Complete blood count |
| • Creatinine, blood electrolyte levels |
| • Transaminases, γ-glutamyltransferase, alkaline phosphatase levels |
| • Total serum protein, albumin level |
| • Fasting serum glucose level |
| • Hepatitis B, hepatitis C, and HIV |
| • Quantiferon gold or PPD level is recommended |
| Recommended, on indication or optional |
| • Serum IgA deficiency should be ruled out prior to IVIG treatment |
| • Analysis of TPMT activity is recommended when azathioprine is considered in countries where genetic polymorphisms for decreased TMPT activity levels are more common |
| • Chest radiograph if Quantiferon gold or PPD level is abnormal |
| • β-HCG test is recommended to exclude pregnancy in women of childbearing potential |
| • Osteodensitometry is recommended prior to corticosteroid treatment |
| • Ocular examination (glaucoma, cataract) is recommended |
DIF, Direct immunofluorescence; ELISA, enzyme-linked immunosorbent assay; β-HCG, human chorionic gonadotropin; IIF, indirect immunofluorescence; IVIG, intravenous immunoglobulin G; PF, pemphigus, foliaceus; PPD, purified protein derivative; PV, pemphigus vulgarus; TPMT, thiopurine methyltransferase.
THERAPEUTIC MANAGEMENT
See Fig 1.
Objectives
To promote healing of blisters and erosions
To improve functional status
To prevent or strictly limit development of new blisters and erosions
To improve the quality of life
To limit common side effects usually associated with long-term immunosuppressive or corticosteroid treatment
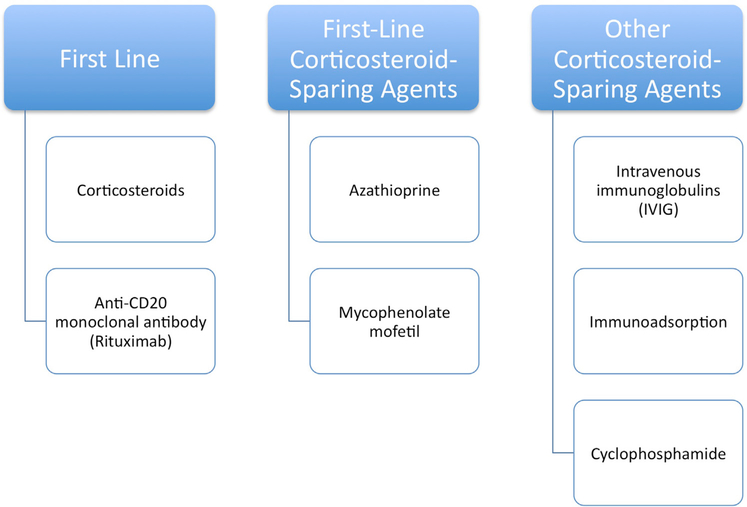
First-line treatment
See Fig 2. The dosing of specific medications is delineated in Table II.11
Fig 2.
Treatment options. The principal objective is to promote the healing of blisters and erosions, prevent development of new lesions, and minimize serious side effects of treatment.
Table II.
Medication dosing
| First-line treatment |
| Corticosteroids |
| • Systemic corticosteroid therapy (predniso(lo)ne at 0.5 mg to 1.5 mg/kg/d) |
| • Systemic corticosteroids (oral or intravenous pulses) can be combined with an immunosuppressive adjuvant at the onset of therapy, especially in cases of increased risk of corticosteroid therapy, complications due to expected prolonged use (>4 months), or dose dependency above minimal therapy (>10 mg/d). However, there is limited evidence that the addition of adjuvants is superior to treatment with corticosteroids alone |
| • Although limited, studies have not shown intravenous corticosteroid pulses to have an additional benefit on top of that of conventional first-line treatment with oral predniso(lo)ne and immunosuppressive adjuvants. Although more evidence is needed, steroid pulse therapy in addition to conventional treatment should be reserved for refractory cases of pemphigus |
| • Treat with the smallest dose for the shortest time possible to minimize risk of adverse events |
| Anti-CD20 monoclonal antibodies |
| Currently there are 2 intravenous CD20 inhibitors available, rituximab and ofatumumab. All the published trials so far have used rituximab |
| • First-line treatment in new-onset moderate-to-severe pemphigus and/or for patients who do not achieve clinical remission with systemic corticosteroids and/or immunosuppressive adjuvants.11 Allows for more rapid tapering of corticosteroid doses and a major corticosteroid-sparing effect |
| • A course of intravenous rituximab consists of 2 × 1000 mg (2 weeks apart) or 4 × 375 mg/m2 (1 week apart) |
| • Treatment can be repeated in cases of clinical relapse or as early as 6 months after treatment. Lower doses are sometimes used for retreatment |
| • Combine with short-term (<4 months) systemic corticosteroids and long-term (>12 months) immunosuppressive treatment, although the need for immunosuppressive adjuvants in rituximab therapy remains unclear |
| • The incidence of unforeseen fatal infections such as progressive multifocal leukoencephalopathy cannot be estimated due to the rarity of pemphigus |
| Corticosteroid-sparing agents |
| First-line corticosteroid-sparing agents |
| • Azathioprine (1–3 mg/kg/d) |
| ■ Start 50 mg/d the first week to detect idiosyncratic reactions such as sudden-onset fevers, oral ulcers, elevated liver function test results and/or drug reaction with eosinophilia and systemic symptoms (and in that case stop immediately), and then raise to desired dose. Although not predictive for idiosyncratic reactions, TPMT activity should be evaluated in countries/ethnicities where there is a higher incidence of polymorphisms before commencing therapy because recommended azathioprine doses vary depending on TPMT activity. In general, adults with pemphigus and high TPMT activity are treated with normal doses of azathioprine (≥2.5 mg/kg/d). Patients with intermediate or low TPMT activity should receive a lower maintenance dose (≥0.5 to 1.5 mg/kg/d) depending on level of enzyme activity. Patients who lack TPMT activity should avoid treatment with azathioprine |
| • Mycophenolate mofetil (30 mg/kg-45 mg/kg/d) or mycophenolic acid (1440 mg/d) |
| Other corticosteroid-sparing agents |
| • IVIG (2g/kg over 2–5 d/mo) |
| ■ Treatment is generally combined with systemic corticosteroids (initially) and immunosuppressive adjuvants |
| ■ Treatment should be performed over several days to avoid side effects |
| ■ Aseptic meningitis is a rare but important side effect of IVIG treatment that needs to be kept in mind in patients who commonly experience episodes of migraine |
| ■ Although uncommon, patients with IgA deficiency should receive IgA-depleted IVIG treatment |
| • Immunoadsorption |
| ■ First-line treatment option in emergency situations where available |
| ■ Second-line corticosteroid-sparing agent where available |
| ■ Contraindications include severe systemic infections, severe cardiovascular diseases, hypersensitivity against components of the immunoadsorption column, treatment with angiotensin-converting enzyme inhibitors and extensive hemorrhagic diathesis |
| • Cyclophosphamide |
| ■ Use in cases of limited resources or in severe cases that have not responded to other treatments |
| ■ Use as a drug of last resort on account of long-term side effects |
IVIG, Intravenous immunoglobulin G; TPMT, thiopurine methyltransferase.
Corticosteroids
Anti-CD20 monoclonal antibodies
Corticosteroid-sparing agents
See Fig 2.
First-line corticosteroid-sparing agents
Azathioprine
Mycophenolate mofetil or mycophenolic acid
Other corticosteroid-sparing agents
Intravenous immunoglobulins
Immunoadsorption
Cyclophosphamide
Supportive treatment that may be recommended
Proper dental care
Intralesional injections of corticosteroids (triamcinolone acetonide) for isolated lesions
Topical treatment with potent corticosteroids (clobetasol propionate) or calcineurin inhibitors applied directly to the lesions, and oral topical corticosteroids (such as triamcinolone acetonide gel) applied directly to oropharyngeal erosions for use in combination with systemic therapy
Antiseptic baths
Covering erosive lesions, if present, using low-adhesive wound dressings or local emollients and compresses
Gels containing local anesthetics for application at the mucosal surfaces
Analgesics (over-the-counter analgesics and opioids)
Nutritional management with the help of a dietician or a nutritionist if malnutrition is related to oral involvement or systemic corticosteroid therapy
Prophylaxis against side effects in prolonged corticosteroid therapy
Osteoporosis baseline screening and prophylaxis
Ophthalmologic evaluation
Vitamin D and calcium supplementation at initiation of corticosteroid treatment
Treatment with bisphosphonates (eg, alendronate, risedronate) in patients at risk of developing osteoporosis (postmenopausal women and men older than 50 years who will be undergoing corticosteroid treatment for more than 3 months)
Systemic antifungal, antiviral, and antibiotic treatment should be used when clinically indicated
H2-blockers or proton pump inhibitor use should be individualized to the patients, given the lack of sufficient evidence
Antithrombotic prophylaxis in cases of high risk of thrombosis
Psychologic support if required
Physiotherapy if prolonged corticosteroid therapy is required
Vaccinations
Adjuvant immunosuppressants and intravenous CD20 inhibitors contraindicate the use of live vaccines
Patients receiving oral corticosteroids or immunosuppressive therapy may be vaccinated against seasonal influenza, H1N1, tetanus, and pneumococci. The level of protection during systemic immunosuppression is questionable
MONITORING
Objectives
To evaluate the efficacy and safety of treatment
To plan the gradual reduction of immunosuppressive treatment and the duration of maintenance therapy or its discontinuation
Definitions for disease outcome parameters
The following definitions have been developed by an international panel of experts.12
Control of disease activity: the time at which new lesions cease to form and established lesions begin to heal
End of consolidation phase : the time at which no new lesions have developed for a minimum of 2 weeks and approximately 80% of lesions have healed. This is when most clinicians start to taper steroids
Complete remission during therapy: the absence of new or established lesions while the patient is receiving minimal therapy
Complete remission off therapy: the absence of new and/or established lesions while the patient has not received any systemic therapy for at least 2 months
Relapse/flare: appearance of 3 or more new lesions in a month that do not heal spontaneously within 1 week, or by the extension of established lesions, in a patient who has achieved disease control
Minimal therapy: prednisolone (or the equivalent) at a dose of 10 mg/d or less and/or minimal adjuvant therapy for at least 2 months
Approach to be maintained after consolidation phase
Expect slow clinical improvement, often requiring a period of 1 to 3 months for complete healing of lesions
Start tapering steroids as soon as disease control is reached or up to the end of the consolidation phase
Decrease predniso(lo)ne by 25% every 2 weeks, until 20 mg/d. Once a dose of 20 mg/d has been reached, decrease predniso(lo)ne by 2.5 mg/wk, and when 10 mg/d has been reached, decrease the dose by 1 mg/d thereafter
Go back to last dose if more than 3 lesions reappear during the tapering of oral corticosteroid therapy
If relapse occurs (ie, the appearance of 3 or more new lesions in a month that do not heal spontaneously within 1 week, or if there is extension of established lesions), increase the oral corticosteroid dose by going back to the second-to-last dose until control of the lesions is achieved within 2 weeks and then resume taper
- If disease control is still not reached despite this, go back to the initial dose
- If oral corticosteroids are given alone, add an immunosuppressant (especially in cases of early-stage relapse occurring despite continued high-dose corticosteroid treatment)
- If oral corticosteroids are already combined with an immunosuppressant, consider a change in immunosuppressant
Scheduling and content of consultations
The frequency of consultations (physical examination, additional examinations) depends on the following:
The patient’s clinical condition, including comorbidities
The severity and disease course specific to the patient’s pemphigus during treatment
The therapeutics used (monitoring, tolerance, side effects)
The level of disease activity measure by the ABSIS and/or PDAI (optional)
Initially, follow-up visits should be offered every 2 weeks until clinical disease control is achieved. In the consolidation phase, patients should be seen every 1 to 2 weeks to determine how soon patients could be started on a steroid taper. Then, during the tapering phase, monthly clinical follow-ups are recommended for the next 3 months. Once the patient is in partial or complete remission while receiving minimal therapy, visits can be less frequent, such as every 3 months.
Clinical evaluation
The clinical follow-up should seek to clarify the following:
Level of disease control
- Presence of adverse effects due to treatment, including
- Diabetes, high blood pressure, cardiac insufficiency, myopathy, osteoporosis, avascular bone necrosis, glaucoma, cataract due to corticosteroids
- Infections, notably, respiratory infections, hepatitis, or hematologic abnormalities (leukopenia) as a result of immunosuppression
- Mental disorders
Serologic monitoring of disease activity
Determination of serum autoantibodies at the initiation of treatment, after 3 months, and every 3 to 6 months on the basis of the evolution or in cases of relapse by the following:
ELISA: anti—desmoglein 1 (Dsgl) and/or desmoglein 3 (Dsg3) IgG
If ELISA is not available: IIF microscopy utilizing monkey esophagus
Overall, serum concentrations of IgG autoantibodies against Dsg1 and Dsg3 correlate with the clinical activity of pemphigus and may thus help in therapeutic decision making
The persistence of high levels of anti-Dsgl by ELISA has a positive predictive value for skin relapses, whereas the persistence of anti- Dsg3 IgG does not necessarily indicate a mucosal relapse
Discontinuation of treatment
Discontinuation of treatment is primarily based on the clinical symptoms but may also be supported by the findings of Dsg ELISA, IIF microscopy, and/or a negative result of DIF microscopy of a skin biopsy specimen
Discontinuation of systemic corticosteroids may be proposed in patients in complete remission while receiving minimal therapy (prednisolone or equivalent at ≤10 mg/d). The adjuvants may be stopped 6 to 12 months after achievement of complete remission during minimal therapy with adjuvants only
Possible sequelae
Pemphigus may cause permanent sequelae not only as a result of the involvement of skin and mucosa but also owing to treatment side effects, justifying a request for recognition or help from departmental disability centers where available. The extent of immunosuppressive therapy increases the risk of side effects
INFORMATION FOR PATIENTS AND THEIR FAMILIES
Education about the disease, its clinical course and prognosis, treatment, relapse signs, and possible side effects of treatment
Awareness of self-support groups, which may help disseminate information regarding the disease, provide comfort, and share the experience of patients regarding daily life. Additionally, it may contribute to a better overall management of the disease by promoting cooperation between patients, patient associations, and health professionals
Information about referral centers
Education about triggers such as drugs, operations, radiation, and physical trauma
Counseling on dietary restrictions is not necessary owing to insufficient evidence
AREAS FOR FUTURE STUDIES
These recommendations are a working document whose purpose is to provide clinicians with the most up-to-date consensus on the diagnosis and management of pemphigus. Further studies are needed to clarify optimal therapeutic regimens and describe their safety and efficacy in the treatment of pemphigus. Some areas identified by the authors include the following:
Intravenous CD20 inhibitors
- Although a recent clinical trial has demonstrated superior efficacy and safety of the intravenous CD20 inhibitor rituximab with short-term lower doses of corticosteroids than the standard dose of systemic corticosteroids initially with slow tapering,9 the following questions remain about how best to use it:
- How should other medications be combined with intravenous CD20 inhibitors?
- Should corticosteroids be used in combination with intravenous CD20 inhibitors from the start to gain disease control and reduce unnecessary iatrogenic morbidity for patients?
- In some patients with comorbidities or mild disease, can CD20 inhibitors be used alone or with a topical corticosteroid?
- What is the role of other immunosuppressives, intravenous immunoglobulins, immunoadsorption, etc, along with CD20 inhibitors?
- Dosing of CD20 inhibitors
- Is there a specific disease activity level at which patients can be treated with only oral steroids and not necessarily with CD20 inhibitors?
- What is the ideal threshold in patients receiving systemic corticosteroids or immune-suppressants to begin CD20 inhibitor therapy?
- What are the optimal dose, frequency, and total number of maintenance infusions to use?
- Are these drugs indicated in patients who test for negative anti-DSG antibodies?
In cases of relapse, is a single dose of 1000 mg/infusion of rituximab (or 375 mg/m2 in the lymphoma protocol) enough to achieve remission instead of a full dose cycle of rituximab (2 × 1000 mg 2 weeks apart or 4 × 375 m2/wk)?
- Long-term side effects
- Will more side effects occur when more patients are treated with multiple maintenance infusions of CD20 inhibitors?
Other treatment options
What role do other treatment options, such as plasmapheresis, play in the treatment of pemphigus?
CONCLUSION
In summary, here we have presented the recommendations arising from a Delphi process involving 39 pemphigus experts. We have made recommendations for evaluation and treatment of pemphigus, including initial evaluation, diagnosis, and management, as well as regarding strategies for maintenance therapy and tapering of medications in remission.
Supplementary Material
CAPSULE SUMMARY.
The European Dermatology Forum and the European Academy of Dermatology and Venereology passed management guidelines for pemphigus.
We present the recommendations of international experts, which have resulted from a Delphi consensus gathering exercise based on the European Dermatology Forum and the European Academy of Dermatology and Venereology guidelines.
This international consensus includes intravenous CD20 inhibitors as a first-line therapy option for moderate-to-severe pemphigus.
Acknowledgments
Funding sources: Supported in part by the Office of Research and Development, Biomedical Laboratory Research and Development, Veterans Health Administration, US Department of Veterans Affairs. Dr Pena’s clinical research is funded by a National Institutes of Health training grant.
Disclosure: Dr Murrell is an investigator and speaker for Roche; she is an investigator for and on an advisory board for Principia Biopharma and is also on an advisory board for Immune Pharmaceuticals and Lilly. Dr Joly is a consultant for Roche, Principia Biopharma, Lillyand Biogen. Dr Payne is a consultant for Syntimmune and TG Therapeutics and receives grants Sanofi. Dr Eming is an investigator for both Biotest AG and Fresenius Medical Care and is a speaker for Biotest AG and Novartis. Dr Jonkman is a monitor for Roche. Dr Prost is an investigator for Roche. Dr Yayli is an investigator for Roche. Dr Zillikens is on the advisory board for Roche and a consultant for Euroimmun, UCB, Fresenius, Almirall, and oGEN-x. Dr Zillikens has received grants and is a speaker for Euroimmun, Miltenyi/Biogen, Fresenius/Roche, Biotest AG, Almirall, and Dompe/Janssen. Dr Amagai receives speaker honoraria and grants from Nihon Pharmaceutical and research support from Medical & Biological Laboratories. Dr Hertl is on an advisory boards for Roche, Biogen, and Novartis; he has received grants from Biotest and Fresenius and is a speaker for Janssen. Dr Schmidt has received grants from Euroimmun and Fresenius and is a speaker for Biotest and Fresenius. Dr Hall is a consultant for Stieffel at GlaxoSmithKline Company, Eli Lilly, Syntimmune, and Immune Sciences and is on the data safety monitoring board for Roche and has received grants from Immune Sciences. Dr Peña, Dr Marinovic, Dr Hashimoto, Dr Diaz, Dr Sinha, Dr Daneshpazhooh, Dr Mimouni, Dr Borradori, Dr Kim, Dr Yamagami, Dr Lehman, Dr Saleh, Dr Culton, Dr Czernik, Dr Zone, Dr Fivenson, Dr Ujiie, Dr Wozniak, Dr Akman-Karakaş, Dr Bernard, Dr Korman, Dr Caux, Dr Drenovska, Dr Vassileva, Dr Feldman, Dr Cardones, Dr Bauer, Dr loannides, Dr Jedlickova, Dr Palisson, Dr Patsatsi, Dr Uzun, Dr Aoki, Dr Grando, Dr Shimizu, Dr Baum, Dr Cianchini, Dr Feliciani, Dr Iranzo, Dr Mascarό Jr, Dr Kowalewski, Dr Groves, Dr Harman, Dr Marinkovich, Dr Maverakis, and Dr Werth have no conflicts of interest to disclose.
Abbreviations used
- ABSIS
Autoimmune Bullous Skin Intensity and Severity Score
- DIF
direct immunofluorescence
- Dsg1
desmoglein 1
- Dsg3
desmoglein 3
- ELISA
enzyme-linked immunosorbent assay
- IIF
indirect immunofluorescence microscopy
- PDAI
Pemphigus Disease and Area Index
REFERENCES
- 1.Mimouni D, Nousari C, Cummins D, Kouba D, David M, Anhalt G. Differences and similarities among expert opinions on the diagnosis and treatment of pemphigus vulgaris. J Am Acad Dermatol. 2003;49(6):1059–1062. [DOI] [PubMed] [Google Scholar]
- 2.Venugopal S, Murrell D. Diagnosis and clinical features of pemphigus vulgaris. Immunol Allergy Clin North Am. 2012; 32(2):233–243. [DOI] [PubMed] [Google Scholar]
- 3.Joly P, Bernard P, Bedane C, Prost C, Ingen-Housz-Oro S. Pemphigus. Guidelines for the diagnosis and treatment. Centres de reference des maladies bulleuses auto-immunes. Societe Francaise de Dermatologie. Ann Dermatol Venereol. 2011;138:252–258. [DOI] [PubMed] [Google Scholar]
- 4.Committee for Guidelines for the Management of Pemphigus Disease, Amagai M, Tanikawa A, Shimizu T, et al. Japanese guidelines for the management of pemphigus. J Dermatol. 2014;41:471–486. [DOI] [PubMed] [Google Scholar]
- 5.Eming R, Sticherling M, Hofmann SC, et al. S2k guidelines for the treatment of pemphigus vulgaris/foliaceus and bullous pemphigoid. J Dtsch Dermatol Ges. 2015;13:833–844. [DOI] [PubMed] [Google Scholar]
- 6.Harman KE, Albert S, Black MM. Guidelines for the management of pemphigus vulgaris. Br J Dermatol. 2003; 149:926–937. [DOI] [PubMed] [Google Scholar]
- 7.Hertl M, Jedlickova H, Karpati S, et al. Pemphigus. S2 guideline for diagnosis and treatment–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2014;29(3): 405–414. [DOI] [PubMed] [Google Scholar]
- 8.Hsu C, Sandford B. The Delphi technique: making sense of consensus. Pract Assess Res Eval. 2007;12(4). Available at: https://scholarworks.umass.edu/pare/vol12/iss1/10. Accessed February 15, 2016. [Google Scholar]
- 9.Shimizu T, Takebayashi T, Sato Y, et al. Grading criteria for disease severity by pemphigus disease area index. J Dermatol. 2014;2014(41):969–973. [DOI] [PubMed] [Google Scholar]
- 10.International Pemphigus Study Group, Boulard C, Duvert Lehembre S, Picard-Dahan C, et al. Calculation of cut-off values based on the autoimmune bullous skin disorder intensity score (ABSIS) and pemphigus disease area index (PDAI) pemphigus scoring systems for defining moderate, significant, and extensive types of pemphigus. Br J Dermatol. 2016;175:142–149. [DOI] [PubMed] [Google Scholar]
- 11.Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line use of rituximab combined with short-term-prednisone versus corticosteroid alone in the treatment of patients with pemphigus: a prospective multicenter randomized trial. Lancet. 2017;389:2031–2040. [DOI] [PubMed] [Google Scholar]
- 12.Murrell DF, Daniel BS, Joly P, et al. Definitions and outcome measures for bullous pemphigoid: recommendations by an international panel of experts. J Am Acad Dermatol. 2012;66(3): 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.